Abstract
Hyperhomocysteinemia is an independent risk factor for atherosclerosis. Uptake of homocysteine induces oxidative stress in macrophages. Antioxidant response elements (AREs) are regulatory elements within promoters of genes, which protect cells against oxidative stress. The current study investigated whether homocysteine induces transcription of glutamate-cysteine ligase (Gcl), via ARE driven gene expression in mouse macrophages. Gcl is the rate-limiting enzyme in the synthesis of glutathione, an important endogenous antioxidant. Gcl is heterodimeric and the genes encoding the subunits of Gcl contain several AREs within their 5′-promoter regions. Treatment of mouse macrophages with d-/l-homocysteine (50 µM) induced depletion of intracellular glutathione and a compensatory increase in Gcl activity. Electro mobiliy shift assays demonstrated increased binding of nuclear proteins to ARE-containing oligonucleotides. Real-time RT-PCR revealed increased mRNA-expression of the catalytic subunit of Gcl (Gclc) after treatment with homocysteine, and this occurred via increased transcription as demonstrated with luciferase promoter reporter constructs for Gclc. Additional site directed mutagenesis demonstrated that ARE4 plays a direct role in mediating induction of Gclc by homocysteine. Supershift analysis and Western blotting revealed that Nrf2 signalling is critical in homocysteine-induced activation of ARE4. Inhibition of MAP kinase activity reduced binding of nuclear proteins to the AREs, nuclear expression of Nrf2 and mRNA expression of Gclc. Western blotting demonstrated phosporylation of ERK1/2 in homocysteine treated macrophages. These data suggest that ARE-driven gene expression of Gclc via a MEK/Nrf2 pathway could help to protect macrophages from oxidative stress due to hyperhomocysteinemia.
Keywords: Homocysteine, Atherosclerosis, Oxidative stress, Glutamate-cysteine ligase, Antioxidant response element
1. Introduction
Elevated plasma homocysteine levels have been implicated as a risk factor for atherosclerosis, resulting in coronary artery disease, cerebrovascular disease and peripheral vascular disease [1–3]. The enzyme 5,10-methyltetrahydrofolate (MTHFR) plays a critical role in regulating plasma homo-cysteine, and a mild deficiency in MTHFR is a common cause for hyperhomocysteinemia (HHcy, [4]). The underlying mechanisms how HHcy contributes to the pathogenesis of atherosclerosis are still poorly understood and may be multifactorial. Several lines of evidence suggest that endothelial dysfunction due to HHcy promotes initiation and progression of atherosclerosis. Impaired endothelium-dependent relaxation caused by a lack of nitric oxide-mediated signalling plays a key role in vascular changes caused by HHcy [5,6]. Recent studies suggest that increased oxidative stress due to HHcy is also a major contributor to the mechanisms involved in atherogenesis. For example,HHcy can increase the production of reactive oxygen species [7]. Although changes regarding endothelial cells have received most of the attention, HHcy can also affect other cell types involved in atherogenesis, such as smooth muscle cells and macrophages [8–10].
It has been hypothesized that macrophages play key roles in the initiation and in the progression of atherosclerosis. Ultimately, macrophages can induce atherosclerotic plaque rupture by secreting proteases that destroy the extracellular matrix which otherwise provides physical strength to the fibrous cap [11]. The uptake of homocysteine and other pro-oxidants, such as oxidized low-density lipoproteins can lead to oxidative stress, resulting in additional activation of macrophages, ultimately leading to macrophage cell death. This is of particular significance since macrophage cell death in late atherosclerotic lesion development can cause a proinflammatory response with subsequent plaque instability [12]. Oxidative stress in macrophages can cause a depletion and a compensatory increase in the synthesis of primary endogenous antioxidants, such as glutathione (GSH). The increase of cellular GSH in response to elevated homocysteine has been demonstrated in various cell types [13,14]. This can either be due to increased transsulfuration, leading to the formation of cysteine, a precursor for GSH synthesis, or it can also be due to increased de novo synthesis of GSH. The rate-limiting step in GSH synthesis is carried out by the heterodimeric enzyme glutamate-cysteine ligase (Gcl), comprised of catalytic (Gclc) and modifier (Gclm) subunits [16,17]. These subunits are products of two different genes, and the promoters for both genes contain consensus antioxidant response elements (AREs). AREs are known to play essential roles in regulating the cellular responses to oxidative stress [17,18]. The current study was designed to investigate whether homocysteine induces the expression of Gclc in murine macrophages, and whether this induction is mediated by AREs present within its promoter.
