Abstract
A non-synonymous, single nucleotide polymorphism (SNP) in the gene coding for steroid 5-α-reductase type 2 (SRD5A2) is associated with reduced conversion of testosterone to dihydrotestosterone (DHT). Because SRD5A2 participates in the regulation of testosterone and cortisol metabolism, hormones shown to be dysregulated in patients with PTSD, we examined whether the V89L variant (rs523349) influences risk for post-traumatic stress disorder (PTSD). Study participants (N = 1,443) were traumatized African-American patients of low socioeconomic status with high rates of lifetime trauma exposure recruited from the primary care clinics of a large, urban hospital. PTSD symptoms were measured with the post-traumatic stress symptom scale (PSS). Subjects were genotyped for the V89L variant (rs523349) of SRD5A2. We initially found a significant sex-dependent effect of genotype in male but not female subjects on symptoms. Associations with PTSD symptoms were confirmed using a separate internal replication sample with identical methods of data analysis, followed by pooled analysis of the combined samples (N = 1,443, sex × genotype interaction P < 0.002; males: n = 536, P < 0.001). These data support the hypothesis that functional variation within SRD5A2 influences, in a sex-specific way, the severity of post-traumatic stress symptoms and risk for diagnosis of PTSD.
Keywords: trauma, African-American, genetic, testosterone, cortisol, male, civilian, human, PTSD
INTRODUCTION
A large body of research has identified abnormalities in the regulation of cortisol and testosterone in patients with post-traumatic stress disorder (PTSD). PTSD is a debilitating, stress-related psychiatric disorder associated with trauma exposure. The lifetime prevalence of PTSD in the general population has been estimated to be 5–10% [Kessler et al., 1995] with higher rates of PTSD observed among combat veterans [Hoge et al., 2004; Dohrenwind et al., 2006] and individuals living in areas of high violence [Alim et al., 2006; Gillespie et al., 2009]. Patients with PTSD exhibit basal levels of urinary cortisol excretion that are lower than those of healthy subjects [Yehuda et al., 1995; Bierer et al., 2006; Wheler et al., 2006] and enhanced negative feedback inhibition on measures of hypothalamic–pituitary–adrenal (HPA) axis function [Yehuda et al., 1993; Newport et al., 2004; Griffin et al., 2005; Yehuda et al., 2006]. However, other studies have found no differences in plasma cortisol [Wheler et al., 2006] or increased cerebrospinal fluid (CSF) [Baker et al., 2005] and salivary [Inslicht et al., 2006] cortisol levels in patients with PTSD. Abnormalities in testosterone secretion have also been identified in clinical studies examining individuals subjected to extreme and prolonged stress or diagnosed with PTSD. Plasma testosterone was reduced in soldiers participating in a physically and psychologically stressful training exercise [Morgan et al., 2000], and CSF testosterone was reduced in patients with PTSD compared to normal controls [Mulchahey et al., 2001]. Conversely, salivary testosterone was elevated in American survivors of the Iranian Hostage Crisis [Rahe et al., 1990] and plasma testosterone was positively correlated with risk for avoidance symptoms in subjects with combat-related PTSD [Spivak et al., 2003], but no difference in serum testosterone was found in East German refugees with PTSD [Bauer et al., 1994]. Given these varied findings, it is possible that enzymatic processes that regulate cortisol and testosterone levels, in addition to the conversion of these steroids to their metabolites, may provide more consistent association with pathology than peripheral hormone levels alone [Yehuda et al., 2009].
Steroid 5-alpha-reductase (5αRD) plays a central role in the metabolism of testosterone, cortisol, and progesterone [Russell et al., 1994; Wilson, 2001]. Best known for its role in the conversion of testosterone (T) to 5-alpha dihydrotestosterone (DHT), 5αRD also reduces cortisol to 5-alpha-tetrahydracortisol and progesterone to 5-alpha-dihydroprogesterone, the precursor of the anxiolytic neurosteroid allopregnanolone. In humans, two major isoforms of 5αRD have been identified that are coded by two separate genes (SRD5A1 & SRD5A2) and display distinct patterns of tissue distribution [Russell et al., 1994]. SRD5A1 codes for type I 5αRD (5αRD1) which is estimated to be responsible for production of approximately one-third of the circulating fraction of DHT [Gisleskog et al., 1998] and is expressed in the sebaceous glands of skin, and in liver, muscle, and brain [Thigpen et al., 1993; Stoffel-Wagner et al., 1998; Stoffel-Wagner et al., 2000; Ellis et al., 2005]. SRD5A2 codes for type II 5αRD (5αRD2) and is expressed in prostate, seminal vesicle, epididymis, hair follicles, and liver [Thigpen et al., 1993] but not in the brain [Stoffel-Wagner et al., 1998; Stoffel-Wagner et al., 2000]. Clinical studies conducted with 5αRD2 deficient pseudohermaphrodite patients have demonstrated the critical role of 5αRD2 in the production of DHT required for virilization during development and cortisol metabolism [Peterson et al., 1985; Andersson et al., 1991].
Considering the role of 5αRD2 in metabolism of these stress and sex steroids, we investigated whether the V89L variant of SRD5A2 influences risk for post-traumatic stress symptoms and diagnosis of PTSD. SRD5A2-V89L (rs523349) is a non-synonymous, functional polymorphism consequent to a G to C transversion that results in a valine to leucine substitution at codon 89. This polymorphism has been associated with a reduced capacity (~33% to 42%) in vitro for the enzymatic conversion of T to DHT in Leu/Leu samples compared to valine carriers [Makridakis et al., 2000]. However, there are no available data describing the effects of SRD5A2-V89L on either cortisol or progesterone metabolism. This SNP has been variably associated with risk for prostate cancer [Li et al., 2010a, b] but association with risk for PTSD or other forms of psychiatric disease has not been reported. We conducted our analysis in a heavily traumatized civilian, African-American population, and the validity of our findings in the primary sample was evaluated by an internal replication sample of similar size.
