Abstract
We examine robotic rehabilitation and assessment of spinalized rats, using robot applied forces at the pelvis, as a prelude to a neurorobotic BMI. Using a surgically implanted frame, a cantilevered phantom robot is attached to the rat pelvis. An isotropic elastic field of constant stiffness is applied and the equilibrium is adjusted to provide a ‘natural’ trunk posture. Rats are trained daily for 20 minutes, 5 days per week in the field. Significant within trial, and long term adaptation occurs. The interaction force assessments from the robot reveal significant differences between spinalized control rats, and rats receiving implants of E14 dorsal raphe tissue to provide a serotonin source. Our system provides an animal model of rehabilitation through robot interaction at the pelvis.
I. introduction
TO date most efforts in neurorobotics and brain machine interface (BMI) design have focused on control of the forelimb, e.g., cortical control of forelimb reaching or virtual reality tasks in rats, monkeys or man in 2 or 3 degrees of freedom tasks [1-3]. Neural circuits for control of forelimbs differ in various ways from those which control the hindlimbs. Our longer range goal is neurorobotic control applied at the pelvis to assist interaction of the trunk and hindlimbs in rats during treadmill locomotion.
Tasks associated with quadrupedal locomotion are distributed through many central nervous system regions. However, our experiments in neonatal injured rats strongly support a major role for cortical control of the trunk in recovery from complete spinal cord transection [4]. In rats with neonatal spinal transection, some manage to achieve hind-limb weight support. In these rats, intracranial microstimulation (ICMS) showed that mid to low trunk representation (and actions at the pelvis) in cortex associated 1:1 with development of functional and autonomous weight support in the hindlimbs. Lesions of the hindlimb/trunk region of cortex also caused major function loss. Accordingly we are focusing on the trunk/hindlimb region of sensorimotor cortex in rats as a source of control information for neurorobotic approaches, to test the organization, plasticity and rehabilitative capacitives in this region in rats. An important control for, and prelude to, any BMI implementation is to understand the responses to assistive interactions with a robotic apparatus without the closed-loop neural control. To explore rehabilitation robotics with our BMI system we used it to train rats spinalized as adults. Our results may have implications for clinical devices and rehabilitation focused at the pelvis.
Trunk control is crucial for upright bipedal locomotion. Human bipedal locomotion is an evolutionary specialization of the quadrupedal locomotor control system seen in the rat, cat or monkey. In the same fashion, it is likely that through evolution the neural circuitry supporting locomotion in quadrupeds was elaborated from and layered onto the trunk locomotor pattern generators of swimming fish. Both undulating swimming in fish and quadrupedal locomotion (especially hindlimb alternating locomotion) have been used as model systems. These have been used to understand basic principles of pattern generation, spinal organization and to model neural repair in spinal cord injury (SCI). In functional behaviors, both trunk and limb control must always be seamlessly integrated. The trunk forms an active platform for limb force development and participates strongly in most, if not all, quadrupeds’ locomotion. However, with a few notable exceptions, many details of thoracicolumbar trunk control in quadruped locomotion are missing. In several ways the axial control in locomotion and stance is still very poorly understood, especially in SCI.
Preliminary work with our system here examines robotic rehabilitation through interaction at the pelvis. Our data show that pelvic force application can provide significant functional improvement and can supply the investigator with novel biomechanical data. These data allow us to identify differences resulting from neural transplant interventions in adult SCI rats.
II. Methods and Rationale
A. System Design and Long Range goals
Our BMI system has been described in Giszter et al. [5]. Our aims in designing the system have been:
To allow neurorobotic BMI training of spinalized rats
To support rapid (1KHz control loop, >150Hz mechanical bandwidth) interaction at the pelvis, applied in different (world frame, or intrinsic body centered frame) coordinate frames.
To allow both fixed and/or neural driven interaction and assistance to rat.
The world-frame fixed interaction robot rehabilitation application of the system are our focus here.
