Abstract
The metabolically active and perpetually remodeling calcium phosphate–based endoskeleton in terrestrial vertebrates sets the demands on whole-organism calcium and phosphate homeostasis that involves multiple organs in terms of mineral flux and endocrine cross talk. The fibroblast growth factor (FGF)-Klotho endocrine networks epitomize the complexity of systems biology, and specifically, the FGF23-αKlotho axis highlights the concept of the skeleton holding the master switch of homeostasis rather than a passive target organ as hitherto conceived. Other than serving as a coreceptor for FGF23, αKlotho circulates as an endocrine substance with a multitude of effects. This review covers recent data on the physiological regulation and function of the complex FGF23-αKlotho network. Chronic kidney disease is a common pathophysiological state in which FGF23-αKlotho, a multiorgan endocrine network, is deranged in a self-amplifying vortex resulting in organ dysfunction of the utmost severity that contributes to its morbidity and mortality.
Keywords: calcium, phosphate, FGF23, Klotho, kidney, bone
BONE-KIDNEY-INTESTINAL HOMEOSTATIC NETWORK
This review covers recent advances in fibroblast growth factor (FGF) 23 and Klotho and discusses bone, kidney, and intestine as homeostatic partners in regulating mineral metabolism. The terms bone-kidney axis and bone-intestinal axis seem to be in vogue in biomedical parlance. With the fashionable appeal of the term axis aside, it is not so clear what criteria must be satisfied before two (or more) organs or hormones actually constitute an axis in biology. It seems that because all organs and hormones function in concert in systems biology, axes are the rule rather than the exception. Nevertheless, there is a multitude of reasons and evidence for coordinated and interactive functions of the bony skeleton, intestine, and kidney. The intestine and kidney are archaic absorptive and excretory organs that confer to the organism critical interfaces with the external world so that their collusion in a network seems quite logical. In contrast, the bony skeleton appeared much later in higher vertebrates and is traditionally considered to be an organ that provides physical structure to support and enable locomotion.
Evolutionary Origin
The FGF23-Klotho system is part of a major milestone in vertebrate evolution that started in the ocean when the early piscine ancestors acquired the bony endoskeleton. The first fish, and indeed the first vertebrates, were the ostracoderms, which emerged in the Cambrian Period some 510 Mya and by the end of the Devonian Period, some 350 Mya, slipped into extinction. The ostracoderms are believed to be the progenitors of the later bony fish. The sharks and rays of the class Chondrichthyes, with their cartilaginous skeletons, appeared approximately 370 Mya in the middle Devonian Period. Cartilage-based skeletons are vastly different from human bones in terms of structure and constituents. Most likely, approximately 390 Mya in the late Silurian or early Devonian Period, the modern bony fishes of the class Osteichthyes made their debut, probably in parallel rather than orthologous to the cartilaginous fish.
The bony endoskeleton brought about revolutionary changes in organ physiology. Various necessities of this massive and important organ mandate complex coordinations with the rest of the organism. Another major landmark occurred when some crossopterygian fish crawled out of the water on the Devonian shorelines to become the first proto-amphibians to explore and subsequently inaugurate terrestrial life. Terrestrial existence imposes extraordinary demands on all the systems compared with life in aquatic habitats. Gas exchange with low gaseous air fluxes across the alveoli is challenging compared with high water flux of soluble gases across the gill. On land, the disappearance of an omnipresent food supply in the water mandates fast versus famine physiology to facilitate caloric storage. The loss of buoyancy introduces new stresses on support structures, and locomotion on land is radically different from propulsion in water. One of the most formidable challenges confronting the early ancestors had to have been the kidneys' task to conserve water while at the same time excreting large quantities of waste. Of all the solutes harbored in the mandatory highly concentrated urine, calcium (Ca2+) and phosphate (Pi) are the most menacing in terms of risk of precipitation. Although humans have evolved the ability to render our urine sodium (Na+) free, there is an obligatory amount of calcium phosphate (CaPi) turnover for the sake of maintenance of the skeleton.
Tri-Organ Calcium Phosphate Regulation
Another truly beguiling aspect of the endoskeleton of higher vertebrates is its inorganic constituents, which distinguish it from the calcareous skeletons of virtually all other metazoans (arthropods, mollusks, and echinoderms). The rigid, inorganic component of bone is primarily CaPi in the form of crystalline calcium hydroxyapatite (Ca10[PO4]6Ca[OH]2). In contrast, the mineral ground substance of almost all calcareous invertebrate endo- or exoskeletons since the early Cambrian Period is principally calcium carbonate as crystalline calcite or aragonite (CaCO3) (1). This deliberate and complete transition has never been adequately explained. Several hypotheses have been submitted, including a sink for Pi disposal (2), long-term Pi storage, and Pi homeostasis by provision of an exchangeable pool (3, 4). Ruben & Bennett (5) proposed a rather different model of superior postactivity acid buffering by CaPi compared with calcium carbonate. Regardless of the reason(s) for a hydroxyapatite skeleton, its existence places Ca2+ and Pi in the center stage of bone biology and mandates the integration of the major organs of Ca2+ and Pi flux into one network with bone.
The impact of the bony skeleton on organ physiology can be illustrated by hormones that regulate caloric homeostasis. Osteocyte-derived leptin that controls caloric intake is also a key regulator of bone remodeling, albeit via convoluted mechanisms (6). Bone-derived osteocalcin is a major regulator of insulin secretion, sensitivity, and energy expenditure (7). Karsenty & Ferron (8) elegantly articulated the logic and evidence for the central role of the bone in the physiology of energy expenditure and that the coappearance of the adipogenic hormone leptin with the bony skeleton in evolution precisely coordinates caloric regulation with bone formation and remodeling.
In the realm of Ca2+ and Pi, the apatite-based bony endoskeleton basically sets the stage for and assumes fundamental roles in Ca2+-Pi homeostasis (see Supplemental Figure 1; follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org). The skeleton has an absolute requirement for Ca2+ and Pi in an exact stoichiometry to maintain structural integrity. It is also the largest pool for chronic storage and acute buffering of Ca2+ and Pi. In the intravascular compartment, Ca2+ exits as ionized, complexed, and albumin-bound forms, but Pi exists in multiple complex forms (see Supplemental Figure 2). The intestine and kidney are the two principal organs that control external balance (Supplemental Figure 1). It will indeed be shocking if there is no coordination among these three organs in terms of flux. The flux across the intestine and kidney has to be concerted to establish positive, negative, or zero external balance. The skeleton is also a master orchestrator for internal balance, as any influx of Ca2+ and Pi has to go where it belongs (i.e., bone) and any ectopic CaPi deposition is emphatically deleterious. In addition to synchronization of flux, there has to be bidirectional hormonal cross talk so that these organs can communicate with each other. Serum Ca2+ and Pi levels can constitute some but cannot be the sole transorgan signaling mechanisms; there undoubtedly has to be hormonal cross talk. The evolution of FGF23, an osteogenic calciophosphoregulatory hormone, in conjunction with the intestine and kidney is akin to osteocalcin's role as an osteogenic, caloric regulatory hormone evolving in conjunction with the pancreas and adipocytes.
FGF FAMILY
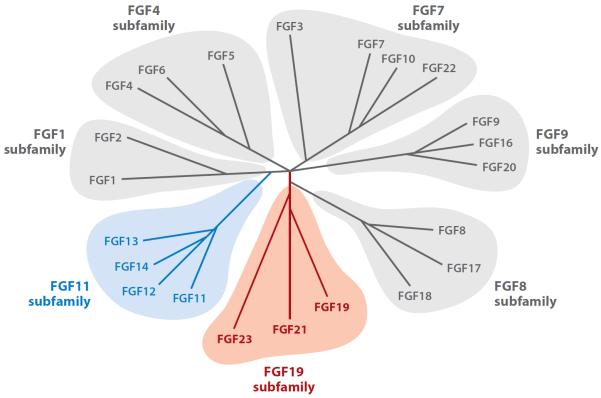
FGFs are a large superfamily of peptides that exert pleiotropic effects on an extremely broad range of biological processes, including development, organogenesis, and metabolism, through binding and activation of FGF receptor (FGFR) tyrosine kinases (9, 10). Twenty-two FGF members, classified in seven subfamilies on the basis of phylogeny, have been identified in humans (11) (Figure 1). Two prototypical FGFs, FGF1 and FGF2 (also known as acidic FGF and basic FGF, respectively), form the FGF1 subfamily. FGFs in the FGF1, FGF4, FGF7, FGF8, and FGF9 subfamilies function basically as paracrine/autocrine factors. The FGFs in the FGF11 subfamily, however, are thought to function as intracellular mediators without binding to FGFRs and are termed nuclear FGFs (12). Of particular interest in this review are the FGFs in the FGF19 subfamily, all of which function as circulating factors and are collectively termed endocrine FGFs.
Figure 1.
Phylogenetic tree of human fibroblast growth factor (FGF). Branch lengths represent the evolutionary distance between each gene. Gray represents the paracrine/autocrine subfamilies. Blue denotes the FGF11 subfamily of intracellular mediators that work independently of FGF receptors. Red represents the endocrine subfamily where the peptide hormone circulates. Adapted from Itoh & Ornitz (182).
Endocrine FGFs
The FGF19 subfamily consists of FGF19, FGF21, and FGF23 (12). It is prudent to point out that FGF15 is the murine ortholog of human FGF19 (13). Distinct from all the other FGFs that are paracrine/autocrine substances, these FGFs exert their specific physiological activities in the regulation of energy and mineral metabolism as endocrine factors or hormones (14). FGF15/19, FGF21, and FGF23 are expressed primarily in small intestine, liver, and bone, respectively (15) (Figure 2). A unique structural feature of these endocrine FGFs is their lack of a heparin-binding domain that is conserved in all paracrine/autocrine FGFs (16). This heparin-binding domain is critical for FGF function in two ways: First, it binds to heparan sulfate (HS) in the extracellular matrix, thereby posing some restriction to the secretion of FGFs and increasing their local concentration to support the paracrine/autocrine mode of action (16). Second, HS is essential for FGFR activation, which requires formation of the 2:2:2 complex of HS, FGF, and FGFR (10). Thus, lack of the heparin-binding domain in endocrine FGFs may be advantageous for facilitating release from their production source but is disadvantageous for FGFR activation at their target organs. Endocrine FGFs overcome this handicap by using Klotho proteins instead of HS to enhance receptor binding (Figure 3). The Klotho family of membrane proteins may have evolved partially to fulfill the need to increase the affinity of endocrine FGFs to FGFRs at their target organs.
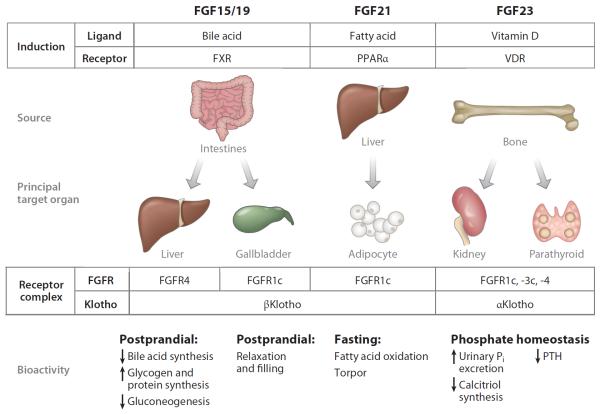
Figure 2.
The endocrine fibroblast growth factor (FGF)-Klotho axes. The FGF15/19-βKlotho axis is essential for postprandial negative feedback regulation of bile acid synthesis and release. FGF15/19 increase hepatic glycogen and protein synthesis (183), induce a lean phenotype (45, 46), and exert postprandial negative feedback of bile acid synthesis, functioning as a satiety hormone (47). In contrast, FGF21 is a fasting hormone (48). FGF21 is secreted from liver upon starvation and acts on white adipose tissue where βKlotho and FGFR1c are coexpressed (39). FGF21 expression is stimulated by ketogenic diet and peroxisome proliferator-activated receptor alpha (PPARα) agonists (49, 50). FGF21 induces resistance to growth hormone and torpor and promotes fatty acid oxidation, gluconeogenesis, and ketogenesis in the liver by increasing hepatic PGC1α expression (49, 52, 53). In white adipose tissue, FGF21 promotes lipolysis, mitochondrial respiration, and thermogenesis (50–53). FGF23 biology primarily concerns mineral metabolism by acting on the kidney and parathyroid glands. Abbreviations: FGFR, fibroblast growth factor receptor; FXR, farnesoid X receptor; Pi, phosphate; PTH, parathyroid hormone; VDR, vitamin D receptor.
