Abstract
Background
Heart failure is one of the most leading cause of death worldwide, but the mechanical characteristics of the pulmonary system in these patients have not been studied enough. The aim of this study was to measure mechanical pulmonary changes in patients with congestive heart failure (CHF) by using impulse oscillometry (IOS), which can obtain data by simpler means and independently from respiratory muscle strength.
Materials and methods
We assessed 24 CHF patients and 24 controls by spirometry and IOS using the Jaeger IOS system. IOS measures central and peripheral airway resistances (R20, R5) and central and peripheral reactances (X20, X5) using sound waves with different frequencies, which superimposed on the patients respiratory tidal volume and then records reflects. P value < 0.05 was taken to be significant.
Results
The mean age of patients and controls was 61 ± 10 and 57 ± 7 years, respectively. The mean ejection fraction (EF) was 37 ± 17% for patients and 55 ± 7% for controls. Patients had a lower X5 (−0.20 ± 0.13 vs −0.13 ± 0.07; P < 0.05), forced expiratory volume in 1 second (FEV1; 2.26 ± 0.68 vs 3.09 ± 0.82: P < 0.01 L/min), and forced vital capacity (FVC; 2.55 ± 0.86 vs 3.32 ± 0.87; P < 0.05) compared to the controls. They also had elevated R5: 0.37 ± 0.21 vs 0.27 ± 0.09; P < 0.06). X5 was correlated with spirometric abnormalities (P < 0.05) and was lower in patients than in controls.
Conclusion
X5 was lower and R5 was higher in patients than in controls. CHF patients can be assessed by IOS more comfortable than by spirometry. IOS can reliably measure peripheral airway resistance in this group of patients.
Keywords: Congestive heart failure, Impulse oscillometry, Spirometry
1. Introduction
Heart failure is the pathophysiological state in which the heart, via an abnormality of cardiac function (detectable or not), fails to pump blood at a rate commensurate with the requirements of the metabolizing tissues or is able to do so only with an elevated diastolic filling pressure.1,2
HF is one of the leading cause of death worldwide, with more than 20 million people affected. The overall prevalence of HF in the adult population in developed countries is 2%. HF prevalence follows an exponential pattern, rising with age, and it affects 6–10% of people over the age of 65 years.2
The cardinal symptoms of HF are fatigue and shortness of breath.3,4 The origin of dyspnea in HF is probably multifactorial. The most important mechanism is pulmonary congestion with accumulation of interstitial or intra-alveolar fluid. Other factors that contribute to dyspnea during exercise include reductions in pulmonary compliance, increased airway resistance, respiratory muscle and/or diaphragm fatigue, and anemia.3,4
Impulse oscillometry (IOS) measures airway resistance by sending a sound wave produced by a loudspeaker into the lungs of a spontaneously breathing subject and then collecting the reflection of that wave.5,6 The reflected wave varies in amplitude and phase according to the resistance and reactance of the lungs. By applying repeated sound waves of varying frequencies at different phases of the respiratory cycle, the instrument can measure airway resistance of the respiratory tree at different levels. Resistance of central and peripheral airways is shown by R20, R5. Reactance (X), in this context, consists of the capacity of the respiratory system (its elasticity) and the inertia of the column of air in the respiratory tree. Central and peripheral reactance are shown by X20, X5.4–7
The impedance (Z) of the respiratory system is the total opposition to “alternating flow” and is a combination of the reactance and resistance [Z = √(R² + X²)].3 Although forced expiratory volume in 1 second (FEV1) measures respiratory function, it requires a forced expiratory maneuver and, therefore, depends on a patient's motivation and respiratory muscle strength. IOS requires only normal respiration and assesses airway resistance during both expiration and inspiration. Increased airway resistance by increasing the work of breathing might contribute to exercise intolerance in congestive heart failure (CHF).7,8 The aim of our study was to measure airway resistance and reactance in patients with CHF by using a noninvasive and simple method. We also assessed which variables of IOS had more correlation with spirometry in systolic heart failure patients.