B. Rationale for Choice of Mechanical Interaction Port with Rat
In most BMI neurorobotics applications focus is on a single limb or cursor control. In locomotion, the motions of both the hindlimbs and their interactions with the trunk are all crucial elements of weight support and stabilization during stepping. Rehabiliation robotics for hindlimbs in rats after SCI has focused on the individual or paired hindlimbs through interaction at the ankle, using experimental or commercial devices [6-8]. One or both limbs have been examined. The major rehabilitative effort and emphasis was placed on the kinematics and coordination of hindlimb swing [6-8]. The pelvis is the nexus of leg/leg and leg and trunk interaction. By moving the mechanical interaction with the rat to the pelvis in our apparatus we potentially achieve several things: (1) we can interact with the limbs during the stance phases through force loading or unloading applied at the pelvis, (2) we can assist interaction with, and control of the pelvis by the trunk, (3) we can apply forces to move the trunk, pelvis and proximal limbs into a kinematically more normal configuration despite lack of adequate hindlimb force production or coordination, (4) we can indirectly assist interaction with forelimbs through trunk for similar reasons.
It seems clear that the trunk/pelvis axial motions can play significant roles in stabilization and energy transfers in vertical, lateral, and head-tail directions, and among rotational degrees of freedom at the pelvis. However, most biomechanical analysis of limb trunk forelimb interaction in locomotion has been restricted to parasagittal planar analysis (forelimb/hindlimb), paired hindlimbs alone, or single limb in 2D or 3D, e.g., [9]. There are thus few predictions available regarding how best to interact at the pelvis to assist and train locomotion in quadrupeds. Our system provides translational forces centered over the pelvis. Reducing the vertical load and providing lateral stabilizating forces are the most obvious initial choices. We do this through isotropic elastic fields (see below).
C. Implant Design and Interaction Issues
A stable implant design with minimal tissue damage and biomechanical perturbation was needed, to interact with the rat at the pelvis in a predictable fashion. We required a connection to bone with long term stability and minimal disruption of the mechanical plant. Over time we have settled on a clamping crossbar system that remains stable for better than two months. We have satisfied ourselves that this implant design does not distrupt the locomotion kinematics of normal rats. After a week of postoperative recovery and a week of treadmill training the normal rat with implant has kinematics indistinguishable from normal.
The robot we used (cantilevered phantom (Sensable Devices Inc)) interacts through a gimbal. The implant was designed to support the gimbal attachment centered on the midline parasagittal plane. The implant also provides a base for simultaneous attachment of 3D markers for 6DOF kinematics measurements.
Frames of interaction. The use of thoracic saddle and pelvic markers allow the rat trunk motion to be assessed with 6DOF measurements of thorax and pelvis. This also allows the use of body centered coordinate frames for control of robot interaction forces, e.g., apparent stiffening of the torso in the yaw plane.
D. Rehabilitation Methods and Adjustment Protocol
To provide simple assistive robotic interaction in an extrinsic coordinate frame we first used isotropic elastic fields.
where r is position, r0 desired position, K stiffness and F applied force.
This scheme allows us to provide adjustable weight support and lateral stabilization. We use a fixed field stiffness K of 80N/m in order to standardize magnitude of interactions among all test rats. To provide weight support to the degree needed, we adjusted the field equilibrium r0. The equilibrium was translated vertically until the trunk posture of the rat was approximately normal, or a little (<1cm) below that of a normal rat. Although field stiffness might arguably be usefully scaled with rat's mass, we avoided this additional complexity. Rostrocaudal position of the field relative to the treadmill frame was fixed. With this arrangement, a normal rat could choose the extent to which it supported itself, or ‘rested’ against the robot elastic field. An injured rat's interaction and position in the field was expected to change with adaptation and rehabilitation throughout training.
Rats were trained 20 minutes per day, 5 days a week, beginning one week after the spinalization. This constitutes what physical therapists term ‘massed’ training and this was readily achieved in our apparatus, while bipedal training of spinalized rats must often be more limited in duration [10].
