Abstract
Study Objective:
To compare the efficacy of bipolar radiofrequency ablation (RFA) and thermal balloon ablation (TBA) using treatment failure and postprocedure amenorrhea as outcome measures.
Design:
A population-based cohort study (Canadian Task Force classification II-2).
Setting:
Two medical centers in the Upper Midwest.
Patients:
Using the medical records linkage system of the Rochester Epidemiology Project, we identified 455 residents of Olmsted County, Minnesota, who underwent global endometrial ablation for menorrhagia from January 1, 1998, through December 31, 2005. Amenorrhea was defined as complete cessation of menstruation that started immediately after ablation and lasted at least 12 months. Treatment failure was defined as re-ablation or hysterectomy for persistent bleeding or pain. Time to treatment failure for each procedure was compared using Kaplan-Meier plots. Relevant clinical data and complications were abstracted from medical records. Risk adjustments were performed using Cox and logistic regression models.
Interventions:
RFA (n=255) and TBA (n=200).
Measurements and Main Results:
Mean (SD) patient age was 43.3 (5.5) years, and median follow-up was 2.2 years. The 3-year cumulative failure rate was 9% (95% confidence interval [CI], 5%-16%) for RFA and 12% (95% CI, 7%-16%) for TBA (P=.26). The difference remained nonsignificant after adjusting for known predictors of treatment failure such as age, parity, pretreatment dysmenorrhea, and tubal ligation (adjusted hazard ratio, 0.7; 95% CI, 0.4-1.4; P=.31). However, women had significantly higher amenorrhea rates after RFA when compared with TBA (32% vs 14%, P<.001). This difference remained significant after adjusting for known predictors of amenorrhea such as age, uterine length, and endometrial thickness (adjusted odds ratio, 2.9; 95% CI, 1.7-4.8; P<.001). Complications were infrequent and similar for the 2 groups.
Conclusion:
RFA and TBA were equally effective treatments for menorrhagia in a population-based cohort. However, women who underwent RFA were 3 times more likely to have postprocedure amenorrhea.
Keywords: endometrial ablation, menorrhagia, population-based cohort
Background
Excessive menstrual bleeding is a common gynecologic disorder that affects 10% to 15% of women and accounts for 3 million gynecologic office visits per year in the United States (1-6). Women with menorrhagia are 50% more likely to visit a physician, 80% more likely to visit the emergency department, and 65% more likely to undergo a surgical procedure (7-9). In addition, women with menorrhagia often have secondary anemia and fatigue. Even in the absence of anemia, excessive menstruation can be socially incapacitating, it often limits productivity, and it frequently has a negative impact on health-related quality of life (4,9-13).
Global endometrial ablation (GEA) procedures were introduced in the mid 1990s and were quickly proven as simple, effective, and safe therapies for menorrhagia, especially when compared with earlier hysteroscopic ablation procedures (14-17). Thermal balloon ablation (TBA) was the first method of GEA approved by the US Food and Drug Administration, and by the end of the 1990s, it had become the most clinically used GEA procedure. Bipolar radiofrequency ablation (RFA), introduced in 2003, has gained popularity because of its shorter procedural time. Currently, TBA and RFA are the most commonly used GEA procedures in the United States (17,18).
Several randomized clinical trials (RCTs) showed that the efficacy of the newer GEA methods was similar to hysteroscopic endometrial ablation but had an improved safety profile (14). Although GEA technologies are widely used in clinical practice, to date, only 2 RCTs have directly compared outcomes after RFA and TBA (19,20). Although these studies were well designed and had good follow-up rates, both had relatively small patient groups from single centers and a relatively short duration of follow-up. The objective of this study was to compare the efficacy of bipolar RFA and TBA for the management of menorrhagia in a population-based cohort of women from Olmsted County, Minnesota.
Materials and Methods
This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. The cohorts of patients included in this study were identified previously in a study from our research group. The methods used for identification and construction of the cohorts were previously published (21).
The Rochester Epidemiology Project
Primary and specialty health care services for residents of Olmsted County, Minnesota, are provided almost exclusively by Mayo Clinic and the Olmsted Medical Center (both in Rochester, Minnesota). The Rochester Epidemiology Project is a unique medical records linkage system that catalogs all aspects of health care delivered to Olmsted residents (22). Medical diagnoses and surgical interventions for each patient are routinely abstracted and coded according to the Hospital Adaptation of the International Classification of Diseases (23). These computerized indices allow the linkage of medical records from all providers and facilitate the evaluation of disease determinants and outcomes after surgical procedures (22). We searched the Rochester Epidemiology Project databases to identify all women who underwent GEA in Olmsted County from January 1, 1998, through December 31, 2005.