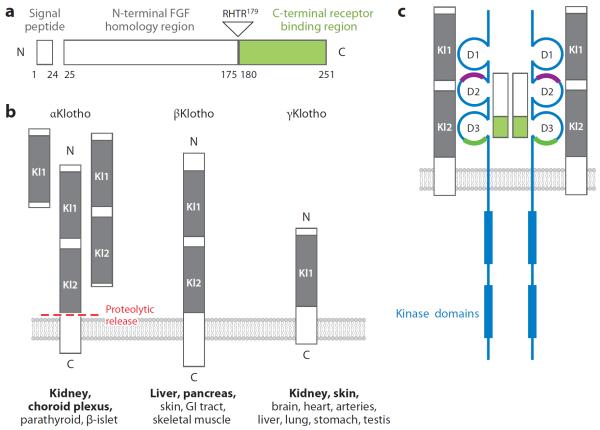
Figure 3.
Fibroblast growth factor (FGF) 23, Klotho family members, and the FGF23-FGF receptor (FGFR)-Klotho complex. (a) Structure of FGF23 showing the three main domains of this small polypeptide. The N terminus shares homology with other FGFs, whereas the C terminus is unique and binds to its cognate receptor (green). FGF23 circulates in the blood as both intact (full-length) and physiologically cleaved fragments. The C terminus binds but does not transactivate the FGFR-Klotho complex and can potentially function as a competitive antagonist. (b) Klotho family showing the three members identified to date in the mammalian genome, all of which are single-transmembrane proteins of varying lengths. Homologous motifs termed Kl domains are conserved. Soluble forms of αKlotho can be generated by alternative splicing of its transcript or by proteolytic cleavage of the transmembrane form by β-secretases into various body fluids. The major organs of expression of αKlotho, βKlotho, and γKlotho are shown in bold. (c) 2FGF23: 2FGFR:2αKlotho complex. The FGFR (blue) has three immunoglobulin-like domains (D1, D2, D3) that are stabilized by internal disulfide bridges. The heparin-binding region in the generic FGFR is shown (purple). For the endocrine FGF ligands, the coreceptor function of heparan sulfate is replaced by Klotho. αKlotho forms complexes with FGFR1c, FGFR3c, and FGFR4 and serves as the high-affinity receptor for FGF23. The ligand-binding region (green) interacts with the C terminus of FGF23. Klotho has a negligible intracellular region, whereas the FGFR has two kinase domains that sustain signal transduction. Abbreviations: GI, gastrointestinal; RHTR, arginine-histidine-threonine-arginine.
Like osteocalcin, FGF23 is a genuine osteogenic endocrine hormone. FGF23 is secreted from the bone (osteocytes and osteoblasts) and acts on kidney to promote excretion of Pi into urine (phosphaturia) and suppression of active vitamin D (1,25-dihydroxyvitamin D3, or calcitriol) synthesis and intestinal Pi absorption, thereby promoting negative external Pi balance. The FGF23 gene encodes a 32-kDa glycoprotein of 251 amino acids, which consist of a hydrophobic signal sequence (24 amino acids), an N-terminal FGF core homology domain (155 amino acids), and a C-terminal domain unique to FGF23 (72 amino acids) (16) (Figure 3). The C-terminal domain is essential for interaction with the FGFR-Klotho complex (17) (Figure 3). Between the N- and C-terminal domains, there is a proteolytic cleavage site (176RXXR179). FGF23 is inactivated when processed at this cleavage site by a protease(s) yet to be identified, resulting in two inactive N- and C-terminal fragments (Figure 3). Of note, the C-terminal fragment competes with intact FGF23 for binding to the FGFR-Klotho complex and has the potential to function as a competitive inhibitor for its parent FGF23 (17).
FGF23 was originally identified as a factor causing Pi wasting, including autosomal dominant hypophosphatemic rickets (ADHR) (18) and tumor-induced osteomalacia (TIO) (19). ADHR patients carry missense mutations at the proteolytic cleavage site of FGF23 (176RXXR179), which confers resistance to inactivation by proteolytic cleavage (20). As a result, ADHR patients exhibit increased blood levels of intact FGF23 and Pi-wasting phenotypes with inappropriately low blood vitamin D levels. Pi wasting in TIO patients is due to FGF23-producing tumors and is cured by removing the tumors.
FGF23 Ligand-Receptor Complex
Identification of Klotho as an obligate coreceptor for FGF23 was prompted by the fact that mice lacking FGF23 (Fgf23−/− mice) (21) and Klotho (Klotho−/− mice) (22) exhibited almost identical phenotypes (23): abnormal mineral metabolism characterized by increased blood Pi, Ca2+, and vitamin D levels associated with aging-like features, including shortened life span, growth retardation, hypogonadism, cognition impairment, hearing loss, vascular calcification, cardiac hypertrophy (24), osteopenia (25), dilatation of airspace (26), and atrophy of thymus, fat, and skeletal muscle. Klotho protein forms constitutive binary complexes with FGFR1c, FGFR3c, and FGFR4 to increase the affinity of these FGFRs selectively to FGF23 (23) (Figure 3). Klotho is required for FGF23 to activate FGFRs and their downstream signaling molecules, including FGF receptor substrate-2α and mitogen-activated protein kinases (MAPKs) such as extracellular signal–regulated kinases (ERK1/2) (23). Klotho−/− mice exhibit extremely high levels of blood FGF23, most likely due to end-organ resistance (27).
Because FGFRs are quite ubiquitous but Klotho expression is restricted, it seems that Klotho's presence governs whether a cell is an FGF23 target. It is currently unclear whether circulating Klotho can serve the role of coreceptor for FGFR, but soluble Klotho is not likely to be an efficient coreceptor for FGFR. One interesting consideration is how the FGFR-Klotho complex sustains FGF23 action on the proximal tubule. FGF23 induces phosphaturia by suppressing Pi reabsorption at the renal proximal tubules (28) via inhibition of Na+-dependent Pi cotransporters (NaPi-2a, NaPi-2c, and possibly Pit-2) on the apical brush border membrane of proximal tubular cells (29). FGF23 also lowers blood vitamin D levels by downregulating Cyp27b1 expression and upregulating Cyp24 expression in renal proximal tubular cells (30). Cyp27b1 encodes 1α-hydroxylase, required for active vitamin D synthesis, whereas Cyp24 encodes 24α-hydrolase that degrades vitamin D (31). Thus, FGF23 suppresses synthesis and promotes degradation of vitamin D. Although all the FGF23 actions seem proximal, Klotho expression is higher in the distal tubules (22, 32). Because proximal tubules also express Klotho, albeit in lower quantities (28), FGF23 may signal directly in proximal tubules to regulate their function with a small number of FGFR-Klotho complexes. Another possibility is that FGF23 acts on distal convoluted tubules where Klotho is most abundantly expressed (32) and initiates release of a paracrine factor(s) that acts on adjacent proximal tubules. These two possibilities are not mutually exclusive and remain to be tested. The parathyroid gland also expresses high levels of Klotho (33), indicating that it is another target organ of FGF23. Indeed, FGF23 suppresses synthesis and secretion of parathyroid hormone (PTH) in an ERK1/2-dependent manner (33, 34).
KLOTHO FAMILY
Klotho Paralogs in the Genome
Klotho was originally identified as an aging suppressor gene in mice that extends life span when overexpressed (35) and induces a premature aging-like syndrome when disrupted (22). Two proteins share homology to Klotho, which are termed βKlotho (36) and Klotho/lactase-phlorizin hydrolase–related protein (Klph) (37), also known as lactase-like protein (Lctl). To distinguish the founder Klotho from βKlotho and Klph/Lctl, Klotho is often termed αKlotho. These three Klotho family members are also termed αKlotho, βKlotho, and γKlotho (Figure 3). Although endocrine FGFs have very low affinity to FGFRs or Klothos individually (23, 38–40), they have high affinity to the FGFR-Klotho complexes (23, 39, 40). Because most tissues and cells express FGFRs, tissue-specific expression of Klotho determines target organs of endocrine FGFs (14). αKlotho is expressed in the kidney and parathyroid glands, where it forms complexes with FGFR1c, FGFR3c, and FGFR4 and serves as the high-affinity receptor for FGF23 (23) (Figure 2). βKlotho forms complexes with FGFR1c and FGFR4 (40); it supports FGF15/19 and FGF21 signaling and is expressed in the liver and fat (39, 40). γKlotho forms complexes with FGFR1b, FGFR1c, FGFR2c, and FGFR4 (15); it increases FGF19 activity and is expressed in the eye, fat, and kidney. The αKlotho and βKlotho genes encode type-I single-pass transmembrane proteins with 41% amino acid identity to each other (Figure 3). Their intracellular domains are very short and have no identifiable functional domains. In contrast, the extracellular domain has two tandem repeats of β-glucosidase-like domains that are also termed Kl domains (22, 36) (Figure 3). The γ Klotho gene encodes a shorter type-I single-pass transmembrane protein with a single β-glucosidase-like extracellular domain and a similarly short cytoplasmic tail (37) (Figure 3).
As the rest of the review focuses much on αKlotho, a brief note on βKlotho is in order to put the two isoforms in perspective. βKlotho contributes to the regulation of energy metabolism as the coreceptor for FGF15/19 and FGF21, analogous to the role of αKlotho for FGF23 (Figure 2). Expression of FGF15/19 in intestine is regulated by bile acid (41). After feeding, bile acid released into the intestinal lumen binds to farnesoid X receptor, which transactivates the FGF15/19 gene (41) (Figure 2). The FGF15/19 peptide secreted from the intestine acts on the liver, where βKlotho and FGFR4 are coexpressed, and suppresses expression of the Cyp7a1 gene (39), which codes for the rate-limiting enzyme of bile acid synthesis. This negative feedback loop is indispensable for proper production of bile acid, because mice lacking βKlotho (42), FGFR4 (43), or FGF15 (41) exhibit increased bile acid synthesis associated with Cyp7a1 overexpression. βKlotho is also expressed in gallbladder (44). Both FGF15−/− and βKlotho−/− mice exhibit empty gallbladders. Injection of FGF19 induces gallbladder relaxation and filling not only in wild-type mice but also in FGFR4−/− mice, suggesting that the FGFR1c-βKlotho complex can mediate FGF19-induced gallbladder relaxation (44). Thus, the FGF15/19-βKlotho endocrine axis is essential for the postprandial negative feedback regulation of bile acid synthesis and release. Recent studies have indicated that overexpression and/or administration of FGF15/19 increases hepatic glycogen and protein synthesis (45) and induces a lean phenotype in mice, including high metabolic rate, improvement of glucose tolerance and insulin sensitivity, increase in brown adipose tissue, and suppression of gluconeogenesis by inhibiting hepatic expression of PGC1α (peroxisome proliferator–activated receptor gamma coactivator protein-1 alpha) (46, 47). Together with its ability to induce postprandial negative feedback of bile acid synthesis, FGF15/19 may be regarded as a satiety hormone (48).
In contrast, FGF21 can be considered a fasting hormone (49). FGF21 is secreted from liver upon starvation and acts on white adipose tissue, where βKlotho and FGFR1c are coexpressed (40). FGF21 expression is also induced by ketogenetic diet and peroxisome proliferator-activated receptor alpha (PPARα) agonists (50, 51). Overexpression and/or administration of FGF21 in mice induces resistance to growth hormone and torpor (temporary hibernation) and promotes fatty acid oxidation, gluconeogenesis, and ketogenesis in the liver. In white adipose tissue, FGF21 promotes lipolysis, mitochondrial respiration, and “browning” of white adipose tissues for thermogenesis (51–54). Recent studies revealed that FGF21 induces these metabolic responses to fasting by increasing hepatic PGC1α expression (50, 53). Presently, little is known about the function of γKlotho except that it supports FGF19 signaling in cultured cells (15). The discovery of endocrine FGFs and Klotho has led to the identification of multiple novel endocrine axes that regulate various metabolic processes.
Transmembrane Versus Soluble αKlotho
Judging from the extremely short intracellular domain of Klotho, it is most unlikely that this tail can sustain any signal transduction function. As such, one may conclude that membrane Klotho solely serves auxiliary functions to FGFRs. However, through alternative splicing of the Klotho gene at exon 3, another shorter-length Klotho protein encompassing only Kl1 of the extracellular domain is released from cells and exists as a soluble extracellular protein (55) (Figure 3).
