Abstract
Heritability estimates of metabolic syndrome traits vary widely across studies. Some studies have suggested that the contribution of genes may vary with age or sex. We estimated the heritability of 11 metabolic syndrome-related traits and height as a function of age and sex in a large population-based sample of twin families (N = 2,792–27,021, for different traits). A moderate-to-high heritability was found for all traits [from H2 = 0.47 (insulin) to H2 = 0.78 (BMI)]. The broad-sense heritability (H2) showed little variation between age groups in women; it differed somewhat more in men (e.g., for glucose, H2 = 0.61 in young females, H2 = 0.56 in older females, H2 = 0.64 in young males, and H2= 0.27 in older males). While nonadditive genetic effects explained little variation in the younger subjects, nonadditive genetic effects became more important at a greater age. Our findings show that in an unselected sample (age range, ∼18–98 years), the genetic contribution to individual differences in metabolic syndrome traits is moderate to large in both sexes and across age. Although the prevalence of the metabolic syndrome has greatly increased in the past decades due to lifestyle changes, our study indicates that most of the variation in metabolic syndrome traits between individuals is due to genetic differences.
Keywords: genetics, lipids, cholesterol, obesity, BMI, cardiovascular, diabetes, sex difference, family study, twin study
The metabolic syndrome refers to a combination of traits, including central obesity, insulin resistance, dyslipidemia, and hypertension (1), associated with an increased risk of cardiovascular disease (CVD) and type 2 diabetes (T2D). The underlying pathophysiological mechanisms are thought to include excess adipose tissue mass, ectopic fat deposition, excessive flux of fatty acids, and inflammation (2–4). Several clinical guidelines have been proposed for the diagnosis of the metabolic syndrome [i.e., ATP III 2001 (5), WHO 1999 (6), EGIR 1999 (7), IDF 2006 (1)], which most commonly include criteria for waist circumference, fasting plasma glucose, systolic blood pressure (SBP), diastolic blood pressure (DBP), HDL cholesterol, and triglycerides. Other traits that have been included in individual guidelines are body mass index [BMI, WHO 1999 (6)], waist-to-hip-ratio [WHR, WHO 1999 (6)], and fasting insulin level [EGIR 1999 (7)]. In all guidelines, metabolic syndrome is defined by the levels of multiple traits exceeding a certain threshold.
Although it has been questioned whether the combination of metabolic characteristics referred to as metabolic syndrome represents a biologically meaningful entity in itself (8–10), the definition is widely applied as a tool for risk prediction. The metabolic syndrome is associated with a doubled risk of developing CVD and a more than 5-fold increased risk of T2D, with risk estimates varying somewhat depending on the classification criteria used to define the metabolic syndrome (2). The metabolic syndrome is also associated with a higher risk of nonalcoholic fatty liver disease, reproductive disorders, depression, sleeping disorders, and other conditions (2, 11). The prevalence of the metabolic syndrome is globally increasing (12), presenting a major health problem, particularly in Western countries [e.g., the prevalence has been estimated at 14% in the Netherlands (13) and at 37% in the United States (14)]. The rise is generally attributed to changes in lifestyle, while at the same time, individual differences in metabolic traits have been shown to be to an important extent heritable. These observations may be explained by the fact that the expression of risk genotypes depends on the environment or that the exposure to lifestyle factors is genetically influenced. Indeed, several important risk factors for the metabolic syndrome that are considered environmental, such as exercise behavior (15) and dietary patterns (16), are moderately heritable.
The importance of genetic influences (“the heritability”) to the variation in susceptibility to the metabolic syndrome and associated traits has been estimated based on the similarity of family members for these traits, using twin and family data (reviewed by Refs. 17–20). If closer relatives resemble each other more for metabolic syndrome traits than more distant relatives do, this indicates that familial factors, including genetic factors and family-shared environmental influences, are important for these traits. The heritability estimates observed in these studies vary widely, with heritability ranging 24–90% for BMI (18, 21–24), 10–75% for fasting glucose (17, 20, 22–25), 20–55% for fasting insulin (17, 20, 22, 23, 26), 0.03–72% for triglycerides (17, 19, 20, 22–25, 27), 25–98% for LDL-cholesterol (17, 19, 20, 22, 23, 27), 30–80% for HDL cholesterol (17, 19, 20, 22, 24, 25, 27), 30–74% for total cholesterol (17, 19, 20, 22–24, 27), 20–71% for SBP (14, 17, 19–21), and 10–50% for DBP (17, 20, 22–24). Such differences may be associated with study designs or may represent meaningful variation because heritability can depend on context, sex (28), or age (29).
Quantitative differences refer to differences in the overall impact of genetic or environmental influences on the variance of a trait, such as differences in heritability between males and females or between different age groups. A study based on twin pairs from eight European countries found consistent sex differences in the heritability of BMI, but the direction of effect was not the same across countries and age strata (30). In a similar comparison, no sex differences were observed for blood pressure (31). For height, the heritability was slightly lower in females compared with males in several countries (32). In a family study of body composition measures, a higher heritability was found for waist circumference and WHR in women, while no significant difference was observed for BMI (33). A study conducted in Sardinian pedigrees found sex differences in the phenotypic variance for many metabolic traits, but a difference in heritability was only evident for weight and triglycerides (for both, the heritability was higher in females) (22). A review of sex differences in the etiology of metabolic syndrome traits concluded that sex differences in heritability are most often reported for body composition and measures related to glucose homeostasis, while most studies of blood pressure and lipid levels found no sex differences in the heritability (28). With regard to age effects, a large meta-analysis reported a decrease of the heritability of BMI with age (21), and age differences in heritability were also reported for several metabolic syndrome traits in the analysis of Sardinian pedigrees (22). In the latter study, the heritability of BMI, lipids, glucose, and insulin was found to be lower in older individuals (age > 42 years), while the heritability of blood pressure was found to be higher in older people.
Qualitative differences arise if a trait is influenced by different genes or aspects of the environment in different groups; for example, different genes may be responsible for the variation of a phenotype in males versus females or at different ages, while the overall impact of genes (the heritability) is the same. Mixed findings have been reported regarding qualitative sex and age effects on metabolic syndrome traits across studies. The results from two large-scale studies that examined a wide range of phenotypes suggested that qualitative genetic differences between the sexes or across age are not common. No evidence was found for qualitative age or sex effects for any of the traits in the study of Sardinian pedigrees (22). Likewise, in a study of a wide range of phenotypes in metabolic, cardiovascular, physiological, and psychiatric domains conducted in a large sample of Dutch twin pairs, no qualitative sex differences were observed for the large majority of traits (34).
If genetic influences depend on age, heritability estimates based on the comparison of twins (who always have the same age and therefore share age-specific genetic influences) may differ from estimates obtained using nontwin relatives (who may share varying degrees of age-specific genetic influences). The mode of action of the underlying genes (additive versus nonadditive genetic effects) may also play a role. The term nonadditive genetic effect is generally used to refer to the effects of interacting alleles at a locus (dominance) or at different loci (epistasis). Related to this distinction, the term “broad-sense heritability” (H2) refers to the variation of a trait due to total heritable genetic effects, while “narrow-sense heritability” (h2) refers to the proportion of variation due to additive genetic effects. Though some studies have indicated that nonadditive genetic influences contribute to metabolic syndrome traits (18, 22, 27), most heritability studies have not separated additive and nonadditive genetic influences. The consequence of not taking genetic dominance into account when estimating the heritability of a trait while dominance effects are present depends on the study design, since dominance effects are shared to some extent by twins and siblings but not between parents and offspring or between more distant relatives. Family studies have reported lower heritability estimates for metabolic syndrome traits compared with twin studies (21), which could be related to age trends in heritability or to differences in the coverage of nonadditive genetic effects between twin and family studies.