E. Spinalization and Surgery Methods
All surgery was conducted in accordance with USDA and NIH guidelines under approval of the Institutional Animal Care and Use Committee.
Pelvic implant
Sterile pelvic implants were constructed in-house. The implants were surgically implanted in normal rats, and in adult spinalized rats at the time of spinal transection.
Spinalization
Rats were fully spinalized by removal of spinal segment T10, between the T9/T11 border. Rats received analgesia post surgery, and spinal rats had bladder expressed twice daily for up to two weeks until automatic bladder voiding returned.
Raphe implant
8 rats, at time of transection, received implants injections into the remaining caudal spinal cord. Dissociated E14 Dorsal Raphe cells prepared by the Drexel Spinal Cord Center from embryos of pregnant dams were injected into the rostral most segments between T11 and L1. Raphe neurons can supply serotonin, a neurotransmitter exogonous to spinal cord, involved in modulation of spinal reflex and locomotor circuits. It was hoped that, with a serotonin supply, the function of the adult spinal cord below the lesion would be improved, and possibly tail-pinch input to promote locomotion in spinalized rats would be unecessary.
F. Assessment Methods
Both adult spinalized rats and adult spinalized rats with raphe required tail pinch to induce stepping. Recovery was assessed from video tape using a modified BBB-type [11] scale due to Antri, Orsin and Barthe [10]. This scored rats based on frequency, amplitude, and coordination of hindlimb stepping motions, presence of weight support, and frequency of plantar stepping. The final score ran from 0 to 22 with a 22 representing normal function. This score was assessed for each training session over two intervals of 2 minutes each, the first at about 3 minutes and the second at about 12 minutes.
Interaction force and motion of interaction point were recorded over a 2-3 minute period around 12 minutes into training. RMS interaction forces along each axis were calculated. In our RH coordinate frame, Z was vertical and X aligned along the length of the treadmill. These measures allowed assessment of weight support provided by the robot, systematic bias in rat robot interaction, and change of these over time through training. Finally, we also used video assessed stepping phase (measured both at toe-off and at toe-touch) of hindlimbs, referenced to the forelimb cycle.
III. Results
A. Robotic Rehabilitation of Spinalized Rats at Pelvis
We found strong effects of robot training both within a session and over multiple sessions.
Qualitative changes
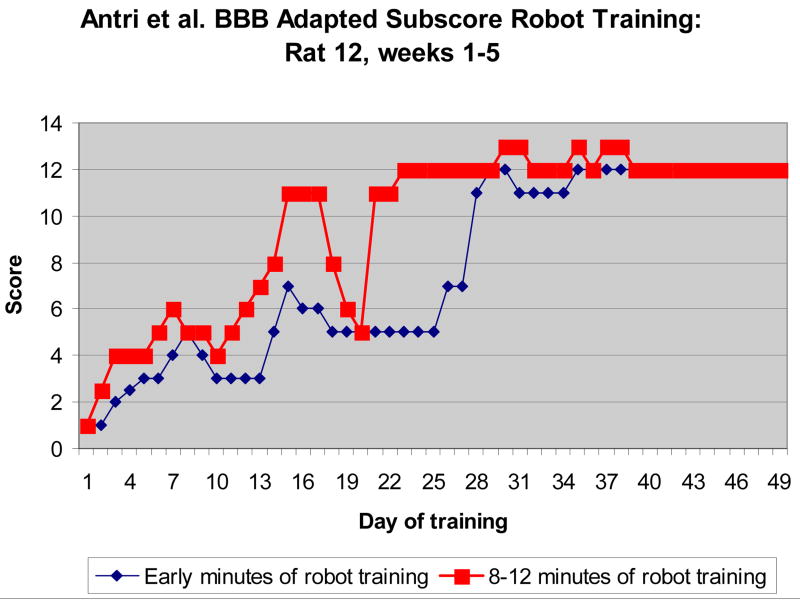
Qualitative scoring improved significantly within each session (e.g., p< 10E-7, paired t-test in Rat 12, shown). There was a cumulative improvement session to session over time until a plateau was reached. However, there were also variations in cumulative response that we could not readily account for. Plateau levels achieved were comparable to the best level achieved with bipedal step training by the originators of the scale used. This could be primarily due to the support of ‘massed’ training in our apparatus, or could represent a difference due to the richer mechanical interaction modes and quadrupedal training enabled by our apparatus.