Alternative splicing is not the only mechanism to produce soluble Klotho. Another unique feature of αKlotho is that it is subjected to ectodomain shedding and its entire extracellular domain is secreted into blood, urine, and cerebrospinal fluid (56), thereby functioning as a humoral factor independently of FGF23 (35). αKlotho is cleaved on the cell surface by membrane-anchored proteases, including ADAM10 (a desintegrin and metalloproteinase 10), ADAM17, and beta-site amyloid precursor protein–cleaving enzyme 1 (BACE1) (57, 58) (Figure 3). Presently, only αKlotho has been shown to be released from the cell into the extracellular fluid, including plasma, cerebrospinal fluid (CSF), and urine (28, 56, 59); there are no data on soluble forms βKlotho and γKlotho. In the plasma, the concentration is approximately 10–50 nM, with urine levels being higher (59); the level in CSF has not been determined. There are some unknown but critical factors: What are the upstream regulators of Klotho shedding? What is the afferent mechanism of sensing and controlling circulating Klotho levels? What are the functions of soluble Klotho? There are some data on the last question. Currently, it is unclear whether soluble Klotho can function as a coreceptor for FGF23. In the CKD model and genetic manipulations, membrane Klotho and soluble Klotho tend to change in unison. We do not yet know if this concordance is universally true.
FGF23-Independent Effects of αKlotho as a Soluble Enzyme
Secreted Klotho has a putative enzymatic activity as sialidase (60), which removes terminal sialic acids from N-linked glycans of several glycoproteins on the cell surface. Secreted Klotho prevents endocytosis of transient receptor potential cation channel, subfamily V, member 5 (TRPV5) (60, 61) and renal outer medullary potassium channel 1 (ROMK1) by modifying their N-linked glycans on the cell surface, resulting in increases in Ca2+ and potassium (K+) currents, respectively. In contrast, secreted Klotho promotes endocytosis and inactivation of NaPi-2a by modifying glycans (28). Furthermore, secreted Klotho suppresses activity of insulin, insulin-like growth factor-1 (IGF-1) (35), Wnt (62), and TGF-β1 (63) by interacting with these growth factors or their receptors. The physiological significance of these pleiotropic activities of secreted Klotho remains to be determined.
The fact that the injection of soluble Klotho into an intact animal produces biological effects supports the notion that circulating Klotho carries regulatory functions (28, 64). Presently, no receptor for circulating Klotho has been identified, although soluble Klotho can theoretically bind to FGFR or self-dimerize with transmembrane Klotho. However, there is no evidence that this type of binding initiates any signaling events. In the absence of a genuine signal-transducing receptor, it is not yet legitimate to term Klotho an endocrine “hormone.” However, there is ample evidence that circulating Klotho functions as a glycan-modifying enzyme. From the time of the cloning of Klotho, bioinformatic analysis suggested possible lactose-phlorizin hydrolase and β-glucosidase activity, but the glucosidase activity was not confirmed by biochemical activity assays (22, 65).
PHYSIOLOGY
Although there is no doubt that both Ca2+ homeostasis and Pi homeostasis are equally important for organisms with apatite skeletons, due to the predominance of recent data on Pi and space considerations, the discussion from here on focuses more on Pi. This should in no way diminish the importance of Ca2+. In fact, FGF23—considered by many as the ultimate hormone dedicated to Pi homeostasis—is likely also regulated by Ca2+ (66, 67), and thus it may be another calciophosphoregulatory hormone. Given the importance of Pi, a short discussion about its various forms in the extracellular fluid is in order and is provided in the Supplemental Text and Supplemental Figure 2.
FGF23 Production in Bone
FGF23 is synthesized by osteocytes (68) and osteoblasts (69), and the present known regulators are PHEX, dentin matrix protein (DMP)-1, sustained Pi load, high 1,25 VD3, low Klotho, PTH, and the recently proposed serum Ca2+ (66, 67, 70). This transforms FGF23 from what was touted as a pure phosphoregulatory hormone into a dual calciophosphoregulatory hormone. Mutations in the Phex gene (a Pi-regulating gene with homology to endopeptidase on the X chromosome) (71–73) and the Dmp-1 (dentin matrix protein-1) gene (74) increase FGF23 expression and induce renal Pi wasting in mice and humans. Activation of FGFR in osteocytes/osteoblasts stimulated FGF23 expression (75) and was required for the Phex and Dmp-1 mutants to increase FGF23 expression (76). PTH stimulates synthesis and secretion of FGF23 through activation of PTH/PTHrP receptor on osteocytes/osteoblasts (68, 77). Quite contrary to FGF23, PTH acts on the kidney to upregulate Cyp27b1 expression and increase blood active vitamin D levels (78). Similar to but independent of FGF23, PTH has phosphaturic activity (79), which prevents positive Pi balance due to increased vitamin D. Other factors that regulate FGF23 expression include vitamin D and Pi.
Vitamin D increases FGF23 expression in a vitamin D receptor (VDR)-dependent manner (80). Pi increases blood FGF23 levels independently of vitamin D (81). Importantly, an acute increase in blood Pi levels by intravenous Pi injection does not increase FGF23 (82), whereas sustained dietary Pi overload increases FGF23 without changes in plasma Pi levels in humans (83). These observations suggest that it is not plasma Pi that regulates FGF23 expression. The mechanism by which osteocytes/osteoblasts sense a state of Pi metabolism is not understood.
Extremely high plasma FGF23 is observed in primary Klotho deficiency (genetic deletion or mutational hypomorph) (22, 84) and the much more common secondary Klotho deficiency in chronic kidney disease (CKD) (59, 85–88). There are no in vitro data to date to support a direct effect of Klotho on FGF23 production, but results from in vivo experiments suggest that Klotho influences FGF23 production in bone (Figure 4). Similar phenotypes were found in Klothodeficient and FGF23-deficient mice (89). Two in vivo models with high blood FGF23—transgenic mice overexpressing FGF23 (90) and Hyp mice with a spontaneously inactivating mutation in the Phex gene (91)—have biochemical and skeletal phenotypes similar to those of Klotho-deficient mice. One explanation is that FGF23-induced signal activation is dependent on the presence of membrane Klotho (23).
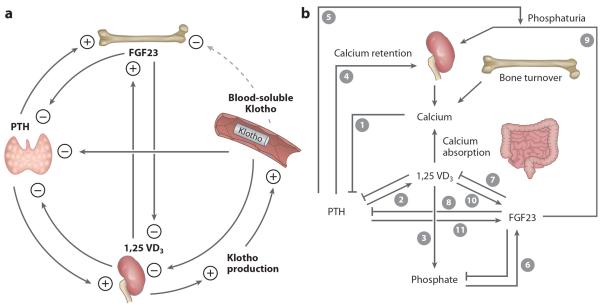
Figure 4.
Endocrine regulation of phosphate (Pi) and calcium metabolism. (a) Multiple negative feedback loops between the principal regulators of mineral metabolism: parathyroid hormone (PTH), fibroblast growth factor (FGF) 23, Klotho, and vitamin D. The kidney is a major contributor to circulating blood Klotho. Klotho is postulated to suppress FGF23 production from bone. Klotho functions as a coreceptor of FGFR allowing FGF23 to suppress PTH. PTH increases plasma levels of FGF23 and vitamin D. Increased vitamin further stimulates FGF23 and directly and indirectly suppresses PTH. Increased vitamin D also stimulates Klotho production in the kidney. Through several negative or positive feedback loops, Klotho functions as both a Pi-regulatory hormone and a calcium-regulatory hormone. (b) A change in one parameter triggers a cascade of events starting with hypocalcemia. Ionized calcium usually inhibits PTH secretion (1). When plasma-ionized calcium levels are low, PTH is stimulated, which promotes synthesis of active vitamin D (2), which in turn increases intestinal absorption of calcium and Pi (3). PTH also leads to renal calcium retention (4) and increased bone turnover (5). These act in concert (3–5) to restore plasma ionized calcium. When blood Pi levels and/or Pi intake is increased, FGF23 is increased (6). FGF23 suppresses vitamin D directly (7) and indirectly by suppressing PTH (2, 8) in a Klotho-dependent manner. Together with its phosphaturic activity (9), FGF23 induces negative Pi balance. FGF23 is also increased by vitamin D (10) and PTH (11), thereby closing negative feedback loops between FGF23, PTH, and vitamin D.
FGF23-Klotho Actions
On the basis of indirect evidence, FGF23 has been proposed to be a regulator of Klotho expression in the kidney. Marsell et al. (93) compared the transcriptome profile of kidneys from FGF23 transgenic mice with that of wild-type littermates. In the transgenic mice, the expression levels of Pi-regulating genes such as Npt2a and Klotho were decreased. This study relied on chronic, sustained systemic elevation of FGF23 and did not examine soluble Klotho in the blood, nor did this study address whether the FGF23 effect is direct. One study reported normal levels of Klotho in the blood of patients with X-linked hypophosphatemia (XLH) with high FGF23 and hypophosphatemia (92). Thus, whether FGF23 increases soluble Klotho in the blood and whether FGF23 directly or indirectly suppresses Klotho mRNA expression are unclear (93). Other physiological effects of FGF23 are better studied. FGF23 acts as a phosphaturic hormone (17, 72), inhibits vitamin D production in the kidney (30) to prevent hypercalcemia (3), and suppresses PTH production in the parathyroid gland (33) to limit Pi wasting.
Modulation of PTH production
Klotho indirectly regulates PTH production through modulation of plasma levels of active vitamin D, Pi, and FGF23. In addition, membrane Klotho may have a direct effect on PTH production and release (Figure 4). Membrane Klotho is expressed in the parathyroid gland with FGFR1 and FGFR3 (22, 33, 94), suggesting that the parathyroid gland is a target organ of FGF23 and that Klotho is a prerequisite for FGF23 action (33). In a physiological setting with normal Klotho and FGFR expression, FGF23 decreases PTH production, increases expression of both the parathyroid Ca2+-sensing receptor and the VDR (both of which contribute to suppression of PTH), and decreases cell proliferation (33, 95). Interestingly, FGF23 seems to also increase Klotho in the parathyroid gland, which positively facilitates FGF23's suppression of PTH production (33). Pit-1, a NaPi-3 isoform, was found in parathyroid gland and was upregulated by vitamin D and low-Pi diet and downregulated by vitamin D–deficient diet (96). Klotho suppresses the activity and expression of Pit-1 in rat vascular smooth muscle cells in vitro (59), but to date there is no evidence for an effect of Klotho on Pit-1 in the parathyroid gland.
Ionized Ca2+ activity is a key modulator of PTH synthesis and release. An intriguing alternative mode of Klotho action on the parathyroid cells was proposed in which intracellular Klotho binds to Na/K-ATPase to form a complex in response to low intracellular [Ca2+] and to bring Na/K-ATPase to the cell surface (97). However, how the Klotho-Na/K-ATPase complex is formed in response to low [Ca2+], how Na/K-ATPase activity is stimulated, and what intracellular signal is required to couple Na/K-ATPase to PTH release remain to be defined.
On the one hand, Klotho stimulates PTH production in the parathyroid glands; on the other hand, Klotho protein expression in the kidney is dependent on PTH levels (66). Lopez et al. (66) found reduced renal Klotho protein in parathyroidectomized rats compared with sham rats and found that low Klotho is reversed by PTH supplementation (66). However, one could not rule out the possibility of this regulatory effect of PTH on Klotho expression in the kidney via a concomitant effect of PTH on vitamin D (Figure 4).
Modulation of vitamin D production
Extremely high plasma levels of active 1,25 vitamin D were noted in homozygous Klotho-deficient (Klotho−/−) mice (98) due to upregulation of 1α-hydroxyase and downregulation of 24-hydrolase (98) (Figure 4). Moreover, normal responses to vitamin D supplementation, such as downregulation of the 1α-hydroxylase gene and upregulation of 24-hydroxylase and VDR transcripts, were impaired in the Klotho−/− mice (99, 100). However, vitamin D supplementation upregulates renal Klotho expression in wild-type mice (100); such expression is dependent on VDR (101). Several vitamin D–responsive elements were identified in the vicinity of both the mouse and the human Klotho genes starting −31 to −46 kb from the Klotho transcriptional start site (101), but whether this is a direct transcriptional effect has not yet been proved. For example, the upregulation of Klotho expression in the kidney by vitamin D was not observed in parathyroidectomized rats, suggesting that the presence of PTH is required for vitamin D–stimulated Klotho expression in the kidney (66).
It is proposed that the phosphatemic actions of vitamin D are opposed by the combined phosphaturic effects of FGF23 and Klotho (101). Like FGF23, which counteracts vitamin D (75), Klotho also forms a negative feedback control loop. High vitamin D levels increase Klotho expression, which in turn promotes phosphaturia and suppresses 1,25 VD3 production (Figure 4).
PATHOPHYSIOLOGY OF PHOSPHATE EXCESS
Concept of Phosphotoxicity
The nephrotoxic potential of enteric Pi overload has been unequivocally demonstrated in animals (102, 103). In humans, acute kidney injury followed by CKD occurs after use of oral Na+-Pi solutions for bowel cleansing (104). A single dose (11.5 g Pi) can push a person with normal kidney function to stages 3–5 CKD in the course of several months, indicating that enteric Pi overload can cause de novo CKD in humans. However, one does not know whether chronic dietary Pi overload at lower doses would increase CKD incidence and/or exacerbate existing CKD. Because hyperphosphatemia is a risk factor for mortality (105, 106), Pi-lowering therapy is prescribed for hyperphosphatemia in CKD and has demonstrated improved clinical outcomes (107). Notably, dietary phosphorus consumption per capita in the United States has continuously increased in the past four decades, exceeding 260% of the recommended intake level (108). Large-scale epidemiological studies have never been performed to confirm the link between dietary phosphorus intake and CKD prevalence/progression. There are several confounding obstacles.