Twin and family studies examine the importance of genetic and environmental influences by comparing the resemblance of individuals who share different degrees of genetic or environmental influences. The classical twin study compares the resemblance of monozygotic (MZ) twins to the resemblance of dizygotic (DZ) twins, while family studies typically include parent-offspring pairs or sibling pairs. Since one chromosome from each chromosome pair of a parent is transmitted to a child, a parent always shares one copy of each gene (allele) with his or her child and thus in total shares 50% of additive genetic effects and no genetic dominance effects. For each chromosome pair, DZ twins and siblings can share 2, 1, or 0 chromosomes with each other that were inherited from the same parent, thus sharing on average 50% of additive genetic effects and 25% of dominance genetic effects. MZ twins share all their genetic material because they are derived from one zygote. Importantly, family members may share both genetic and environmental influences. The classical twin design allows distinguishing between heritable genetic influences and influences of the shared family environment (“common environment”): a larger phenotypic correlation in MZ twins than in DZ twins indicates that a phenotype is influenced by genetic factors, because influences of the family environment are shared equally in both types of twins. Most twin studies of metabolic syndrome traits have found no significant effect of the common environment in adults (18, 19, 27, 30, 31, 35). It should be noted that in classical twin studies, the assessment of common environment, by definition, tends to be limited to the effects of early family environment and does not necessarily capture all environmental influences common to people who share a household because adult twins often live separately. Insights into the role of shared household effects in adult subjects may be obtained by studying, for example, the similarity of spouse pairs.
To summarize, variation in heritability estimates of metabolic syndrome traits across previous studies may be related to differences in study population and design. To obtain a representative estimate of heritability in the population and to examine interactions with age and sex, studies should cover a broad age range and include multiple types of familial relations. Therefore, we examined the etiology of metabolic syndrome traits using an extended twin-family design (36–38), combining data from a large population-based sample of MZ twins, DZ twins, nontwin siblings, and parents registered with the Netherlands Twin Register (NTR) with a broad age range (18–98 years). Measurements included BMI, waist circumference, WHR, LDL, HDL, total cholesterol, triglycerides, fasting glucose, fasting insulin, SBP, DBP, and height. This large dataset with a variety of familial relations allowed us to assess the contribution of additive and nonadditive genetic influences (i.e., estimating narrow-sense and broad-sense heritability) and environmental influences (including shared household effects) to the variation in metabolic syndrome traits and height, and to examine variation in the heritability and expression of genes (qualitative effects) across age groups and sex. This study comprises one of the most extensive family-based datasets on metabolic syndrome traits described thus far and represents an elaborate assessment of quantitative and qualitative variation in genetic and environmental effects on metabolic syndrome traits across age and sex.
MATERIALS AND METHODS
Subjects
The subjects in this study are Dutch twin families registered with the NTR (39). Most twins were recruited through City Councils between 1990 and 1993 when the twins were adolescents or young adults. Since 1993, adult twins have been recruited through a variety of other approaches as well. Every two to five years since 1991, twins and their families are invited to complete a survey (i.e., in 1991, 1993, 1995, 1997, 2000, 2002, 2004, and 2009) (39, 40). In each survey, participants were asked to report their height and current weight. Twin families are also regularly invited to participate in projects in which biological samples, anthropometric traits, and cardiovascular measures are collected. The current analyses are based on data from adult participants (age ≥ 18), including twins (maximum one pair per family), brothers (maximum two per family), sisters (maximum two per family), and parents. For a detailed description of the characteristics of subjects, see supplementary Table I. Informed consent was obtained from participants, and study protocols were approved by the Medical Ethics Committee of the VU University Medical Center. Zygosity determination was based on DNA markers in 84.2–88.7% of twins (range is for different phenotypes), except for BMI (47% of twins) and height (45%), for which a larger proportion of the data came from subjects who had only participated in survey studies. If DNA was not available, zygosity determination was based on validated questionnaire items. Only subjects with complete information on sex and age (or birth year, for the analysis of height) were included in the analyses.
Procedure biobank project
Metabolic biomarkers and anthropometric measures were collected in a large-scale biobank project in which 9,530 individuals participated, including 4,259 twins, 2,704 biological parents, and 2,052 biological siblings. Data from nonbiological parents and siblings, spouses of twins and siblings, children of twins and siblings, and second-degree relatives (e.g., grandparents, uncles, and aunts) were not included in the current analyses (N = 515). Participants were visited in the morning, usually at their home. At the visit, weight, waist circumference, and hip circumference were measured, information about health, medication use, fasting status, and height was collected, and fasting blood and morning urine samples were collected, from which cell lines, biomarkers, DNA and RNA were obtained. For a detailed description of the study procedure, see Willemsen et al. (41).
Lipid profiles and glucose metabolism
Total cholesterol, HDL-cholesterol, and triglyceride levels were measured in heparin plasma using the Vitros 250 total cholesterol assay, the Vitros 250 direct HDL-cholesterol assay and the Vitros 250 triglycerides assay (Johnson and Johnson, Rochester, NY). LDL-cholesterol (LDL) was calculated using the Friedewald equation (42). Glucose and insulin were measured in blood plasma using the Vitros 250 glucose assay (Johnson and Johnson) and the Immulite 1,000 insulin method (Diagnostic Product Corporation, Los Angeles, CA). The following numbers of subjects had missing data due to sampling issues or technical reasons: glucose, N = 253; insulin, N = 315; total cholesterol, N = 160; LDL, N = 180; HDL, N = 161; and triglycerides, N = 159.
Because the distributions of insulin and triglycerides were skewed, an LN-transformation was applied. For the analyses of all lipids, glucose, and insulin, individuals who had not fasted from 12 PM the evening before blood collection were excluded (N = 706, 7.4%). For the analyses of lipids, individuals using lipid-lowering medication were excluded (N = 642, 6.7%). For the analyses of glucose and insulin, individuals were excluded if they used diabetes medication (N = 249, 2.6%) or if they had a fasting glucose level higher than 7 (N = 400, 4.2%). For HDL, one individual with an extreme value was excluded (HDL = 6.56 mmol/l, i.e., >13 SD above the mean). Based on the above inclusion criteria, the following sample sizes were obtained: triglycerides N = 7,469 (3,105 families); total cholesterol: N = 7,468 subjects (3,105 families); HDL: N = 7,466 subjects (3,104 families); LDL: N = 7,453 subjects (3,102 families); glucose: N = 7,563 (3,102 families); and insulin: N = 7,510 subjects (3,088 families).