Quantitative interaction changes
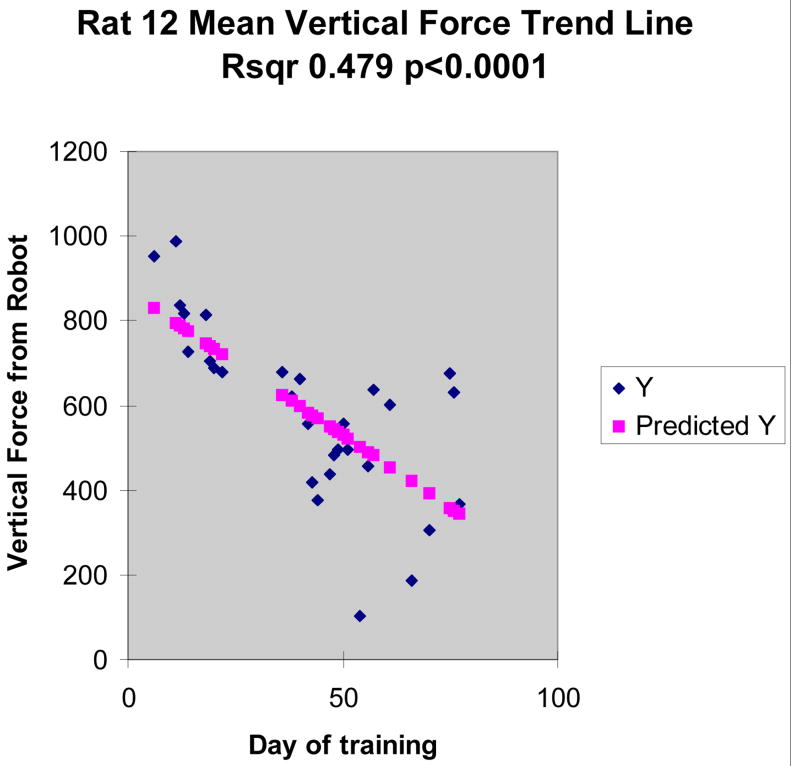
RMS lateral and rostral forces did not change significantly in our spinalized rats and were not significantly different from zero as a group. RMS vertical forces were significant and consistent with strong intial robot support contributions, but they also showed very significant trend. Linear regression to training session showed a progressive reduction of vertical force in all spinalized rats (e.g., trend r2 0.48, p<0.0001 in Rat 12 shown in Figure 2 and 3). RMS robot vertical force dropped by 50%, from initial values of 800 units (∼80g) to under 400 units in Rat 12. Standard deviation of RMS force in this rat also increased, slightly but significantly.
Fig. 2.
Behavioral Score improvement during training of a spinalized rat. Scores were obtained over 2 minutes early in training with the robot applied isotropic field (blue, circle) and later in training in the same session (red, square). Improvement was usually seen each day, until plateau. The red and blue curves are significantly different.
Fig. 3.
Gradual reduction in vertical interaction force between rat and robot over progressive days of training in spinalized rats. This significant linear trend indicates the rat is supporting more of its weight with the hindlimbs, over repeated sessions (days), and bringing the pelvis closer the applied field equilibrium.
B. Robotic Rehabilitation of Raphe Implant Rats
We implanted spinalized rats with embryonic dorsal raphe to replicate experiments of Privat and colleagues. We hoped to obtain spinalized rats that did not require tail pinch to step. However, this was not routinely the case in our hands. Raphe rats on average achieved greater function (plateau scores of 15 vs 12 in spinalized). However all required tail pinch to induce stepping on all trials. Within trial improvement was once again significant (e.g., p<0.0001 for paired t-test, in rat 24 with Raphe transplant).