Estimation of dietary phosphorus intake is inaccurate in existing nutrition surveys, e.g., the National Health and Nutrition Examination Survey, which does not account for the large, poorly specified, and variable amounts of added phosphorus (primarily as Pi) in processed foods (meats, cheeses, dressings, colas, bakery products, fast foods, etc.) for preservation, leavening, buffering, emulsification, color maintenance, flavor enhancement, and cryoprotection (109). Current regulations do not require the amounts of phosphorus-containing additives to be revealed, and such additives can increase phosphorus intake by as much as 1.0 g day−1 (109). In addition, phosphorus intake does not correlate with net phosphorus absorption from intestine. Phosphorus in natural foods exists as various forms of organic Pi, and its bioavailability varies from 0% to 60% (110). In contrast, phosphorus in food additives is universally inorganic Pi and 100% absorbable. At zero balance, Pi absorption from intestine is equal to excretion into urine, but 24-h urine data are rarely available in epidemiological studies.
Another impediment is the fact that we do not know what to measure to evaluate kidney damage induced by dietary Pi overload. Effects of dietary Pi overload on kidney have been studied in rodents since the 1930s (102). Dietary Pi loading induces tubulointerstitial fibrosis, tubular atrophy, and tubular dilatation in normal animals and, to a more severe extent, in uninephrectomized mice, suggesting that kidney damage correlates with Pi load excreted per nephron (103). Evidence for kidney damage, even without decreases in glomerular filtration rate (GFR), can be associated with increased mortality in humans (111). Sensitive and accurate biomarkers for histological kidney damage and dietary Pi intake, respectively, must be established before launching epidemiological studies to explore links between dietary Pi intake and CKD prevalence/progression.
Histological renal damage occurs when the estimated Pi excretion per nephron exceeds ~1.0 μg day−1 for several weeks, which is achievable in aged and CKD patients. Normal adults ingesting a “normal” diet excrete 0.8–1.5 g day−1 of Pi into urine (112). With the nephron number normally at ~0.9–1 million per kidney (the full range is 0.2 to 2.5 million) (113), Pi excretion is estimated at 0.64 μg day−1 nephron−1 in healthy young adults. A decrease in the nephron number by 36% increases Pi excretion to 1.0 μg day−1 nephron−1, a level that can induce kidney damage in rodents. A decrease in the nephron number by 36% also occurs during normal aging in humans: The nephron number of humans >55 years old is 30–46% less than that of people <45 years old (114). It is plausible that Pi excretion per nephron in CKD patients is higher than 1.0 g day−1. The mechanism by which increased Pi induces kidney damage remains to be determined. One hypothesis is that Pi in the luminal fluid of renal tubules is deleterious. This can be shown in cell culture by increasing Pi concentrations in the medium. When Pi concentrations are increased from 1 to 2.0–5.0 mM, various cellular responses can be induced, including activation of MAPKs, increases in reactive oxygen species, calcification, cell cycle arrest, and cell death (115, 116). In cultured vascular smooth muscle cells, increased Pi uptake via type III Na-dependent Pi transporters (Pit-1) was shown to be essential for in vitro calcification induced by high-Pi medium (117). Recent studies demonstrated that high extracellular Pi triggered cell death and calcification only when Pi formed insoluble nanocrystals with Ca2+ when Ca2+ and Pi concentrations exceeded the solubility limit. CaPi crystals of a particular size (<1,000 nm) are highly bioactive that can promote calcification by inducing expression of osteopontin and bone morphogenic protein-2 (118). These cellular responses were abolished by pyrophosphate, an inhibitor of CaPi crystal formation. Phosphonophormic acid (PFA) also interferes with CaPi crystal formation and calcification (119) but has been erroneously used as an inhibitor for Pit-1. PFA has dual activity that inhibits CaPi crystal formation and type II Na+-dependent Pi cotransporters but not Pit-1 (119).
CaPi nanocrystals can be endocytosed and dissolved in lysosomes, resulting in Ca2+ leak into cytosol and induction of apoptosis. In renal proximal tubular cells, CaPi crystals induced production of reactive oxygen species and oxidative cellular damage (115). Pi concentrations in tubular fluid measured by micropuncture in rats predicted that supersaturation of Ca2+ and Pi would occur in the proximal tubular lumen when Pi excretion per nephron is increased (120). CaPi crystals cause oxidative damage and induce expression of monocyte chemotactic protein-1 in proximal tubular cells (121), which promotes leukocyte infiltration and release of inflammatory cytokines. If this cascade of events indeed takes place in vivo, it may contribute to renal fibrosis induced by increased Pi excretion per nephron.
Phosphate and Aging
An important concept to at least entertain is whether individuals with normal renal function suffer any ill effects from dietary Pi loading. Serum Pi levels positively correlate with all-cause mortality in the general population, even when levels are within the normal range (105). Mice lacking FGF23 (Fgf23−/− mice) (21) or Klotho (Klotho−/− mice) (22) suffer a syndrome resembling human aging and abnormal mineral metabolism characterized by high blood levels of Pi, Ca2+, and vitamin D. The critical question is: Who is the guilty party? Vitamin D–deficient diet (100, 122) or disruption of the VDR (123) or Cyp27b1 gene (124, 125) rescued many aging-like features in Fgf23−/− and/or Klotho−/− mice. This suggests that vitamin D intoxication might be responsible for the premature aging syndrome in Fgf23−/− and Klotho−/− mice. These interventions reduced not only blood vitamin D levels but also blood Ca2+ and Pi levels, which raised the possibility that Ca2+ and/or Pi might be the true culprit(s). However, Pi-deficient diet (122) or induction of renal Pi wasting by disruption of the Npt2a gene (126) also rescued the aging-like features in Fgf23−/− and/or Klotho−/− mice. These interventions reduced blood Pi levels but increased vitamin D and Ca2+ levels. Pi deficiency caused by either low intake (dietary restriction) or high excretion (Npt2a knockout) increases vitamin D, which also increases intestinal Ca2+ absorption and results in increased blood Ca2+ levels. Despite the high blood vitamin D and Ca2+ levels, these interventions rescued the Fgf23−/− and/or Klotho−/− mice. Furthermore, Npt2a−/− Klotho−/− double-knockout mice regained the aging-like phenotypes by dietary Pi overload (126). These observations provide unequivocal evidence that Pi retention can induce a premature aging syndrome in mice and revealed an unexpected link between Pi and aging. It remains to be determined whether a certain level of Ca2+ and/or vitamin D is a prerequisite for Pi to induce a premature aging syndrome.
CHRONIC KIDNEY DISEASE: A SPECIAL SCENARIO
CKD is a global public health problem affecting 5–10% of the world's population. Because the components of the bone-kidney-intestine network are inseparable in vertebrates with hydroxyapatite skeletons, as kidney function declines, there is unavoidable progressive derangement in mineral homeostasis, with a disruption of both blood and tissue concentrations of Pi and Ca2+ and changes in circulating levels of calciophosphotropic hormones. The term chronic kidney disease–mineral and bone disease (CKD-MBD) refers to a constellation of features due exclusively to renal dysfunction and is used to describe (a) the broader clinical syndrome encompassing altered levels of Ca2+, phosphorus, PTH, and vitamin D; (b) disturbances in bone modeling and remodeling, with the associated development of fractures or impaired linear bone growth (in children); and (c) extraskeletal calcification in soft tissues and arteries (127).
In early CKD with decline in Klotho, compensatory mechanisms in the form of elevated FGF23 occur and, in patients with advanced CKD, elevated PTH, and decreased calcitriol levels, result in normal to near-normal blood Ca2+ and Pi levels. These compensatory mechanisms become overwhelmed in end-stage CKD, eventually resulting in a group of abnormalities encompassed by CKD-MBD (128).
Mineral Disturbances and Morbidity and Mortality
Current evidence is still largely associative in nature in terms of mineral disturbance and high morbidity and mortality in CKD. The severity and number of each component of CKD-MBD increase with advancing CKD. Numerous cohort studies have shown associations between parameters of deranged mineral metabolism with fractures, cardiovascular disease, and overall mortality in CKD (129, 130). At this point, the epidemiological database will keep growing and is no doubt informative, but what the field needs direly is the identification of pathophysiological mechanisms that establish causality between mineral disturbances and cardiovascular disease and mortality.
CKD-MBD Complex: An Evolving Concept
Although an official definition for this entity exists, the full scope of its constitution is still evolving. The hormonal disturbances include PTH, vitamin D and its metabolites, FGF23, and growth hormone (127). It was recommended that the term renal osteodystrophy be restricted to bone histopathology associated with CKD. An expanded classification system was developed on the basis of parameters of bone turnover, mineralization, and volume (127). The mineral and endocrine functions disrupted in CKD are critically important in the regulation of both initial bone formation during growth (bone modeling) and bone structure and function during adulthood (bone remodeling). As a result, bone abnormalities are found universally in patients with CKD requiring dialysis and in the majority of patients with CKD stages 3–5.
Cardiovascular disease is a leading cause of mortality in CKD, and cardiovascular calcification is universal in cardiovascular disease in CKD (131, 132). Cardiovascular calcification is a heterogeneous disorder with overlapping yet distinct mechanisms of initiation and progression (133, 134). Vascular calcification is a dynamic process resulting from the imbalance between promoters and inhibitors (133, 135). In addition to traditional factors, FGF23 and Klotho are novel contributors to ectopic calcification in soft tissues, including cardiac valves and aorta (22, 59, 89). In rats with CKD induced by adenine and high protein diet, severe bone loss is associated with medial calcifications in the aorta (136). An intriguing finding is that cortical rather than trabecular bone loss correlated more with the severity of vascular calcification in CKD rats (136). It is proposed that Ca2+ and Pi release from disturbed PTH, low 1,25 VD3, high FGF23, and low soluble Klotho may trigger or accelerate vascular calcification (136).
Perhaps it is appropriate that the CKD-MBD complex be broadened to include organs other than bone, serum chemistry, and soft tissue. It is increasingly convincing that the cardiovascular disturbances of CKD are coupled to mineral disturbances. In fact, CKD-MBD may very well encompass the bulk of the metabolic abnormalities and end-organ damage seen in uremia, including those affecting the heart. Regardless of how one defines CKD-MBD, one certain fact is the necessity to understand the underlying causes of this syndrome. We commence this exercise by considering Klotho.
CKD: A State of Klotho Deficiency
As a general principle, if the organ of origin of an endocrine substance is diseased, it is logical to suspect that endocrine deficiency of that substance will ensue. There are many similar features between the clinical manifestations of CKD and the phenotype of Klotho−/− mice (Table 1). Experimental data from in vivo and in vitro studies and clinical findings have, by and large, supported this view (87, 88, 137–141) (Table 2).
Table 1.
Comparison of phenotypes between primary (genetic loss-of-function mutation) and secondary (chronic kidney disease) states of Klotho deficiencya
| Phenotype | Klotho deficiency | Chronic kidney disease |
|---|---|---|
|
| ||
| Blood chemistry | ||
|
| ||
| Phosphate | Extreme increase | Increase |
| GFR | Mild increase | Decrease with stage |
| FGF23 | Extreme increase | Severe increase |
| Renal Klotho | Disappear | Decrease with decline in GFR |
| Systemic Klotho | Disappear | Decrease with decline in GFR |
|
| ||
| Gross phenotype | ||
|
| ||
| Body weight | Severe decrease | Decrease |
| Growth | Severe retardation | Retardation mainly in children |
| Physical activity | Severe decrease | Decrease |
| Fertility | Lost | Decrease |
| Life span | Much shorter | High mortality |
|
| ||
| Cardiovascular disease | ||
|
| ||
| Heart | Hypertrophy and fibrosis | Hypertrophy and fibrosis |
| Vasculature | Calcification | Calcification |
| Blood pressure | Mild hypertension | Severe hypertension |
| Atherosclerosis | Mild | Moderate or severe |
|
| ||
| Anemia | Mild | Severe |
|
| ||
| Bone | Osteoporosis | Renal bone disease |
Table 2.