Interventions
Part of the cohort underwent GEA with TBA (ThermaChoice, Gynecare, Somerville, New Jersey), a method introduced in 1998 (24). The others were treated with bipolar RFA (NovaSure, Cytyc Surgical Products, Palo Alto, California), a method introduced in 2003 (25). Before the procedure, all women had a Papanicolaou test, endometrial sampling, pelvic ultrasonography, and office hysteroscopy if structural uterine lesions were suspected. Only women with benign polyps or submucous leiomyomas not distorting the endometrial cavity or less than 2 cm in size were offered endometrial ablation. Removal was by dilation and curettage or ablation in situ.
Baseline and Procedural Data
Baseline data were obtained for each patient, including age, parity, body mass index, pattern of bleeding, presence of dysmenorrhea, uterine length, uterine position, presence of fibroids or polyps, endometrial thickness, and endometrial pathology. Previous operations, including cesarean births and tubal sterilization, were recorded. For procedural data, we noted the type of anesthesia used, total procedural time, balloon fluid volume and pressure for TBA, and the power setting for RFA.
Follow-up and Measurement of Different Outcomes
Treatment failure was defined as the need for another ablation procedure or hysterectomy at any point during follow-up, and time to treatment failure was the primary end point for the evaluation of outcome after GEA. Patients with treatment failure were identified by using the relevant International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes; records were reviewed individually to confirm failure and performance of re-ablation or hysterectomy. To avoid the confounding effect of menopause, the menstrual outcome of primary interest was amenorrhea; this was defined as the documented complete cessation of menstruation that began immediately after GEA and lasted for at least 12 months. Other secondary outcomes included postprocedural change in duration of bleeding, hemoglobin levels, and ferritin levels. Intraprocedural and postprocedural complications were recorded.
To minimize measurement bias, an independent ICD-9-CM code search for postprocedure endometrial cancer, pregnancy, and pelvic pain was conducted. Postprocedural mortality rates were determined by searching the Rochester Epidemiologic Project database. In addition, to test the reliability of outcome measurements, 50 women were randomly selected using the random row selection function of JMP version 6.0 (SAS Institute Inc, Cary, North Carolina). The outcomes of these 50 women were reviewed by one of the investigators (M.R.H.), who was masked to the outcome determined by the initial chart review. The agreement was evaluated using κ statistics. κ statistics is an index that compares the observed agreement against the agreement that might be expected by chance. κ can be considered the chance-corrected proportional agreement, and possible values range from +1 (perfect agreement) to 0 (no agreement above that expected by chance) to −1 (complete disagreement). The estimates of κ agreement were interpreted according to Landis and Koch (26) (<0, no agreement; 0.0-0.19, poor agreement; 0.20-0.39, fair agreement; 0.40-0.59, moderate agreement; 0.60-0.79, substantial agreement; and 0.80-1.00, almost perfect agreement).
Statistical Analysis
Continuous data with normal distribution are presented as mean (SD); median and interquartile range (IQR) are used for skewed data. Normality was assessed by evaluating the shape of the frequency histogram. Categorical data are presented as number and percent of patients. Baseline characteristics of treatment cohorts were compared using the t test or Wilcoxon rank sum (Mann-Whitney) test for continuous variables according to the distribution, and the χ2 test or Fisher exact test were used for comparison of categorical variables as appropriate.
Time to treatment failure was evaluated with Kaplan-Meier curves and tested with the log-rank test. The plots showing cumulative failure rates were used to calculate 3-year failure rates and corresponding 95% confidence intervals (CI). The proportion of women who had amenorrhea in each group were compared using the χ2 test. For other secondary outcomes and complications, comparisons between the 2 groups were based on the difference in the means for continuous variables and on the proportion of women with a specific finding for categorical variables; the paired t test, Wilcoxon signed rank test, and McNemar test were used as appropriate. Paired testing was used to compare postprocedure changes in hemoglobin and ferritin for each patient. A subgroup analysis was performed to explore whether TBA patient characteristics differed after the introduction of RFA in 2003; differences that might have affected outcomes could be considered evidence of selection bias.
Regression models were used to adjust for potential bias caused by known confounders. For treatment failure, Cox proportional hazard modeling was used to adjust for age, parity, pretreatment dysmenorrhea, and tubal ligation. These 4 variables were identified previously as significant pretreatment predictors of treatment failure in our population (21). Similarly, for evaluation of postablation amenorrhea, logistic regression modeling was used to adjust for age, uterine length, and endometrial thickness. These variables were significantly associated with amenorrhea in our population (26). Adjusted hazard ratios and odds ratios (ORs) were used to evaluate the likelihood of treatment failure and immediate amenorrhea. All statistical analyses were 2-sided, and P values less than .05 were considered significant. All statistical analyses were performed using JMP version 6.0 (SAS Institute Inc, Cary, North Carolina).