2. Materials and methods
2.1. Cell culture
The murine macrophage cells (RAW264.7; ATCC, Manassas, VA) were grown in DMEM (Gibco BRL, Karlsruhe, Germany) and supplemented with 10% heat-inactivated FCS. Before the experiments, cells were plated in this growth medium and after attachment, they were cultured in serum-free medium (0.1% BSA in DMEM) for 24 h. For transfection assays, activity assay, flow cytometry and RNA isolations, cells were seeded at a density of 5×105 per well in six well culture plates. For isolation of nuclear extracts, the cells were plated at a density of 2× 106 in 10 cm culture dishes. Homocysteine was used as d-/l-homocysteine (Sigma–Aldrich, Taufkirchen, Germany) at a concentration of 50 µM, which corresponds to 25µM l-homocysteine. This concentration can be found in hyperhomocysteinemic individuals [19].
2.2. Intracellular glutathione content
Glutathione (GSH) content of individual cells was measured by flow cytometry as previously reported [20,21]. Subsequent to homocysteine exposure for the indicated times, cells were harvested from plates and stained with monochlorobimane (Invitrogen/Molecular Probes, Eugene, OR; 10µM final concentration in PBS) for 10 min at 37 °C. Propidium iodide (Invitrogen; 2 µM final) was then added and the cells were analyzed on a Beckman–Coulter Elite flow cytometer using 350 nm excitation and 475±30 nm fluorescence emission for the glutathione-bimane adduct and >605 nm (PI emission). Analysis of cellular GSH content was restricted to PI negative (intact) cells.
2.3. Glutamate cysteine ligase activity
Gcl activity was measured using monobromobimane derivitization and HPLC analysis with fluorescence detection as previously reported [22].
2.4. RNA-isolation and quantitive RT-PCR
Total RNA was extracted from RAW cells using Tripure reagent (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s instructions. Two micrograms of total RNA was subjected to reverse transcription, followed by real-time quantitative PCR for β-actin and murine Gclc as described earlier [18]. The transcript for the constitutive gene product β-actin was used for data normalization.
2.5. Preparation of nuclear extracts
Cells were washed three times with ice-cold PBS and lysed directly on the culture dish in 1.0 ml cold lysis buffer, then transferred to a micro-centrifuge tube and incubated on ice for 5 min. The nuclei were pelleted (1250 × g, 4 °C, 5 min), and the supernatant was discarded. Nuclear proteins were extracted in 50 µl cold extraction buffer. Extraction time was 20 min on ice. Cellular debris was removed by centrifugation (1250 × g, 4 °C, 5 min). Supernatants containing the nuclear proteins were stored at −80 °C. Protein concentrations were measured by the method of Bradford (Bio-Rad, München, Germany).
2.6. Electrophoretic mobility shift assay (EMSA)
Double stranded oligonucleotides were radiolabeled with γ-32P-ATP using T-4 polynucleotide kinase. The sequences for the probes were as follows. Bold letters indicate the core consensus ARE sequences.
Gclc-ARE3: 5′-CGG GCCGATGGTCAC GTC GCC-3′
Gclc-ARE4: 5′-GCA GAG TGC TGA GTC ACG GTG AG-3′
Five micrograms of nuclear protein were incubated with the labeled oligonucleotides and loaded on a 4% non-denaturating polyacrylamide gel for separation from the unbound oligonucleotides according to the manufacturer’s specifications (Promega, Madison, WI, USA). Gels were exposed to X-ray film for 12–24 h. For the supershift experiments, anti-Nrf2 (Santa Cruz, Santa Cruz, CA, USA) antibody was added at a dilution of 1:200 subsequent to the labeled oligonucleotide. Densitometry analysis was performed using Bio-rad instruments and was measured in arbitrary units.
2.7. Western blotting
Nuclear protein extracts (15 µg/lane) were separated by SDS-PAGE using a 12% separating gel and transferred to a nitrocellulose membrane for immunoblotting. Rabbit anti-phospho p44/42 Map Kinase, p44/42 Map Kinase, p38 MAPK (Cell Signaling, Beverly, MA) and Nrf2 (Santa Cruz) antibodies were used. Densitometry analysis was performed using Bio-rad instruments and was measured in arbitrary units.
2.8. Plasmids and transfections
Luciferase reporter constructs containing the murine 6.5 kb Gclc promoter have been described elsewhere [18]. Cells were transfected with 1.5µg DNA/well using Lipofectamine reagent (Gibco, Gaithersburg, MD, USA) according to the manufacturer’s protocol. All samples were co-transfected with a CMV-Renilla luciferase construct to compensate for variances in transfection efficiency (less then 10%). Cells were harvested and luciferase assays were performed using the Dual Luciferase Reporter System (Promega) according to the manufacturer’s protocol.
2.9. Statistics
Statistical analysis was performed using the unpaired Student t-test. Data are presented as mean±S.E.M., and values of p < 0.05 were considered statistically significant. All experiments were performed at least three times and representative results are shown.