Summary of evidence for systemic and renal Klotho deficiency in chronic kidney disease
| Etiology | Renal Klotho | Systemic Klotho | Comment | Reference | |||
|---|---|---|---|---|---|---|---|
| Protein | mRNA | Plasma | Urine | ||||
| Animal model | |||||||
| CKD | Npx + IRI | ↓ | ↓ | ↓ | ↓ | Klotho protein assayed by IB | 59 |
| CKD | 5/6 Npx | ↓ | Assayed by NB | 88 | |||
| CKD | ICGN | ↓ | Assayed by RT-qPCR | 138 | |||
| CKD | Npx | ↓ | ↓ | ↓ | Subtotal Npx in apo-E−/− mice Plasma Klotho assayed by ELISA |
184 | |
| DM | Streptozotocin | ↓ | Assayed by RT-qPCR | 142 | |||
| DM | OLETF | ↓ | Assayed by NB | 141 | |||
| DM | db/db | ↓ | ↓ | Klotho decreases inflammation in the kidney of db/db | 139 | ||
| Hypertension | SHR | ↓ | Assayed by NB | 141 | |||
| Hypertension | DOCA | ↓ | Assayed by NB | 141 | |||
| Hypertension | Ang II | ↓ | ↓ | Klotho mRNA assayed by NB | 180 | ||
| Hypertension | Ang II | ↓ | ↓ | Klotho mRNA assayed by RT-qPCR | 185 | ||
| Hypertension | SHR | ↓ | ↓ | Klotho gene delivery decreases blood pressure | 140 | ||
| Human subject | |||||||
| CKD | CGN | ↓ | ↓ | Etiologies are not shown; assayed by NB, IB, and IHC | 88 | ||
| CKD | DN | ↓ | ↓ | Assayed by NB, IB, and IHC | 88 | ||
| Graft rejection | CGR | ↓ | ↓ | Assayed by NB, IB, and IHC | 88 | ||
| CKD | N/A | ↓ | Assayed by ELISA and starts to reduce in early CKD | 85 | |||
| CKD | N/A | ↑ | Assayed by ELISA and increases in early CKD | 144 | |||
| CKD | N/A | ↑ | Assayed by ELISA and increases in CKD | 186 | |||
| CKD | DN | ↓ | Assayed by ELISA | 186 | |||
| CKD | N/A | ↓ | Assayed by IB and starts to decrease in early CKD | 59 | |||
| CKD | DN | ↓ | RT-qPCR and starts to decrease in early CKD | 142 | |||
| CKD | IgA nephropathy | ↓ | Only in advanced CKD | 142 | |||
| CKD | MCD | ↓ | Only in advanced CKD | 142 | |||
| ADPKD | ↓ | Assayed by ELISA and starts to reduce in early CKD | 86 | ||||
| CKD | N/A | ↔ | No reduction in early CKD | 86 | |||
Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; Ang II, angiotensin II; apo-E, apolipoprotein E; CKD, chronic kidney disease; CGN, chronic glomerulonephritis; CGR, chronic glomerulonephritis; DM, diabetes mellitus; DN, diabetic nephropathy; DOCA, deoxycorticosterone acetate; ELISA, enzyme-linked immunosorbent assay; IB, immunoblot; ICGN, Institute for Cancer Research–derived (spontaneous) glomerulonephritis; IgA, immunoglobulin A; IHC, immunohistochemistry; IRI, ischemia-reperfusion injury; MCD, minimal change disease; N/A, no information available; NB, Northern blot; Npx, nephrectomy; OLETF, Otsuka Long-Evans Tokushima fatty (rat); RT-qPCR, reverse transcription quantitative polymerase chain reaction; SHR, spontaneous hypertension.
Thus far, measurements of renal Klotho mRNA and protein in human CKD have been limited. Renal expression of the Klotho gene is markedly decreased in patients with CKD with different etiologies (88, 142) (Table 2). In the last two to three years, data on plasma Klotho levels in human CKD have begun to emerge (86) by using a recently generated ELISA (143). The initial data showed that plasma Klotho correlated with serum creatinine, blood urea nitrogen, and FGF23, suggesting that plasma Klotho might be affected by renal function, even though that study did not enroll any CKD patients (143). However, soluble plasma Klotho assayed with the same kit was three- to fourfold higher in CKD patients compared with healthy volunteers (144) (Table 2). Although reproducible and valid clinical data in human plasma Klotho are yet to be acquired, urinary Klotho levels in CKD patients were shown to decrease at very early stages and were sustainably reduced with the progression of CKD (59) (Table 2). In a rodent CKD model, Klotho levels in plasma, urine, and kidney are decreased in parallel (59) (Table 1). This relationship remains to be defined in humans. Furthermore, almost all CKD models, including renal tissue ablation, glomerulonephritis, nephrotoxin, diabetic nephropathy, and hypertensive kidney damage, show considerable downregulation of Klotho mRNA and protein in the kidney with low levels of soluble Klotho in plasma or in urine (Table 2). Whether plasma Klotho is also decreased in patients with early CKD who have decreased Klotho expression in the kidney remains to be confirmed. Animal experiments have clearly and consistently shown that CKD is a state of systemic and renal Klotho deficiency and that maintenance of higher Klotho levels by genetic means is overall beneficial (59) (Table 2).
Mineral Parameters in CKD Progression
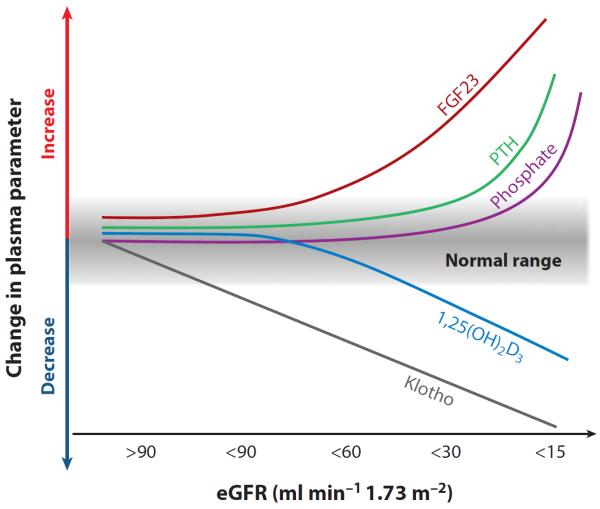
Determination of blood-soluble Klotho has several potential uses in CKD. The first is to simply understand its pathophysiology and how downregulation of circulating Klotho triggers disturbs mineral metabolism and causes imbalance of calciophosphotropic hormones (PTH, FGF23, and 1,25 VD3). In addition, plasma Klotho may be an early, sensitive, and specific diagnostic biomarker for kidney disease and may also bear prognostic value in predicting progression and complications of CKD. The most promising data thus far are that urinary Klotho is reduced at a very early stage of CKD (stages 1 and 2) and its levels are progressively lowered with declining estimated glomerular filtration rate (eGFR) (59). Figure 5 illustrates a hypothetical model. Systemic Klotho declines very early in CKD. Rodent data indicate that the lower is the Klotho level, the more severe is the soft tissue calcification (59), suggesting that Klotho may be a useful prognostic factor. A cross-sectional observational study showed that plasma Klotho is decreased in stage 2 CKD and is negatively correlated with eGFR (85).
Figure 5.
Proposed time profile of changes in blood phosphate (Pi), calcium, Klotho, and hormones relevant to mineral metabolism in chronic kidney disease (CKD). The decrease in Klotho protein in the kidney and blood is an early event in CKD and is sustainably and progressively reduced along with the decline of renal function. Low Klotho partially induces fibroblast growth factor (FGF) 23 resistance, causing an initial compensatory increase in blood FGF23 to maintain Pi homeostasis. Increase in FGF23 decreases vitamin D levels and is followed by elevation of parathyroid hormone (PTH). Hyperphosphatemia is a relatively late event in advanced CKD; normal range is shown in gray. The scale is not meant to be truly proportionate; e.g., the elevation of FGF23 is massive in CKD compared with the elevation of parathyroid hormone. The x axis represents decline in renal function from stage 1 to 5 of CKD based on estimated glomerular filtration rate (eGFR).
In autosomal dominant polycystic kidney disease patients with low Klotho, the high FGF23 does not lead to the expected phosphaturia despite normal renal function (86). As more functional nephrons are lost during the progression of CKD, Klotho in the kidney, blood, and urine decreases further, causing further FGF23 elevation. Increasing blood levels of FGF23 will suppress active vitamin D synthesis in the kidney. Decreases in functional nephrons also contribute to decreases in blood 1,25 VD3. In fact, decreases in Klotho, increases in FGF23, and decreases in vitamin D precede overt hyperphosphatemia during the progression of CKD (Figure 5). Because 1,25 VD3 is a potent stimulator of Klotho (100), decreases in 1,25 VD3 can further reduce Klotho expression. Because 1,25 VD3 also functions as a potent suppressor of PTH (31), decreases in 1,25 VD3 can increase PTH (31), which may be synergistically enhanced by low Klotho expression and FGFR expression in the parathyroid (145). Serum PTH levels increase prior to increases in serum Pi levels (146). Increases in PTH can further increase FGF23 (77, 147). The ability of the kidneys to appropriately excrete a Pi load is diminished, leading to hyperphosphatemia, elevated PTH, and decreased vitamin D with further increases in blood FGF23. The kidney fails to respond adequately to PTH or to FGF23, which normally enhances Pi excretion. In addition, there is evidence at the tissue level of downregulation of VDR and of resistance to the actions of PTH. Once hyperphosphatemia sets in, it exacerbates all the other abnormalities. Thus, these changes in Pi-regulating hormones and Klotho form a vicious positive feed-forward downhill cycle, leading to high FGF23, high PTH, low vitamin D, and low Klotho in patients with end-stage renal disease (Figure 6a). Klotho deficiency may initiate or aggravate dysregulated mineral metabolism, including high FGF23, high PTH, low vitamin D, and hyperphosphatemia. All these abnormalities contribute to CKD progression and development of extrarenal complications (148) (Figure 6b).
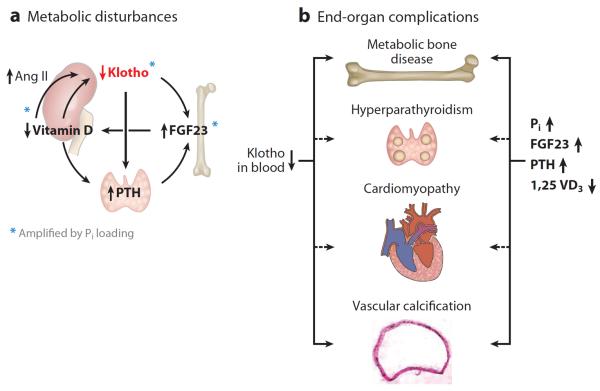
Figure 6.
Pathophysiological role of Klotho deficiency in disturbed mineral metabolism and complications of chronic kidney disease-mineral and bone disease (CKD-MBD). (a) A sequence of events that constitutes a positive feedback, self-exacerbating downhill spiral. In CKD and end-stage kidney disease, the normal interactive network is deranged. Each of the hormonal disturbances is amplified by phosphate (Pi) loading (blue asterisks). Both renal and plasma Klotho are decreased. The downregulation of Klotho increases fibroblast growth factor (FGF) 23 production, which in turn suppresses vitamin D production in the kidney. Low blood Klotho will blunt the suppressive effect of FGF23 on parathyroid hormone (PTH) production. In addition, decreased FGFR1 and Klotho in the uremic parathyroid gland render the gland resistant to the suppressive effect of FGF23 and triggers and/or promote secondary hyperparathyroidism. High blood PTH further stimulates FGF23 production. Hyperphosphatemia amplifies the high FGF23 and PTH and low Klotho. Low vitamin D downregulates renal Klotho expression directly and indirectly via intrarenal angiotensin II (Ang II), which further reduces renal Klotho production. (b) High plasma PTH, Pi, and FGF23 and low plasma vitamin D and Klotho contribute in concert to the development of complications such as metabolic bone disease, secondary hyperparathyroidism, cardiomyopathy, and vascular calcification. Dashed lines depict proposed but unproven roles of the metabolic disturbances.
Klotho Deficiency: A Principal Perpetrator in CKD-MBD
Secondary hyperparathyroidism is an important component of the CKD-MBD spectrum (149). The low circulating Klotho might be a novel pathogenic contributor to secondary hyperparathyroidism in addition to the other mineral disturbances (150, 151), causing failure of FGF23 to inhibit PTH production in parathyroid gland in the uremic setting (95, 145). CKD patients have low FGFR1 and Klotho expression in parathyroid gland (145). FGF23 fails to suppress PTH in rats with advanced CKD (152). This is further compounded by the low expression of Ca2+-sensing receptor and VDR (Figure 6b).
PTH may play a role in upregulation of blood FGF23 because parathyroidectomy decreases blood Ca2+ and FGF23 but parathyroid glands do not express FGF23, suggesting that PTH is involved in modulation of FGF23 production in bone (147, 153). Direct addition of PTH to osteoblast-like UMR106 cells increased FGF23 mRNA levels (77). The effect of PTH on FGF23 completes a bone-parathyroid endocrine feedback loop (Figure 4).
