Abstract
Background
Wald and law in their landmark paper published in BMJ in 2003 hypothesized that the use of fixed dose combination of statins, beta blockers, angiotensin-converting-enzyme inhibitor (ACE) inhibitor, and aspirin (Pollypill) may decrease cardiovascular disease by >80% if Pollypills are used as primary prevention. Many clinical trials were started to test this hypothesis. The present systematic review and meta-analysis aims to assess the available clinical trials to see the effect of Pollypill on cardiovascular mortality and on other risk factors that linked with increase in cardiovascular events.
Materials and methods
Available databases were searched with different specific terms and combination of key words. All randomized clinical trials exploring the effect of Pollypill on various cardiovascular parameters were included in the analysis. Primary endpoints as decided were cardiovascular mortality, systolic blood pressure, diastolic blood pressure, and low-density lipoprotein (LDL) cholesterol. Effect of Pollypill on high-density lipoprotein (HDL) cholesterol, total cholesterol, triglycerides, the number of participants who discontinued treatment, and the number of participants who experienced side effects were measured and analyzed as secondary outcomes. Both fixed and random models were used for analysis. Analysis was performed by comprehensive meta-analysis software.
Results
Six trials were included in systematic review. It was observed that Pollypill decreases systolic and diastolic blood pressure (P = 0.000). Pollypill was also found to decrease LDL cholesterol, total cholesterol, and triglyceride as compared to the control (all P = 0.000); however, there was no significant improvement in HDL (P = 0.39). The number of participants in whom side effects were observed were found to be more in the Pollypill group (odds ratio = 1.73, P = 0.000). It was also observed that dropouts were more in the Pollypill group than in the control group (odds ratio = 1.48, P = 0.02). Due to the lack of sufficient data effect of Pollypill, cardiovascular mortality could not be assessed.
Conclusion
Pollypill decreases various surrogate endpoints related to cardiovascular outcome, but with the increased chance of side effects as compared to control.
Keywords: Clinical trials, Diastolic blood pressure, Low-density lipoprotein cholesterol, Pollypill, Systolic blood pressure
1. Introduction
Cardiovascular disease (CVD) is a leading multi-factorial disease in developing countries.1 Aspirin, antihypertensive, and statins have been documented to reduce the risk of CVD both in patient with high risk of developing CVD as well as in patients with established CVD.2–4 in developing countries where adherence of drug is always an issue, combining these drugs may be an attractive option.5 Pollypill is usually a fixed dose combination of drugs with multiple active components. As Pollypill reduces the pill count of tablets and capsule to be taken by an individual; hence, it eases the administration and handling of drugs and improves compliance.6
Wald and law hypothesized the term Pollypill in 2003 with the aim that Pollypill can reduce the risk of CVD and mortality by 80%, while maintaining safety profile.7 It can be administered to patients > 55 years of age irrespective of whether there have risk factors for CVD. Several formulation of Pollypill have been developed thereafter, and clinical trials were also initiated regardless of emerging controversy on Pollypill to an extent that few researchers suggested that Pollypill might be detrimental for modern medicine.8
This article aims to present the current best evidences regarding the use of Pollypill in CVD by systematic review and meta-analysis of available clinical trials on Pollypills.
2. Materials and methods
2.1. Search methods
The present study aimed to include all relevant clinical trials exploring the effect of Pollypill on various cardiovascular parameters. Two reviewer (J.K and J. G) searched PubMed, Cochrane clinical trial registry, Web of Science, Google Scholar, and Google, independently. PubMed search was done based on different medical subject headings (MeSH) singularly or in different combinations. Various cross references mentioned in other review articles related to Pollypill were also searched to find clinical trials. Full text article were download, and another reviewer (D.S) decided about the inclusion or exclusion of clinical trials based on predefined inclusion and exclusion criteria. Relevant information was noted in a predesigned proforma by the first reviewer (J.K), which was later checked by the second reviewer (J.G). Corresponding authors of few clinical trials were contacted for some queries related to published data and to get raw unpublished data.
