Abstract
Hydatid cyst (HC) is a human parasitic disease caused by the larval stage of Echinococcus granulosus. Cardiac involvement is rare and occurs in 0.5%–2% of patients with hydatid cyst, but isolated pericardial hydatid cyst is rarer still. We present two cases of isolated pericardial hydatid cyst who presented with precordial chest pain and dyspnea. In both the cases, HC were diagnosed by transthoracic echo (TTE), Computed Tomography/Magnetic Resonance and positive hydatid serology. Intraoperatively transesophageal echo (TEE) revealed unilocular transitional cystic lesion the transverse pericardial sinus in one case and multilobulated active cystic lesion in another. The report highlights the role of TEE in diagnosis and evaluation of cardiac HC. Both the cases underwent surgical resection followed by albendazole therapy to prevent recurrence.
Keywords: Cardiac echinococcosis, Echinococcus pericardial effusion, Isolated pericardial hydatid cyst, Pericardial location, Transesophageal echocardiography
1. Introduction
Hydatid cyst (HC) is a human parasitic disease caused by the larval stage of Echinococcus granulosus. Cardiac involvement is rare and occurs in 0.5%–2% of patients with hydatid cyst.1 The most common locations of cardiac HC are the left ventricular free wall (52%), interventricular septum (42%), the right ventricle (31%), pericardium (10%), and the atrium.1 Isolated pericardial localization without myocardial involvement is also extremely uncommon. TEE determination of location of the cysts involving the heart and great blood vessels is of great help for a successful surgical treatment, making it the imaging method of choice for the diagnosis of cardiac echinococcosis.2
2. Case report
2.1. Case 1
A 56-year-old woman living in rural area, presented with history of progressively increasing dyspnea for last one year. She was diagnosed as having pericardial effusion (PE) nine months back and had received anti tubercular treatment (ATT) for the same duration without any relief in symptoms, presuming the effusion to be tuberculous. Her chest X-ray revealed cardiomegaly. Routine investigations were normal with strongly positive hydatid serology (ELISA). Transthoracic echo window was suboptimal revealing only moderate PE. CT and MRI demonstrated multiple pericardial cysts and ruled out any other organ involvement. The intraoperative TEE examination confirmed the presence of pericardial cysts. Following midline sternotomy, after opening of pericardium and complete surgical evacuation of pericardial effusion and visible cysts, TEE examination revealed another transitional lesion – unilocular anechoic cystic lesion round in shape with a clearly visible cyst wall (laminated layer) and floating laminated membranes (water lily sign) in the transverse pericardial sinus (between aorta and pulmonary artery) Fig. 1. Doppler examination did not reveal any compression of the great arteries.
Fig. 1.
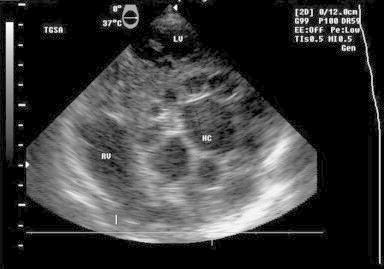
TEE image – mid esophageal right ventricle inflow outflow view: showing cystic lesion in transverse pericardial sinus – transitional cyst.
2.2. Case 2
A 37-year-old man living in rural area, presented with history of non-specific chest pain for three months. His physical examination was normal but the chest radiograph displayed a well-defined smooth bulge over left cardiac border and positive hydatid serology (ELISA). The electrocardiogram (ECG) showed non-specific ST–T changes. Transthoracic echo (TTE) showed solitary cystic lesion with honeycomb appearance abutting the left ventricle wall. CT and MRI demonstrated solitary pericardial cyst and ruled out any other organ involvement. Intraoperative TEE findings showed an active lesion – single, multilobulated cystic lesion with well-defined cyst wall and multiple daughter cysts demonstrating ‘rosette’ appearance. Doppler examination did not reveal any alteration in mitral inflow/left ventricle outflow tract velocities ruling out compression and intracavitary communication of the cyst [Figs. 2 and 3, Videos S1 and S2]. Though there was a confusion in case II [Fig. 2, Video 1] regarding whether the cyst is pericardial or intramyocardial on TEE; however lack of a well defined cardiac tissue rim around the cyst, restricted cyst mobility on real time echocardiography favored the pericardial location of cyst which was consistent with preoperative computed tomography and intraoperative findings and was confirmed by post cyst excision TEE [Fig. 4, Video S2].
Fig. 2.
TEE image – transgastric short axis view: multiloculated cystic lesion indenting anteroseptal, anterior left ventricle wall showing rosette appearance – active cyst.