3. Results
3.1. Homocysteine depletes intracellular glutathione
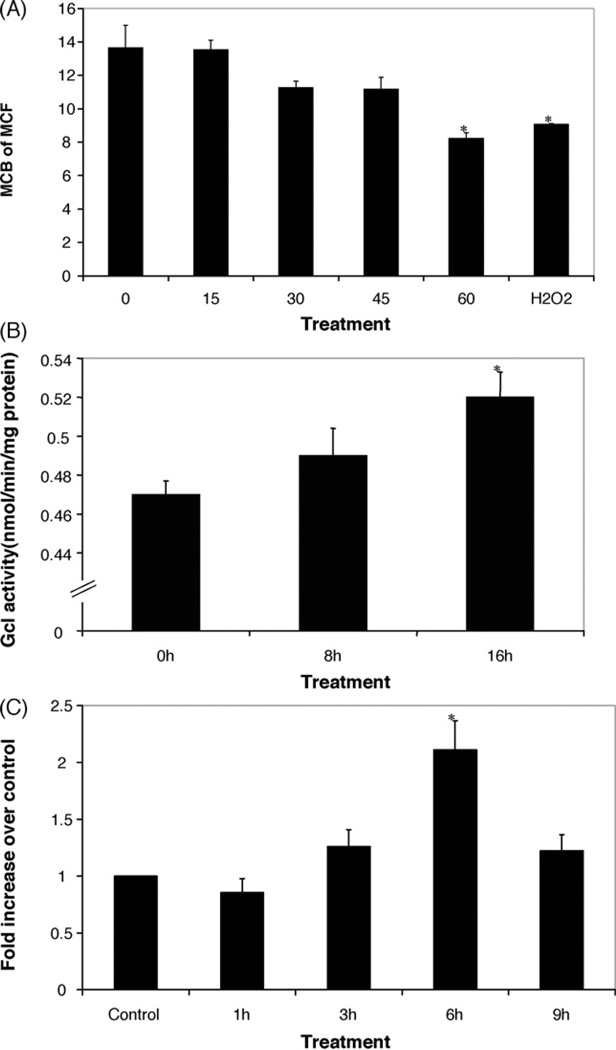
To determine if homocysteine causes oxidative stress in macrophages, we evaluated the intracellular levels of the antioxidant thiol glutathione (GSH) using flow cytometry and monochlorobimane fluorescence (MCB). Incubations with 50µM d-/l-homocysteine resulted in a reduction in MCB fluorescence comparable to that seen in RAW 264.7 cells treated with100 µM H2O2 (Fig. 1A). We did not observe any toxicity using this concentration of homocysteine.
Fig 1.
Effect of homocysteine on intracellular glutathione content, Gcl activity and Gclc mRNA expression. (A) RAW 267.4 cells were treated with d-/l-homocysteine (50µM) for the indicated times, or H2O2 (100µM) for 30 min, and harvested for flow cytometric analysis of intracellular glutathione content using mean channel fluorescence (MCF) of monochlorobimane fluorescence (MCB). Analysis of cellular GSH content was restricted to PI negative (intact) cells. Values are means±S.E.M. *Statistically different from control (p < 0.05). (B) Homocysteine increases the levels of Gcl activity in mouse macrophages. RAW cells were treated with d-/lhomocysteine at 50µM for various times. Values are means±S.E.M. *Statistically different from control (p < 0.05). (C) RAW 267.4 cells were treated with d-/l-homocysteine (50µM) for the indicated time points and Gclc mRNA levels were determined using real time RT-PCR analysis. Values are normalized to β-actin expression and presented as mean±S.E.M. *Statistically different from control (p < 0.05).
3.2. Homocysteine increases Gcl activity
RAW mouse macrophages were treated with 50 µM d- /l-homocysteine for various time points and Gcl activity was measured using monobromobimane derivitization and HPLC analysis. As shown in Fig. 1B treatment with homocysteine increased the activity of Gcl in these cells. Taken together, these results suggest that the depletion of intracellular GSH causes a compensatory increase in Gcl activity.
3.3. Homocysteine induces Gclc mRNA expression
To evaluate whether treatment of RAW 264.7 macrophages with homocysteine induces an increase in Gclc mRNA expression, cells were treated with 50 µM d-/l-homocysteine and quantitative real-time RT-PCR analysis was performed. We were able to observe a time dependent increase in mRNA of Gclc expression of more than twofold after 6 h of treatment with homocysteine (Fig. 1C).