Anthropometric traits
BMI was calculated from height and weight obtained in laboratory-based NTR projects, or if no lab-based data were available, from data obtained in surveys: BMI = weight (kg) / height2 (m). For subjects who completed multiple surveys at age 18+, height data were checked for consistency over time. If the difference in height reported by an individual across time did not exceed 2 cm, reported values were averaged to obtain one measure of adult body height. If different self-reports differed by 3 cm or more, the most deviating report was removed and the remaining values were averaged if the difference between remaining values was smaller than 3 cm. If the difference in height reported by an individual at different surveys was 3 or 4 cm after removal of two outlier values, remaining values were averaged to obtain one measure of adult height; if the difference still exceeded 4 cm after removal of two outlier values, height data for that subject were considered unreliable and were excluded. Waist and hip circumference were measured in various projects. For weight, waist circumference, and hip circumference, the most recent measure was selected for subjects with multiple data points. Data from twins were selected from the same project or survey where possible. WHR was calculated as: waist (cm) / hip (cm). For WHR, three individuals with a WHR > 1.5 (i.e., > 8 SD above the mean) were excluded. Data from the following number of subjects were analyzed: height: N = 24,904 (9,513 families); BMI: N = 27,021 (9,793 families); waist circumference: N = 8,965 (3,834 families); and WHR: N = 8,962 (3,834 families).
Blood pressure
Blood pressure was measured in a subset of NTR participants as part of several projects that used similar methodology [e.g., Hottenga et al. (43)]. Here, we analyze SBP and DBP measured at rest. For subjects who participated in multiple projects, the first measure was selected. SBP and DBP were corrected for antihypertensive medication use by adding drug-class specific average treatment effects to the measured values (44–47). In total, data from 2,792 subjects (1,334 families) were analyzed.
Statistical analysis
The variance of a trait (phenotypic variance, or VP) can be divided into genetic variance (VG), due to genetic differences between individuals, common environmental variance (VC), due to environmental factors that are shared within families, and unique environmental variance (VE), caused by environmental factors that are not shared within families (48). VE also includes measurement error. Genetic variance can be subdivided into variance due to additive effects of alleles (additive genetic variance, VA) and variance due to nonadditive effects of alleles, which includes interactions among alleles at a single locus (dominance variance, VD) or at different loci (epistasis). Thus, the variance of a trait may be represented as: VP = VG + VC + VE, where VG = VA + VD. The proportion of the phenotypic variance that is due to additive genetic effects is called narrow-sense heritability (h2 = VA / VP), and the proportion of variance due to all genetic effects is called broad-sense heritability (H2= (VA + VD) / VP). Using model-fitting approaches, VA, VD, VC, and VE can be estimated from the covariance or correlation of a trait between different types of relatives who differ in genetic relatedness (see supplementary methods).
In total, 12 traits were studied: 11 metabolic syndrome traits and height. For metabolic syndrome traits, we examined the heritability and variation with age and sex, and for height, we examined the heritability and variation with birth year and sex. Phenotypic correlations among family members were estimated from the observed data after taking sex and age (for metabolic syndrome traits) or birth year (for height) effects into account in Mx (49). Mx was also used to test for sex differences in means and variances, to test for age (or birth year) effects on means, and to test whether the effect of age (or birth year) differed between males and females. To get a first impression of differences in heritability across age and sex (for metabolic syndrome traits) or across birth year and sex (for height), we used SPSS version 17.0 to obtain sex- and age -stratified phenotypic correlations among twins, using the median age or birth year as a cut-off (birth year < 1973 or birth year ≥ 1973 for height, and age < 32 years and age ≥ 32 years for all other traits). Next, three types of analyses were performed in POLY (http://www.sph.umich.edu/csg/chen/public/software/poly) (22, 50). In a first set of analyses, VA, VD, and VE were estimated for each trait in the entire cohort to obtain an overall estimate of heritability. In the second set of analyses, age differences in heritability were examined. VA, VD, and VE were estimated and VA and VE were allowed to differ between age groups, and the correlation between additive genetic effects among family members belonging to different age groups was estimated to test whether different genes are expressed at different ages. Age groups were defined according to the median age of the subjects (age < median and age ≥ median age) for each trait. For height, the heritability was examined as a function of birth year instead of age (birth year < median and birth year ≥ median). In the third series of analyses, we assessed age-specific sex effects; here data from two age groups were analyzed separately. Between age groups, VA, VD, and VE could differ and within age groups, and VA and VE could differ between males and females. Qualitative sex effects were assessed by estimating the genetic correlation among family members of different sex. This third set of analyses provided four estimates of heritability (for younger and older males and females) and an assessment of qualitative sex differences, separately for younger and older subjects. Height was analyzed as a function of sex and birth year instead of age. In all analyses, observed trait values were adjusted for sex and age [using standardized age scores (z-scores)] or birth year (height) by linear regression.
Statistical significance of effects was assessed by comparison of the log likelihood of submodels (e.g., to assess qualitative effects, the log likelihood of a model in which the correlation between additive genetic effects was estimated was compared with the log likelihood of a model in which it was constrained at its theoretical value when there were no qualitative differences). An α level of 0.01 was applied to assess the statistical significance of correlations and to assess the significance of sex differences in means, phenotypic variances, and age trends in trait values.
Finally, the data from spouses were used to examine the importance of shared household effects (environmental effects that contribute to the similarity of people who share a household). For each metabolic syndrome trait, the correlation between the mean age of spouses (as a measure of the duration of their relationship) and the absolute trait difference between spouses (as a measure of their similarity) was computed. A negative correlation suggests that spouses become more similar (the difference between spouses becomes smaller) with increasing duration of their relationship, which is suggestive of shared household effects.
RESULTS
Variation of metabolic syndrome traits with age and sex
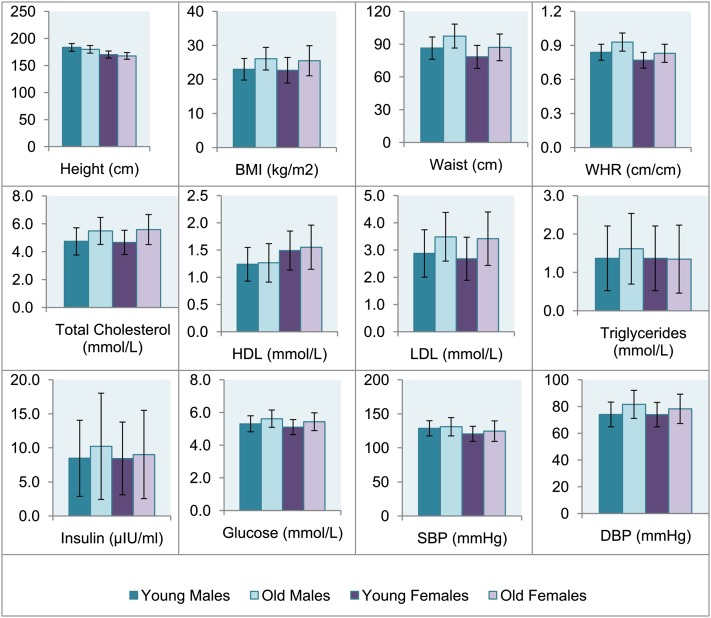
The mean age of subjects varied slightly for different traits; for parents, the mean age ranged from 45.28 (SD = 5.70) to 61.88 (SD = 7.05) years and the age of the offspring (twins and siblings) ranged from 28.79 (SD = 11.93) to 42.12 (SD = 10.58) years for different traits. For a detailed description of the age, sex, and numbers of family members for which data on different traits were available, see supplementary Table I. All metabolic syndrome traits showed a significant increase with age (P < 0.001, Table 1). Significant sex differences in means (P < 0.01, Table 1) were observed for all traits except for insulin (P = 0.315). On average, males had an unfavorable metabolic profile compared with females, with a higher mean BMI, waist circumference, WHR, SBP, and DBP, and higher levels of LDL, triglycerides, and glucose. Females had higher levels of HDL and total cholesterol. Many traits showed a significant sex difference in the effect of age on trait level (P < 0.01, Table 1), with males having on average a steeper increase in WHR and DBP with age compared with females, and females showing a steeper increase in the levels of total cholesterol, LDL, triglycerides, and glucose with age. Sex differences in the total variance (P < 0.01, Table 1) were evident for BMI, waist, and HDL (larger variance in females) and for triglycerides (larger variance in males). No significant sex difference in variance was observed for WHR, total cholesterol, LDL, glucose, insulin, SBP, or DBP. Height showed a significant increase with birth year, and the mean and variance of height and the effect of birth year on height were larger in males compared with females (P < 0.01, Table 1). Fig. 1 shows the means of each trait in males and females, separately for the two age groups in which heritability analyses were conducted.