MATERIALS AND METHODS
Sample and Sample Recruitment
We have previously described the demographic characteristics of our study population, a primary care sample of convenience, in genetic association and epidemiological studies of trauma exposure [Binder et al., 2008; Bradley et al., 2008; Gillespie et al., 2009; Ressler et al., 2011]. Subjects were approached in the waiting rooms of the primary care or obstetrical-gynecological clinics of a large urban, public hospital while waiting for their own or their companion's medical appointments. Participating subjects provided written informed consent, completed a verbal interview, and provided a salivary sample. At the conclusion of the interview, subjects were given the option of further study participation involving donation of a blood sample and collection of additional phenotypic data. Data and DNA from the subjects in Samples 1 and 2 were collected concurrently in the manner described above. The samples are located on separate genotyping plates and the subjects comprising Sample 1 are those that we use as a discovery sample and for whom we have a blood and saliva sample as well as more extensive phenotype data. Subjects in Sample 2 are individuals for whom we have saliva samples only and a less extensive set of phenotype data and are used as an internal replication data set. All procedures in this study were approved by the Institutional Review Boards of Emory University School of Medicine and Grady Memorial Hospital.
Subject Assessment Procedure
Participants completed a battery of verbal self-report measures that are described below. To mitigate the effects of illiteracy on subject questionnaire comprehension, each instrument was read to the subjects. Each subject was paid $15.00 for participation in this phase of the study.
Demographics Form
The demographics form is locally developed and assesses subject age, self-identified race, education, income, employment, and disability status.
Personal Medical History Form
The personal medical history form is locally developed and assesses subject past medical, surgical and psychiatric/substance abuse history as well as allergies and currently prescribed medications.
Childhood trauma questionnaire (CTQ)
The CTQ [Bernstein et al., 1994, 1997] is a 28-item, self-report inventory assessing three types of child abuse: sexual, physical, and emotional. Cutoff scores for each category have shown excellent sensitivity and specificity in correctly classifying cases of abuse [Bernstein et al., 1997, 2003]. The CTQ yields a total score and subscale scores for each of the three types of child abuse. Bernstein and Fink [1998] established scores for mild, moderate, and severe for each type of abuse. To summarize the level of exposure to child abuse trauma, we summed the total number of different types of child abuse trauma to create a 3-level categorical variable (0, 1, ≥2) reflecting number of types of child abuse trauma because in our prior work [Binder et al., 2008; Bradley et al., 2008; Gillespie et al., 2009] it relates in a predictable and consistent manner with a number of measures of adaptive functioning and trauma related functioning.
Traumatic events inventory (TEI)
The TEI [Schwartz et al., 2005] is a 14-item instrument for lifetime history of traumatic events. For each event, the TEI assesses experiencing, witnessing, and confrontation of traumatic events where appropriate. For our analysis, we did not utilize the TEI items assessing trauma exposure during childhood since we used the CTQ to assess childhood trauma exposure. To summarize the level of exposure to trauma other than child abuse, we summed the total number of different types of non-child abuse trauma exposure reported by each subject and created a 3-level categorical variable (0, 1, ≥2) reflecting number of types of non-child abuse trauma experienced. The total number or types of trauma exposure variable was created and used in our data analysis because in our prior work [Binder et al., 2008; Bradley et al., 2008; Gillespie et al., 2009] and in other research on the impact of trauma exposure [Anda et al., 2006] it relates in a consistent manner with a number of measures of adaptive and trauma related functioning.
PTSD symptom scale
The PSS is a psychometrically valid 17-item self-report scale assessing the extent of PTSD symptom severity [Falsetti et al., 1993; Coffey et al., 1998; Foa and Tolin, 2000; Schwartz et al., 2005; Schwartz et al., 2006] over the prior 2 weeks. Consistent with prior literature, we summed the PSS frequency items (“0: not at all” to “3: ≥5 times a week”) to obtain a continuous measure of PTSD symptom severity ranging from 0 to 51. DSM-IV diagnosis of PTSD was made on the basis of presence of DSM-IV criteria A–E based on response to the PSS questionnaire.
Beck depression inventory
Depressive symptoms were assessed with the 21-item BDI [Beck et al., 1961], a commonly used continuous measure of level of depressive symptoms [Beck et al., 1988]. We used the summation of BDI score as our primary measure of depressive symptom severity.
Genetic Methods
DNA extraction
DNA was collected from saliva, using Oragene DNA sample collection kits (DNA Genotek), or from blood collected in EDTA vacuum tubes. DNA was extracted from saliva using the Purelink Genomic DNA kit (Invitrogen Corp., Carlsbad, CA or from blood using MagAttract DNA Blood M48 kit (Qiagen, Valencia, CA). All DNA was quantified using PicoGreen (Invitrogen Corp.) and normalized to a concentration of 5–10 ng/μl.
Genotyping and quality control
DNA was plated into 384 well plates at 5 ng for Taqman genotyping and 20 ng for Sequenom genotyping. All DNAs were dried down prior to performing reactions. Sequenom genotypes were collected using the iPLEX chemistries and the MassARRAY system (Sequenom, Inc., San Diego, CA). Taqman reactions were performed using Taqman SNP Genotyping Assays along with Taqman Genotyping Master Mix (Applied Biosystems, Inc., Foster City, CA). Alleles were discerned using the 7900HT fast real-time PCR system. Negative controls and within and across plate duplicates were used for quality control. Discordant samples were removed prior to analysis. For all Sequenom genotypes there was a 41.4% duplication rate and 0.04% discordance rate. The duplication rate for Taqman genotypes was 30.0%. There were no within method discordants. SNP rs523349 was genotyped using both Taqman and Sequenom with an across method duplication rate of 32.6%, an across method discordance rate of <0.05%, and an overall call rate of 95.2%. All negative controls passed QC. Prior to performing association studies with SRD5A2, we examined SRD5A2-V89L genotype frequencies (n, %) in African-American subjects (N=1,443) which were GG-Val/Val (779, 54.0%), GC-Val/Leu (565, 39.1%), CC-Leu/Leu (99, 6.9%); minor allele frequency (C-Leu) was 26.5%, which is consistent with the frequency of this allele in other studies [Febbo et al., 1999; Makridakis et al., 2000]. These genotypes were in Hardy Weinberg Equilibrium (HWE P > 0.8).