Results
Study Subjects
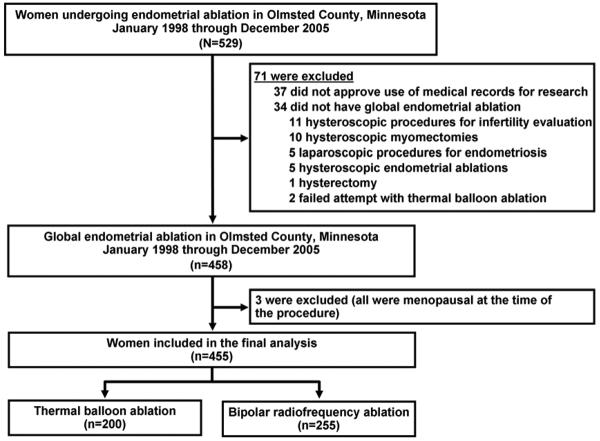
Of the 458 women identified, 3 women were excluded because of menopause at the time of the procedure. Thus, 455 women were included in the analyses: 255 underwent RFA and 200 underwent TBA (Figure 1). The mean age was 43.3 (5.6) years and the median parity was 2 (IQR, 2-3). The median follow-up was 2.2 years (IQR, 1.3-3.5 years), and only 3 patients (<1%) were lost to follow-up. Baseline patient characteristics and clinical data were similar for the RFA and TBA groups (Table 1). Comparing baseline characteristics of patients who underwent TBA before and after the 2003 introduction of bipolar RFA showed statistically significant differences in the preprocedure ferritin levels, use of ultrasound, and number of patients with submucous fibroids. Other preoperative variables were similar for both cohorts of patients, including known confounders such as age, parity, preprocedure dysmenorrhea, tubal ligation, uterine sounding length, and endometrial thickness (Table 2).
Figure 1.
Study Flow Chart. Adapted from El-Nashar et al (21). Used with permission.
Table 1.
Baseline Patient Characteristics
| Characteristica | Radiofrequency Ablation (n=255) |
Thermal Balloon Ablation (n=200) |
P Values |
|---|---|---|---|
| Age, y | 43.4 (5.5) | 43.0 (5.6) | .44b |
| Body mass index, kg/m2 | 29.4 (7.6) | 29.3 (7.4) | .91b |
| Parity, median (IQR) | 2 (2-3) | 2 (2-3) | .97c |
| Previous cesarean delivery | 45 (18%) | 29 (15%) | .53d |
| Tubal ligation | 86 (34%) | 62 (31%) | .54d |
| Metrorrhagia | 129 (51%) | 88 (44%) | .16d |
| Preprocedure bleeding, d | 9.9 (6.0) | 9.8 (5.8) | .88c |
| Preoperative dysmenorrhea | 13 (5%) | 9 (5%) | .77e |
| Uterine length (uterine sounding), cm | 9.1 (1.2) | 9.0 (1.3) | .74b |
| Retroverted uterus | 14 (6%) | 13 (7%) | .69e |
| Hemoglobin, g/dL | 12.4 (1.4) | 12.0 (1.7) | .003b |
| Ferritin, mcg/L | 20.2 (33.3) | 20.0 (41.9) | .98b |
| Preprocedure US | 202 (79%) | 147 (74%) | .15d |
| Endometrial thickness, mm | 9.2 (6.1) | 9.1 (4.2) | .88c |
| Adenomyosis (US) | 10 (4%) | 10 (5%) | .49e |
| Intracavitary lesions (hysteroscopy) | 85 (33%) | 57 (29%) | .76d |
| Polyps (US and/or hysteroscopy) | 63 (25%) | 45 (23%) | .58d |
| Submucous fibroids (US and/or hysteroscopy) | 25 (10%) | 13 (7%) | .24e |
| Fibroids other than submucous (US) | 52 (20%) | 42 (21%) | .56d |
| Lost to follow-up (no visits after ablation) | 3 (1%) | 0 (0%) | .26e |
Abbreviations: IQR, interquartile range; US, ultrasound.
Data are shown as mean (SD) or number of patients (percentage of sample) unless otherwise indicated.
Independent sample t-test.
Wilcoxon rank sum test.
χ2 Test.
Fisher exact test.
Table 2.