TABLE 1.
Sex differences in mean values, standard deviation, and the effect of age on metabolic syndrome traits and height
| N | Meana | SDa | β ageb | ||
| Height (cm) | F | 15,095 | 168.85* | 6.54* | 1.40*,c |
| M | 9,809 | 181.31 | 7.37 | 2.12c | |
| BMI (kg/m2) | F | 16,312 | 24.290* | 4.389* | 1.636 |
| M | 10,709 | 24.772 | 3.606 | 1.583 | |
| Waist (cm) | F | 5,730 | 83.400* | 12.350* | 0.532 |
| M | 3,235 | 92.170 | 11.940 | 0.556 | |
| WHR (cm/cm) | F | 5,728 | 0.802* | 0.081 | 0.039* |
| M | 3,234 | 0.891 | 0.084 | 0.044 | |
| Total cholesterol (mmol/l) | F | 4,840 | 5.185* | 1.088 | 0.542* |
| M | 2,628 | 5.142 | 1.045 | 0.376 | |
| HDL (mmol/l) | F | 4,839 | 1.521* | 0.385* | 0.025 |
| M | 2,627 | 1.255 | 0.335 | 0.012 | |
| LDL (mmol/l) | F | 4,833 | 3.097* | 0.972 | 0.445* |
| M | 2,620 | 3.207 | 0.936 | 0.310 | |
| Triglycerides (mmol/l, LN) | F | 4,841 | 0.094* | 0.450* | 0.124* |
| M | 2,628 | 0.269 | 0.514 | 0.088 | |
| Glucose (mmol/l) | F | 4,866 | 5.293* | 0.536 | 0.197* |
| M | 2,697 | 5.483 | 0.533 | 0.145 | |
| Insulin (µIU/ml, LN) | F | 4,835 | 1.975 | 0.703 | 0.056 |
| M | 2,675 | 2.003 | 0.738 | 0.093 | |
| SBP (mmHg)d | F | 1,665 | 122.689* | 13.301 | 2.764 |
| M | 1,127 | 129.844 | 12.347 | 1.496 | |
| DBP (mmHg)d | F | 1,665 | 76.097* | 10.263 | 2.495* |
| M | 1.127 | 77.986 | 10.511 | 4.044 |
*P < 0.01 in estimate between males and females. F, female; M, male; LN, LN-transformed values.
Obtained from a model in Mx without correction for age.
Beta for the regression of metabolic syndrome traits on age. The effect of age was significant for all traits (P < 0.001, for dropping β age in both sexes).
For height only, the β in this column represents the effect of birth year rather than age.
Corrected for medication use.
Fig. 1.
Mean trait values stratified by age and sex. Age categories were defined based on the median age of subjects (young = subjects younger than the median and older = subjects older than the median). The median age was 35 years for SBP and DBP; 39 years for BMI; 40 years for HDL, LDL, total cholesterol, and triglycerides; and 41 for insulin, glucose, waist, and WHR. For height only, birth year (median = 1966) instead of age was used to define categories. Error bars represent standard deviations.
Familial resemblance for metabolic syndrome traits
The familial correlations (Table 2) indicated a substantial role of genetic influences on metabolic syndrome traits, with higher correlations in MZ twins than in DZ twins, nontwin siblings, and parent-offspring pairs. Scatterplots illustrating the similarity of sib-pairs are shown in supplementary Fig. I. When looking at the correlations of MZ twin pairs, male MZ twins were slightly more similar compared with female MZ twins for WHR, total cholesterol, HDL, LDL and triglycerides, and insulin, while female MZ twins showed slightly larger similarity for SBP and glucose.
TABLE 2.
Familial correlations and spousal resemblance for metabolic syndrome traits and height
| Height | BMI | Waist | WHR | TC | HDL | LDL | TG | Insulin | Glucose | SBP | DBP | |
| MZ twins | ||||||||||||
| MZ males | 0.90 | 0.79 | 0.76 | 0.60 | 0.74 | 0.75 | 0.72 | 0.67 | 0.53 | 0.49 | 0.58 | 0.60 |
| MZ females | 0.90 | 0.78 | 0.76 | 0.49 | 0.65 | 0.65 | 0.67 | 0.54 | 0.46 | 0.52 | 0.61 | 0.60 |
| Male twins/sibs | ||||||||||||
| DZ males | 0.50 | 0.46 | 0.45 | 0.48 | 0.30 | 0.57 | 0.27 | 0.38 | 0.39 | 0.34 | 0.25 | 0.21 |
| Brother-brothera | 0.50 | 0.28 | 0.33 | 0.27 | 0.30 | 0.34 | 0.30 | 0.27 | 0.33 | 0.33 | 0.28 | 0.41 |
| Female twins/sibs | ||||||||||||
| DZ females | 0.51 | 0.40 | 0.37 | 0.25 | 0.43 | 0.36 | 0.39 | 0.23 | 0.20 | 0.34 | 0.51 | 0.46 |
| Sister-sistera | 0.47 | 0.33 | 0.34 | 0.09 | 0.31 | 0.29 | 0.37 | 0.19 | 0.22 | 0.33 | 0.24 | 0.42 |
| Opposite-sex twins/sibs | ||||||||||||
| DZ opposite-sex | 0.46 | 0.30 | 0.26 | 0.25 | 0.25 | 0.24 | 0.26 | 0.18 | 0.30 | 0.32 | 0.32 | 0.32 |
| Sister-brothera | 0.46 | 0.23 | 0.20 | 0.10 | 0.20 | 0.25 | 0.23 | 0.19 | 0.12 | 0.24 | 0.33 | 0.32 |
| Parent-offspring | ||||||||||||
| Mother-daughter | 0.50 | 0.27 | 0.22 | 0.21 | 0.29 | 0.16 | 0.30 | 0.17 | 0.19 | 0.21 | −0.08 | 0.01 |
| Mother-son | 0.48 | 0.20 | 0.23 | 0.16 | 0.23 | 0.16 | 0.20 | 0.20 | 0.23 | 0.14 | 0.04 | 0.11 |
| Father-son | 0.49 | 0.21 | 0.20 | 0.17 | 0.28 | 0.26 | 0.25 | 0.18 | 0.22 | 0.22 | 0.26 | 0.19 |
| Father-daughter | 0.46 | 0.24 | 0.24 | 0.14 | 0.32 | 0.29 | 0.31 | 0.19 | 0.16 | 0.27 | 0.04 | 0.27 |
| Spouses | ||||||||||||
| Mother-Father | 0.26 | 0.22 | 0.22 | 0.18 | 0.13 | 0.10 | 0.13 | 0.09 | 0.31 | 0.24 | 0.01 | −0.07 |
| Age-abs. differenceb | NA | −0.04 | −0.05 | 0.01 | 0.01 | 0.01 | −0.03 | −0.05 | 0.08 | 0.02 | −0.08 | −0.03 |
NA, not applicable; TC, total cholesterol; TG, triglyceride.