2.2. Inclusion and exclusion criteria
All randomized clinical trials exploring the effect of Pollypill on various cardiovascular parameters were considered for analysis. Non-randomized clinical trials or observational/epidemiological studies were not included in the present analysis. Clinical trials exploring the effect of multiple drugs separately but not as fixed dose combinations in the form of Pollypill were also not included in the analysis.
2.3. Outcomes
The primary outcome was the effect of Pollypill on cardiovascular mortality, systolic blood pressure, diastolic blood pressure, and low-density lipoprotein (LDL) cholesterol. While the secondary outcomes were effect of Pollypill on high-density lipoprotein (HDL) cholesterol, total cholesterol, and triglycerides. The number of participants who discontinued treatment and the number of participants who experienced side effects were also measured and analyzed as secondary outcomes.
2.4. Critical appraisal of included studies
Critical appraisal of included trials was done by methods given in the Cochrane handbook. This appraisal is based on the generation of allocation sequence, allocation concealment, blinding, and missing data reporting.9
2.5. Statistics
The statistical analysis was performed with the help of comprehensive meta-analysis software 2 (CAM 2). Quantitative data were pooled together in the form of as standardized mean difference with 95% confidence interval (CI), and, in the case of qualitative data, it was odds ratio with 95% CI. Analysis was done by both random and fixed model. In forest plot, only fixed model is shown. Heterogeneity was measured by Q statistics, where P < 0.05 was considered as significant for showing heterogeneity. I squared was also measured for measuring the heterogeneity. Funnel plot was plotted to access the publication bias.
3. Results
A total of six randomized trials were included in this systematic review.10–15 Characteristics of included trials are given in Table 1. Biases observed in clinical trials are given in Table 2. For most of the primary and secondary endpoints, only placebo controlled trials were included in the meta-analysis.
Table 1.
Characteristics of trials included in systematic review.
| Author/year/place of trial/type of trial | Participants | Intervention | Duration of study (months) | |
|---|---|---|---|---|
| 1 | Malekzadeh et al (2010), Iran, Double blind placebo controlled parallel group trial |
Men (age 50–79), women (age 55–79) No diagnosed CVD, not taking any antihypertensive medications or statins | Pollypill group (one Pollypill containing aspirin 81 mg, enalapril 2.5 mg, atorvastatin 20 mg, hydrochlorothiazide 12.5 mg) (n = 241) Placebo group (similar looking placebo) (n = 234) |
12 |
| 2 | Yusuf et al (2012) (Multicentric, India) Double blind parallel group trial |
Men and women >40 year with previous vascular disease or diabetes mellitus | Full dose group (two capsule each containing simvastatin 20 mg, ramipril 5 mg, atenolol 50 mg, hydrochlorothiazide 12.5 mg, and aspirin 100 mg) with potassium 30 mEq/L (n = 257) Low dose group (two capsules; one containing simvastatin 20 mg, ramipril 5 mg, atenolol 50 mg, hydrochlorothiazide 12.5 mg, aspirin 100 mg, and another cap and one capsule is similar looking placebo) (n = 261) |
2 |
| 3 | Soliman et al (2011) (Muticentric, Sri Lanka) Parallel group open label trial |
Men aged > 40 years and women > 50 years 10-year CVD risk score > 20% | Pollypill group (75 mg aspirin, 20 mg simvastatin, 10 mg lisinopril, and 12.5 mg hydrochlorothiazide) (n = 99) Standard practice (n = 104) |
3 |
| 4 | PILL Collaborative Group (2011) (Multicentric, international) Double blind placebo controlled parallel group trial |
Men and women aged > 18 years Cardiovascular risk over 5years > 7.5% | Pollypill group (aspirin 75 mg, lisinopril 10 mg, hydrochlorothiazide 12.5 mg, and simvastatin 20 mg) (n = 189) Placebo – identical pill (n = 189) |
|
| 5 | Wald et al (2012) Double blind placebo controlled cross over trial |
Men and women aged > 50 years No self reported cardiovascular event Already taking simvastatin or BP lowering drug in cardiovascular preventive program | Pollypill group (amlodipine 2.5 mg, losartan 25 mg, and hydrochlorothiazide 12.5 mg) (n = 84) Placebo – identical pill (n = 84) |
3 |
| 6 | The Indian Polycap Study (TIPS) Investigators (2009) (Multicentric, India) Double blind parallel group trial |
Men and women aged > 45 years and < 08 year without cardiovascular disease, having atleast one risk factor | Pollypill (thiazide 12.5 mg, atenolol 50 mg, ramipril 5 mg, simvastatin 20 mg, and aspirin 100 mg) (n = 412) Aspirin (n = 205) Thiazide (n = 205) Thiazide + ramipril (n = 209) Thiazide + atenolol (n = 207) Ramipril + atenolol (n = 205) Thiazide + ramipril + atenolol (n = 204) Thiazide + ramipril + atenolol + aspirin (n = 204) Simvastatin (n = 202) |
3 |
Table 2.