Baseline Patient Characteristics of Women who Underwent Thermal Balloon Ablation Before and After Introduction of Bipolar Radiofrequency Ablation in 2003
| Characteristica | Treatment from 1998-2002 (n=143) |
Treatment from 2003-2005 (n=57) |
P Values |
|---|---|---|---|
| Age, y | 42.7 (5.8) | 43.7 (5.1) | .28b |
| Body mass index, kg/m2 | 29.6 (7.5) | 28.7 (7.3) | .43b |
| Parity, median (IQR) | 2 (2-3) | 2 (2-3) | .34c |
| Previous cesarean delivery | 21 (16%) | 8 (14%) | .89d |
| Tubal ligation | 48 (34%) | 14 (25%) | .21d |
| Metrorrhagia | 66 (46%) | 22 (39%) | .33d |
| Preprocedure bleeding, d | 9.8 (5.9) | 9.8 (5.6) | .98c |
| Preoperative dysmenorrhea | 8 (6%) | 1 (2%) | .45e |
| Uterine length (uterine sounding), cm | 9.1 (1.2) | 9.0 (1.5) | .66b |
| Retroverted uterus | 8 (6%) | 5 (9%) | .41e |
| Hemoglobin, g/dL | 12.0 (1.6) | 12.0 (2.1) | .81b |
| Ferritin, mcg/L | 24.0 (46.8) | 5.9 (3.8) | .02b |
| Preprocedure US | 99 (69%) | 48 (84%) | .03d |
| Endometrial thickness, mm | 8.6 (4.4) | 10.0 (3.6) | .09c |
| Adenomyosis (US) | 5 (4%) | 5 (9%) | .30e |
| Intracavitary lesions (hysteroscopy) | 40 (28%) | 17 (30%) | .22d |
| Polyps (US and/or hyeroscopy) | 33 (23%) | 45 (21%) | .76d |
| Submucous fibroids (US and/or hysteroscopy) | 5 (3%) | 8 (14%) | .01e |
| Fibroids other than submucous (US) | 26 (18%) | 16 (28%) | .38d |
| Lost to follow-up (no visits after ablation) | 0 (0%) | 0 (0%) | … |
Abbreviations: IQR, interquartile range; US, ultrasound.
Data are shown as mean (SD) or number of patients (percentage of sample) unless otherwise indicated.
Independent sample t-test.
Wilcoxon rank sum test.
χ2 Test.
Fisher exact test.
Treatment Failure
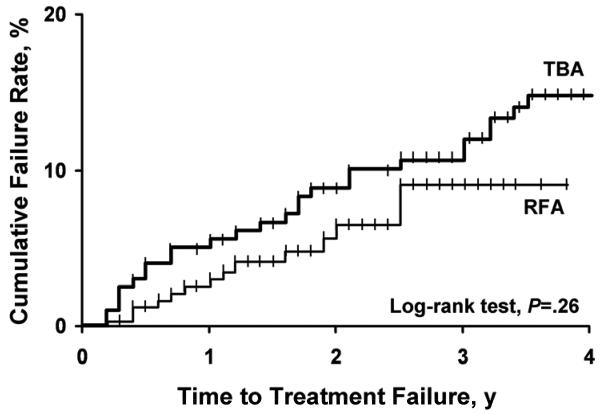
The Kaplan-Meier plot showed no difference between RFA and TBA for time to treatment failure (P=.26) (Figure 2). At 3 years, the cumulative failure rate was 9.3% (95% CI, 5.1%-16.0%) in the RFA group and 11.9% (95% CI, 7.0%-16.0%) in the TBA group (Table 3). Age younger than 45 years, parity of 5 or higher, pretreatment dysmenorrhea, and tubal ligation were previously identified as factors significantly associated with treatment failure (21). After adjusting for these variables, the likelihood of treatment failure was similar for RFA and TBA, with an adjusted hazard ratio of 0.70 (95% CI, 0.4-1.4; P=.31).
Figure 2.
Cumulative treatment failure rate after bipolar radiofrequency ablation (RFA) or thermal balloon ablation (TBA). Treatment failure was defined by the performance of re-ablation or hysterectomy.
Table 3.
Cumulative Failure Ratesa
| RFA (n=255) |
TBA (n=200) |
|||
|---|---|---|---|---|
| Time | No. of Patients at Risk |
CFR (95% CI), % |
No. of Patients at Risk |
CFR (95% CI), % |
| 1 year | 197 | 2.6 (1.1-5.6) | 178 | 5.0 (3.0-9.0) |
| 2 years | 103 | 5.7 (3.0-10.0) | 156 | 8.8 (4.7-13.8) |
| 3 years | 34 | 9.3 (5.1-16.0) | 133 | 11.9 (7.0-16.0) |
Abbreviations: CFR, cumulative failure rate per 100 patients; CI, confidence interval; RFA, radiofrequency ablation; TBA, thermal balloon ablation.