Correlations among nontwin sibling pairs and between twins and their nontwin sibling.
Correlations between the mean age of spouses and the absolute difference in their trait values.
Correlations between parents and offspring were generally a bit smaller compared with correlations between DZ twins and siblings for all metabolic traits, particularly for SBP and DBP. This pattern is suggestive age-specific expression of effects or the presence of nonadditive genetic effects. Age-stratified twin correlations (supplementary Table II) overall did not suggest large differences in heritability in the younger and older age group for most variables, though some variation between age groups was present. For HDL, total cholesterol, and LDL, dizygotic opposite-sex (DOS) correlations were consistently smaller in the younger group of twins, which is suggestive of qualitative sex effects (e.g., differences in the set of genes influencing a trait in males and females).
Spousal resemblance for metabolic syndrome traits
Significant spouse correlations were observed for most traits. Spouses were most similar for anthropometric traits, glucose, and insulin (correlations ranging from r = 0.18 for WHR to r = 0.31 for insulin, P < 0.01). For lipids, spouse correlations ranged from 0.10 to 0.13. Spouse correlations were not statistically significant for triglycerides (r = 0.09, P = 0.026), SBP (r = 0.01, P = 0.911), or DBP (r = −0.07, P = 0.408). Correlations between the mean age of spouses and the difference in their trait values (Table 2) were not significant for any trait, which suggests that spousal resemblance for metabolic syndrome traits does not increase with increasing duration of the relationship.
Heritability of metabolic syndrome traits
Table 3 shows the heritability estimates based on all data (sexes and age groups combined). Moderate to high heritabilities were evident for all traits, with the highest estimates for height (H2 = 0.90), BMI (H2 = 0.78), and waist circumference (H2 = 0.76). The heritability of WHR was lower compared with other body composition measures (H2 = 0.49). For lipids, broad heritability estimates ranged from 0.59 (triglycerides) to 0.67 (total cholesterol). Heritabilities were estimated at 0.47 and 0.53 for insulin and glucose and at 0.61 and 0.60 for DBP and SBP. While the narrow-sense heritability (h2 = 0.81) and broad-sense heritability (H2 = 0.90) of height indicated that additive genetic effects explain most of the total heritability of height, metabolic syndrome traits generally showed a larger discrepancy between broad- and narrow-sense heritability. For BMI and waist circumference, almost half of the broad-sense heritability was ascribed to nonadditive genetic effects.
TABLE 3.
Heritability estimates of metabolic syndrome traits and height
| Height | BMI | Waist | WHR | TC | HDL | LDL | TG | Insulin | Glucose | SBP | DBP | |
| NSs | 24,904 | 27,021 | 8,965 | 8,962 | 7,468 | 7,466 | 7,453 | 7,469 | 7,510 | 7,563 | 2,792 | 2,792 |
| VP | 42.89 | 14.14 | 119.58 | 0.0051 | 0.93 | 0.13 | 0.77 | 21.25 | 0.50 | 0.25 | 161.21 | 97.48 |
| h22 | 0.81 | 0.41 | 0.39 | 0.31 | 0.51 | 0.40 | 0.51 | 0.33 | 0.31 | 0.38 | 0.37 | 0.53 |
| d22 | 0.09 | 0.37 | 0.37 | 0.18 | 0.16 | 0.27 | 0.18 | 0.25 | 0.16 | 0.15 | 0.24 | 0.08 |
| H22 | 0.90 | 0.78 | 0.76 | 0.49 | 0.67 | 0.67 | 0.69 | 0.59 | 0.47 | 0.53 | 0.60 | 0.61 |
| SE | 0.01 | 0.02 | 0.03 | 0.06 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.03 | 0.08 | 0.09 |
All trait values were corrected for age and sex by linear regression, and overall heritability estimates were obtained based on the entire sample, assuming no differences in heritability between sexes or age groups. d2, proportion of variation due to nonadditive genetic effects (VD / VP); h2, narrow-sense heritability (VA / VP); H2, broad-sense heritability [(VA + VD) / VP]; NSs, total number of subjects included in the analysis; SE, robust standard error of the broad-sense heritability estimate from poly; TC, total cholesterol, TG, triglyceride; Vp, total phenotypic variance after taking out age and sex effects.
For all traits except for insulin, the phenotypic variance was larger in the older subjects (see Materials and Methods for definition) compared with younger subjects (supplementary Table III). The median age was 35 years for SBP and DBP, 39 years for BMI, 40 years for HDL, LDL, total cholesterol and triglycerides, and 41 for insulin, glucose, waist, and WHR. Comparing the heritability estimates in the younger versus older group, no large differences were evident, although broad-sense heritability estimates were a bit lower in the older group for BMI, waist circumference, total cholesterol, LDL, and glucose, while the heritabilities of WHR, HDL, and triglycerides were estimated a bit higher in the older age group. No significant qualitative genetic differences between age groups were found.
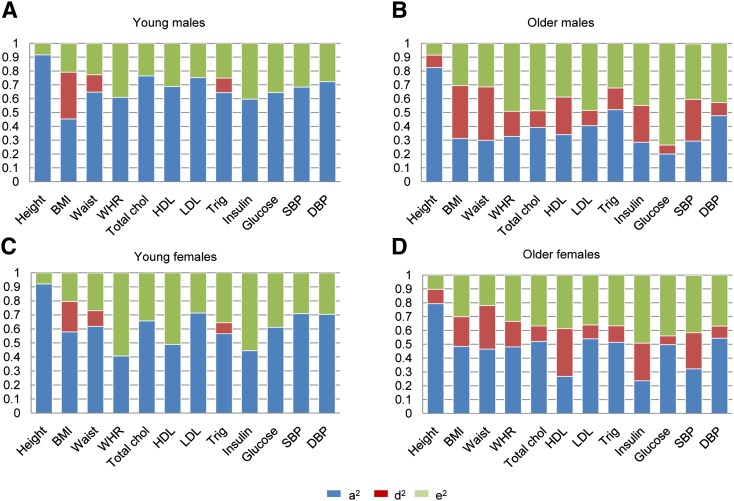
When the heritability was analyzed separately by age and sex (allowing for differences in additive genetic influence (A), dominance genetic influence (D), and unique environmental influence (E) between age groups and differences in A and E between males and females), age-related differences were more pronounced in males compared with females (Fig. 2). This pattern was most evident for LDL (young males: H2 = 0.75, older males: H2 = 0.52), total cholesterol (young males: H2 = 0.77, older males: H2 = 0.51), and glucose (young males: H2 = 0.64, older males: H2 = 0.27) and was primarily due to larger unique environmental variance in the older group. Consequently, these traits showed slightly lower heritability in males compared with females in the older age group, while this pattern was not observed in the younger age group (e.g., for glucose, young females: H2 = 0.61, older females: H2 = 0.56). The results suggested no important role of qualitative sex differences; significant qualitative sex differences were only observed in the younger age group for BMI, total cholesterol, and HDL, and in the older age group, only for insulin. The most consistent finding across traits that emerged when analyzing the two age groups separately was a divergence of broad- and narrow-sense heritability estimates (Fig. 2). Whereas most of the heritable variation across traits in the younger age group was explained by additive genetic effects, nonadditive genetic effects accounted for a considerable portion of the heritability of all traits in the older age group.