Vascular calcification is prominent in mice with Klotho gene deletion, similarly to CKD subjects (Table 1). Vascular calcification observed in Klotho−/− mice is reversed by Klotho overexpression through adenoviral delivery of Klotho gene (154). In addition, recombinant Klotho protein suppresses Pit1 expression and Pi uptake induced by a high-Pi medium in rat vascular smooth cells (59). The suppressive influence of Klotho on vascular calcification is multifactorial, including the indirect effect of phosphaturia. In addition, there are direct effects of Klotho on the vasculature independent of systemic effects. High Pi uptake induces vascular smooth muscle cell reprogramming to osteoblasts/osteochondrocytes through the NaPi-3 transporters Pit-1 or/and Pit-2, and Klotho inhibits all these changes (155). Klotho also suppresses cell senescence, apoptosis, and death in vascular endothelial cells and smooth muscle cells induced by a variety of insults, including Pi (59, 156, 157). Finally, Klotho serves as an anti-inflammatory modulator and restricts the inflammatory process to protect the vasculature (139). All these experiments of direct Klotho addition in vitro occurred in the absence of FGF23.
The fact that Klotho-deficient mice have high blood FGF23 and ectopic calcification in soft tissues brought forth the hypothesis that vascular calcification might be an off-target effect of FGF23 in CKD (158, 159). Lim et al. (158) proposed that CKD is a state of vascular Klotho deficiency that potentiates the development of accelerated calcification through a Runx2- and myocardin-serum response factor–dependent pathway. Vascular cells may be a Klotho-dependent target tissue for FGF23 because Klotho knockdown abrogated FGF23-mediated cell signaling and proliferative effects. The restoration of Klotho and FGFRs by VDR activators renders human vascular smooth muscle cells FGF23 responsive, which may exert anticalcification effects (158).
Uremic cardiomyopathy is characterized by cardiac hypertrophy and fibrosis and is a principal pathological feature of cardiovascular complications in CKD. Clinically, uremic cardiomyopathy is characterized by cardiac arrest or sudden death, left ventricular hypertrophy, diastolic dysfunction, and congestive heart failure, which is in addition to and distinct from hypertensive and ischemic cardiomyopathy (160). Novel risk factors in CKD include inappropriate activation of the reninangiotensin-aldosterone system (RAS) (161), vitamin D deficiency (162), high plasma FGF23 (24, 163), and more recently, low plasma Klotho in blood (24) (Figure 6b). Klotho-deficient mice have left ventricular hypertrophy (24). Because there is normally no Klotho expression in the ventricles, the cardiac phenotype in Klotho−/− mice is due to other abnormal parameters brought about by deficiency of circulating Klotho. Left ventricular hypertrophy in CKD appears to be FGF23 dependent because intramyocardial and intravenous administration of FGF23 results in cardiac hypertrophy in wild-type mice, and an FGFR blocker attenuates cardiac hypertrophy in 5/6 nephrectomized CKD but does not change blood pressure (24).
Metabolic bone disease is the skeletal component of CKD-MBD and is characterized by either high or low turnover and either the presence or absence of mineralization defect in CKD patients (164, 165). Secondary hyperparathyroidism, low blood Ca2+, Pi retention, deficiency of vitamin D, high FGF23, and low Klotho may all potentially contribute to CKD-MBD (166) (Figure 6 b). Klotho−/− mice have abnormal elongation of the trabecular bone(s) in the epiphyses of long bones, with lower density compared with the wild type. Cancellous bone volume in the epiphyses of Klotho−/− mice is three times that of the wild-type mice, suggesting that the elongation of the trabecular bone apparently results from the relatively low levels of bone resorption. Therefore, bone turnover is low in Klotho−/− mice (167). A direct effect of Klotho on osteoblast was shown in mouse osteoblast cell line MC3T3.E1. Shalhoub et al. (168) proposed that soluble Klotho enables FGF23 signaling, likely through FGFR1 in physiological condition, whereas supraphysiological FGF23 can directly impair bone independently of soluble Klotho. This result supports the hypothesis that elevated FGF23 with low blood Klotho in CKD may contribute to bone pathologies by direct action on bone cells (168).
Future Therapeutic Strategies in CKD
The proposed model in Figure 6 predicts that a number of abnormalities in CKD amplify themselves; hence, beyond a certain stage, even if the original insult is ameliorated or removed, this downhill spiral will bring about the eventual demise of the kidney and various extrarenal sites. On the basis of this paradigm, it is important to interrupt this vortex early and, most desirably, at multiple loci because a single manipulation is much less likely to forestall or stop this vortex. Pi binders have been used for a long time, and their scope is being broadened, at least in terms of a variety of blockers. The clinical benefits of Pi binders have been verified (107, 169), and hopefully, with the advent of intestinal transport inhibitors, this mode of therapy will be more effective. Vitamin D replacement is successfully instituted to treat both the vitamin D deficiency itself and the secondary hyperthyroidism, and its positive impact has been documented (170, 171). Hyperparathyroidism can also be controlled with calcimimetics (172, 173). The spiral presented in Figure 6 is admittedly too simple, but even with this caveat, there are still two remaining culprits, namely, high FGF23 and low Klotho. Pharmacological or peptide blockade of FGF23 is being studied in the preclinical stage (17, 174). The use of therapeutic anti-FGF23 monoclonal antibodies was founded on the assumption that high FGF23 is detrimental in CKD. The animal data thus far show that complete neutralization of FGF23 improved serum and bone mineral parameters (175, 176) but considerably worsened vascular calcification and mortality (176). This study illustrates that, although high FGF23 may very well be harmful, complete annihilation is clearly not the solution (176).
The last component of the downward spinning vortex proposed in Figure 6 is Klotho deficiency, which may well be one of the earliest events. Thus far, no study has directly documented the therapeutic effect of Klotho in humans. Establishing whether Klotho protein has therapeutic potential for CKD and its complications is a critical next step. Because Klotho is a multifunctional protein, its action on CKD may result from modulation of circulating hormone or/and mineral parameters or from its direct influence on target organs. The potential favorable effects of Klotho on CKD are related to induction of phosphaturia, restoration of the on-target effect of FGF23, anti–soft tissue calcification, antifibrosis, antioxidation, anti–cell senescence, and antagonism of angiotensin II effects.
Administration of exogenous recombinant Klotho to CKD patients is one simple and effective means to correct an endocrine deficiency similar to the replacement of erythropoietin and active vitamin D. Klotho administration has proved successful in acute kidney injury in animals, which is a state of acute Klotho deficiency induced by ischemia-reperfusion injury (177) and by unilateral ureteral ligation (178), and also in CKD in mice that have systemic and renal Klotho deficiency induced by unilateral nephrectomy and contralateral ischemia-reperfusion (59). Furthermore, Klotho may potentially reverse or retard the progression of CKD. Even in advanced stages of CKD, Klotho supplementation may alleviate extrarenal complications of CKD. In addition to Klotho protein replacement, stimulation or reversal of suppression of endogenous Klotho production may be an alternative to increase Klotho expression in the kidney. Furthermore, strategies to increase extrarenal Klotho production will be of more particular importance for end-stage renal disease patients whose functional kidney tissue is totally lost. Thus far, the possible strategies that can be used to increase endogenous Klotho include control of hyperphosphatemia (179), vitamin D repletion (100), angiotensin II blockade (180), and the use of PPARγ agonists (181).
EPILOGUE
The FGF23-Klotho system represents a highly specialized endocrine network that evolved under the demand of the apatite endoskeleton to maintain skeletal health and external and internal Ca2+ and Pi balance. This network involves the calciophosphoregulatory fluxes in the intestine, bone, and kidney and invokes multiple feedback loops that transcend many organs. In addition to serving as an obligatory coreceptor for FGF23, the soluble form of Klotho also carries a multitude of functions that are above and beyond those of a coreceptor for FGF23. It is unclear at the moment whether functional soluble Klotho pre- or postdated the FGF23-FGFR-Klotho complex in evolution. As there is no identified receptor for soluble Klotho, it is enigmatic how this circulating protein exerts its effects, other than through the known glycan-modifying properties of Klotho. How can such far-reaching effects of soluble Klotho be achieved without some form of intracellular signal transduction?
Derangements of mineral metabolism can have universal impact on all cell functions. The most subtle form can be witnessed in conditions that fall on the borderline between health and disease, such as accelerated aging from very subtle but chronic, sustained Ca2+ and/or Pi overload. The elucidation of possible causality is clearly worthwhile.
Although the impact of mineral derangement on healthy subjects remains to be proved, CKD represents the most common and blatant state of severe and global alterations in mineral metabolism. If the term CKD-MBD was intended to embrace the spectrum of mineral disorders, it may in fact end up encompassing most of the morbid conditions seen in uremia, perhaps with the exception of Na+ and K+ overload. Single disturbances in mineral regulation do not produce multisystemic catastrophes even near the magnitude of CKD. Vitamin D deficiency causes rickets or osteomalacia; monogenic causes of high FGF23 lead to Pi wasting and osteomalacia; and primary hyperparathyroidism causes osteitis fibrosa cystica, hypercalcemia, and hypercalciuric urolithiasis. The combinatorial defects and self-amplifying nature of the ensemble of mineral disturbances in CKD are what beget the disastrous calamity.
If evolution of the apatite skeleton endowed us with the intestine-bone-kidney network of mineral metabolism, CKD exemplifies the delicacy and consequence of disruption of this system. Treatment requires sound knowledge of the physiology so that specific interventions of each pathophysiological derangement can be deployed.
Supplementary Material
Chronic kidney disease-mineral and bone disease.
systemic disorder in CKD with (a) abnormal blood calcium, phosphorus, parathyroid hormone, vitamin D, or fibroblast growth factor 23; (b) disturbed bone turnover, mineralization, volume, or strength; and (c) vascular or soft-tissue calcification
ACKNOWLEDGMENTS
The authors are supported by the National Institutes of Health, the Charles and Jane Pak Foundation, and the Simmons Family Foundation.