Calculated by using the Kaplan-Meier method.
Amenorrhea After the Procedure
Of the 455 GEA procedures performed, evaluation of amenorrhea was possible for 412 women (91%). Of those, 96 (23%) met the criteria for amenorrhea. However, subgroup analysis showed a higher amenorrhea rate in the RFA group (70/221 patients) than the TBA group (26/191 patients) (unadjusted OR, 2.94; 95% CI, 1.78-4.86; P<.001). Age greater than 45 years, uterine length less than 9 cm, and endometrial thickness less than 4 mm were previously identified as significant predictors of immediate amenorrhea in our cohort (21). After adjusting for these variables, the likelihood of postprocedure amenorrhea remained significantly higher for women undergoing RFA (adjusted OR, 2.86; 95% CI, 1.67-4.76; P<.001). The agreement in the evaluation of postablation amenorrhea between the masked investigator and the initial chart reviewer was substantial (κ =0.717; 95% CI, 0.489-0.945).
Secondary End Points
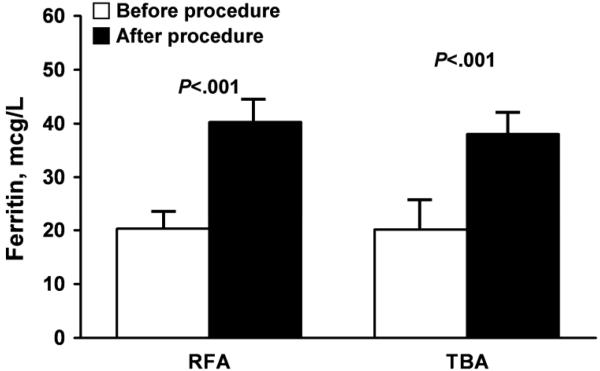
Significantly fewer patients needed general anesthesia in the RFA group compared with the TBA group (P<.001), and the mean procedure time was significantly shorter for women undergoing RFA (P=.006) (Table 4). Table 5 shows that both cohorts had significantly reduced bleeding after treatment (measured in days per month). For the RFA group, median bleeding was reduced from 7 (IQR, 6-10) to 0 (IQR, 0-2) days (P<.001); for the TBA group, bleeding was reduced from 7 (IQR, 7-10) to 3 (IQR, 0-5) days. The reduction in days of bleeding was significantly greater (ie, reduced by more days) in the RFA group (P=.006). Statistically significant increases in hemoglobin levels and ferritin levels were observed after the procedure in both groups, with a mean increase of 1.2 (1.4) g/dL for hemoglobin and 19.1 (33.1) mcg/L for ferritin. The relative increases in hemoglobin and ferritin were similar for both groups (Figure 3).
Table 4.
Surgical Procedure Variables
| Variablea | RFA (n=255) | TBA (n=200) | P values |
|---|---|---|---|
| General anesthesia | 108 (42%) | 174 (88%) | <.001 |
| ASA physical status III or higher | 6 (2%) | 9 (5%) | .29 |
| Admission to postanesthesia care unit | 113 (44%) | 76 (38%) | .18 |
| Duration of the procedure, min | 35.6 (22) | 43.5 (26) | .006 |
| Preprocedure dilation and curettage | 177 (69%) | 142 (71%) | .68 |
| RFA power setting, watts | 128.5 (30.8) | NA | … |
| RFA coagulation, s | 81.0 (22.1) | NA | … |
| TBA balloon fluid volume, mL | NA | 16.3 (7.6) | … |
| TBA balloon pressure, mm Hg | NA | 163.2 (16.6) | … |
Abbreviations: ASA, American Society of Anesthesia; NA, not applicable; RFA, radiofrequency ablation; TBA, thermal balloon ablation.
Categorical data are presented as number of patients (percentage of sample); continuous data are presented as mean (SD).
Table 5.
Secondary End Points
| RFA (n=255) |
TBA (n=200) |
||||||
|---|---|---|---|---|---|---|---|
| Characteristica | Before Treatment |
After Treatment |
Paired P Valueb |
Before Treatment |
After Treatment |
Paired P Valueb |
P Valuec |
| Bleeding or spotting, d | 7 (6-10) | 0 (0-2) | <.001 | 7 (7-10) | 3 (0-5) | <.001 | .006 |
| Menstrual accidents, No. of patients | 107 (42%) | 4 (2%) | <.001 | 71 (36%) | 10 (5%) | <.001 | .09 |
| Hemoglobin, g/dL | 12.6 (1.4) | 13.4 (1.1) | <.001 | 12.0 (1.7) | 13.4 (1.3) | <.001 | .19 |
| Ferritin, mcg/L | 20.2 (33.3) | 40.3 (32.4) | <.001 | 20.0 (41.9) | 37.9 (26.6) | <.001 | .96 |
Abbreviations: IQR, interquartile range; RFA, radiofrequency ablation; TBA, thermal balloon ablation.