Bias observed in clinical trials included in systematic review.
| Adequate sequence generation? | Allocation concealment? | Blinding? | Incomplete outcome data? | Selective reporting? | Free of other bias? | |
|---|---|---|---|---|---|---|
| Malekzadeh et al (2010) | Yes | Yes | Yes | No | No | Unclear |
| Yusuf et al (2012) (Multicentric, India) |
Yes | No | No | No | No | Yes |
| Soliman et al (2011) (Muticentric open label, Sri Lanka) |
Yes | No | No | Yes | Yes | Yes |
| PILL Collaborative Group (2011) (Multicentric, international) | Yes | Yes | Yes | No | Yes | Unclear |
| Wald et al (2012) Cross over | Yes | Yes | Yes | Yes | Yes | Unclear |
| The Indian Polycap Study (TIPS) Investigators (2009) (Multicentric, India) | Yes | Yes | Yes | Yes | Yes | Unclear |
The first trial exploring the effect of fixed dose combination of various drugs affecting cardiovascular parameters (Pollypill) was published by TIPS Investigators. It was a phase two multicentric trial in India. In this trial, five drug fixed dose combination Pollypill was compared with eight different drugs/drug combinations [Table 1]. The follow-up period was 3 months. At the end of trial, it was observed that Pollypill reduced the systolic blood pressure by 7.4 mmHg (95% CI 6.1–8.1) and diastolic blood pressure by 5.6 mmHg (4.7–6.4) as compared to drug groups having no effect on blood pressure such as aspirin and simvastatin. This was comparable to three blood pressure reducing drugs used together with or without aspirin. Pollypill reduced LDL cholesterol significantly as compared to the groups in which simvastatin was absent, but this reduction in LDL cholesterol was less as compared to the groups with simvastatin. Pollypill also decreased heart rate similar to that by atenolol. It was also observed that tolerability was similar among Pollypill and other groups.10
In a trial done by Soliman et al, (2011) Pollypill group was compared with the groups that were given standard treatment based on the judgment of the treating physician. Both groups were followed up for 3 months. It was a feasibility trial, and 94% subjects completed the trial showing high acceptability. At the end of trial, no significant difference was found between both the groups for systolic and diastolic blood pressure, total cholesterol, and estimated 10-year cardiovascular risk. In the Pollypill group, the mean systolic blood pressure was decreased from baseline 165.6 mmHg–136.8 mmHg. It was observed that subjects in the standard treatment group received extra care, which may be the reason for non-significant difference.12
Yusuf et al [The Second Indian Polycap Study (TIPS-2) Investigators] conducted a trial comparing risk factor reduction and tolerability between subjects taking full dose of Pollypill (two capsule of Pollypill plus potassium) and subjects taking low dose of Pollypill (one drug capsule and one placebo capsule) [Table 1]. After 2 months, it was observed that full dose Pollypill reduces more systolic blood pressure (2.8 mm Hg, P = 0.003) and diastolic blood pressure (1.7 mmHg, P = 0.001) as compared to low dose. In the full dose