2. Materials and methods
This was a case-control study conducted in Imam Hospital in Ahwaz from October 2011 to February 2012. We performed IOS on 24 patients with CHF and 24 control subjects. CHF was defined as the presence of symptoms of fatigue or shortness of breath on exertion and a left ventricular ejection fraction (EF) on echocardiography of <40% with no other cause of dyspnea. The condition had to have, at least, 6 months duration with no recent exacerbation or change in medication. Patients with a previous diagnosis of chest disease or symptoms and signs suggestive for primary chest disease were excluded. All patients took aspirin, one drug from angiotensin converting enzyme inhibitor (ACE inh) group and one drug from ß blocker group. They also had no evidence of primary lung pathology on a chest radiograph. We also excluded patients with neurologic conditions, which could possibly affect pulmonary function or induce ischemia while exercising. The study included a control group, which was chosen randomly from the local staff. This group included individuals who did not have any pulmonary disease or other general conditions and none of them were on regular medications; they were matched with patient group for age, sex, height, and weight. All the subjects underwent echocardiography, and subjects with EF > 50% were selected. An appropriate informed consent was obtained from all the subjects.
The selected patients and controls were evaluated with spirometry. The patients, after placing the mouth piece in their mouth and closing their nose with a clip, were asked to take a deep inspiration and then perform a deep forced expiration. Two parameters: forced vital capacity (FVC) and FEV1 were recorded by the instrument. After spirometry, each subject underwent IOS test. Both tests, spirometry and IOS, were performed by using the IOS instrument, JAEGER model, made in Germany. After following the above steps, the test and control subjects were asked to breath for 60 s normally through the mouth piece and the following parameters were assessed:
• R at 5 Hz: Resistance of the distal parts of the pulmonary system.
• R at 20 Hz: Resistance of the proximal parts of the pulmonary system.
• X at 5 Hz: Reactance of the distal parts of the pulmonary system.
• X at 20 Hz: Reactance of proximal parts of the pulmonary system.
• Zr5: Impedance amplitude of pulmonary system at 5 Hz.
The resistive component of IOS parameters (R) mainly reflects the frictional loss occurring during airflow in the bronchus, while the non-resistive component of IOS parameters (X) reflects the energy mostly stored by peripheral parts of the respiratory system. Results are reported as means (standard deviations). We used unpaired student t-test for “between-groups” comparisons and paired t-test for “within group” analysis. P value < 0.05 was taken to be significant. We used linear regression analysis to explore the relation between continuous variables and the chi-squared test to compare nominal variables.
3. Results
Anthropometric, clinical, and functional characteristics of the patients and controls are shown in Table 1. There are no significant differences between the patient and control groups in terms of age, weight, and height. All patients in the study were under treatment with drugs including aspirin, β blockers, ACE inhibitors, and diuretics.
Table 1.
Subject characteristics.
| Patients (n = 24) | Controls (n = 24) | P-value | |
|---|---|---|---|
| Age (year) | 61 ± 10 | 57 ± 7 | 0.6 |
| Height (cm) | 1.66 ± 9 | 1.67 ± 10 | 0.9 |
| Weight (kg) | 71 ± 11 | 68 ± 9 | 0.54 |
| Sex | |||
| M | 17 (71%) | 13 (54%) | |
| F | 7 (29%) | 11 (46%) | |
| Smokers | 10 (41%) | 2 (8%) | |
| Hypertension | 7 (29%) | 2 (8%) | |
| Diabetes mellitus | 10 (41%) | 3 (12.5%) | |
| Old MI | 7 (29%) | ||
| Rales | 10 (41%) | ||
| Ischemia in ECG | 24 (100%) | ||
| Cardiomegaly in CxR | 21 (87.5%) | ||
| Elevated JVP | 14 (58.3%) | ||
| Pulmonary congestion | 14 (58.3%) | ||
| NYHA functional class | |||
| I | 0 | ||
| II | 15 (62.5%) | ||
| III | 7 (29.1%) | ||
| IV | 2 (8.4%) | ||
| Heart rhythm in ECG | NL: 22 (91.6%) | ||
| Arrhythmia: 2 (8.4%) | |||
Values are means (%); MI, Myocardial infarction; NYHA, New York Heart Association; EKG: Electrocardiography.