Statistical Analysis
We assessed differences in the demographic variables using SPSS (v. 19). Because the variables were not normally distributed, non-parametric methods were used. Mann–Whitney U-tests were performed to assess differences in continuous demographic (age), post-traumatic stress symptom (PSS total score and PSS subscale scores), and depressive symptom (BDI total score) variables between study samples. For categorical variables (sex, income, education, employment, childhood and adult trauma exposure, substance abuse, and suicide attempts), we used chi-squared tests to determine statistical differences between study samples. All tests of association were run using PLINK software version 1.07 [Purcell et al., 2007]. We used linear regression to model the effect of genotype and other covariates on PSS and other quantitative outcome variables, and logistic regression for categorical outcome variables. Except where noted, we tested for association under an additive (allelic) model where the number of copies of the C allele was allowed to influence the outcome variable linearly. To ensure that our results did not depend on distributional assumptions, we verified key results with permutation tests. We used PLINK to carry out 100,000 permutations; in each permutation, trait values were randomly shuffled across individuals, the association test was re-performed, and the t-statistic was recorded. Permutation P values were then estimated as the proportion of permutations for which the t-statistic exceeded the original in magnitude.
RESULTS
Demographics of Participants
This study utilized two different, concurrently obtained, cohorts (Samples 1 and 2) from the Grady Trauma Project [e.g., Binder et al., 2008; Bradley et al., 2008; Gillespie et al., 2009; Ressler et al., 2011]. Since PTSD symptoms were the primary outcome variables, we limited analyses to subjects who had experienced at least one criterion A trauma based on the traumatic events inventory. Subjects in this study were founSd to have experienced levels and types of traumas as we have previously described. Study Sample 1 consisted of 834 African American individuals, 780 of whom met criterion A for experience of trauma. Study Sample 2 consisted of 713 African American subjects, 663 of whom met trauma criteria. Thus the two samples combined consisted of 1,443 subjects that had experienced at least one severe trauma. The demographic characteristics, childhood and adult trauma exposure histories, substance abuse history, post-traumatic, and depressive symptom severity for the separate and combined samples are listed in Table I.
TABLE 1.
Demographic and Clinical Characteristics of the Primary and Replication Samples
| N | Mean | Std. error | P-value | |
|---|---|---|---|---|
| Sex (% female) | ||||
| Sample 1 | 780 | 61.8% | ||
| Sample 2 | 663 | 64.1% | ||
| Total | 1,443 | 62.9% | 0.37 | |
| Age | ||||
| Sample 1 | 775 | 40.3 | 0.5 | |
| Sample 2 | 661 | 38.3 | 0.5 | |
| Total | 1,436 | 39.4 | 0.4 | 0.01 |
| House monthly income (%<$500/month) | ||||
| Sample 1 | 780 | 59.2% | ||
| Sample 2 | 663 | 64.4% | ||
| Total | 1,443 | 61.6% | 0.05 | |
| Highest grade completed (%>high school) | ||||
| Sample 1 | 779 | 76.0% | ||
| Sample 2 | 662 | 77.9% | ||
| Total | 1,441 | 76.9% | 0.92 | |
| Currently employed (%) | ||||
| Sample 1 | 780 | 27.6% | ||
| Sample 2 | 663 | 34.2% | ||
| Total | 1,443 | 30.6% | 0.01 | |
| Current substance problem (%) | ||||
| Sample 1 | 780 | 6.4% | ||
| Sample 2 | 663 | 5.0% | ||
| Total | 1,443 | 5.8% | 0.24 | |
| Suicide attempt (%) | ||||
| Sample 1 | 758 | 17.0% | ||
| Sample 2 | 659 | 15.6% | ||
| Total | 1,417 | 16.4% | 0.48 | |
| Childhood trauma (%>1 trauma) | ||||
| Sample 1 | 780 | 21.4% | ||
| Sample 2 | 663 | 19.3% | ||
| Total | 1,443 | 20.4% | 0.47 | |
| Adult trauma (%>1 trauma) | ||||
| Sample 1 | 780 | 68.1% | ||
| Sample 2 | 663 | 66.4% | ||
| Total | 1,443 | 67.3% | 0.38 | |
| PSS total score | ||||
| Sample 1 | 780 | 13.3 | 0.4 | |
| Sample 2 | 663 | 12.9 | 0.5 | |
| Total | 1,443 | 13.1 | 0.3 | 0.47 |
| PSS intrusive score | ||||
| Sample 1 | 780 | 3.2 | 0.1 | |
| Sample 2 | 663 | 3.2 | 0.2 | |
| Total | 1,443 | 3.2 | 0.1 | 0.77 |
| PSS avoidance/numbing score | ||||
| Sample 1 | 780 | 5.4 | 0.2 | |
| Sample 2 | 663 | 5.1 | 0.2 | |
| Total | 1,436 | 5.3 | 0.1 | 0.37 |
| PSS hyperarousal score | ||||
| Sample 1 | 777 | 4.7 | 0.2 | |
| Sample 2 | 663 | 4.6 | 0.2 | |
| Total | 1,440 | 4.7 | 0.1 | 0.68 |
| Current PTSD diagnosis (%; caps subsample) | ||||
| Sample 1 | 780 | 33.8% | ||
| Sample 2 | 663 | 32.8% | ||
| Total | 1,443 | 33.4% | 0.70 | |
| BDI total score | ||||
| Sample 1 | 780 | 14.5 | 0.4 | |
| Sample 2 | 663 | 14.7 | 0.5 | |
| Total | 1,436 | 14.6 | 0.3 | 0.86 |
We examined sample differences with respect to demographic variables of age, sex, income, education, and employment. Of these, the only differences between Samples 1 and 2 were age (Sample 1 was older) and that a greater percentage of Sample 2 subjects were employed with marginally lower incomes (Table I). There were 37% men in the total sample, with 38% in Sample 1 and 36% in Sample 2. We examined level of trauma history between groups and found that there was a tremendous amount of trauma exposure in this population, with subjects experiencing, on average, five criterion A traumas in their lifetime. There were no significant differences between samples with respect to childhood or adult trauma exposure (Table I). We also examined prevalence of current substance abuse, history of suicide attempt, extent of depressive and post-traumatic stress symptoms, and percent of subjects with current PTSD based on the DSM-IV criteria of the PTSD symptoms. Approximately 33% of the subjects met criteria for current PTSD, and there were no differences between the groups on any of these variables (Table I).