Continuous data are presented as mean (SD) for normally distributed data and as median (IQR) for skewed data. Categorical data are presented as number of patients (percentage of sample).
Comparisons within each group were performed by using the paired t test for continuous variables and the McNemar test for categorical variables.
Comparisons between the 2 groups were based on the difference in mean values. Continuous variables were compared by using the independent sample t test. Categorical variables were compared by using the Fisher exact test.
Figure 3.
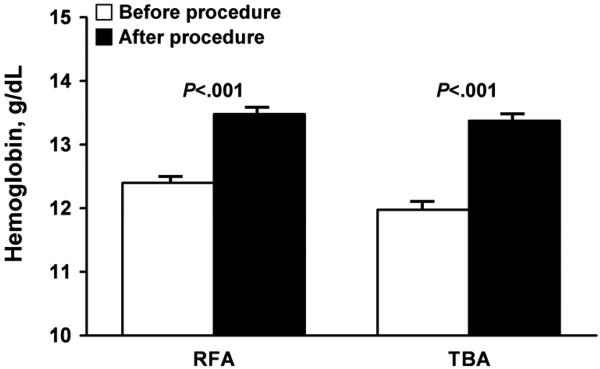
Secondary treatment outcomes after bipolar radiofrequency ablation (RFA) or thermal balloon ablation (TBA). A, Hemoglobin levels (difference between procedures, P=.19). B, Ferritin levels (difference between procedures, P=.96). TBA and RFA outcomes were compared by assessing the differences in the mean change.
Procedure-Related Complications
Intraprocedural and postprocedural complications were minor, infrequent, and generally similar for the RFA and TBA groups. Postprocedural pregnancy occurred for 3 patients (1%); all had first-trimester spontaneous abortions. No cases of endometrial cancer after ablation were reported, and no deaths were reported during follow-up.
Discussion
In this study, we directly compared the effectiveness of bipolar RFA and TBA in a population-derived cohort. Outcome measures included treatment failure and postablation amenorrhea. We reported procedural characteristics and other secondary outcomes, including duration of menstruation and change in hemoglobin and ferritin levels after ablation. Procedure-related complications were also documented and reported.
We observed no significant differences in treatment failure rates between RFA and TBA (unadjusted 3-year cumulative failure rate of 9% for RFA and 12% for TBA). The similar likelihood of treatment failure was unchanged after adjusting for known confounders of treatment failure, including age, parity, pretreatment dysmenorrhea, and history of tubal ligation. To date, only the RCT conducted by Abbott and colleagues (20) has examined treatment failure in RFA and TBA; however, their trial was not statistically powered to compare treatment failure in the 2 groups, and they did not observe a significant difference in the rate of additional surgery within 12 months. In addition, at least 3 years of follow-up are needed for adequate evaluation of treatment failure after ablation (27).
Another important finding of this study was that the postablation amenorrhea rate in the RFA group was approximately 3 times higher than that observed in the TBA group (unadjusted OR, 2.94). This finding was still true after adjusting for known confounders of amenorrhea, including age, uterine length, and endometrial thickness (adjusted OR, 2.86). Our results were consistent with the 2 previous RCTs that directly compared RFA and TBA and reported higher amenorrhea rates after RFA (Table 6) (19,20).
Table 6.
Comparison of Outcomes With Previously Published Studiesa
| RFA |
TBA |
||||
|---|---|---|---|---|---|
| Study | No. | % | No. | % | RR (95% CI) |
| Treatment failure at 12 months | |||||
| Abbott et al (20), 2003 | 6/37 | 16 | 0/18 | 0 | 6.50 (0.39, 109.40) |
| Bongers et al (19), 2004 | 4/83 | 5 | 4/43 | 9 | 0.52 (0.14, 1.97) |
| Current study | 5/197 | 3 | 9/178 | 5 | 0.50 (0.17, 1.47) |
| Postablation amenorrhea | |||||
| Abbott et al (20), 2003 | 16/37 | 43 | 2/18 | 11 | 3.89 (1.00, 15.13) |
| Bongers et al (19), 2004 | 34/83 | 41 | 3/43 | 7 | 5.87 (1.91, 18.02) |
| Current study | 70/221 | 32 | 26/191 | 14 | 2.33 (1.55, 3.49) |
Abbreviations: CI, confidence interval; RFA, bipolar radiofrequency ablation; RR, risk ratio; TBA, thermal balloon ablation.