3.4. Homocysteine stimulates binding of nuclear proteins to the AREs within the Gclc promoter
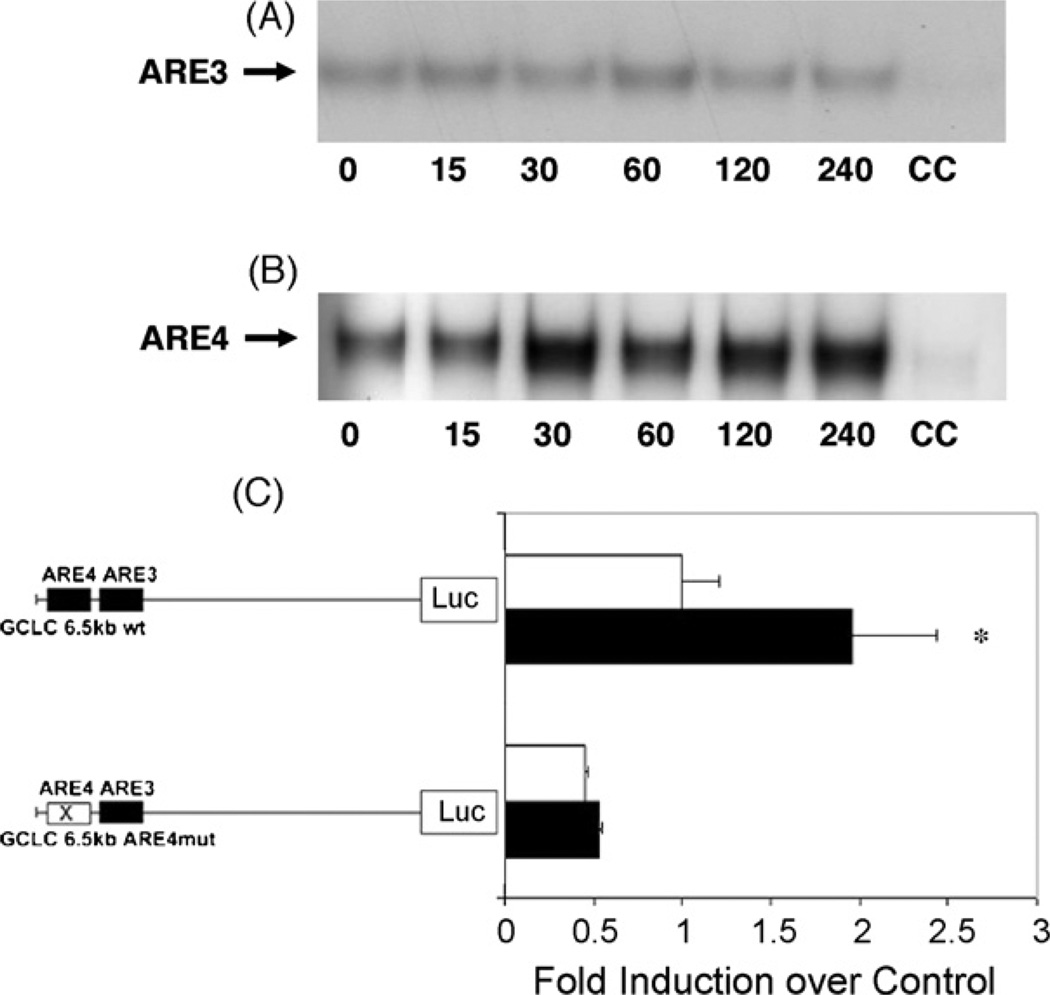
We conducted electromobility shifts assays with the oligonucleotides containing the consensus sequences of the ARE3 and ARE4 to assess whether homocysteine induces binding of nuclear proteins to these AREs, which are present within the murine Gclc subunit promoter. RAW cells were treated with homocysteine, and cells were harvested at various time points. As demonstrated in Fig. 2A, homocysteine induced only a weak binding of nuclear proteins to the ARE3 after 15 min. However, at ARE4, binding of nuclear proteins is strongly induced by 30 min and is still induced at 1 and 2 h of homocysteine treatment (Fig. 2B).
Fig 2.
Induction of the Gclc promoter by homocysteine involves activated ARE4. Electromobility shift assays with RAW 264.7 cell nuclear extracts for ARE3 (A) and ARE4 (B). Cells were treated with 50µMd-/l-homocysteine for the indicated time periods (minutes). CC indicates cold competition, addition of 100-fold excess unlabeled ARE. (C) RAW 267.4 cells were transiently transfected with murine wild-type or mutated 6.5 kb Gclc/pGL3 constructs for 16 h. Cells were then treated with DMSO (open bars) or 50µMd-/l-homocysteine (closed bars) for another 16 h. Luciferase activity in cell lysates was then determined. Values are presented as mean± S.E.M. *Statistically different from control (p < 0.05).