SRD5A2 V89L Association With Total PTSD Symptoms in Men
We initially examined whether there was an association between the SRD5A2-V89L polymorphism and the extent of PTSD symptoms in Sample 1 (Table II). Because we anticipated sex-dependent effects of the SRD5A2-V89L variant considering its prominent role in testosterone metabolism, we examined male and female subjects separately. We observed an association between the C allele and total PTSD symptoms, as measured by PSS, in male subjects (n=298, T=2.169, P=0.03) but not in female subjects (n=482, T=−0.452, P>0.6). We next performed the same analysis in Sample 2 (Table II) and again observed an association between the C allele and PSS score in male subjects (n=238, T=2.604, P=0.01) but not female subjects (n=425, T=−0.703, P>0.4).
TABLE II.
PTSD Symptom Score in Male and Female Subjects as a Function of Genotype in Samples 1 and 2
| N | Mean | Std. error | |
|---|---|---|---|
| Sample 1 | |||
| Male | |||
| Val/Val | 167 | 11.3 | 0.9 |
| Va I/Leu | 116 | 15.4 | 1.2 |
| Leu/Leu | 15 | 12.7 | 3.7 |
| Female | |||
| Val/Val | 265 | 13.7 | 0.8 |
| Va I/Leu | 181 | 13.0 | 0.9 |
| Leu/Leu | 36 | 13.3 | 2.3 |
| Sample 2 | |||
| Male | |||
| Val/Val | 121 | 11.2 | 1.1 |
| Va I/Leu | 102 | 14.3 | 1.3 |
| Leu/Leu | 15 | 18.9 | 4.4 |
| Female | |||
| Val/Val | 226 | 12.9 | 0.8 |
| Va I/Leu | 166 | 13.7 | 1.0 |
| Leu/Leu | 33 | 9.4 | 1.7 |
PTSD, post-traumatic stress disorder.
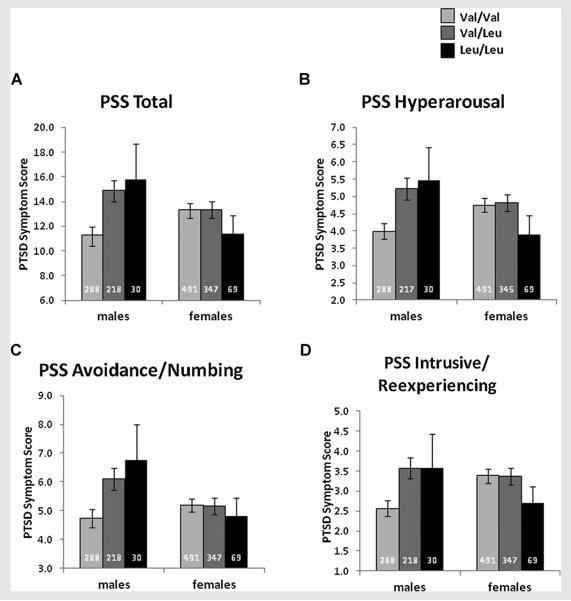
For the remainder of the studies, we performed all analyses on the combined study population (N=1,443), stratifying by sex. When we combined Samples 1 and 2 (N=1,443) in a stratified analysis, we observed the same pattern (Fig. 1A and Supplementary Figs. 1 and 2) in which the C allele was associated with PSS score in male subjects (n=536, T=3.372, P=0.0008, permutation P=0.0010) but not female subjects (n=907, T=−0.811, P>0.4). This result was robust to a sensitivity analysis that excluded the 20.4% of subjects exposed to >1 childhood trauma (P=5.1×10−5 in males). To test whether the association between SRD5A2-V89L and PSS differed significantly by sex, we performed an interaction analysis on males and females combined controlling for study sample. We observed a significant interaction between sex and genotype (N=1,443, T=3.206, P=0.0014), suggesting a sex-dependent association between SRD5A2-V89L and PSS.
FIG. 1.
Sex and genotype-dependent (allelic model) effects in the combined sample on total post-traumatic stress symptoms (A) and symptom cluster-specificpost-traumatic stress symptoms (B–D). PSS, post-traumatic stressdisorder symptomscale; PTSD, post-traumatic stress disorder. Bars represent the means±SE, and sample sizes are represented at the base of each bar.
We also assessed whether level of trauma (including childhood trauma) was responsible for the association of SRD5A2-V89L and PSS score in male subjects. Even though trauma (as measured by the variable TEI) was a significant predictor of PSS score in our model (T=11.19), the significant association between genotype and PSS score remained (N=559, T=3.648, P=0.00029). We did not observe an interaction between SRD5A2-V89L and TEI when predicting PSS score in males (P>0.1). When including childhood trauma (as measured by the continuous CTQ variable) in our model, again the association between genotype and PSS score in males remained significant (N=559, T=2.663, P=0.0010). The interaction between SRD5A2-V89L and CTQ when predicting PSS score in males was suggestive but not significant (P=0.052).
Controlling for Admixture Within the Sample Population
To adjust for potential confounding due to admixture in our all-African-American sample, we relied on a published panel of ancestry informative markers (AIMs) that was developed for admixture mapping in African Americans [Tian et al., 2006]. For a subset of our sample (N=587) for which genotypes for 1,019 of these AIMs were available, we re-fit the above models both including and excluding the first two principal components of these AIMs as covariates (further principal components were not significant according to a Tracy–Widom test [Patterson et al., 2006]). In each case, the estimated effect size did not change substantially with inclusion versus exclusion of the principal components, suggesting that the associations reported above are not the result of confounding due to admixture.