RevMan software (version 4.2.8; The Cochrane Collaboration, Oxford, United Kingdom) was used to calculate risk ratios and 95% confidence intervals using the random effects statistical method.
Compared with previous trials, the strengths of this study were the inclusion of a larger, population-based sample, longer duration of follow-up, and the use of regression models to adjust for known confounders. Women in both groups had a significant reduction in the duration of bleeding and in the number of menstrual accidents after ablation. The observed reduction in the duration of menstruation was significantly greater (ie, reduced by more days) in the RFA group. This finding further confirmed the superiority of RFA in reducing menstrual blood loss (19,20). Nonetheless, both groups had similar increases in hemoglobin and ferritin levels. Finally, our results further support the safety of GEA technologies. Complications were generally minor and infrequent, and the complication rates were similar for both groups. In our cohort, pregnancy was reported by 3 women (<1%). Preprocedural contraception counseling is warranted.
The main limitation of this study was its retrospective nature. Objective measures of treatment outcomes (eg, validated bleeding scores, evaluation of menorrhagia-related quality of life) were documented inconsistently in our cohort. However, we used hysterectomy and re-ablation rates as surrogate measures of treatment failure, and hemoglobin levels before and after ablation were used as an indirect indicator of bleeding severity. Because nonrandomized studies can falsely show significant differences between groups for baseline characteristics, we used multivariate Cox and logistic regression models to adjust for known confounders of treatment failure and amenorrhea, respectively. We also acknowledge the potential for selection bias in favor of RFA after its introduction in 2003. We compared patient characteristics in the TBA group before and after introduction of RFA and observed no significant differences between the groups for confounders of treatment failure or amenorrhea. Thus, we believe the effect of selection bias was minimal.
In summary, we reported the first population-based cohort study that compared the long-term effectiveness of 2 GEA technologies. When using treatment failure as an outcome measure, RFA and TBA were equally effective options for management of menorrhagia. However, women who underwent RFA were 3 times more likely to have postprocedure amenorrhea compared with women who underwent TBA.
Abbreviations
- CI
confidence interval
- GEA
global endometrial ablation
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IQR
interquartile range
- OR
odds ratio
- RCT
randomized clinical trial
- RFA
radiofrequency ablation
- TBA
thermal balloon ablation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 37th Global Congress of Minimally Invasive Gynecology, Las Vegas, Nevada, October 28-November 1, 2008.
Conflict of interest: None
References
- 1.Hallberg L, Hogdahl AM, Nilsson L, Rybo G. Menstrual blood loss: a population study: variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand. 1966;45(3):320–51. doi: 10.3109/00016346609158455. [DOI] [PubMed] [Google Scholar]
- 2.Cole SK, Billewicz WZ, Thomson AM. Sources of variation in menstrual blood loss. J Obstet Gynaecol Br Commonw. 1971 Oct;78(10):933–9. doi: 10.1111/j.1471-0528.1971.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Eijkeren MA, Christiaens GC, Sixma JJ, Haspels AA. Menorrhagia: a review. Obstet Gynecol Surv. 1989 Jun;44(6):421–9. doi: 10.1097/00006254-198906000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Prentice A. Fortnightly review: medical management of menorrhagia. BMJ. 1999 Nov 20;319(7221):1343–5. doi: 10.1136/bmj.319.7221.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warner PE, Critchley HO, Lumsden MA, Campbell-Brown M, Douglas A, Murray GD. Menorrhagia II: is the 80-mL blood loss criterion useful in management of complaint of menorrhagia? Am J Obstet Gynecol. 2004 May;190(5):1224–9. doi: 10.1016/j.ajog.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Warner PE, Critchley HO, Lumsden MA, Campbell-Brown M, Douglas A, Murray GD. Menorrhagia I: measured blood loss, clinical features, and outcome in women with heavy periods: a survey with follow-up data. Am J Obstet Gynecol. 2004 May;190(5):1216–23. doi: 10.1016/j.ajog.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Cote I, Jacobs P, Cumming DC. Use of health services associated with increased menstrual loss in the United States. Am J Obstet Gynecol. 2003 Feb;188(2):343–8. doi: 10.1067/mob.2003.92. [DOI] [PubMed] [Google Scholar]