Differences in Quantitative Interaction
Raphe rats did not exhibit the change in vertical support forces that were seen uniformly in the spinalized rats without Raphe. Instead we saw significant alterations in rostrocaudal force components. The Raphe rats adapted propulsive but not weight support forces over time. The trends in X forces were significant in 4/8 rats, with variance accounted for by the trend lines ranging from 30% to 60% in these 4 rats, and remaining around 0 in the rest. The Z force variances accounted for by trends in Raphe implant rats were all under 15%.
C. Comparisons of Interventions in Adult Spinalized Rats
Raphe implant rats often adapted more rapidly, e.g. rat 24 (Raphe) surpassed a behavioral score of 10 within 10 training days (two weeks), while rat 12 (spinal alone) took more than 15 days (three weeks). Final plateau score was also higher. The differences in variance accounted for and slopes of X force trends and Z force trends between the spinalized rats and those that received raphe implants in addition were highly significant (post-hoc t-tests p< 0.005 for X, and p< 10E-7 for Z). The serotonergic implant clearly altered the biomechanical adaptation process exhibited by the lumbar spinal cord below the injury, in our rehabilitation regime. The presence of serotonergic Raphe transplants changed the adaptation process from adaptation of weight support forces (increasing leg load carried over training) to propulsive force adaptation (increasing rostral propulsion over training).
IV. Conclusions
A. Robotic Rehabilitation at the Pelvis in Rats
We have presented data on application of an animal model of robot rehabilitation using pelvic support. This is complementary to the approaches of Reinkensmeyer and colleagues [12], and Hogan and colleagues in robotic rehabilitation of locomotion in man. The application of assistive and stabilizing elastic fields at the rat pelvis allows massed training and leads to significant improvement in treadmill locomotor stepping over time in adult spinalized rats. Transfer of these gains to locomotion off the robot was not addressed here.
B. Assessment of Function using the Robot System
The robotic rehabilitation used here allows assessment of the adaptation processes. Our data show there are highly significant differences between spinalized rats receiving serotonergic transplants and rats without. The ability to quantify weight support and propulsive force coordination and hence differences among treatments, through the robotic training system, is a significant feature of our approach. The basic results from the methods here may transfer to less invasive approaches, or to clinical therapy and assessment.
Fig. 1.
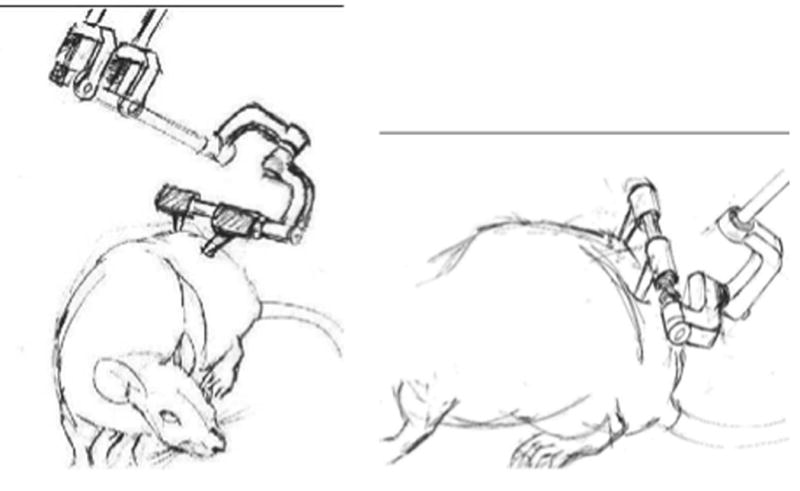
Sketch of rat and pelvic implant arrangement. Left: front view. Right: lateral view of rat and implant structure attached to pelvis. Force application in the world frame occurs at the gimbal center which is at the crossbar center, despite the sketch appearance.