3.5. Homocysteine induction of Gclc mRNA expression is regulated by ARE4
Transfection experiments were conducted to determine if the increase in Gclc mRNA expression was due to increased transcription and if ARE3 and ARE4 had a functional significance in this induction in response to homocysteine treatment. Transient transfections with 6.5 kb Gclc promoter/luciferase reporter constructs revealed that homocysteine increases transcription of the Gclc promoter. A twofold increase in luciferase activity relative to DMSO-treated cells occurred 16 h after treatment with homocysteine (Fig. 2C). To evaluate the functional significance of the AREs within the Gclc promoter site directed mutagenesis was performed. The introduced mutation targets the core TGAC sequence of ARE3 and ARE4 and contains a T >G single base pair transversion. Mutations within the ARE4 of the constructs revealed a strong reduction in luciferase activity after transfection for 16 h and treatment with homocysteine for another 16 h (Fig. 2C). Site directed mutagenesis of the ARE3 had no significant effect on constitutive and inducible activity of the Gclc promoter (data not shown).
3.6. The transcription factor Nrf2 plays a critical role in homocysteine-induced activation of Gclc AREs
We conducted supershift gel shift experiments to evaluate what transcription factors bind to Gclc AREs after stimulation with homocysteine. Addition of an anti-Nrf2 antibody partially displaced the protein:DNA complex, indicating involvement of the Nrf2 transcription factor at this site (Fig. 3A). Western blots analysis using nuclear extracts revealed that macrophages also had an increased Nrf2 nuclear protein content after stimulation with homocysteine (Fig. 3B).
Fig 3.
Nrf2 mediates homocysteine induced increased binding-activity to ARE4. (A) Electromobility shift assay of protein binding to ARE4 oligonucleotide by nuclear extracts from control RAW 267.4 macrophages exposed to d-/l-homocysteine (50µM) for the indicated time periods (minutes). Supershift analysis was performed using an Nrf2-antibody. Subsequent densitometric evaluation demonstrating a significant reduction in ARE4 binding activity due to the use of the Nrf2-antibody after 1 h treatment with homocysteine (*p < 0.05) and after 2 h treatment (†p < 0.05). (B)Western blot and densitometric evaluation showing increased nuclear accumulation of Nrf2 after treatment of RAW267.4 macrophages with homocysteine (50µM) for the indicated time periods (minutes, *p < 0.05 in comparison to control).
3.7. The homocysteine-induced Nrf2/ARE-dependent expression of Gclc is driven by the ERK1/2 MAPK pathway
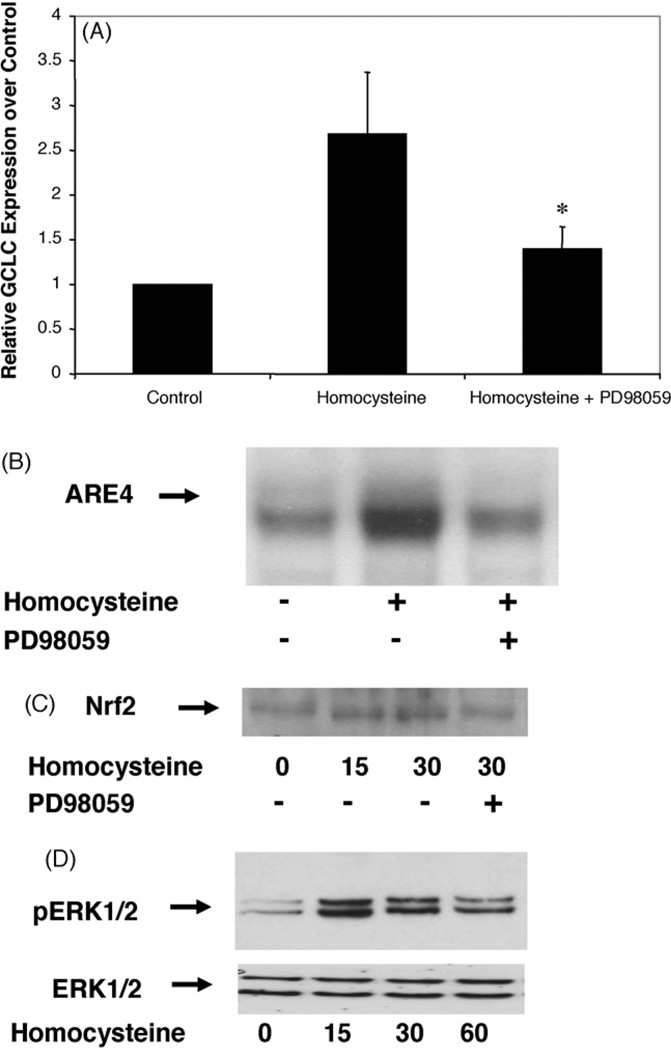
To address the potential involvement of ERK1/2 and p38 MAPK in homocysteine induced activation of Gclc, we used specific inhibitors. As shown in Fig. 4A, PD98059 (30µmol/l, 30 min), a selective inhibitor of the MAPK kinase (MEK), inhibited mRNA induction of Gclc activation by approximately 50%. The use of the p38 MAPK inhibitor SB203580 did not show any inhibitory effect on homocysteine-induced Gclc expression (data not shown).