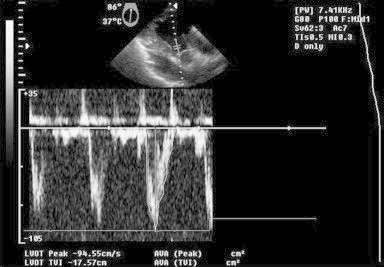
Fig. 3.
TEE image – deep transgastric long axis view: pulse wave Doppler across left ventricle outflow tract – no compression/impedance to outflow.
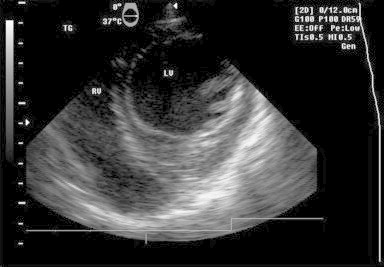
Fig. 4.

TEE image – Transgastric view: anteroseptal wall free of indentation after cyst excision.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jcdr.2012.11.004.
The following are the supplementary video related to this article:
Video 1Transgastric basal short axis loop showing rosette cystic lesion indenting anterior and anteroseptal left ventricle walls. Right and left ventricles are seen well contracting.
Video 2Mid esophageal long axis loop showing color Doppler interrogation of cyst to rule out intracavitary communication. The cyst contents do not reveal any abnormal color pattern.
In both the cases, following thoracotomy and opening of cyst, the cyst cavity was thoroughly washed with hypertonic saline solution and repeat TEE before closure of the thorax ruled out any residual cysts. Histopathological analysis confirmed the diagnosis of hydatid cyst. The patients were discharged with albendazole prophylaxis a week later. The cases were followed up by annual TTE and did not reveal any recurrence at one year follow-up.
3. Discussion
The present cases highlight the impact of perioperative TEE in assessment of cardiac HC and adequacy of cyst removal intraoperatively. Cardiac HC is associated with increased risk of fatal complications such as intracardiac/pericardial rupture which may result in anaphylactic shock, myocardial infarction, conduction abnormalities, cardiac arrest, tamponade, acute/chronic constrictive pericarditis and pulmonary/systemic hydatid embolism.2–6 Rupture of HC into the pericardial cavity may lead to pericarditis with effusion (as in the first case), cardiac tamponade and formation of secondary cysts.7 TTE can detect HC as small as 0.5 cm8
Transesophageal echocardiographic (TEE) determination of number, size, location of the cysts involving the heart and great blood vessels is of great help for a successful surgical treatment and makes it the imaging method of choice for the diagnosis of cardiac echinococcosis.2–4,9–11 As per echocardiographic classification of cardiac echinococcosis (ce) by Tufekcioglu et al12 CE lesions are classified into:
Active: Uni/multilocular cyst, double-layered cyst wall, viable, may feature ‘hydatid sand’. Multilocular HC contain internal septae that produce a characteristic wheel appearance or multiple daughter cysts that create a honeycomb/rosette appearance.
Transitional: Viable cyst. The pericystium is detached from the cyst wall and appears as a floating membrane, producing the characteristic ‘water lily’ sign.
Inactive: Daughter cysts absent, lesion features degenerative structures producing the ‘ball-of-wool’ sign.
4. Tee examination
Pre-resection examination should focus on the number, size, location of the mass, attachment to intracardiac structures which might be important for deciding cannulation site in intracavitary lesions, morphology (uni/multilocular), viability (active/transitional/inactive), intracavitary communication, compression of surrounding structure and evidence of embolism in pulmonary artery/aorta if intracavitary cyst.3 While examining for intracavitary communication the color Doppler gain should be kept minimal, the Doppler signal in such cases would be a low velocity signal.
Ongoing examination during surgical manipulation of the lesion can detect any embolic events or complications like intramyocardial rupture.
Post resection examination should focus on any residual cyst which may escape surgical attention.
The role of TEE cannot be duplicated by any other investigating modality thus making it superior even to CT/MRI which is at a disadvantage as compared to TEE in terms of availability, portability, and intraoperative assessment in the settings where CE is common. Post surgery transthoracic echocardiographic examination should be done annually to detect recurrence if any. TEE is limited by the fact that it is invasive as compared to CT/MRI, it's difficult to distinguish inactive CE/degenerated cyst from other cardiac tumors on echo and TEE can not rule out extra cardiac HC.