Acknowledgments
Supported by NIH NS24707 and NS40412.
Tim Himes, Theresa Connors, Carla Tyler-Polsz contributed invaluable technical assistance in spinal transection and Raphe implantation surgeries. Jonie Young was a codesigner of the pelvic orthosis used. Supported by NIH NS24707, system developed under NIH NS24707 and NS40412.
Contributor Information
Ubong I Udoekwere, Email: uiu22@drexel.edu, School of Bioengineering, Drexel University.
Arun Ramakrishnan, Email: ar88@drexel.edu, School of Bioengineering, Drexel University.
Lollise Mbi, Email: lollise.mbi@drexel.edu, Department of Neurobiology, Drexel Univ College of Medicine, Drexel University.
Simon F. Giszter, Email: simon.giszter@drexel.edu, Department of Neurobiology, Drexel Univ College of Medicine, Drexel University.
References
- 1.Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296(5574):1829–32. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 2.Nicolelis MA. Brain-machine interfaces to restore motor function and probe neural circuits. Nat Rev Neurosci. 2003;4(5):417–22. doi: 10.1038/nrn1105. [DOI] [PubMed] [Google Scholar]
- 3.Donoghue JP. Connecting cortex to machines: recent advances in brain interfaces. Nat Neurosci. 2002 Nov;(5 Suppl):1085–8. doi: 10.1038/nn947. [DOI] [PubMed] [Google Scholar]
- 4.Giszter SF, Kargo WJ, Davies MR, Shibayama M. Fetal transplants rescue axial muscle representations in M1 cortex of neonatally transected rats that develop weight support. J Neurophysiol. 1998 Dec;80(6):3021–30. doi: 10.1152/jn.1998.80.6.3021. [DOI] [PubMed] [Google Scholar]
- 5.Giszter SF, Hart CB, Udoekwere UI, Markin S, Barbe C. A Real-Time System for Small Animal Neurorobotics at Spinal or Cortical Levels. 2nd International IEEE EMBS Conference on Neural Engineering; March 16-19, 2005; 2005. pp. 450–453. [Google Scholar]
- 6.DeLeon RD, Kubasak MD, Phelps PE, Timoszyk WK, Reinkensmeyer DJ, Roy RR, Edgerton VR. Using robotics to teach the spinal cord to walk. Brain Res Reviews. 2002;40:267–273. doi: 10.1016/s0165-0173(02)00209-6. [DOI] [PubMed] [Google Scholar]
- 7.Reinkensmeyer DJ, Timoszyk WK, deLeon RD, Joynes R, Kwak E, Minakata K, Edgerton VR. (2000) A robotic stepper for retraining locomotion in spinal-injured rodents. Proc IEEE Intl Conf on Robotics and Automation; San Francisco, CA. 2000. [Google Scholar]
- 8.Reinkensmeyer DJ, Emken JL, Cramer SC. Robotics, motor learning and neurological recovery. Ann Rev Biomed Eng. 2004;6:497–525. doi: 10.1146/annurev.bioeng.6.040803.140223. [DOI] [PubMed] [Google Scholar]
- 9.Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change, and ankle extensor EMG patterns. Journal of Neurophysiology. 2006;95:1397–1409. doi: 10.1152/jn.01300.2004. [DOI] [PubMed] [Google Scholar]
- 10.Antri M, Orsal D, Barthe JY. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. European Journal of Neuroscience. 2002;16:467. doi: 10.1046/j.1460-9568.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- 11.Basso DM, Beattie MS, Bresnehan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 12.Ichinose WE, Reinkensmeyer DJ, Aoyagi D, Lin JT, Ngai K, Edgerton VR, Harkema SJ, Bobrow JE. A robotic device for measuring and controlling pelvic motion during locomotor rehabilitation (man). Proc 25th Ann Intl Conf IEEE EMBS; Cancun, Mexico. 2003. [Google Scholar]