Footnotes
DISCLOSURE STATEMENT The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Lowenstam HA. Minerals formed by organisms. Science. 1981;211:1126–31. doi: 10.1126/science.7008198. [DOI] [PubMed] [Google Scholar]
- 2.Berrill NJ. Origin of Vertebrates. Clarendon; Oxford: 1955. [Google Scholar]
- 3.Haltstead LB. Vertebrate Hard Tissues. Wykeham; London: 1974. [Google Scholar]
- 4.Pautard FG. Calcium, phosphorus and the origin of backbones. New Sci. 1961;260:364. [Google Scholar]
- 5.Ruben JA, Bennett AA. The evolution of bone. Evolution. 1987;41:1187–97. doi: 10.1111/j.1558-5646.1987.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 6.Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, et al. Leptin in human physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 2011;301:E567–84. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011;54:1291–97. doi: 10.1007/s00125-011-2155-z. [DOI] [PubMed] [Google Scholar]
- 8.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–20. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–49. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–37. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev. Dyn. 2008;237:18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- 12.Beenken A, Mohammadi M. The structural biology of the FGF19 subfamily. Adv. Exp. Med. Biol. 2012;728:1–24. doi: 10.1007/978-1-4614-0887-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones SA. Physiology of FGF15/19. Adv. Exp. Med. Biol. 2012;728:171–82. doi: 10.1007/978-1-4614-0887-1_11. [DOI] [PubMed] [Google Scholar]
- 14.Kuro-o M. Endocrine FGFs and Klothos. Landes Biosci./Springer; Austin, TX/New York: 2012. p. 233. [Google Scholar]
- 15.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 2010;24:2050–64. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, et al. Molecular insights into the Klotho-dependent, endocrine mode of action of FGF19 subfamily members. Mol. Cell. Biol. 2007;27:3417–28. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc. Natl. Acad. Sci. USA. 2010;107:407–12. doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White KE, Evans WE, O'Riordan JLH, Speer MC, Econs MJ, et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000;26:345–48. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 19.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl. Acad. Sci. USA. 2001;98:6500–5. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–86. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 21.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004;113:561–68. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 23.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006;281:6120–23. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011;121:4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J. Clin. Investig. 1999;104:229–37. doi: 10.1172/JCI5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suga T, Kurabayashi M, Sando Y, Ohyama Y, Maeno T, et al. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am. J. Respir. Cell Mol. Biol. 2000;22:26–33. doi: 10.1165/ajrcmb.22.1.3554. [DOI] [PubMed] [Google Scholar]
- 27.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–74. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 28.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–50. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murer H, Hernando N, Forster I, Biber J. Regulation of Na/Pi transporter in the proximal tubule. Annu. Rev. Physiol. 2003;65:531–42. doi: 10.1146/annurev.physiol.65.042902.092424. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 2006;17:1305–15. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 31.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am. J. Physiol. Ren. Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 32.Farrow EG, Davis SI, Summers LJ, White KE. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J. Am. Soc. Nephrol. 2009;20:955–60. doi: 10.1681/ASN.2008070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, et al. The parathyroid is a target organ for FGF23 in rats. J. Clin. Investig. 2007;117:4003–8. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1α-hydroxylase expression in cultured bovine parathyroid cells. J. Endocrinol. 2007;195:125–31. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 35.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–33. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, et al. Molecular cloning and expression analyses of mouse βklotho, which encodes a novel Klotho family protein. Mech. Dev. 2000;98:115–19. doi: 10.1016/s0925-4773(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 37.Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y. Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim. Biophys. Acta. 2002;1576:341–45. doi: 10.1016/s0167-4781(02)00281-6. [DOI] [PubMed] [Google Scholar]
- 38.Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, et al. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–56. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, et al. Tissue-specific expression of βKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 2007;282:26687–95. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, et al. βKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl. Acad. Sci. USA. 2007;104:7432–37. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–25. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Ito S, Fujimori T, Furuya A, Satoh J, Nabeshima Y, Nabeshima Y. Impaired negative feedback suppression of bile acid synthesis in mice lacking βKlotho. J. Clin. Investig. 2005;115:2202–8. doi: 10.1172/JCI23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu C, Wang F, Kan M, Jin C, Jones RB, et al. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J. Biol. Chem. 2000;275:15482–89. doi: 10.1074/jbc.275.20.15482. [DOI] [PubMed] [Google Scholar]
- 44.Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, et al. Identification of a hormonal basis for gallbladder filling. Nat. Med. 2006;12:1253–55. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 45.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–24. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomlinson E, Fu L, John L, Hultgren B, Huang X, et al. Transgenic mice expressing human fibro-blast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–47. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 47.Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 2011;13:729–38. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26:312–24. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kliewer SA, Mangelsdorf DJ. Fibroblast growth factor 21: from pharmacology to physiology. Am. J. Clin. Nutr. 2010;91:254S–57S. doi: 10.3945/ajcn.2009.28449B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, et al. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA. 2009;106:10853–58. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–25. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, et al. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology. 2011;152:2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–81. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–37. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted Klotho protein. Biochem. Biophys. Res. Commun. 1998;242:626–30. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 56.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–47. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 57.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natl. Acad. Sci. USA. 2007;104:19796–801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, et al. Klotho is a substrate for α-, β- and γ-secretase. FEBS Lett. 2009;583:3221–24. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011;22:124–36. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro-o M, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natl. Acad. Sci. USA. 2008;105:9805–10. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–93. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 62.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–6. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 63.Doi S, Zou Y, Togao O, Pastor JV, John GB, et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 2011;286:8655–65. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL. Regulation of renal outer medullary potassium channel and renal K+ excretion by Klotho. Mol. Pharmacol. 2009;76:38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, et al. Klotho is a novel β-glucuronidase capable of hydrolyzing steroid β-glucuronides. J. Biol. Chem. 2004;279:9777–84. doi: 10.1074/jbc.M312392200. [DOI] [PubMed] [Google Scholar]
- 66.Lopez I, Rodriguez-Ortiz ME, Almaden Y, Guerrero F, de Oca AM, et al. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011;80:475–82. doi: 10.1038/ki.2011.107. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez-Ortiz ME, Lopez I, Munoz-Castaneda JR, Martinez-Moreno JM, Ramirez AP, et al. Calcium deficiency reduces circulating levels of FGF23. J. Am. Soc. Nephrol. 2012;23:1190–97. doi: 10.1681/ASN.2011101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49:636–43. doi: 10.1016/j.bone.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, et al. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J. Clin. Investig. 2006;116:3150–59. doi: 10.1172/JCI29463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haussler MR, Whitfield GK, Kaneko I, Forster R, Saini R, et al. The role of vitamin D in the FGF23, klotho, and phosphate bone-kidney endocrine axis. Rev. Endocr. Metab. Disord. 2011;13:57–69. doi: 10.1007/s11154-011-9199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J. Clin. Endocrinol. Metab. 2002;87:4957–60. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 72.Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J. Bone Miner. Res. 2003;18:1227–34. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 73.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N. Engl. J. Med. 2003;348:1656–63. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 74.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006;38:1310–15. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wohrle S, Bonny O, Beluch N, Gaulis S, Stamm C, et al. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF-23 signaling and regulating FGF-23 expression in bone. J. Bone Miner. Res. 2011;26:2486–97. doi: 10.1002/jbmr.478. [DOI] [PubMed] [Google Scholar]
- 76.Martin A, Liu S, David V, Li H, Karydis A, et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25:2551–62. doi: 10.1096/fj.10-177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am. J. Physiol. Ren. Physiol. 2010;299:F882–89. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 78.Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu. Rev. Physiol. 2007;69:341–59. doi: 10.1146/annurev.physiol.69.040705.141729. [DOI] [PubMed] [Google Scholar]
- 79.Yuan Q, Sato T, Densmore M, Saito H, Schuler C, et al. FGF-23/Klotho signaling is not essential for the phosphaturic and anabolic functions of PTH. J. Bone Miner. Res. 2011;26:2026–35. doi: 10.1002/jbmr.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barthel TK, Mathern DR, Whitfield GK, Haussler CA, Hopper HA, 4th, et al. 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J. Steroid Biochem. Mol. Biol. 2007;103:381–88. doi: 10.1016/j.jsbmb.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 81.Yu X, Sabbagh Y, Davis SI, Demay MB, White KE. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone. 2005;36:971–77. doi: 10.1016/j.bone.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Ito N, Fukumoto S, Takeuchi Y, Takeda S, Suzuki H, et al. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J. Bone Miner. Metab. 2007;25:419–22. doi: 10.1007/s00774-007-0779-3. [DOI] [PubMed] [Google Scholar]
- 83.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J. Bone Miner. Res. 2006;21:1187–96. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 84.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Investig. 2007;117:2684–91. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, et al. Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin. Exp. Nephrol. 2012;16:722–29. doi: 10.1007/s10157-012-0621-7. [DOI] [PubMed] [Google Scholar]
- 86.Pavik I, Jaeger P, Ebner L, Poster D, Krauer F, et al. Soluble klotho and autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2012;7:248–57. doi: 10.2215/CJN.09020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu MC, Kuro-o M, Moe OW. Secreted klotho and chronic kidney disease. Adv. Exp. Med. Biol. 2012;728:126–57. doi: 10.1007/978-1-4614-0887-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem. Biophys. Res. Commun. 2001;280:1015–20. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 89.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, et al. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23)-mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–41. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bai X, Dinghong Q, Miao D, Goltzman D, Karaplis AC. Klotho ablation converts the biochemical and skeletal alterations in FGF23 (R176Q) transgenic mice to a Klotho-deficient phenotype. Am. J. Physiol. Endocrinol. Metab. 2009;296:E79–88. doi: 10.1152/ajpendo.90539.2008. [DOI] [PubMed] [Google Scholar]
- 91.Nakatani T, Ohnishi M, Razzaque MS. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. FASEB J. 2009;23:3702–11. doi: 10.1096/fj.08-123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carpenter TO, Insogna KL, Zhang JH, Ellis B, Nieman S, et al. Circulating levels of soluble Klotho and FGF23 in X-linked hypophosphatemia: circadian variance, effects of treatment, and relationship to parathyroid status. J. Clin. Endocrinol. Metab. 2010;95:E352–57. doi: 10.1210/jc.2010-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marsell R, Krajisnik T, Goransson H, Ohlsson C, Ljunggren O, et al. Gene expression analysis of kidneys from transgenic mice expressing fibroblast growth factor-23. Nephrol. Dial. Transplant. 2008;23:827–33. doi: 10.1093/ndt/gfm672. [DOI] [PubMed] [Google Scholar]
- 94.Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–82. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- 95.Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, et al. FGF23 fails to inhibit uremic parathyroid glands. J. Am. Soc. Nephrol. 2010;21:1125–35. doi: 10.1681/ASN.2009040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tatsumi S, Segawa H, Morita K, Haga H, Kouda T, et al. Molecular cloning and hormonal regulation of PiT-1, a sodium-dependent phosphate cotransporter from rat parathyroid glands. Endocrinology. 1998;139:1692–99. doi: 10.1210/endo.139.4.5925. [DOI] [PubMed] [Google Scholar]
- 97.Nabeshima Y. The discovery of α-Klotho and FGF23 unveiled new insight into calcium and phosphate homeostasis. Cell. Mol. Life Sci. 2008;65:3218–30. doi: 10.1007/s00018-008-8177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoshida T, Fujimori T, Nabeshima Y. Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1α-hydroxylase gene. Endocrinology. 2002;143:683–89. doi: 10.1210/endo.143.2.8657. [DOI] [PubMed] [Google Scholar]
- 99.Carpinelli MR, Wise AK, Burt RA. Vitamin D-deficient diet rescues hearing loss in Klotho mice. Hear. Res. 2011;275:105–9. doi: 10.1016/j.heares.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 100.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 2003;17:2393–403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 101.Forster RE, Jurutka PW, Hsieh JC, Haussler CA, Lowmiller CL, et al. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem. Biophys. Res. Commun. 2011;414:557–62. doi: 10.1016/j.bbrc.2011.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mackay EM, Oliver J. Renal damage following the ingestion of a diet containing an excess of inorganic phosphate. J. Exp. Med. 1935;61:319–34. doi: 10.1084/jem.61.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haut LL, Alfrey AC, Guggenheim S, Buddington B, Schrier N. Renal toxicity of phosphate in rats. Kidney Int. 1980;17:722–31. doi: 10.1038/ki.1980.85. [DOI] [PubMed] [Google Scholar]
- 104.Ori Y, Herman M, Tobar A, Chernin G, Gafter U, et al. Acute phosphate nephropathy—an emerging threat. Am. J. Med. Sci. 2008;336:309–14. doi: 10.1097/MAJ.0b013e318167410c. [DOI] [PubMed] [Google Scholar]