SRD5A2-V89L Association With Individual PTSD Symptom Clusters
We next examined whether the number of C alleles was associated with severity of individual PTSD symptom clusters, as measured by the three subscales of the PSS (avoidance/numbing, hyperarousal, and intrusive/re-experiencing). In males, we found a significant association with PTSD symptom severity on all three PSS subscale scores (Fig. 1B–D). The hyperarousal subscale had the most robust association (n=535, T=3.151, P=0.0017, permutation P=0.0018) followed by the avoidance/numbing subscale (n=536, T=3.111, P=0.0020, permutation P=0.0020) and the intrusive/re-experiencing subscale (n=536, T=2.934, P=0.0035, permutation P=0.0038); there were no associations with PSS subscales in females (P>0.3).
SRD5A2-V89L Association With PTSD Diagnosis
As our prior data suggest that continuous measures of PTSD symptoms may be a more powerful measure of biological risk than categorical diagnostic measures, we initially examined PTSD symptoms with total score on PSS or its subscales as our outcome. However, we also wanted to assess the association between SRD5A2-V89L genotype and categorical diagnosis of PTSD as an outcome. To do this, we tested for an association between SRD5A2-V89L genotype and the presence versus absence of PTSD-proxy diagnosis based on responses to PSS following DSM-IV criteria (see Table III for SRD5A2-V89L genotype frequencies in subjects with/without a categorical proxy-diagnosis of PTSD). We observed a significant association between SRD5A2-V89L genotype and proxy-diagnosis of PTSD in male subjects (N=536, P=0.013, permutation P=0.013; OR=1.456, CI=1.083–1.957). Conversely, SRD5A2-V89L genotype did not predict PTSD proxy-diagnosis in females (N=907, P=0.207; OR=0.8671, CI=0.6949–1.082). As above, we observed a significant sex by genotype interaction (N=1,443, T=2.788, P=0.005).
TABLE III.
Genotype Frequency in Male and Female Subjects With and Without PTSD Diagnosis Based on DSM-IV Diagnosis From the PSS Scale
| N | Percent | Std. error | |
|---|---|---|---|
| No PTSD | |||
| Male | |||
| Val/Val | 208 | 58.4% | 2.6 |
| Va I/Leu | 128 | 36.0% | 2.5 |
| Leu/Leu | 20 | 5.6% | 1.2 |
| Female | |||
| Val/Val | 321 | 53.1% | 2.0 |
| Val/Leu | 233 | 38.5% | 2.0 |
| Leu/Leu | 51 | 8.4% | 1.1 |
| PTSD | |||
| Male | |||
| Val/Val | 80 | 44.4% | 3.7 |
| Val/Leu | 90 | 50.0% | 3.7 |
| Leu/Leu | 10 | 5.6% | 1.7 |
| Female | |||
| Val/Val | 170 | 56.3% | 2.9 |
| Val/Leu | 114 | 37.7% | 2.8 |
| Leu/Leu | 18 | 6.0% | 1.4 |
PSS, Post-traumatic stress disorder symptom scale; PTSD, post-traumatic stress disorder.
SRD5A2-V89L Is Not Associated With Depression Symptoms
PTSD is often comorbid with other stress-related psychiatric disorders such as depression and substance abuse. Thus we analyzed whether the associations reported above for PTSD also occur with depression or whether the observed effects may actually be due to depression comorbidity. When controlling for study sample, there was no relationship between SRD5A2-V89L genotype and depression symptoms (BDI total score) in men or women; furthermore, there was no interaction with sex (N=1,443, T=−0.536, P>0.5). We also examined rates of current substance abuse (not shown) and again found no relationship between SRD5A2-V89L genotype and substance abuse in men or women and no interaction effect (N=1,443, T=0.487, P>0.6).
DISCUSSION
The central finding of our study is that functional variation at the SRD5A2-V89L locus exerts a sex-dependent influence on the severity of post-traumatic stress symptoms and risk for PTSD diagnosis. Male subjects with a Val/Leu or Leu/Leu genotype had more PTSD symptoms and an increased rate of PTSD diagnosis using the PSS measure, compared with male subjects with a Val/Val phenotype. Conversely, the V89L genotype did not significantly contribute to the severity of PTSD symptoms or rate of PTSD diagnosis in female subjects.
SRD5A2 is an important modulator of androgen levels. Comparative research conducted across a variety of species has demonstrated the anxiolytic effects of androgen administration [Archer, 1976; Vandenheede and Bouissou, 1993; Boissy and Bouissou, 1994; Aikey et al., 2002; Frye et al., 2008]. Acute testosterone administration also reduces subcortical fear [van Honk et al., 2005] and fear-potentiated startle in human female experimental subjects [Hermans et al., 2006]. Preclinical research using rodents has shown that reduction in the expression of fear examined with contextual (hippocampally mediated), but not cued (amygdala-mediated), models of fear learning is predominantly dependent on the DHT metabolite 3-alpha androstanediol glucuronide (3AG) [Edinger et al., 2004; Frye et al., 2004, 2008]. 3AG is synthesized from DHT through the action of 3-alpha hydroxysteroid dehydrogenase [Stoffel-Wagner, 2003] and although it has low affinity for the androgen receptor, 3AG is a potent neurosteroid exerting inhibitory effects through actions on the GABA-A receptor [Frye et al., 1996a, b].
Since SRD5A2 is not expressed, or only expressed at very low levels, in the brain [Stoffel-Wagner, 2003], variation in SRD5A2-V89L must act through the alteration of peripheral steroid metabolism to affect PTSD. This could occur through increased peripheral production of 3AG that might then act centrally to alter PTSD symptoms. Clinical studies examining 5αRD activity, or the functional effects of the SRD5A2-V89L genotype on 5αRD2 activity, commonly use circulating levels of 3AG as a quantitative trait measure of 5αRD activity [Ross et al., 1992; Wong et al., 1995; Gann et al., 1996; Ahn et al., 2009]. Ethnicity appears to interact strongly with V89L genotype to moderate plasma concentration of 3AG as circulating levels of 3AG are lowest in Asian men with the Leu/Leu genotype [Makridakis et al., 1997; Hsing et al., 2001] but do not appear to vary significantly by genotype in Caucasian men [Makridakis et al., 1997; Febbo et al., 1999; Allen et al., 2001] or African-American men [Makridakis et al., 1997]. In our study, which was African-American in composition, we did not measure plasma 3AG so we are unable to address the effect of genotype on 3AG levels alone or as a mediating variable with respect to PTSD symptoms.