- 8.Shapley M, Jordan K, Croft PR. An investigation in primary care of the relationship between consultation behaviour, increased vaginal bleeding and mental disorder. J Obstet Gynaecol. 2004 Sep;24(6):684–6. doi: 10.1080/01443610400008040. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health. 2007 May-Jun;10(3):183–94. doi: 10.1111/j.1524-4733.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 10.Cote I, Jacobs P, Cumming D. Work loss associated with increased menstrual loss in the United States. Obstet Gynecol. 2002 Oct;100(4):683–7. doi: 10.1016/s0029-7844(02)02094-x. [DOI] [PubMed] [Google Scholar]
- 11.Clark TJ, Khan KS, Foon R, Pattison H, Bryan S, Gupta JK. Quality of life instruments in studies of menorrhagia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2002 Sep 10;104(2):96–104. doi: 10.1016/s0301-2115(02)00076-3. [DOI] [PubMed] [Google Scholar]
- 12.Jones GL, Kennedy SH, Jenkinson C. Health-related quality of life measurement in women with common benign gynecologic conditions: a systematic review. Am J Obstet Gynecol. 2002 Aug;187(2):501–11. doi: 10.1067/mob.2002.124940. [DOI] [PubMed] [Google Scholar]
- 13.Thomas SL, Ellertson C. Nuisance or natural and healthy: should monthly menstruation be optional for women? Lancet. 2000 Mar 11;355(9207):922–4. doi: 10.1016/S0140-6736(99)11159-0. [DOI] [PubMed] [Google Scholar]
- 14.Lethaby A, Hickey M, Garry R. Endometrial destruction techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005 Oct 19;(4):CD001501. doi: 10.1002/14651858.CD001501.pub2. [DOI] [PubMed] [Google Scholar]
- 15.McGurgan P, O'Donovan P. Endometrial ablation. Curr Opin Obstet Gynecol. 2003 Aug;15(4):327–32. doi: 10.1097/01.gco.0000084244.09900.94. [DOI] [PubMed] [Google Scholar]
- 16.Sharp HT. Assessment of new technology in the treatment of idiopathic menorrhagia and uterine leiomyomata. Obstet Gynecol. 2006 Oct;108(4):990–1003. doi: 10.1097/01.AOG.0000232618.26261.75. [DOI] [PubMed] [Google Scholar]
- 17.Vilos GA. Hysteroscopic and nonhysteroscopic endometrial ablation. Obstet Gynecol Clin North Am. 2004 Sep;31(3):687–704. doi: 10.1016/j.ogc.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Cooper J, Gimpelson RJ. Summary of safety and effectiveness data from FDA: a valuable source of information on the performance of global endometrial ablation devices. J Reprod Med. 2004 Apr;49(4):267–73. [PubMed] [Google Scholar]
- 19.Bongers MY, Bourdrez P, Mol BW, Heintz AP, Brolmann HA. Randomised controlled trial of bipolar radio-frequency endometrial ablation and balloon endometrial ablation. BJOG. 2004 Oct;111(10):1095–102. doi: 10.1111/j.1471-0528.2004.00253.x. [DOI] [PubMed] [Google Scholar]
- 20.Abbott J, Hawe J, Hunter D, Garry R. A double-blind randomized trial comparing the Cavaterm and the NovaSure endometrial ablation systems for the treatment of dysfunctional uterine bleeding. Fertil Steril. 2003 Jul;80(1):203–8. doi: 10.1016/s0015-0282(03)00549-1. [DOI] [PubMed] [Google Scholar]
- 21.El-Nashar SA, Hopkins MR, Creedon DJ, Sauver JL, Weaver AL, McGree ME, et al. Prediction of treatment outcomes after global endometrial ablation. Obstet Gynecol. 2009 Jan;113(1):97–106. doi: 10.1097/AOG.0b013e31818f5a8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996 Mar;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 23.Commission on Professional and Hospital Activities . H-ICDA, hospital adaptation of ICDA. 2nd ed. Commission on Professional and Hospital Activities; Ann Arbor (MI): 1973. [Google Scholar]
- 24.Neuwirth RS, Duran AA, Singer A, MacDonald R, Bolduc L. The endometrial ablator: a new instrument. Obstet Gynecol. 1994 May;83(5 Pt 1):792–6. [PubMed] [Google Scholar]
- 25.Gallinat A, Nugent W. NovaSure impedance-controlled system for endometrial ablation. J Am Assoc Gynecol Laparosc. 2002 Aug;9(3):283–9. doi: 10.1016/s1074-3804(05)60405-7. [DOI] [PubMed] [Google Scholar]
- 26.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–74. [PubMed] [Google Scholar]
- 27.Parkin DE. Prognostic factors for success of endometrial ablation and resection. Lancet. 1998 Apr 18;351(9110):1147–8. doi: 10.1016/S0140-6736(05)79116-9. [DOI] [PubMed] [Google Scholar]