group, mean systolic blood pressure was decreased from 130.5 mmHg to 126.6 mmHg while, in the half dose group, it was decreased to 129.4 mmHg. In the full dose group, diastolic blood pressure is decreased from baseline 78.6 to 77 mm Hg, while in half dose it decreased to 78.7 mmHg. High dose Pollypill also found to be reducing more total cholesterol (7.2 mg/dl, P = 0.014) and LDL cholesterol (6.6 mg/dl, P = 0.006) as compared to low dose. Discontinuation of treatment was comparable in both the groups.15
Three trials comparing Pollypill with the placebo were considered for meta-analysis.11,13,14 Baseline systolic and diastolic blood pressure in Malekzadeh et al, (2010) was 124.8 and 78.4 mmHg, which reduced to 121.1 and 77.6 mmHg. In a trial by PILL Collaborative Group (2011), baseline mean systolic blood pressure was 134 mmHg, which decreased to 123.9 mm Hg and baseline mean diastolic blood pressure was 82 mmHg, which decreased to 73.4 mmHg. In the case of Wald et al, (2012) initial mean blood pressures were 143 and 86 mmHg systolic and diastolic blood pressure, respectively, which decreased to 125.6 and 76.6 mmHg, respectively. For blood pressure, we also included comparison between Pollypill and aspirin plus simvastatin group as later have no effect on blood pressure. Durations of almost all trials were around 3 months; hence, it was not possible to analyze prevention of cardiovascular mortality and only surrogate endpoints were considered for meta-analysis.
3.1. Effect of Pollypill on systolic and diastolic blood pressures
Four trials were included in the meta-analysis for systolic and diastolic blood pressures [Malekzadeh et al, (2010) PILL Collaborative Group (2011), Wald et al, (2012) and The Indian Polycap Study (TIPS) Investigators (2009)]. There were 926 subjects in the Pollypill group and 714 in the control group. Pollypill significantly reduced the systolic blood pressure as compared to control group by both fixed and random method of analysis (standard mean difference = −0.356 (95% CI −0.455 to −0.256), Z = −6.993, P = 0.00). Same results were obtained for random effect model. There was no heterogeneity observed (Q value = 1.77, df = 3, P = 0.62, and I Square = 0) [Fig. 1]. Funnel plot does not show publication bias [Fig. 2].
Fig. 1.
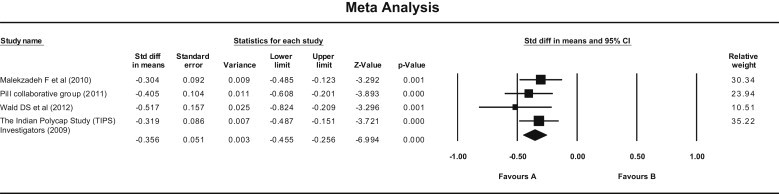
Forest plot showing effect of Pollypill on systolic blood pressure (Fixed effect model). Standard mean difference = −0.356 (95% CI −0.455 to −0.256), Z = −6.993, P = 0.00). (Q = 1.77, df = 3, P = 0.62, and I Square = 0).
Fig. 2.
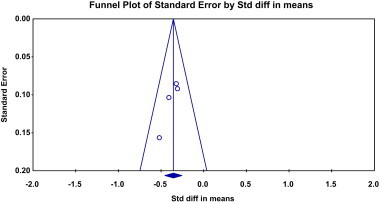
Funnel plot for clinical trials included to explore the effect of Pollypill on systolic blood pressure.
In the case of diastolic blood pressure, Pollypill significantly reduced it as compared to control by both fixed and random model (Fixed—standard mean difference = −0.308, 95% CI −0.407 to −0.208, P = 0.000; Random—standard mean difference = −0.310, 95% CI −0.416 to −0.204, P = 0.000). Heterogeneity was not observed (Q value = 3.33, df = 3, P = 0.34, and I Square = 10.12) [Fig. 3]. No publication bias was observed in funnel plot [Fig. 4].