A group of 24 patients [17 (71%) men] and 24 controls [13 (54%) men] were studied. The mean ages of patients and controls were 61 ± 10 and 7 ± 57 years, respectively. The mean EF was 37 ± 17% for patients and 55 ± 7% for controls. The average body weight of the patient group was 71 ± 11 kg and that of the control group was 68 ± 9 kg, differences were not statistically significant (P > 0.05). The co-morbidities included hypertension (29% in patients vs 8% in controls), hyperlipidemia (87.5% in patients vs 21% in controls), and smoking (41% in patients vs 8% in controls). The most common abnormal finding in the physical examination of the patients was elevated jugular vein pressure (JVP) (58.3%) and the most common pathological finding in their chest X-ray imaging was pulmonary congestion (58.3%). Only 2 patients had AF rhythm. Also, the patients were evaluated according to the New York Heart Association (NYHA) functional class (FC); 15 (62.5%) patients were classified as FCII, seven (29.1%) patients as FCIII, and two (8.4%) patients as FCIV.
All the analyzed spirometric parameters were significantly lower in the patients with CHF (FEV1 2.26 ± 0.68% vs 3.09 ± 0.82%, P = 0.01; FVC 2.55 ± 0.86 vs 3.32 ± 0.87, P = 0.04). It was also found a trend for IOS parameters of total resistance (higher) and reactance (lower) to be different in patients than in controls (R5 0.37 ± 0.21 vs 0.27 ± 0.09, P = 0.06; X5 −0.20 ± 0.13 vs −0.13 ± 0.07, P = 0.05; Zr5 0.43 ± 0.22 vs 0.31 ± 0.09, P = 0.06). There was no significant difference between patients and controls for R20 (0.26 ± 0.1 vs 0.24 ± 0.08, P = 0.39) and X20 (−0.04 ± 0.02 vs −0.04 ± 0.02, P = 0.57) [Table 2].
Table 2.
Spirometry and impulse oscillometry findings in patient and control groups.
| Patients (n = 24) |
Controls (n = 24) |
P-value | |
|---|---|---|---|
| FEV1 (L/min) | 2.26 ± 0.68 | 3.09 ± 0.82 | 0.01 |
| FVC (L) | 2.55 ± 0.86 | 3.32 ± 0.87 | 0.04 |
| R5 Hz (kPa/(L/s)) | 0.37 ± 0.21 | 0.27 ± 0.09 | 0.06 |
| R2O Hz (kPa/(L/s)) | 0.26 ± 0.10 | 0.24 ± 0.08 | 0.39 |
| X5 Hz (kPa/(L/s)) | −0.20 ± 0.13 | −0.13 ± 0.08 | 0.05 |
| X2O Hz (kPa/(L/s)) | −0.04 ± 0.02 | −0.04 ± 0.02 | 0.28 |
| Z (kPa/(L/s)) | 0.43 ± 0.22 | 0.31 ± 0.09 | 0.03 |
Values are means ± SD; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; R5 Hz, resistance at 5 Hz; R2O Hz, resistance at 20 Hz; X5 Hz, reactance at 5 Hz; X2O Hz, reactance at 20 Hz; Z, impedance of the respiratory system at 5 Hz.
For all spirometric and IOS parameters, except for R5, the presence of previous smoking history was associated with no significant differences between smokers and non-smokers (FEV1 2.43 ± 0.64 vs 0.81 ± 0.83, P = 0.3; FVC 2.83 ± 0.93 vs 3.04 ± 0.87, P = 0.6; R20 0.26 ± 0.11 vs 0.23 ± 0.08, P = 0.6; X5 −0.16 ± 0.06 vs −0.14 ± 0.08, P = 0.5; X20 −0.05 ± 0.035 vs −0.03 ± 0.01, P = 0.4; Z 0.47 ± 0.14 vs 0.30 ± 0.08, P = 0.06), but R5 was significantly greater in smokers (0.43 ± 0.14 vs 0.25 ± 0.09, P = 0.03) [Table 3].