5αRD is also involved in cortisol metabolism and preclinical research suggests that 5-alpha reduced glucocorticoids may activate the glucocorticoid receptor [McInnes et al., 2004]. Research conducted in rodents suggests that even small changes in the peripheral metabolism of cortisol have the capacity to alter the activity of the HPA axis [Paterson et al., 2007]. Recent findings in survivors of the Holocaust show reduced cortisol metabolism by 5αRD and 11-β-hydroxysteroid dehydrogenase type 2 [Yehuda et al., 2009]. Finally, increased cortisol metabolism was found in patients with schizophrenia spectrum disorders mediated by a different SRD5A2 variant (rs6732223) [Steen et al., 2010]. With regards to our findings of rs523349 association with male, but not female, PTSD, it has also been shown that sexual dimorphism may occur in the activity of 5αRD [Finken et al., 1999].
Progesterone, like cortisol, is also a substrateof 5αRD and may be reduced to 5-α-dihydroprogesterone, the immediate precursor of the anxiolytic neurosteroid, allopregnanolone. Reduced levels of allopregnanolone have been reported in the CSF of premenopausal women with PTSD that are negatively correlated with the intensity of re-experiencing symptoms [Rasmusson et al., 2006]. Preclinical research using a mouse social isolation model (reviewed in [Pinna et al., 2008; Pinna, 2010]) has identified a sexually dimorphic effect of social isolation on the development of impulsive aggression and increased contextual fear conditioning in male mice. These changes in behavior and learning correspond in a time-dependent manner with downregulation of corticolimbic allopregnanolone and the decay of 5αRD1 isozyme mRNA expression within the hippocampus and amygdala. With respect to our present findings related to PTSD risk, we believe that it is unlikely that our results are due to an effect of the SRD5A2-V89L polymorphism on the progesterone pathway and allopregnanolone synthesis since SRD5A2 is expressed minimally within the brain and we are not aware of any data demonstrating a kinetic effect of this polymorphism on the synthesis of 5-α-dihydroprogesterone.
Considered broadly, the current findings complement our recent report of a polymorphism in the gene coding for the PAC1 receptor which showed a differential association of an estrogen-sensitive gene on PTSD symptoms in women but not men [Ressler et al., 2011]. Others have demonstrated that estrogen and DHEA levels may also be important mediators of symptoms in traumatized women [Rasmusson et al., 2004; Milad et al., 2010]. Taken together, these data provide evidence that gonadal hormones and other sex-specific biological modulators may be critical factors leading to different mechanistic pathways to PTSD in men and women. Within the current analysis, we did not find an interaction of trauma exposure severity with SRD5A2-V89L, but future studies should analyze this possibility in more detail.
In summary, we describe a robust association between PTSD and a functional polymorphism in the SRD5A2 gene, which has previously been shown to result in altered levels of steroid metabolism. We find that SRD5A2-V89L is strongly associated with PTSD symptoms and PTSD diagnosis in traumatized men but not women. Further appreciation of the biological role of SRD5A2 will likely enhance our understanding of the pathogenesis of PTSD and suggest new diagnostic, prevention, and treatment approaches.
Supplementary Material
ACKNOWLEDGMENTS
This work was primarily supported by National Institutes of Mental Health (MH071537). Support was also received from National Institute of Mental Health (MH082256 to C.F.G.), Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01RR00039 and P20RR16435), NARSAD (C.F.G.), the American Foundation for Suicide Prevention (B.B.) and the Burroughs Wellcome Fund (K.J.R.). We thank staff of the Grady Trauma Project, especially Allen Graham and Angelo Brown, as well as Emily Reiser, Caitlin Fitzgerald, and Richard Barfield for excellent technical support. Portions of the data described in this manuscript were previously presented at the 2011 and 2012 meetings of the Society of Biological Psychiatry and the 2011 meeting of the American College of Neuropsychopharmacology.
Grant sponsor: National Institutes of Mental Health; Grant number: MH071537; Grant sponsor: National Institute of Mental Health; Grant number: MH082256; Grant sponsor: Emory and Grady Memorial Hospital General Clinical Research Center; Grant sponsor: NIH National Centers for Research Resources; Grant numbers: M01RR00039, P20RR16435; Grant sponsor: NARSAD; Grant sponsor: American Foundation for Suicide Prevention; Grant sponsor: Burroughs Wellcome Fund.
Financial Disclosure Statement: There were no commercial sponsors or commercial relationships related to the current work. All additional financial ties of the investigators within the last 3 years are disclosed herein: Dr. Gillespie has received funding from APIRE/Wyeth, NARSAD, NIDA, NCCAM, and NIMH. Dr. Bradley has received funding from AFSP. Dr. Binder receives funding from NIMH, the Behrens-Weise foundation and PharmaNeuroBoost. Dr. Ressler has received awards and/or funding support related to other studies from Burroughs Wellcome Foundation, NARSAD, NIMH, NIDA, and is a cofounder of Extinction Pharmaceuticals for use of NMDA-based therapeutics with Psychotherapy.