Fig. 3.
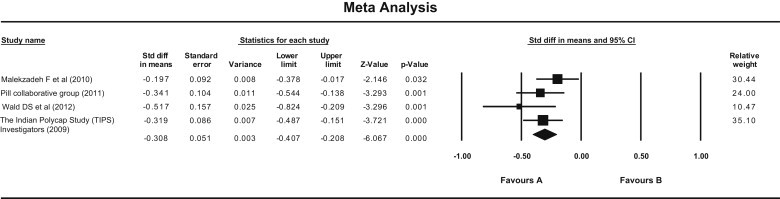
Forest plot showing effect of Pollypill on diastolic blood pressure (Fixed effect model). (Fixed—standard mean difference = −0.308, 95% CI −0.407 to −0.208, P = 0.000, Random—standard mean difference = −0.310, 95% CI −0.416 to −0.204), P = 0.000). (Q value = 3.33, df = 3, P = 0.34, and I Square = 10.12).
Fig. 4.
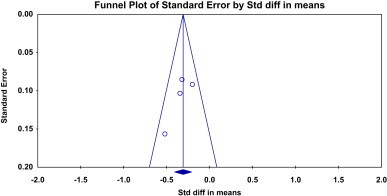
Funnel plot for clinical trials included to explore the effect of Pollypill on diastolic blood pressure.
3.2. Effect of Pollypill on lipid parameters
Three trials that were comparing the Pollypill with placebo were included in the analysis of lipid parameters [Malekzadeh et al, (2010) PILL Collaborative Group (2011), and Wald et al (2012)]. There were 514 subjects in the Pollypill group and 507 in the control group.
LDL cholesterol was significantly decreased in the Pollypill group as compared to placebo. According to fixed model, standard mean difference was −0.376 (95% CI −0.499 to −0.252) with P = 0.000 [Fig. 5]. Same results were obtained through random method. Heterogeneity was not observed (Q = 1.49, df = 2, P = 0.47, I Square = 0). No publication bias was seen [Fig. 6]. Similarly, Pollypill significantly reduced total cholesterol and triglycerides (P = 0.000) as compared to the placebo group. There was no significant difference observed for HDL Cholesterol (P = 0.397) (forest plots and funnel plots are not shown for these data). Heterogeneity and publication bias was not found for any parameter.
Fig. 5.
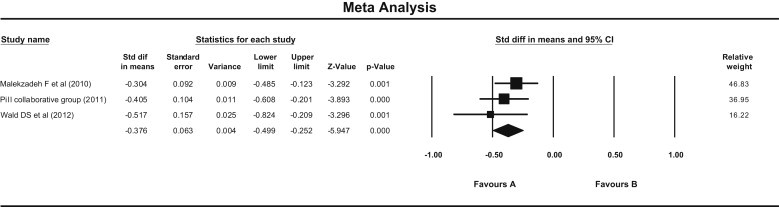
Forest plot showing effect of Pollypill on LDL Cholesterol (Fixed effect model). (Fixed—standard mean difference = −0.376 (95% CI −0.499 to −0.252), P = 0.000. Random—same result. Q = 1.49, df = 2, P = 0.47, I Square = 0.
Fig. 6.
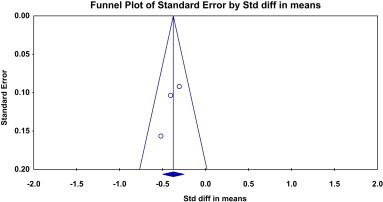
Funnel plot for clinical trials included to explore the effect of Pollypill on LDL cholesterol.
3.3. Compliance and safety of Pollypill
Compliance was assessed by comparison of dropouts from both the groups. The dropouts were reported in only two trials [Malekzadeh et al, (2010) and PILL Collaborative Group (2011)]. There were 430 subjects in the Pollypill group and 423 subjects in the placebo group. Dropouts were significantly more in the Pollypill group than in the placebo group (odds ratio = 1.480, 95% CI 1.044–2.089) and P = 0.028, according to fixed model [Fig. 7]. Same results were obtained with random model, and there was no heterogeneity found between clinical trials (Q = 0.97, df = 1, P = 0.3, I Square = 0.000). Funnel plot could not be plotted because of less number of trials.