Fig 4.
Homocysteine induction of Gclc mRNA expression via Nrf2 involves the MEK-ERK1/2 kinase pathway. (A) RAW267.4 cells were preincubated with or without the MEK inhibitor PD98059 (30µM, 30 min) prior to treatment with d-/l-homocysteine (50µM). Total RNA was extracted 6 h after treatment and Gclc mRNA levels were determined by real-time RT-PCR. Values are presented as mean± SEM. *Statistically different from homocysteine treated cells (p < 0.05). (B) Cells were treated with d-/l-homocysteine (50µM, 30 min) in the presence or absence of PD 98059 (30µM, 30 min) and electromobility shift assay using a labeled ARE4 probe was performed. (C) Western blot showing the nuclear accumulation of Nrf2 in cells treated with d-/l-homocysteine (50µM, 15 min) with or without pre-treatment with PD98059 (30µM, 30 min). (D) RAW cells were treated with 50µM d-/lhomocysteine for the indicated time period (minutes) and nuclear extracts were harvested. Phosphorylation of ERK1/2was visualized by Western blotting using antibodies against phosphorylated ERK1/2 and unphosphorylated ERK1/2 as controls.
Furthermore, we evaluated whether the homocysteine-induced binding of nuclear proteins to the ARE4 was dependent on the ERK1/2 Kinase Pathway. Cells were pretreated with 30 µM of the MEK inhibitor PD98059 for 30 min before treatment with homocysteine for 30 min, and binding reactions were evaluated by EMSA. The presence of PD98059 significantly inhibited the binding by approximately 60%, suggesting that the MEK-ERK1/2 MAPK pathway plays a role in induction of the ARE after treatment with homocysteine (Fig. 4B). The increased enhanced nuclear translocation of the transcription factor Nrf2was also dependent on the MEK-ERK1/2 MAPK pathway. Pretreatment of the macrophages with the MEK-ERK1/2 MAPK inhibitor with PD98059 (30µM) for 30 min demonstrated reduced nuclear accumulation of Nrf2 (Fig. 4C).
3.8. Homocysteine induces phosphorylation of ERK1/2 but not p38
To demonstrate if homocysteine phosphorylates ERK1/2 and p38 MAPK in homocysteine treated macrophages, we performed Western blot analysis with phospho-specific ERK1/2 and p38 MAPK antibodies. Phosphorylation of ERK1/2 was induced in a time-dependent manner after treatment with homocysteine. Maximum phosphorylation of ERK1/2 was observed 15 min after homocysteine treatment (Fig. 4D). No phoshrylation of p38 could be observed (data not shown).
4. Discussion
In the present study, we identified oxidative stress caused by exposure to homocysteine at micromolar levels as a novel inducer of glutamate-cysteine ligase (Gcl) in mouse macrophages. We found that this occurs via an antioxidant response element within the Gclc promoter. Furthermore, we discovered that this induction is dependent on an MEK-ERK1/ 2/Kinase-Nrf2 pathway.
Homocysteine-induced oxidative stress and depletion of GSH have been described in many cell types [13]. These changes in GSH levels are not specific to homocysteine, but also have been described for other oxidative metabolites, such as oxLDL [18]. After an acute depletion of GSH due to exposure to oxidative stress, a compensatory increase in production of GSH can be observed [23]. This may at least in part be due to the relief in the feedback inhibition of the catalytic subunit of Gcl and the induction of this enzyme [24,25]. In the present study, homocysteine was shown to cause partial depletion of GSH followed by a compensatory increase in Gcl activity. Using quantitative real-time RT-PCR analysis, an increase in mRNA levels for the catalytic subunit of Gcl could be demonstrated.