Fig. 2.
Heritability estimates stratified by age and sex. a2 = narrow-sense heritability = VA / VP, d2 = VD / VP, e2 = VE / VP. Age categories were defined based on the median age of subjects (young = subjects younger than the median and older = subjects older than the median). The median age was 35 years for SBP and DBP; 39 years for BMI; 40 years for HDL, LDL, total cholesterol, and triglycerides; and 41 for insulin, glucose, waist, and WHR. For height only, birth year (median = 1966) instead of age was used to define categories. A: Young males. B: Older males. C: Young females. D: Older females.
DISCUSSION
We examined the contribution of genetic and environmental influences to the variation in metabolic syndrome traits in a large population-based sample of twin families, representing one of the most extensive family-based datasets on metabolic syndrome traits described thus far. Our twin-family design allowed for representative estimation of narrow-sense and broad-sense heritability in the general population and allowed us to examine whether genetic influences on metabolic syndrome traits interact with age and sex. In summary, moderate to high broad-sense heritability estimates were evident for all traits, ranging from H2 = 0.47 for insulin to H2 = 0.78 for BMI. Averaging the estimates of broad-sense heritability over all metabolic syndrome traits, genetic variation accounted for 62% of the phenotypic variation of metabolic syndrome traits on average. Although these heritability estimates showed some variation across age groups and sex, it can be concluded that overall, heritability estimates were consistently high. These findings emphasize the importance of heritable influences on individual differences in susceptibility to the metabolic syndrome.
The rising prevalence of the metabolic syndrome is most likely related to changes in lifestyle, including an increase in the consumption of high-energy food and a decrease in physical activity. Our findings indicate that even though lifestyle changes are driving an increase in the prevalence of metabolic syndrome, the heritability of the underlying traits continues to be high. This finding may reflect that within wealthy countries, including the Netherlands, environmental conditions contributing to these traits are homogenously distributed, with, for example, high-caloric meals being available to every adult individual. Even in this uniform “obesogenic” environment, not every individual develops an unhealthy metabolic profile, and our study indicates that these individual differences in metabolic syndrome traits are largely explained by genetic differences between individuals.
Analysis of qualitative genetic differences across age and sex suggested that the same set of genes accounts for the variation in metabolic syndrome traits in subjects with an age above the median age in our sample (around 40 years) and subjects with an age below the median age. We also found that the same genes generally accounted for metabolic syndrome traits in men and women, with a few exceptions: For BMI, HDL, and total cholesterol, the data from opposite-sex relatives suggested that the set of genes influencing variation in these traits was not entirely the same for men and women in the younger age group, and for insulin, different genes contributed to the variation in men and women in the older age group. One possible explanation for our finding that the genes influencing BMI in the younger age group differs between males and females could be that this finding is related to pregnancy-related changes in BMI in women, as most women in the reproductive age range were categorized within the younger age group.
While thus far only a few studies have reported that nonadditive genetic effects contribute to the variation in metabolic syndrome traits (18, 22, 27), an important part of the heritability of all metabolic syndrome traits was ascribed to nonadditive genetic effects in our study, particularly in the older age group. To our knowledge, our study is the first that specifically looked at the variation of nonadditive genetic effects across age groups. The overall estimates of narrow-sense and broad-sense heritability from our study are largely similar to the estimates reported previously based on an analysis of extended Sardinian pedigrees that included siblings, parent-offspring pairs, grandparent-grandchild pairs, avuncular relationships, and more distant relatives [e.g., for BMI: H2 = 0.78 and h2 = 0.41 in our study, versus H2 = 0.78 and h2 = 0.36 in the study by Pilia et al. (19)]. In our study, the information about nonadditive genetic effects comes from the difference between the phenotypic correlation in MZ twins compared with DZ twins and siblings, and from the difference between the phenotypic correlation among parents and offspring compared with DZ twins and siblings. The variation ascribed to nonadditive genetic effects may include genetic dominance effects and epistasis, as well as other genetic effects not acting in an additive manner (causing, e.g., lower parent-offspring similarity compared with sib-pair similarity), such as interactions between genetic effects and age or generation. We found no evidence for a difference in the set of genes influencing metabolic syndrome traits in subjects of different ages, suggesting that interactions between genetic effects and age or generation are not a likely explanation for the nonadditive effects observed in this study. Our finding that part of the broad-sense heritability of metabolic syndrome traits is ascribed to nonadditive genetic effects may explain some of the variation between heritability estimates reported by previous studies.
When looking at the data from spouse pairs, we noticed significant spouse correlations for all traits except for triglycerides, and we hypothesized that the similarity between spouses may reflect shared household effects (e.g., similarity in diet, leisure time activities, etc., in subjects who live together). To explore this hypothesis, we tested whether the similarity of spouses increases over time, but we found no evidence for an increase in the similarity of spouse pairs with increasing duration of their relationship. This observation does not rule out, however, that shared household effects do account for the similarity of metabolic syndrome traits in spouse pairs without inducing an increase in the resemblance of spouses over time, which could be interesting to assess in future studies. In addition to shared household effects, spousal similarity for anthropometric traits may, at least to some extent, be related to assortative mating. Assortative mating refers to the phenomenon that individuals tend to choose a partner whose phenotype is similar to their own, and it has been demonstrated to contribute to spousal similarity in height and BMI (51).
To summarize, our findings indicate that in a representative population-based sample including multiple types of family relationships, individual metabolic syndrome traits are moderately to highly heritable. Representative heritability estimates are informative to obtain an estimate of the total genetic variation of traits that can be explained by currently identified loci based, for example, on genome-wide association studies (GWAS). Significant GWAS hits identified to-date together explain between 1% and 2% of the variation in BMI, 10% of the variation in height, and 10% of the variation in HDL cholesterol (52). Accordingly, a large part of the heritability of these traits still remains to be identified.
Supplementary Material
Acknowledgments
The authors like to thank all the twins and family members.