Fig. 7.
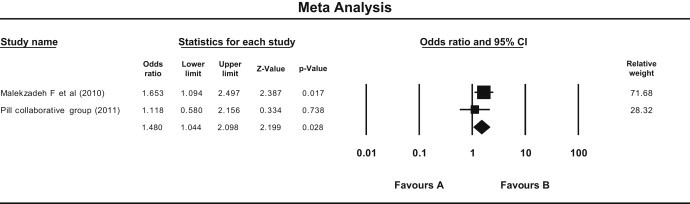
Forest plot showing effect of Pollypill on participants dropouts from clinical trials (Fixed effect model). (Fixed—odds ratio = 1.480, 95% CI 1.044–2.089), P = 0.028), Random—same results. (Q = 0.97, df = 1, P = 0.3, I Square = 0.000).
Safety was assessed on the basis of the number of subject experienced side effects in both the groups. Three trials reported side effects [Malekzadeh et al, (2010) PILL Collaborative Group (2011), and Wald et al (2012)]. There were 514 subjects in the Pollypill group and 507 in the control group. It was found that side effects were significantly more in Pollypill group than in the control group by both random and fixed model (P = 0.000) [Fig. 8]. There was no heterogeneity according to Q statistic, but I squared reported moderate heterogeneity (I Square = 34.69). We could not compare specific side effects because of the unavailability of proper data about specific side effects.
Fig. 8.
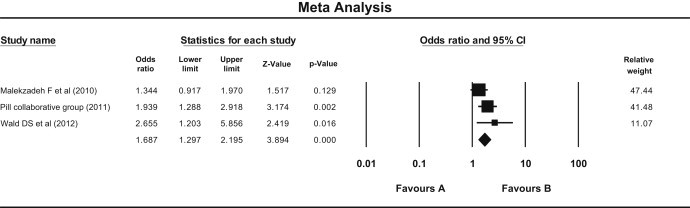
Forest plot showing comparison in side effects observed in Pollypill group as compared to the control (Fixed effect model). Fixed—odds ratio = 1.68 (95% CI 1.29–2.19), P = 0.000. Random—odds ratio = 1.73 (95% CI 1.23–2.44), P = 0.000. Q = 3.06, df = 2, P = 0.21, I Square = 34.69).
4. Discussion
In this systematic review and meta-analysis, it was observed that Pollypill decreases the cardiovascular surrogate endpoints with increased chances of having side effects/adverse effects. However, due to the lack of available data, effect of Pollypill on clinical endpoints like cardiovascular mortality could not be assessed.
Although results of meta-analysis are reported by both random and fixed method, there was no heterogeneity observed in most of the analysis. Heterogeneity was assessed by Q statistic and I Square value. Publication bias was also not observed in majority of analysis.
This systematic review has some limitations. The numbers of clinical trials included in this review are few; one of the possible reasons is paucity of available clinical trials on Pollypill. Components and strength of components as well as subjects recruited in these trials were not the same in all clinical trials that may increase the bias and heterogeneity. However, many clinical trials are being conducted to explore the effect of Pollypill on clinical and surrogate cardiovascular endpoints, and possibly more information will be generated in the future. Clinical trials included in the present systematic review were conducted for a very short duration; hence, effect on only surrogate endpoints could be explored. Surrogate endpoints many times may mislead researchers and may not show true prediction for clinical endpoints.16,17 In this review, almost all clinical trials were of similar (2–3 months) duration, except one trial [Malekzadeh et al, (2010)], which was of 12-months duration. This clinical trial was pooled together with other clinical trials during meta-analysis; this may be the reason for some heterogeneity observed for analysis of side effects. Funnel plots were plotted to see publication bias, but interpretation is always questionable when the number of clinical trials are less.18 In meta-analysis, crossover trial is also included with parallel group trial, possibly leading to “unit of analysis error”.19
On the basis of available clinical trials, it can be said that Pollypill decreases risk factors that are known to be responsible for cardiovascular morbidity and mortality with increased chances of side effects and decreased compliance as compared to control. Availability of very few trial is indeed a serious limitation of this systematic review; however, in the coming years, when results of large scale clinical trials (based on clinical endpoints) will be available, more clarity will emerge.