The observed increase in Gclc expression in response to homocysteine is in part due to increased de novo gene expression as revealed by an increased luciferase activity from Gclc promoter/luciferase reporter construct. Earlier studies have demonstrated the presence of several putative consensus response elements within the Gclc promoter, including two potential antioxidant response elements, ARE3 and ARE4 [15]. The constitutive and β-naphthoflavone-induced expression of the Gclc gene is mediated by a distal ARE sequence, ARE4, in which an embedded AP-1 site is well conserved [18,26]. Using electromobility shift assays, we were able to demonstrate that homocysteine induces increased binding of nuclear proteins in macrophages at these AREs. The induced increase in binding was much greater for the ARE4 site. This is in agreement with a recent study where homocysteine induced increased binding activity of the heme oxygenase- 1-ARE in smooth muscle cells [10]. Our transfection studies using luciferase reporter constructs confirmed the inducing function of ARE4 within the Gclc promoter in response to homocysteine treatment. AREs are present in the 5′-flanking regions of several genes encoding enzymes involved in phase II metabolism of xenobiotics and other antioxidant proteins including glutathione S-transferases, NAD(P)H:quinone oxidoreductase, and glucuronosyl-transferases. Furthermore, AREs are also involved in the regulation of heme oxygenase- 1, ferritin (heavy and light chains) and microsomal epoxide hydrolase [27]. Thus, the activation of AREs in macrophages by homocysteine is novel and is likely to have a much broader effect on macrophage antioxidative defense mechanisms than simply increasing Gclc expression.
The present study demonstrates that Nrf2 is required for homocysteine induced ARE-driven gene expression of Gclc in macrophages. Nrf2 plays a critical role in ARE-mediated gene expression in response to oxidative stress [10,28]. Consistent with this, we found that stimulation of the Gclc promoter is dependent on binding of Nrf2 at ARE4. We furthermore found that this was mediated by enhanced nuclear accumulation of Nrf2.
It has been demonstrated that homocysteine can activate the MAP kinase pathway. The activation of the MEK-ERK1/ 2 kinase in response to homocysteine treatment has been demonstrated in smooth muscle cells and endothelial cells [29,30], but little is known about the activation of the ERK1/2 kinase in macrophages in response to homocysteine treatment. In this study we found that homocysteine transiently activates the MEK-ERK1/2 kinase pathway. We were furthermore able to demonstrate that the inhibition of the MEK-ERK1/2 kinase pathway blocked Nrf2 nuclear accumulation and ARE-driven gene expression of Gclc after treatment with homocysteine.
In conclusion, the present data demonstrate that homocysteine induces the expression of Gclc in macrophages and that this induction involves ARE4 and the MEK-ERK1/2 kinase pathway. We have also shown that this induction is dependent on nuclear Nrf2 accumulation. Increased expression of antioxidant and cytoprotective genes via ARE-driven gene regulation could help to protect macrophages against hyper-homocysteinemia induced oxidative stress, and ultimately provide protection against further inflammatory changes and instability within atherosclerotic plaques.
Acknowledgments
We thank Annette Buttler and Anne Sterzer for excellent technical assistance. This work was supported by a grant from the Deutsche Forschungsgemeinschaft Be3188/2-2 and NIH grants HL58954, 1R01ES10849, 1T32ES07032 and 1P30ES07033.
References
- 1.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202–1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 3.Rasouli ML, Nasir K, Blumenthal RS, Park R, Aziz DC, Budoff MJ. Plasma homocysteine predicts progression of atherosclerosis. Atherosclerosis. 2005;181:159–165. doi: 10.1016/j.atherosclerosis.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Mikael LG, Genest J, Jr, Rozen R. Elevated homocysteine reduces apolipoprotein A-I expression in hyperhomocysteinemic mice and in males with coronary artery disease. Circ Res. 2006;98:564–571. doi: 10.1161/01.RES.0000204825.66410.0b. [DOI] [PubMed] [Google Scholar]
- 5.Ungvari Z, Csiszar A, Bagi Z, Koller A. Impaired nitric oxide-mediated flow-induced coronary dilation in hyperhomocysteinemia: morphological and functional evidence for increased peroxynitrite formation. Am J Pathol. 2002;161:145–153. doi: 10.1016/S0002-9440(10)64166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberhardt RT, Forgione MA, Cap A, et al. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virdis A, Iglarz M, Neves MF, et al. Effect of Hyperhomocystinemia and hypertension on endothelial function in methylenetetrahydrofolate reductase-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1352–1357. doi: 10.1161/01.ATV.0000083297.47245.DA. [DOI] [PubMed] [Google Scholar]