Footnotes
Abbreviations:
- A
- additive genetic influence
- ATP III
- Third Report of the National Cholesterol Education Program's Adult Treatment Panel
- BMI
- body mass index
- C
- common environmental influence
- CVD
- cardiovascular disease
- D
- dominance genetic influence
- d2
- proportion of trait variance due to nonadditive genetic effects
- DBP
- diastolic blood pressure
- DOS
- dizygotic opposite-sex
- DZ
- dizygotic
- E
- unique environmental influence
- EGIR
- European Group for the Study of Insulin Resistance
- G
- genetic influence
- h2
- narrow-sense heritability
- H2
- broad-sense heritability
- IDF
- International Diabetes Federation
- MZ
- monozygotic
- NTR
- Netherlands Twin Register
- SBP
- systolic blood pressure
- T2D
- type 2 diabetes
- VA
- additive genetic variance
- VC
- common environmental variance
- VD
- dominance genetic variance
- VE
- unique environmental variance
- VG
- genetic variance
- VP
- phenotypic variance
- WHO
- World Health Organization
- WHR
- waist-to-hip ratio
This work was supported by Netherlands Organization for Scientific Research Grants 56-464-14192, 400-03-330, 480-04-004, 400-07-080, and 911-09-032; Netherlands Organization for Health Research and Development Grant ZonMW 31160008; EMGO+ Institute for Health and Care Research, Neuroscience Campus Amsterdam, Center for Medical Systems Biology (CMSB), BBRMI-NL Grant 184.021.007 Biobanking and Biomolecular Resources Research Infrastructure; National Institutes of Health Grants 5R37-DA-018673-03 and 4R37-DA-018673-06; FP7 ENGAGE Grant FP7-HEALTH-F4-2007-201413; and European Research Council Grants 230374-GMI and 284167).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of methods, one figure, and three tables.
REFERENCES
- 1.Alberti K. G., Zimmet P., Shaw J. 2006. Metabolic syndrome: a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet. Med. 23: 469–480 [DOI] [PubMed] [Google Scholar]
- 2.Cornier M. A., Dabelea D., Hernandez T. L., Lindstrom R. C., Steig A. J., Stob N. R., Van Pelt R. E., Wang H., Eckel R. H. 2008. The metabolic syndrome. Endocr. Rev. 29: 777–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckel R. H., Grundy S. M., Zimmet P. Z. 2005. The metabolic syndrome. Lancet. 365: 1415–1428 [DOI] [PubMed] [Google Scholar]
- 4.Grundy S. M. 2011. The metabolic syndrome. In Atlas of Atherosclerosis and Metabolic Syndrome. S. M. Grundy, editor. Springer, New York. 1–26. [Google Scholar]
- 5.National Institutes of Health 2011. Third Report of the National Cholesterol Education Program Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). NIH publication 1. 3670 [Google Scholar]
- 6.World Health Organization 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. World Health Organization, Geneva. [Google Scholar]
- 7.Balkau B., Charles M. A. 1999. Comment on the provisional report from the WHO consultation. Diabetic Med. 16: 442–443 [DOI] [PubMed] [Google Scholar]
- 8.Després J. P., Lemieux I. 2006. Abdominal obesity and metabolic syndrome. Nature. 444: 881–887 [DOI] [PubMed] [Google Scholar]
- 9.Ding E. L., Smit L. A., Hu F. B. 2010. The metabolic syndrome as a cluster of risk factors: is the whole greater than the sum of its parts?: comment on “The metabolic syndrome, its component risk factors, and progression of coronary atherosclerosis”. Arch. Intern. Med. 170: 484–485 [DOI] [PubMed] [Google Scholar]
- 10.Kahn R., Buse J., Ferrannini E., Stern M. 2005. The metabolic syndrome: time for a critical appraisal. Diabetes Care. 28: 2289–2304 [DOI] [PubMed] [Google Scholar]
- 11.Vogelzangs N., Beekman A. T., Boelhouwer I. G., Bandinelli S., Milaneschi Y., Ferruci L., Penninx B. W. 2011. Metabolic depression: a chronic depressive subtype? Findings from the InCHIANTI study of older persons. J. Clin. Psychiatry. 72: 598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron A. J., Shaw J. E., Zimmet P. Z. 2004. The metabolic syndrome: prevalence in worldwide populations. Endocrinol. Metab. Clin. North Am. 33: 351–375 [DOI] [PubMed] [Google Scholar]
- 13.Bos M. B., De Vries J. H., Wolffenbuttel B. H., Verhagen H., Hillege J. L., Feskens E. J. 2007. The prevalence of the metabolic syndrome in the Netherlands: increased risk of cardiovascular diseases and diabetes mellitus type 2 in one quarter of persons under 60. Ned. Tijdschr. Geneeskd. 151: 2382–2388 [PubMed] [Google Scholar]
- 14.Ford E. S. 2005. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the US. Diabetes Care. 28: 2745–2749 [DOI] [PubMed] [Google Scholar]
- 15.Stubbe J. H., Boomsma D. I., Vink J. M., Cornes B. K., Martin N. G., Skytthe A., Kyvik K. O., Rose R. J., Kujala U. M., Kaprio J. 2006. Genetic influences on exercise participation in 37,051 twin pairs from seven countries. PLoS ONE. 1: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teucher B., Skinner J., Skidmore P. M., Cassidy A., Fairweather-Tait S. J., Hooper L., Roe M. A., Foxall R., Oyston S. L., Cherkas L. F., et al. 2007. Dietary patterns and heritability of food choice in a UK female twin cohort. Twin Res. Hum. Genet. 10: 734–748 [DOI] [PubMed] [Google Scholar]
- 17.Argyropoulos G., Smith S., Bouchard C. 2005. Genetics of the metabolic syndrome. In Insulin Resistance: Insulin Action and Its Disturbances in Disease. S. Kumar and S. O'Rahilly, editors. John Wiley & Sons, Chichester, UK. 401–450. [Google Scholar]
- 18.Maes H. H. M., Neale M. C., Eaves L. J. 1997. Genetic and environmental factors in relative body weight and human adiposity. Behav. Genet. 27: 325–351 [DOI] [PubMed] [Google Scholar]
- 19.Snieder H., van Doornen L. J. P., Boomsma D. I. 1999. Dissecting the genetic architecture of lipids, lipoproteins, and apolipoproteins: lessons from twin studies. Arterioscler. Thromb. Vasc. Biol. 19: 2826–2834 [DOI] [PubMed] [Google Scholar]
- 20.Teran-Garcia M., Bouchard C. 2007. Genetics of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 32: 89–114 [DOI] [PubMed] [Google Scholar]
- 21.Elks C. E., den Hoed M., Zhao J. H., Sharp S. J., Wareham N. J., Loos R. J., Ong K. K. 2012. Variability in the heritability of body mass index: a systematic review and meta-regression. Front. Endocrinol. (Lausanne). 3: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilia G., Chen W. M., Scuteri A., Orru M., Albai G., Dei M., Lai S., Usala G., Lai M., Loi P., et al. 2006. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet. 2: e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elder S. J., Lichtenstein A. H., Pittas A. G., Roberts S. B., Fuss P. J., Greenberg A. S., McCrory M. A., Bouchard T. J., Jr, Saltzman E., Neale M. C. 2009. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J. Lipid Res. 50: 1917–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S., Duan H., Pang Z., Zhang D., Duan H., Hjelmborg J. V., Tan Q., Kruse T. A., Kyvik K. O. Heritability of eleven metabolic phenotypes in Danish and Chinese twins: a cross-population comparison. Obesity (Silver Spring).Epub ahead of print. December 12, 2012; 10.1002/oby.20217. [DOI] [PubMed] [Google Scholar]