Table 3.
Spirometry and impulse oscillometry findings in two groups of smokers and non-smokers.
| Smokers n = 9 (18.75%) |
Non-smokers n = 39 (81.25%) |
P-value | |
|---|---|---|---|
| Age (year) | 68 ± 13 | 59 ± 10 | 0.4 |
| Weight (kg) | 72.4 ± 8 | 71.1 ± 13 | 0.8 |
| Height (cm) | 1.69 ± 13 | 1.66 ± 9 | 0.7 |
| FEV1 (L/min) | 2.43 ± 0.64 | 2.81 ± 0.83 | 0.3 |
| FVC (L) | 2.83 ± 0.93 | 3.04 ± 0.87 | 0.6 |
| R5 Hz (kPa/(L/s)) | 0.43 ± 0.14 | 0.25 ± 0.09 | 0.03 |
| R2O Hz (kPa/(L/s)) | 0.26 ± 0.11 | 0.23 ± 0.08 | 0.6 |
| X5 Hz (kPa/(L/s)) | −0.16 ± 0.06 | −0.14 ± 0.08 | 0.5 |
| X2O Hz (kPa/(L/s)) | −0.05 ± 0.03 | −0.03 ± 0.01 | 0.4 |
| Z (kPa/(L/s)) | 0.47 ± 0.147 | 30 ± 0.08 | 0.06 |
Values are means ± SD; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; R5 Hz, resistance at 5 Hz; R2O Hz, resistance at 20 Hz; X5 Hz, reactance at 5 Hz; X2O Hz, reactance at 20 Hz; Z, impedance of the respiratory system at 5 Hz.
In our study, we found no relationship between IOS parameters and spirometric parameters except for the FEV1 and X5 values with correlation 0.60 and P = 0.004 and FEV1 and Z values with correlation 0.43 and P = 0.04 [Table 4].
Table 4.
Correlation between spirometry and IOS in patients and controls.
| FEV1 (L/min) | FVC (L) | |
|---|---|---|
| R5 Hz (kPa/(L/s)) | −0.32 | 0.29 |
| R2O Hz (kPa/(L/s)) | −0.17 | 0.23 |
| X5 Hz (kPa/(L/s)) | 0.60∗ | 0.64† |
| X2O Hz (kPa/(L/s)) | 0.33 | 0.34 |
| Z (kPa/(L/s)) | 0.43* | 0.43 |
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; R5 Hz, resistance at 5 Hz; R2O Hz, resistance at 20 Hz; X5 Hz, reactance at 5 Hz; X2O Hz, reactance at 20 Hz; Z, impedance of the respiratory system at 5 Hz. ∗P < 0.05; †P < 0.001.
4. Discussion
In patients with systolic heart failure, even before the presentation of respiratory complaints, mixed ventilatory abnormalities are common, which make early diagnosis and continuous follow-up necessary.1–3 Shortness of breath might be a consequence of the increased work of breathing, which is resulting from increased airway's resistance and reduced pulmonary compliance. Abnormalities of pulmonary function are common in CHF patients.4,5 The measurement of the degree of broncho constriction is normally measured by using forced spirometry. This technique can be even used in patients who are too weak to perform spirometry. But it also avoids forced maneuvers, which can affect bronchial tone.5–7 IOS measures total airway resistance throughout the respiratory cycle and by altering the frequency of the applied sound wave, allows localization of the pathology. It depends much less on a patient's motivation and respiratory muscle strength than on forced maneuvers. Resistance, which was measured by IOS, had a good correlation with assessments of resistance, which were measured by the plethysmographic or oesophageal methods.6,7 Oscillometry is accurate enough to identify changes in the respiratory system of asymptomatic subjects who exposed to inhaled irritants when maximal expiratory flow volume measurements are normal, and it is also more accurate than spirometry in the assessment of responsiveness to methacholine in the normal population. It can identify the presence or absence of extrathoracic obstruction as well. Patients with chronic obstructive lung disease showed marked frequency dependence of pulmonary impedance.9–14
Our study confirms the presence of abnormalities of pulmonary function in patients with CHF. In this study, measurements of the inspiratory and expiratory spirometric parameters (FEV1, P = 0.01 and FVC, P = 0.04) were lower in patients than in controls, meanwhile IOS parameters (X5, P = 0.05; R5, P = 0.06; Z, P = 0.03) were greater significantly.