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- Ahn J, Schumacher FR, et al. Quantitative trait loci predicting circulating sex steroid hormones in men from the NCI-Breast and Prostate Cancer Cohort Consortium (BPC3) Hum Mol Genet. 2009;18(19):3749–3757. doi: 10.1093/hmg/ddp302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikey JL, Nyby JG, et al. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm Behav. 2002;42(4):448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- Alim TN, Graves E, et al. Trauma exposure, posttraumatic stress disorder and depression in an African-American primary care population. J Natl Med Assoc. 2006;98(10):1630–1636. [PMC free article] [PubMed] [Google Scholar]
- Allen NE, Forrest MS, et al. The association between polymorphisms in the CYP17 and 5alpha-reductase (SRD5A2) genes and serum androgen concentrations in men. Cancer Epidemiol Biomarkers Prev. 2001;10(3):185–189. [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S, Berman DM, et al. Deletion of steroid 5 alpha-reductase 2 gene in male pseudohermaphroditism. Nature. 1991;354(6349):159–161. doi: 10.1038/354159a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J. Testosterone and fear behavior in male chicks. Physiol Behav. 1976;17(4):561–564. doi: 10.1016/0031-9384(76)90151-7. [DOI] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, et al. Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 2005;162(5):992–994. doi: 10.1176/appi.ajp.162.5.992. [DOI] [PubMed] [Google Scholar]
- Bauer M, Priebe S, et al. Psychological and endocrine abnormalities in refugees from East Germany: Part II. Serum levels of cortisol, prolactin, luteinizing hormone, follicle stimulating hormone, and testosterone. Psychiatry Res. 1994;51(1):75–85. doi: 10.1016/0165-1781(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, et al. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Bernstein DP, Fink L. Childhood trauma questionnaire manual. Psychological Corporation; San Antonio, TX: 1998. [Google Scholar]
- Bernstein DP, Fink L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, et al. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bierer LM, Tischler L, et al. Clinical correlates of 24-h cortisol and norepinephrine excretion among subjects seeking treatment following the world trade center attacks on 9/11. Ann N Y Acad Sci. 2006;1071:514–520. doi: 10.1196/annals.1364.055. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy A, Bouissou MF. Effects of androgen treatment on behavioral and physiological responses of heifers to fear-eliciting situations. Horm Behav. 1994;28(1):66–83. doi: 10.1006/hbeh.1994.1006. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Dansky BS, et al. Screening for PTSD in a substance abuse sample: psychometric properties of a modified version of the PTSD Symptom Scale Self-Report Posttraumatic stress disorder. J Trauma Stress. 1998;11(2):393–399. doi: 10.1023/A:1024467507565. [DOI] [PubMed] [Google Scholar]
- Dohrenwind BP, Turner JB, Turse NA, Adams BG, Koenen KC. The psychological risks of Vietnam for U.S. veterans: A revisit with new data and methods. Science. 2006;313:979–982. doi: 10.1126/science.1128944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger KL, Lee B, et al. Mnemonic effects of testosterone and its 5alpha-reduced metabolites in the conditioned fear and inhibitory avoidance tasks. Pharmacol Biochem Behav. 2004;78(3):559–568. doi: 10.1016/j.pbb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Ellis JA, Panagiotopoulos S, et al. Androgenic correlates of genetic variation in the gene encoding 5alpha-reductase type 1. J Hum Genet. 2005;50(10):534–537. doi: 10.1007/s10038-005-0289-x. [DOI] [PubMed] [Google Scholar]
- Falsetti SA, Resnick HS, et al. The Modified PTSD Symptom Scale: A brief self-report measure of posttraumatic stress disorder. Behav Ther. 1993;16:161–162. [Google Scholar]
- Febbo PG, Kantoff PW, et al. The V89L polymorphism in the 5alpha-reductase type 2 gene and risk of prostate cancer. Cancer Res. 1999;59(23):5878–5881. [PubMed] [Google Scholar]
- Finken MJ, Andrews RC, et al. Cortisol metabolism in healthy young adults: sexual dimorphism in activities of A-ring reductases, but not 11beta-hydroxysteroid dehydrogenases. J Clin Endocrinol Metab. 1999;84(9):3316–3321. doi: 10.1210/jcem.84.9.6009. [DOI] [PubMed] [Google Scholar]
- Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale-Interview Version and the Clinician Administered PTSD Scale. J Traum Stress. 2000;13(2):181–191. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- Frye CA, Van Keuren KR, et al. Behavioral effects of 3 alpha-androstanediol. I: Modulation of sexual receptivity and promotion of GABA-stimulated chloride flux. Behav Brain Res. 1996a;79(1–2):109–118. doi: 10.1016/0166-4328(96)00004-6. [DOI] [PubMed] [Google Scholar]
- Frye CA, Van Keuren KR, et al. Analgesic effects of the neurosteroid 3 alpha-androstanediol. Brain Res. 1996b;709(1):1–9. doi: 10.1016/0006-8993(95)01118-8. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL. Testosterone's metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol Biochem Behav. 2004;78(3):473–481. doi: 10.1016/j.pbb.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger K, et al. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology. 2008;33(5):1049–1061. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gann PH, Hennekens CH, et al. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118–1126. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31(6):505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisleskog PO, Hermann D, et al. A model for the turnover of dihydrotestosterone in the presence of the irreversible 5 alpha-reductase inhibitors GI198745 and finasteride. Clin Pharmacol Ther. 1998;64(6):636–647. doi: 10.1016/S0009-9236(98)90054-6. [DOI] [PubMed] [Google Scholar]
- Griffin MG, Resick PA, et al. Enhanced cortisol suppression following dexamethasone administration in domestic violence survivors. Am J Psychiatry. 2005;162(6):1192–1199. doi: 10.1176/appi.ajp.162.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, et al. A single administration of testosterone reduces fear-potentiated startle in humans. Biol Psychiatry. 2006;59(9):872–874. doi: 10.1016/j.biopsych.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Hsing AW, Chen C, et al. Polymorphic markers in the SRD5A2 gene and prostate cancer risk: A population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2001;10(10):1077–1082. [PubMed] [Google Scholar]
- Inslicht SS, Marmar CR, et al. Increased cortisol in women with intimate partner violence-related posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31(7):825–838. doi: 10.1016/j.psyneuen.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Li J, Coates RJ, et al. Steroid 5-{alpha}-reductase Type 2 (SRD5a2) gene polymorphisms and risk of prostate cancer: A HuGE review. Am J Epidemiol. 2010a;171(1):1–13. doi: 10.1093/aje/kwp318. [DOI] [PubMed] [Google Scholar]
- Li X, Huang Y, et al. Meta-analysis of three polymorphisms in the steroid-5-alpha-reductase, alpha polypeptide 2 gene (SRD5A2) and risk of prostate cancer. Mutagenesis. 2010b;26(3):371–383. doi: 10.1093/mutage/geq103. [DOI] [PubMed] [Google Scholar]