- 8.Song YS, Rosenfeld ME. Methionine-induced hyperhomocysteinemia promotes superoxide anion generation and NFkappaB activation in peritoneal macrophages of C57BL/6 mice. J Med Food. 2004;7:229–234. doi: 10.1089/1096620041224021. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Werstuck GH, Lhotak S, et al. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation. 2004;110:207–213. doi: 10.1161/01.CIR.0000134487.51510.97. [DOI] [PubMed] [Google Scholar]
- 10.Liu XM, Peyton KJ, Ensenat D, et al. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle. Role in cell survival. J Biol Chem. 2005;280:872–877. doi: 10.1074/jbc.M410413200. [DOI] [PubMed] [Google Scholar]
- 11.Hansson GK. Inflammation atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 12.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 13.Vitvitsky V, Dayal S, Stabler S, et al. Perturbations in homocysteine-linked redox homeostasis in a murine model for hyperhomocysteinemia. Am J Physiol Regul Integr Comp Physiol. 2004;287:R39–R46. doi: 10.1152/ajpregu.00036.2004. [DOI] [PubMed] [Google Scholar]
- 14.Upchurch GR, Welch GN, Jr, Fabian AJ, et al. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 1997;272:17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 15.Mulcahy RT, Wartman MA, Bailey HH, Gipp JJ. Constitutive and β-naphthoflavone- induced expression of the human γ-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J Biol Chem. 1997;272:7445–7454. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- 16.Hudson FN, Kavanagh TJ. Cloning and characterization of the proximal promoter region of the mouse glutamate-l-cysteine ligase regulatory subunit gene. Biochim Biophys Acta. 2000;1492:447–451. doi: 10.1016/s0167-4781(00)00128-7. [DOI] [PubMed] [Google Scholar]
- 17.Dickinson DA, Levonen AL, Moellering DR, et al. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic Biol Med. 2004;37:1152–1159. doi: 10.1016/j.freeradbiomed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Bea F, Hudson FN, Chait A, Kavanagh TJ, Rosenfeld ME. Induction of glutathione synthesis in macrophages by oxidized low-density lipoproteins is mediated by consensus antioxidant response elements. Circ Res. 2003;92:386–393. doi: 10.1161/01.RES.0000059561.65545.16. [DOI] [PubMed] [Google Scholar]
- 19.Nygård O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 20.Botta D, Franklin CC, White CC, et al. Glutamate-cysteine ligase attenuates TNF-induced mitochondrial injury and apoptosis. Free Radic Biol Med. 2004;37:632–642. doi: 10.1016/j.freeradbiomed.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Thompson SA, Roellich KL, Grossmann A, Gilbert SG, Kavanagh TJ. Alterations in immune parameters associated with low level methylmercury exposure in mice. Immunopharmacol Immunotoxicol. 1998;20:299–314. doi: 10.3109/08923979809038546. [DOI] [PubMed] [Google Scholar]
- 22.White CC, Krejsa CM, Eaton DL, Kavanagh TJ. HPLC-based assays for enzymes of glutathione biosynthesis. In: Maines M, Costa LG, Hodgson E, Reed DJ, Sipes IG, editors. Current protocols in toxicology. New York: John Wiley & Sons, Inc; 1999. pp. 651–654. [DOI] [PubMed] [Google Scholar]
- 23.Shen L, Sevanian A. OxLDL induces macrophage γ-GCS-HS protein expression: a role for oxLDL-associated lipid hydroperoxide in GSH synthesis. J Lipid Res. 2001;42:813–823. [PubMed] [Google Scholar]
- 24.Li MH, Jang JH, Na HK, Cha YN, Surh YJ. Carbon monoxide produced by upregulated heme oxygenase-1 in response to nitrosative stress induces expression of glutamate cysteine ligase in PC12 cells via activation of PI3K-Akt and Nrf2-ARE signalling. J Biol Chem. 2007;282:28577–28586. doi: 10.1074/jbc.M701916200. [DOI] [PubMed] [Google Scholar]
- 25.Krzywanski DM, Dickinson DA, Iles KE, et al. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch Biochem Biophys. 2004;423:116–425. doi: 10.1016/j.abb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Go YM, Gipp JJ, Mulcahy RT, Jones DP. H2O2-dependent activation of GCLC-ARE4 reporter occurs by mitogen-activated protein kinase pathways without oxidation of cellular glutathione or thioredoxin-1. J Biol Chem. 2004;279:5837–5845. doi: 10.1074/jbc.M307547200. [DOI] [PubMed] [Google Scholar]
- 27.Chen XL, Varner SE, Rao AS, et al. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem. 2003;278:703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 28.Papaiahgari S, Kleeberger SR, Cho HY, Kalvakolanu DV, Reddy SP. NADPH oxidase and ERK signaling regulates hyperoxia-induced Nrf2- ARE transcriptional response in pulmonary epithelial cells. J Biol Chem. 2004;279:42302–42312. doi: 10.1074/jbc.M408275200. [DOI] [PubMed] [Google Scholar]
- 29.Brown JC, Rosenquist TH, Monaghan DT. ERK2 activation by homo-cysteine in vascular smooth muscle cells. Biochem Biophys Res Commun. 1998;251:669–676. doi: 10.1006/bbrc.1998.9535. [DOI] [PubMed] [Google Scholar]
- 30.Moshal KS, Sen U, Tyagi N, et al. Regulation of homocysteine-induced MMP-9 by ERK1/2 pathway. Am J Physiol Cell Physiol. 2006;290:C883–C891. doi: 10.1152/ajpcell.00359.2005. [DOI] [PubMed] [Google Scholar]