In our study, changes of FEV1 and FVC between patients and controls were very similar to spirometric findings in the study performed by Klaus et al [Fig. 1].6,7 While in the study of Klaus R5 and R2O changed more significantly between CHF patients and controls in comparison to that in our study, in which X5 and R5 changed more significantly between the two groups.
Fig. 1.
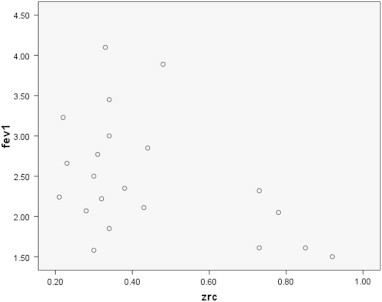
Correlation between FEV1 and Zrc.
As shown in Table 2, Z (P = 0.03) was changed more significantly in comparison to all other parameters. Then, X5 and R5 were changed more, respectively. According to the fact that the total Z reflects the total opposition of conducting flow and it is the combination of the resistance and reactance; these findings (of greater levels of Z in the patient group than then in the control group) shows increased resistance in the lungs of the patients. Between the determining factors of Z (elasticity and resistance), the rate of elasticity of peripheral airways has changed more than peripheral resistance.
Reactance and resistance in the central large airways does not show significant differences in two CHF group and controls [X20 (P = 0.29) and R20 (P = 0.39)]. Therefore, we can suggest that elasticity and resistance of the central airways in CHF patients has not changed markedly.
Klaus et al reported higher changes in R5 and R2O between CHF patients and controls than what we found in our study, which could be due to higher average age of that patients.6,7 Another variable that can affect R5 and R2O is diastolic dysfunction, but as it has not been determined in these studies, differences in the rate of diastolic dysfunction could be a possible reason for higher rates of R5 and R20 in the Klaus study.6,7
As shown in Table 3, although it was a trend toward reduction in spirometric data (FEV1 and FVC) in smokers, this was not a significant point. But R5 (the resistance parameter of IOS) was significantly different (P = 0.03) between two groups. Therefore, we can suggest that IOS measures the changes of pulmonary system with more sensitivity than does spirometry [Fig. 2].
Fig. 2.
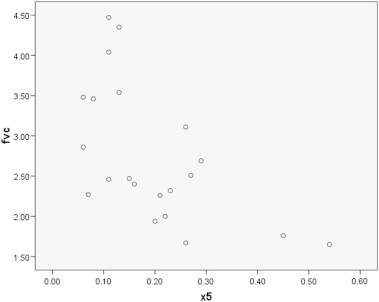
Correlation between fvc and X5.
About the association between the spirometry and IOS findings, in Klaus et al,6,7 the relationship between all parameters of IOS and spirometry were observed [(X2O, X5, R5, Zr5, R20) and FEV1], but, in our study, the highest correlation existed between FEV1 and X5 (P < 0.001) and between FEV1 and R5 (P = 0.07), respectively, where it seems more compatible with pathophysiology of heart failure, which affects the peripheral parts of bronchial tree more [Table 4].
In conclusion, CHF patients can be assessed by IOS more comfortable than by spirometry, and it can reliably measure peripheral airway resistance in this group of patients.
Conflicts of interest
All authors have none to declare.