- 105.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–33. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 106.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J. Am. Soc. Nephrol. 2001;12:2131–38. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 107.Martin KJ, Gonzalez EA. Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: What is normal, when to start, and how to treat? Clin. J. Am. Soc. Nephrol. 2011;6:440–46. doi: 10.2215/CJN.05130610. [DOI] [PubMed] [Google Scholar]
- 108.Hiza H, Bente L, Fungwe T. Nutrient content of the U.S. food supply, 2005. US Dep. Agric.; Washington, DC: 2008. Home Econ. Res. Rep. No. 58. [Google Scholar]
- 109.Uribarri J. Phosphorus additives in food and their effect in dialysis patients. Clin. J. Am. Soc. Nephrol. 2009;4:1290–92. doi: 10.2215/CJN.03950609. [DOI] [PubMed] [Google Scholar]
- 110.Uribarri J. Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin. Dial. 2007;20:295–301. doi: 10.1111/j.1525-139X.2007.00309.x. [DOI] [PubMed] [Google Scholar]
- 111.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–82. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 112.Bell RR, Draper HH, Tzeng DY, Shin HK, Schmidt GR. Physiological responses of human adults to foods containing phosphate additives. J. Nutr. 1977;107:42–50. doi: 10.1093/jn/107.1.42. [DOI] [PubMed] [Google Scholar]
- 113.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr. Nephrol. 2011;26:1529–33. doi: 10.1007/s00467-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 114.Tan JC, Busque S, Workeneh B, Ho B, Derby G, et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 2010;78:686–92. doi: 10.1038/ki.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aihara K, Byer KJ, Khan SR. Calcium phosphate-induced renal epithelial injury and stone formation: involvement of reactive oxygen species. Kidney Int. 2003;64:1283–91. doi: 10.1046/j.1523-1755.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- 116.Di Marco GS, Hausberg M, Hillebrand U, Rustemeyer P, Wittkowski W, et al. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am. J. Physiol. Ren. Physiol. 2008;294:F1381–87. doi: 10.1152/ajprenal.00003.2008. [DOI] [PubMed] [Google Scholar]
- 117.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000;87:E10–17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 118.Sage AP, Lu J, Tintut Y, Demer LL. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2011;79:414–22. doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Villa-Bellosta R, Sorribas V. Phosphonoformic acid prevents vascular smooth muscle cell calcification by inhibiting calcium-phosphate deposition. Arterioscler. Thromb. Vasc. Biol. 2009;29:761–66. doi: 10.1161/ATVBAHA.108.183384. [DOI] [PubMed] [Google Scholar]
- 120.Bank N, Su WS, Aynedjian HS. A micropuncture study of renal phosphate transport in rats with chronic renal failure and secondary hyperparathyroidism. J. Clin. Investig. 1978;61:884–94. doi: 10.1172/JCI109014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin. Exp. Nephrol. 2004;8:75–88. doi: 10.1007/s10157-004-0292-0. [DOI] [PubMed] [Google Scholar]
- 122.Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J. Am. Soc. Nephrol. 2007;18:2116–24. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 123.Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26:75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 124.Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–22. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1α-hydroxylase. Kidney Int. 2009;75:1166–72. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ohnishi M, Razzaque MS. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010;24:3562–71. doi: 10.1096/fj.09-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int. Suppl. 2009;76(Suppl. 113):1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 128.Moorthi RN, Moe SM. CKD-mineral and bone disorder: core curriculum 2011. Am. J. Kidney Dis. 2011;58:1022–36. doi: 10.1053/j.ajkd.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chmielewski M, Carrero JJ, Stenvinkel P, Lindholm B. Metabolic abnormalities in chronic kidney disease that contribute to cardiovascular disease, and nutritional initiatives that may diminish the risk. Curr. Opin. Lipidol. 2009;20:3–9. doi: 10.1097/mol.0b013e32831ef234. [DOI] [PubMed] [Google Scholar]
- 130.Hruska KA, Choi ET, Memon I, Davis TK, Mathew S. Cardiovascular risk in chronic kidney disease (CKD): the CKD-mineral bone disorder (CKD-MBD) Pediatr. Nephrol. 2010;25:769–78. doi: 10.1007/s00467-009-1337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coresh J, Astor B, Sarnak MJ. Evidence for increased cardiovascular disease risk in patients with chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2004;13:73–81. doi: 10.1097/00041552-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 132.Kendrick J, Chonchol MB. Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat. Clin. Pract. Nephrol. 2008;4:672–81. doi: 10.1038/ncpneph0954. [DOI] [PubMed] [Google Scholar]
- 133.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2008;19:213–16. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 134.Cannata-Andia JB, Rodriguez-Garcia M, Carrillo-Lopez N, Naves-Diaz M, Diaz-Lopez B. Vascular calcifications: pathogenesis, management, and impact on clinical outcomes. J. Am. Soc. Nephrol. 2006;17:S267–73. doi: 10.1681/ASN.2006080925. [DOI] [PubMed] [Google Scholar]
- 135.Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J. Am. Soc. Nephrol. 2009;20:1453–64. doi: 10.1681/ASN.2008070692. [DOI] [PubMed] [Google Scholar]
- 136.De Schutter TM, Neven E, Persy VP, Behets GJ, Postnov AA, et al. Vascular calcification is associated with cortical bone loss in chronic renal failure rats with and without ovariectomy: the calcification paradox. Am. J. Nephrol. 2011;34:356–66. doi: 10.1159/000331056. [DOI] [PubMed] [Google Scholar]
- 137.Hu MC, Kuro-o M, Moe OW. Klotho and kidney disease. J. Nephrol. 2010;23(Suppl. 16):136–44. [PMC free article] [PubMed] [Google Scholar]
- 138.Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc. Natl. Acad. Sci. USA. 2007;104:2331–36. doi: 10.1073/pnas.0611079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, et al. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes. 2011;60:1907–16. doi: 10.2337/db10-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–17. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, et al. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem. Biophys. Res. Commun. 1998;249:865–71. doi: 10.1006/bbrc.1998.9246. [DOI] [PubMed] [Google Scholar]
- 142.Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, et al. Decreased renal α-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012;81:539–47. doi: 10.1038/ki.2011.423. [DOI] [PubMed] [Google Scholar]
- 143.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, et al. Establishment of sandwich ELISA for soluble α-Klotho measurement: age-dependent change of soluble α-Klotho levels in healthy subjects. Biochem. Biophys. Res. Commun. 2010;398:513–18. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sugiura H, Tsuchiya K, Nitta K. Circulating levels of soluble α-Klotho in patients with chronic kidney disease. Clin. Exp. Nephrol. 2011;15:795–96. doi: 10.1007/s10157-011-0511-4. [DOI] [PubMed] [Google Scholar]
- 145.Krajisnik T, Olauson H, Mirza MA, Hellman P, Akerstrom G, et al. Parathyroid Klotho and FGF-receptor 1 expression decline with renal function in hyperparathyroid patients with chronic kidney disease and kidney transplant recipients. Kidney Int. 2010;78:1024–32. doi: 10.1038/ki.2010.260. [DOI] [PubMed] [Google Scholar]
- 146.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J. Am. Soc. Nephrol. 2005;16:2205–15. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 147.Kobayashi K, Imanishi Y, Miyauchi A, Onoda N, Kawata T, et al. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur. J. Endocrinol. 2006;154:93–99. doi: 10.1530/eje.1.02053. [DOI] [PubMed] [Google Scholar]
- 148.Goldsmith DJ, Cunningham J. Mineral metabolism and vitamin D in chronic kidney disease—more questions than answers. Nat. Rev. Nephrol. 2011;7:341–46. doi: 10.1038/nrneph.2011.53. [DOI] [PubMed] [Google Scholar]
- 149.Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–18. doi: 10.1038/ki.2009.464. [DOI] [PubMed] [Google Scholar]
- 150.Drueke TB. Klotho, FGF23, and FGF receptors in chronic kidney disease: A yin-yang situation? Kidney Int. 2010;78:1057–60. doi: 10.1038/ki.2010.339. [DOI] [PubMed] [Google Scholar]
- 151.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J. Clin. Endocrinol. Metab. 2010;95:578–85. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lafage-Proust MH. Does the downregulation of the FGF23 signaling pathway in hyperplastic parathyroid glands contribute to refractory secondary hyperparathyroidism in CKD patients? Kidney Int. 2010;77:390–92. doi: 10.1038/ki.2009.512. [DOI] [PubMed] [Google Scholar]
- 153.Saji F, Shiizaki K, Shimada S, Okada T, Kunimoto K, et al. Regulation of fibroblast growth factor 23 production in bone in uremic rats. Nephron. Physiol. 2009;111:59–66. doi: 10.1159/000210389. [DOI] [PubMed] [Google Scholar]
- 154.Shiraki-Iida T, Iida A, Nabeshima Y, Anazawa H, Nishikawa S, et al. Improvement of multiple pathophysiological phenotypes of klotho (kl/kl) mice by adenovirus-mediated expression of the klotho gene. J. Gene Med. 2000;2:233–42. doi: 10.1002/1521-2254(200007/08)2:4<233::AID-JGM110>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 155.Lau WL, Festing MH, Giachelli CM. Phosphate and vascular calcification: emerging role of the sodium-dependent phosphate co-transporter PiT-1. Thromb. Haemost. 2010;104:464–70. doi: 10.1160/TH09-12-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.de Oliveira RM. Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett. 2006;580:5753–58. doi: 10.1016/j.febslet.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 157.Nakano-Kurimoto R, Ikeda K, Uraoka M, Nakagawa Y, Yutaka K, et al. Replicative senescence of vascular smooth muscle cells enhances the calcification through initiating the osteoblastic transition. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1673–84. doi: 10.1152/ajpheart.00455.2009. [DOI] [PubMed] [Google Scholar]
- 158.Lim K, Lu TS, Molostvov G, Lee C, Lam F, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to FGF-23. Circulation. 2012;125:2243–55. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 159.Donate-Correa J, Mora-Fernandez C, Martinez-Sanz R, Muros-de-Fuentes M, Perez H, et al. Expression of FGF23/KLOTHO system in human vascular tissue. Int. J. Cardiol. 2011 doi: 10.1016/j.ijcard.2011.08.850. In press; doi:10.1016/j.ijcard.2011.08.850. [DOI] [PubMed] [Google Scholar]
- 160.London GM, Marchais SJ, Guerin AP, Metivier F. Contributive factors to cardiovascular hypertrophy in renal failure. Am. J. Hypertens. 1989;2:261S–3S. doi: 10.1093/ajh/2.11.261s. [DOI] [PubMed] [Google Scholar]
- 161.Burrell LM, Johnston CI. Angiotensin II receptor antagonists. Potential in elderly patients with cardiovascular disease. Drugs Aging. 1997;10:421–34. doi: 10.2165/00002512-199710060-00003. [DOI] [PubMed] [Google Scholar]
- 162.Achinger SG, Ayus JC. The role of vitamin D in left ventricular hypertrophy and cardiac function. Kidney Int. Suppl. 2005;67(Suppl. 95):37–42. doi: 10.1111/j.1523-1755.2005.09506.x. [DOI] [PubMed] [Google Scholar]
- 163.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–51. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 164.Carter JL, O'Riordan SE, Eaglestone GL, Delaney MP, Lamb EJ. Bone mineral metabolism and its relationship to kidney disease in a residential care home population: a cross-sectional study. Nephrol. Dial. Transplant. 2008;23:3554–65. doi: 10.1093/ndt/gfn302. [DOI] [PubMed] [Google Scholar]
- 165.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin. J. Am. Soc. Nephrol. 2010;5:286–91. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fukagawa M, Kazama JJ. FGF23: its role in renal bone disease. Pediatr. Nephrol. 2006;21:1802–6. doi: 10.1007/s00467-006-0230-3. [DOI] [PubMed] [Google Scholar]
- 167.Yamashita T, Nifuji A, Furuya K, Nabeshima Y, Noda M. Elongation of the epiphyseal trabecular bone in transgenic mice carrying a klotho gene locus mutation that leads to a syndrome resembling aging. J. Endocrinol. 1998;159:1–8. doi: 10.1677/joe.0.1590001. [DOI] [PubMed] [Google Scholar]
- 168.Shalhoub V, Ward SC, Sun B, Stevens J, Renshaw L, et al. Fibroblast growth factor 23 (FGF23) and α-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif. Tissue Int. 2011;89:140–50. doi: 10.1007/s00223-011-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Isakova T, Gutierrez OM, Chang Y, Shah A, Tamez H, et al. Phosphorus binders and survival on hemodialysis. J. Am. Soc. Nephrol. 2009;20:388–96. doi: 10.1681/ASN.2008060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kandula P, Dobre M, Schold JD, Schreiber MJ, Jr, Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin. J. Am. Soc. Nephrol. 2011;6:50–62. doi: 10.2215/CJN.03940510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wolf M, Betancourt J, Chang Y, Shah A, Teng M, et al. Impact of activated vitamin D and race on survival among hemodialysis patients. J. Am. Soc. Nephrol. 2008;19:1379–88. doi: 10.1681/ASN.2007091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N. Engl. J. Med. 2004;350:1516–25. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 173.Drueke TB, Ritz E. Treatment of secondary hyperparathyroidism in CKD patients with cinacalcet and/or vitamin D derivatives. Clin. J. Am. Soc. Nephrol. 2009;4:234–41. doi: 10.2215/CJN.04520908. [DOI] [PubMed] [Google Scholar]
- 174.Razzaque MS. Therapeutic potential of klotho-FGF23 fusion polypeptides: WO2009095372. Expert Opin. Ther. Pat. 2010;20:981–85. doi: 10.1517/13543771003774100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–80. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 176.Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, et al. FGF23 neutralization improves secondary hyperparathyroidism and osteodystrophy parameters yet exacerbates vascular calcification in chronic kidney disease rats. J. Clin. Investig. 2012;122:2543–53. doi: 10.1172/JCI61405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–51. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Doi S, Zou Y, Togao O, Pastor JV, John GB, et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 2011;286:8655–65. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Morishita K, Shirai A, Kubota M, Katakura Y, Nabeshima Y, et al. The progression of aging in klotho mutant mice can be modified by dietary phosphorus and zinc. J. Nutr. 2001;131:3182–88. doi: 10.1093/jn/131.12.3182. [DOI] [PubMed] [Google Scholar]
- 180.Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, et al. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension. 2002;39:838–43. doi: 10.1161/01.hyp.0000013734.33441.ea. [DOI] [PubMed] [Google Scholar]
- 181.Zhang H, Li Y, Fan Y, Wu J, Zhao B, et al. Klotho is a target gene of PPAR-γ. Kidney Int. 2008;74:732–39. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 182.Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J. Biochem. 2011;149:121–30. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, et al. Identification of a hormonal basis for gallbladder filling. Nat. Med. 2006;12:1253–55. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 184.Yu J, Deng M, Zhao J, Huang L. Decreased expression of klotho gene in uremic atherosclerosis in apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 2010;391:261–66. doi: 10.1016/j.bbrc.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 185.Zhou Q, Lin S, Tang R, Veeraragoo P, Peng W, Wu R. Role of fosinopril and valsartan on klotho gene expression induced by angiotensin II in rat renal tubular epithelial cells. Kidney Blood Press. Res. 2010;33:186–92. doi: 10.1159/000316703. [DOI] [PubMed] [Google Scholar]
- 186.Devaraj S, Syed B, Chien A, Jialal I. Validation of an immunoassay for soluble klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am. J. Clin. Pathol. 2012;137:479–85. doi: 10.1309/AJCPGPMAF7SFRBO4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.