- 25.Zarkesh M., Daneshpour M. S., Faam B., Fallah M. S., Hosseinzadeh N., Guity K., Hosseinpanah F., Momenan A. A., Azizi F. 2012. Heritability of the metabolic syndrome and its components in the Tehran Lipid and Glucose Study (TLGS). Genet. Res. (Camb.). 94: 331–337 [DOI] [PubMed] [Google Scholar]
- 26.Poulsen P., Kyvik K. O., Vaag A., Beck-Nielsen H. 1999. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance - a population-based twin study. Diabetologia. 42: 139–145 [DOI] [PubMed] [Google Scholar]
- 27.Rahman I., Bennet A. M., Pedersen N. L., de Faire U., Svensson P., Magnusson P. K. 2009. Genetic dominance influences blood biomarker levels in a sample of 12,000 Swedish elderly twins. Twin Res. Hum. Genet. 12: 286–294 [DOI] [PubMed] [Google Scholar]
- 28.McCarthy J. J. 2007. Gene by sex interaction in the etiology of coronary heart disease and the preceding metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 17: 153–161 [DOI] [PubMed] [Google Scholar]
- 29.Snieder H., van Doornen L. J., Boomsma D. I. 1997. The age dependency of gene expression for plasma lipids, lipoproteins, and apolipoproteins. Am. J. Hum. Genet. 60: 638–650 [PMC free article] [PubMed] [Google Scholar]
- 30.Schousboe K., Willemsen G., Kyvik K. O., Mortensen J., Boomsma D. I., Cornes B. K., Davis C. J., Fagnani C., Hjelmborg J., Kaprio J. 2003. Sex differences in heritability of BMI: a comparative study of results from twin studies in eight countries. Twin Res. 6: 409–421 [DOI] [PubMed] [Google Scholar]
- 31.Evans A., Van Baal G. C., McCarron P., DeLange M., Soerensen T. I., De Geus E. J., Kyvik K., Pedersen N. L., Spector T. D., Andrew T., et al. 2003. The genetics of coronary heart disease: the contribution of twin studies. Twin Res. 6: 432–441 [DOI] [PubMed] [Google Scholar]
- 32.Silventoinen K., Sammalisto S., Perola M., Boomsma D. I., Cornes B. K., Davis C., Dunkel L., De L. M., Harris J. R., Hjelmborg J. V., et al. 2003. Heritability of adult body height: a comparative study of twin cohorts in eight countries. Twin Res. 6: 399–408 [DOI] [PubMed] [Google Scholar]
- 33.Zillikens M. C., Yazdanpanah M., Pardo L. M., Rivadeneira F., Aulchenko Y. S., Oostra B. A., Uitterlinden A. G., Pols H. A., van Duijn C. M. 2008. Sex-specific genetic effects influence variation in body composition. Diabetologia. 51: 2233–2241 [DOI] [PubMed] [Google Scholar]
- 34.Vink J. M., Bartels M., van Beijsterveldt T. C., van Dongen J., van Beek J. H., Distel M. A., de Moor M. H., Smit D. J., Minica C. C., Ligthart L., et al. 2012. Sex differences in genetic architecture of complex phenotypes? PLoS One. 7: e47371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snieder H. 2004. Familial aggregation of blood pressure. In Clinical hypertension and vascular disease: pediatric hypertension. R. J. Portman, J. M. Sorof, and J. R. Ingelfinger, editors. Humana Press, Totowa, NJ. 265–277. [Google Scholar]
- 36.Eaves L., Heath A., Martin N., Maes H., Neale M., Kendler K., Kirk K., Corey L. 1999. Comparing the biological and cultural inheritance of personality and social attitudes in the Virginia 30,000 study of twins and their relatives. Twin Res. 2: 62–80 [DOI] [PubMed] [Google Scholar]
- 37.Maes H. H., Neale M. C., Medland S. E., Keller M. C., Martin N. G., Heath A. C., Eaves L. J. 2009. Flexible Mx specification of various extended twin kinship designs. Twin Res. Hum. Genet. 12: 26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neale M. C., Walters E. E., Eaves L. J., Maes H. H., Kendler K. S. 1994. Multivariate genetic analysis of twin-family data on fears: Mx models. Behav. Genet. 24: 119–139 [DOI] [PubMed] [Google Scholar]
- 39.Boomsma D. I., De Geus E. J., Vink J. M., Stubbe J. H., Distel M. A., Hottenga J. J., Posthuma D., van Beijsterveldt T. C., Hudziak J. J., Bartels M., et al. 2006. Netherlands Twin Register: from twins to twin families. Twin Res. Hum. Genet. 9: 849–857 [DOI] [PubMed] [Google Scholar]
- 40.Willemsen G., Vink J. M., Abdellaoui A., den Braber A., van Beek J. H., Draisma H. H., van Dongen J., van't Ent D., Geels L. M., van Lien R., et al. 2013. The Adult Netherlands Twin Register: twenty-five years of survey and biological data collection. Twin Res. Hum. Genet. 16: 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willemsen G., De Geus E. J., Bartels M., van Beijsterveldt C. E., Brooks A. I., Estourgie-van Burk G. F., Fugman D. A., Hoekstra C., Hottenga J. J., Kluft K., et al. 2010. The Netherlands Twin Register biobank: a resource for genetic epidemiological studies. Twin Res. Hum. Genet. 13: 231–245 [DOI] [PubMed] [Google Scholar]
- 42.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502 [PubMed] [Google Scholar]
- 43.Hottenga J. J., Boomsma D. I., Kupper N., Posthuma D., Snieder H., Willemsen G., De Geus E. J. 2005. Heritability and stability of resting blood pressure. Twin Res. Hum. Genet. 8: 499–508 [DOI] [PubMed] [Google Scholar]
- 44.de Geus E. J., Kupper N., Boomsma D. I., Snieder H. 2007. Bivariate genetic modeling of cardiovascular stress reactivity: does stress uncover genetic variance? Psychosom. Med. 69: 356–364 [DOI] [PubMed] [Google Scholar]
- 45.Kupper N., Willemsen G., Riese H., Posthuma D., Boomsma D. I., de Geus E. J. C. 2005. Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension. 45: 80–85 [DOI] [PubMed] [Google Scholar]
- 46.Mancia G., Parati G. 2004. Office compared with ambulatory blood pressure in assessing response to antihypertensive treatment: a meta-analysis. J. Hypertens. 22: 435–445 [DOI] [PubMed] [Google Scholar]
- 47.Wright J. M., Lee C. H., Chambers G. K. 1999. Systematic review of antihypertensive therapies: does the evidence assist in choosing a first-line drug? CMAJ. 161: 25–32 [PMC free article] [PubMed] [Google Scholar]
- 48.Falconer D. S. 1960. Introduction to Quantitative Genetics. Ronald Press Co., New York. [Google Scholar]
- 49.Neale M. C., Boker S. M., Xie G., Maes H. H. 2006. Mx: Statistical Modeling. Department of Psychiatry, Virginia Commonwealth University, Richmond, VA. [Google Scholar]
- 50.Chen W. M., Abecasis G. R. 2006. Estimating the power of variance component linkage analysis in large pedigrees. Genet. Epidemiol. 30: 471–484 [DOI] [PubMed] [Google Scholar]
- 51.Silventoinen K., Kaprio J., Lahelma E., Viken R. J., Rose R. J. 2003. Assortative mating by body height and BMI: Finnish twins and their spouses. Am. J. Hum. Biol. 15: 620–627 [DOI] [PubMed] [Google Scholar]
- 52.Visscher P. M., Brown M. A., McCarthy M. I., Yang J. 2012. Five years of GWAS discovery. Am. J. Hum. Genet. 90: 7–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.