- Makridakis N, Ross RK, et al. A prevalent missense substitution that modulates activity of prostatic steroid 5alpha-reductase. Cancer Res. 1997;57(6):1020–1022. [PubMed] [Google Scholar]
- Makridakis NM, di Salle E, et al. Biochemical and pharmacogenetic dissection of human steroid 5 alpha-reductase type II. Pharmacogenetics. 2000;10(5):407–413. doi: 10.1097/00008571-200007000-00004. [DOI] [PubMed] [Google Scholar]
- McInnes KJ, Kenyon CJ, et al. 5alpha-reduced glucocorticoids, novel endogenous activators of the glucocorticoid receptor. J Biol Chem. 2004;279(22):22908–22912. doi: 10.1074/jbc.M402822200. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168(3):652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA, III, Wang S, et al. Hormone profiles in humans experiencing military survival training. Biol Psychiatry. 2000;47(10):891–901. doi: 10.1016/s0006-3223(99)00307-8. [DOI] [PubMed] [Google Scholar]
- Mulchahey JJ, Ekhator NN, et al. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology. 2001;26(3):273–285. doi: 10.1016/s0306-4530(00)00052-4. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Heim C, et al. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biol Psychiatry. 2004;55(1):10–20. doi: 10.1016/s0006-3223(03)00692-9. [DOI] [PubMed] [Google Scholar]
- Paterson JM, Holmes MC, et al. Liver-selective transgene rescue of hypothalamic-pituitary-adrenal axis dysfunction in 11beta-hydroxysteroid dehydrogenase type 1-deficient mice. Endocrinology. 2007;148(3):961–966. doi: 10.1210/en.2006-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson N, Price AL, et al. Population structure and eigenanalysis. PLoS Genet. 2006;2(12):e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RE, Imperato-McGinley J, et al. Urinary steroid metabolites in subjects with male pseudohermaphroditism due to 5 alpha-reductase deficiency. Clin Endocrinol (Oxf) 1985;23(1):43–53. doi: 10.1111/j.1365-2265.1985.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Pinna G. In a mouse model relevant for post-traumatic stress disorder, selective brain steroidogenic stimulants (SBSS) improve behavioral deficits by normalizing allopregnanolone biosynthesis. Behav Pharmacol. 2010;21(5–6):438–450. doi: 10.1097/FBP.0b013e32833d8ba0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa RC, et al. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem Res. 2008;33(10):1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahe RH, Karson S, et al. Psychological and physiological assessments on American hostages freed from captivity in Iran. Psychosom Med. 1990;52(1):1–16. doi: 10.1097/00006842-199001000-00001. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Vasek J, et al. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology. 2004;29(8):1546–1557. doi: 10.1038/sj.npp.1300432. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, et al. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60(7):704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470(7335):492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RK, Bernstein L, et al. 5-alpha-reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet. 1992;339(8798):887–889. doi: 10.1016/0140-6736(92)90927-u. [DOI] [PubMed] [Google Scholar]
- Russell DW, Wilson JD. Steroid 5 alpha-reductase: Two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- Schwartz A, Bradley RG, et al. Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatr Serv. 2005a;56(2):212–215. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- Schwartz AC, Bradley R, et al. Pain medication use among patients with posttraumatic stress disorder. Psychosomatics. 2006;47(2):136–142. doi: 10.1176/appi.psy.47.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak B, Maayan R, et al. Plasma testosterone levels in patients with combat-related posttraumatic stress disorder. Neuropsychobiology. 2003;47(2):57–60. doi: 10.1159/000070009. [DOI] [PubMed] [Google Scholar]
- Steen NE, Tesli M, et al. SRD5A2 is associated with increased cortisol metabolism in schizophrenia spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1500–1506. doi: 10.1016/j.pnpbp.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann N Y Acad Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Watzka M, et al. Expression of 5alpha-reductase in the human temporal lobe of children and adults. J Clin Endocrinol Metab. 1998;83(10):3636–3642. doi: 10.1210/jcem.83.10.5157. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Beyenburg S, et al. Expression of 5alpha-reductase and 3alpha-hydroxisteroid oxidoreductase in the hippocampus of patients with chronic temporal lobe epilepsy. Epilepsia. 2000;41(2):140–147. doi: 10.1111/j.1528-1157.2000.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Thigpen AE, Silver RI, et al. Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. J Clin Invest. 1993;92(2):903–910. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Hinds DA, et al. A genomewide single-nucleotide-polymorphism panel with high ancestry information for African American admixture mapping. Am J Hum Genet. 2006;79(4):640–649. doi: 10.1086/507954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Honk J, Peper JS, et al. Testosterone reduces unconscious fear but not consciously experienced anxiety: Implications for the disorders of fear and anxiety. Biol Psychiatry. 2005;58(3):218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Vandenheede M, Bouissou MF. Effect of androgen treatment on fear reactions in ewes. Horm Behav. 1993;27(4):435–448. doi: 10.1006/hbeh.1993.1032. [DOI] [PubMed] [Google Scholar]
- Wheler GH, Brandon D, et al. Cortisol production rate in posttraumatic stress disorder. J Clin Endocrinol Metab. 2006;91(9):3486–3489. doi: 10.1210/jc.2006-0061. [DOI] [PubMed] [Google Scholar]
- Wilson JD. The role of 5alpha-reduction in steroid hormone physiology. Reprod Fertil Dev. 2001;13(7–8):673–678. doi: 10.1071/rd01074. [DOI] [PubMed] [Google Scholar]
- Wong IL, Morris RS, et al. A prospective randomized trial comparing finasteride to spironolactone in the treatment of hirsute women. J Clin Endocrinol Metab. 1995;80(1):233–238. doi: 10.1210/jcem.80.1.7829618. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, et al. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry. 1993;150(1):83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, et al. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. Am J Psychiatry. 1995;152(7):982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Yang RK, et al. Alterations in cortisol negative feedback inhibition as examined using the ACTH response to cortisol administration in PTSD. Psychoneuroendocrinology. 2006;31(4):447–451. doi: 10.1016/j.psyneuen.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, et al. Enduring effects of severe developmental adversity, including nutritional deprivation, on cortisol metabolism in aging Holocaust survivors. J Psychiatr Res. 2009;43(9):877–883. doi: 10.1016/j.jpsychires.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.