Acknowledgments
This study was a joint postgraduate thesis of Dr Gholampoor and Dr Nourizadeh and was supported by a grant from Artesh University and Ahwaz Jundishapur University of Medical Sciences.
References
- 1.McCullough P.A., Nowak R.M., McCord J. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106:416–422. doi: 10.1161/01.cir.0000025242.79963.4c. [DOI] [PubMed] [Google Scholar]
- 2.Wasserman K., Zhang Y.Y., Gitt A. Lung function and exercise gas exchange in chronic heart failure. Circulation. 1997;96:2221–2227. doi: 10.1161/01.cir.96.7.2221. [DOI] [PubMed] [Google Scholar]
- 3.Witte K.K., Clark A.L. Beta-blockers and inspiratory pulmonary function in chronic heart failure. J Card Fail. 2005;11:112–116. doi: 10.1016/j.cardfail.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Duiverman E.J., Clement J., van de Woestijne K.P., Neijens H.J., van den Bergh A.C., Kerrebijn K.F. Forced oscillation technique. Reference values for resistance and reactance over a frequency spectrum of 2–26 Hz in healthy children aged 2.3–12.5 years. Bull Eur Physiopathol Respir. 1985;21:171–178. [PubMed] [Google Scholar]
- 5.Meraz E., Nazeran H., Ramos C.D. Analysis of impulse oscillometric measures of lung function and respiratory system model parameters in small airway-impaired and healthy children over a 2-year period. Biomed Eng Online. 2011;10:21. doi: 10.1186/1475-925X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witte K.K., Morice A., Clark A.L., Cleland J.G. Airway resistance in chronic heart failure measured by impulse oscillometry. J Card Fail. 2002;8:225–231. doi: 10.1054/jcaf.2002.126916. [DOI] [PubMed] [Google Scholar]
- 7.Klaus K.A., Morice A., Cleland J.G., Clark A.L. The reversibility of increased airways resistance in CHF measured by impulse oscillometry. J Card Fail. 2004;10:149–154. doi: 10.1016/j.cardfail.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Làndsér F.J., Nagels J., Demedts M., Billiet L., van de Woestijne K.P. A new method to determine frequency characteristics of the respiratory system. J Appl Physiol. 1976;41:101–106. doi: 10.1152/jappl.1976.41.1.101. [DOI] [PubMed] [Google Scholar]
- 9.Schmekel B., Smith H.J. The diagnostic capacity of forced oscillation and forced expiration techniques in identifying asthma by isocapnic hyperpnoea of cold air. Eur Resp J. 1997;10:2243–2249. doi: 10.1183/09031936.97.10102243. [DOI] [PubMed] [Google Scholar]
- 10.Orehek J., Nicoli M.M., Delpierre S., Beaupré A. Influence of the previous deep inspiration on the spirometric measurement of provoked bronchoconstriction in asthma. Am Rev Respir Dis. 1981;123:269–272. doi: 10.1164/arrd.1981.123.3.269. [DOI] [PubMed] [Google Scholar]
- 11.Mead J., Lindgren I., Gaensler E.A. The mechanical properties of the lungs in emphysema. J Clin Invest. 1955;34:1005–1016. doi: 10.1172/JCI103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimby G., Takishima T., Graham W., Macklem P., Mead J. Frequency dependence of flow resistance in patients with obstructive lung disease. J Clin Invest. 1968;47:1455–1465. doi: 10.1172/JCI105837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brochard L., Pelle G., de Palmas J. Density and frequency dependence of resistance in early airway obstruction. Am Rev Respir Dis. 1987;135:579–584. doi: 10.1164/arrd.1987.135.3.579. [DOI] [PubMed] [Google Scholar]
- 14.Pairon J.C., Iwatsubo Y., Hubert C. Measurement of bronchial responsiveness by forced oscillation technique in occupational epidemiology. Eur Respir J. 1994;7:484–489. doi: 10.1183/09031936.94.07030484. [DOI] [PubMed] [Google Scholar]