Conflicts of interest
All authors have none to declare.
References
- 1.World Health Organization . 2005. Preventing Chronic Diseases: A Vital Investment. WHO Global Report.http://www.who.int/chp/chronic_disease_report/en/ Available from: [Last Accessed on 18.09.12] [Google Scholar]
- 2.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baigent C., Keech A., Kearney P.M. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 patients in 14 randomized trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 4.Psaty B.M., Lumley T., Furberg C.D. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289:2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 5.Kulkarni S.P., Alexander K.P., Lytle B., Heiss G., Peterson E.D. Long-term adherence with cardiovascular drug regimens. Am Heart J. 2006;151:185–191. doi: 10.1016/j.ahj.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Lonn E., Bosch J., Teo K.K., Pais P., Xavier D., Yusuf S. The polypill in the prevention of cardiovascular diseases: key concepts, current status, challenges, and future directions. Circulation. 2010;122:2078–2088. doi: 10.1161/CIRCULATIONAHA.109.873232. [DOI] [PubMed] [Google Scholar]
- 7.Wald N.J., Law M.R. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326:1419. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green L.W. Prospects and possible pitfalls of a preventive polypill: confessions of a health promotion convert. Eur J Clin Nutr. 2005;59(suppl 1):S4–S8. doi: 10.1038/sj.ejcn.1602167. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Indian Polycap Study (TIPS) Yusuf S., Pais P., Afzal R. Effects of a polypill (polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trial. Lancet. 2009;373:1341–1351. doi: 10.1016/S0140-6736(09)60611-5. [DOI] [PubMed] [Google Scholar]
- 11.Malekzadeh F., Marshall T., Pourshams A. A pilot double-blind randomised placebo-controlled trial of the effects of fixed-dose combination therapy ('polypill') on cardiovascular risk factors. Int J Clin Pract. 2010;64:1220–1227. doi: 10.1111/j.1742-1241.2010.02412.x. [DOI] [PubMed] [Google Scholar]
- 12.Soliman E.S., Mendis S., Dissanayake W.P. A polypill for primary prevention of cardiovascular disease: a feasibility study of the World Health Organization. Trials. 2011;12:3. doi: 10.1186/1745-6215-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collaborative Group P.I.L.L., Rodgers A., Patel A. An international randomised placebo-controlled trial of a four-component combination pill (“polypill”) in people with raised cardiovascular risk. PLoS One. 2011;6:e19857. doi: 10.1371/journal.pone.0019857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wald D.S., Morris J.K., Wald N.J. Randomized polypill crossover trial in people aged 50 and over. PLoS One. 2012;7:e41297. doi: 10.1371/journal.pone.0041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yusuf S., Pais P., Sigamani A. Comparison of risk factor reduction and tolerability of a full-dose polypill (with potassium) versus low-dose polypill (polycap) in individuals at high risk of cardiovascular diseases: the second Indian Polycap Study (TIPS-2) Investigators. Circ Cardiovasc Qual Outcomes. 2012;5:463–471. doi: 10.1161/CIRCOUTCOMES.111.963637. [DOI] [PubMed] [Google Scholar]
- 16.Fleming T.R., DeMets D.L. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 17.Jaykaran Surrogate end points and their role in clinical trial. Indian J Pharmacol. 2009;41:54. doi: 10.4103/0253-7613.48880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne J.A., Sutton A.J., Ioannidis J.P. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 19.Cochrane Collaboration Open Learning Material. Available from: http://www.cochrane-net.org/openlearning/HTML/mod0-3.htm; Accessed 07.06.13.