The differential diagnosis of cardiac echinococcosis on echocardiography includes myocardial aneurysms, infected intracardiac thrombus, congenital pericardial cysts, pleuropericardial cysts, peritoneal venous shunt pseudocyst, myocardial abscess, cystic degenerating tumors, and pericardial hematomas.12 The cardiophrenic angle is the typical location for pericardial and pleuropericardial cysts. Albendazole treatment (15 mg/kg/day) for 12 weeks is recommended for prophylaxis.13 Surgical excision is the definitive treatment for CHC even in asymptomatic cases to prevent rupture.
Conflicts of interest
All authors have none to declare.
References
- 1.Dighiero J., Canabal E.J., Aguirre C.V., Hazan J., Horjales J.O. Echinococcus disease of the heart. Circulation. 1958;17:127–132. doi: 10.1161/01.cir.17.1.127. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Gomez F., Duran H., Tamames S., Perotte J.L., Blaner A. Cardiac echinococcosis: clinical picture and complications. Br Heart J. 1973;35:1326–1331. doi: 10.1136/hrt.35.12.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Bello R., Menendez H. Intracardiac rupture of hydatid cysts of the heart: a study based on 3 personal observations and 101 cases in the world literature. Circulation. 1963;27:366–374. doi: 10.1161/01.cir.27.3.366. [DOI] [PubMed] [Google Scholar]
- 4.Kardaras F., Kardara D., Tselikos D. Fifteen year surveillance of echinococcal heart disease from a referral hospital in Greece. Eur Heart J. 1996;17:1265–1270. doi: 10.1093/oxfordjournals.eurheartj.a015045. [DOI] [PubMed] [Google Scholar]
- 5.Ozer N., Aytemir K., Kuru G. Hydatid cyst of the heart as a rare cause of embolization: report of 5 cases and review of published reports. J Am Soc Echocardiogr. 2001;14:299–302. doi: 10.1067/mje.2001.108474. [DOI] [PubMed] [Google Scholar]
- 6.Charet E., Roudaut R., Lafitte S., Laffort P., Madonna F., de Mascarel A. Echocardiographic demonstration of rupture of intraseptal hydatid cyst. J Am Soc Echocardiogr. 2000;13:955–958. doi: 10.1067/mje.2000.106824. [DOI] [PubMed] [Google Scholar]
- 7.De Martini M., Nador F., Binda A., Arpesani A., Odero A., Lotto A. Myocardial hydatid cyst ruptured into the pericardium: cross-sectional echocardiographic study and surgical treatment. Eur Heart J. 1988;9:819–824. doi: 10.1093/eurheartj/9.7.819. [DOI] [PubMed] [Google Scholar]
- 8.Oliver J.M., Sotillo José F., Dominguez F.J. Two-dimensional echocardiographic features of echonococcosis of the heart and great blood vessels: clinical and surgical implications. Circulation. 1988;78:327–337. doi: 10.1161/01.cir.78.2.327. [DOI] [PubMed] [Google Scholar]
- 9.Miralles A., Bracamonte L., Pavie A. Cardiac echinococcosis: surgical treatment and results. J Thorac Cardiovasc Surg. 1994;107:184–190. [PubMed] [Google Scholar]
- 10.Rey M., Alfonso F., Torrecilla E.G. Diagnostic value of two–dimensional echocardiography in cardiac hydatid disease. Eur Heart J. 1991;12:1300–1307. doi: 10.1093/eurheartj/12.12.1300. [DOI] [PubMed] [Google Scholar]
- 11.Atilgan D., Kudat H., Tukek T. Role of transesophageal echocardiography in diagnosis and management of cardiac hydatid cyst: report of three cases and review of the literature. J Am Soc Echocardiogr. 2002;15:271–274. doi: 10.1067/mje.2002.120507. [DOI] [PubMed] [Google Scholar]
- 12.Tufekcioglu O., Birincioglu C.L., Arda K., Fansa I., Sarıtas A., Karahan M. Echocardiography findings in 16 cases of cardiac echinococcosis: proposal for a new classification system. J Am Soc Echocardiogr. 2007;20:895–904. doi: 10.1016/j.echo.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Birincioğlu C.L., Tarcan O., Bardakç H., Sarıtas A., Tasdemir O. Off-pump technique for the treatment of ventricular myocardial echinococcosis. Ann Thorac Surg. 2003;75:1232–1237. doi: 10.1016/s0003-4975(02)04709-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1Transgastric basal short axis loop showing rosette cystic lesion indenting anterior and anteroseptal left ventricle walls. Right and left ventricles are seen well contracting.
Video 2Mid esophageal long axis loop showing color Doppler interrogation of cyst to rule out intracavitary communication. The cyst contents do not reveal any abnormal color pattern.


