Abstract
A method was developed that allowed simultaneous monitoring of the acute secretory dynamics of insulin and islet amyloid polypeptide (IAPP) from islets of Langerhans using a microfluidic system with two-color detection. A flow-switching feature changes in the perfusion media within 5-s allowing rapid exchange of the glucose concentrations delivered to groups of islets. The perfusate was continuously by electroosmotic flow and mixed online with Cy5-labeled insulin, fluorescein isothiocyanate (FITC)-labeled IAPP, anti-insulin, and anti-IAPP antibodies in an 8.15-cm reaction channel maintained at 37 °C. The immunoassay mixture was injected for 0.3-s onto a 1.5-cm separation channel at 11.75-s intervals and immunoassay reagents detected using 488- and 635-nm lasers with two independent photomultiplier tubes for detection of the FITC and Cy5 signal. RSD of the bound-to-free immunoassay ratios ranged from 2–7% with LODs of 20 nM for insulin and 1 nM for IAPP. Simultaneous secretion profiles of the two peptides were monitored from groups of 4–10 islets during multiple step changes in glucose concentration. Insulin and IAPP were secreted in an approximately 10:1 ratio and displayed similar responses to step changes from 3 mM to 11 mM or 20 mM glucose. The ability to monitor the secretory dynamics of multiple peptides from islets of Langerhans in a highly automated fashion is expected to be a useful tool for investigating hormonal regulation of glucose homeostasis.
Keywords: electrophoresis, immunoassay, two-color, detection
INTRODUCTION
Peptide hormones released into the bloodstream by the islets of Langerhans, located in the pancreas, play a key role in the regulation of glucose homeostasis. While the role of glucose lowering by insulin is well established, that of other islet hormones, such as islet amyloid polypeptide (IAPP), is less well known.1 IAPP is co-secreted with insulin from islet β-cells, and is a regulator of glucose appearance in circulation by slowing the rate of gastric emptying2 and may also act as an autocrine signal within the islet.3 Under certain conditions, IAPP has a tendency to aggregate in a fibrillar conformation called amyloid. In type 2 diabetes, islets typically contain extracellular deposits of mature amyloid fibrils;4 however, smaller intracellular IAPP oligomers may be an early complication of, or a contribution to, β-cell failure.5
It is the complex interplay of the islet peptides that help to tightly regulate blood glucose levels; therefore, a detailed knowledge of not only the quantitative levels of islet hormone secretion, but also the dynamic features of the secretion patterns of multiple hormones is critical to understanding the process of normal regulation of blood glucose and the defects that may lead to various metabolic diseases. However, because each of these peptides are released in response to glucose and may respond to both autocrine and paracrine interactions from other islet-derived peptides6, ideally, multiple peptides should be monitored simultaneously to provide detailed quantitative and qualitative information about the secretion profiles.
The ability to monitor single peptide secretion from single or multiple islets has been widely demonstrated with traditional islet assays such as RIA or ELISA.7 These techniques have also been multiplexed for simultaneous monitoring of multiple peptides,8 but the temporal resolution of such strategies is limited by the expense and labor of collection, storage, and offline processing of potentially hundreds of samples. Solution-phase capillary electrophoresis (CE) immunoassays provide advantages in minimizing reagent consumption and repeatability, in addition to sensitive and rapid laser-induced fluorescence detection, but online sample collection, reagent mixing, and analysis are complex.9,10 Transfer of the electrophoresis-based immunoassay to a microfluidic platform allows online mixing of immunoassay reagents with continuous sampling of islet secretions and has been applied to the measurement of insulin11 or glucagon secretion.12 Perfusing media through the islet chamber and replenishing reagent buffer solutions allows long-term monitoring,13,14 and multiplexed microfluidic networks enable parallel islet assays.15,16
A difficulty with the measurement of multiple islet-secreted proteins is that the amount of insulin released from islets is 10–100-fold larger than the other peptides, which necessitates the use of a larger concentration of insulin immunoassay reagents compared to the other peptides. This disparity in immunoassay reagents then requires a single detector to be responsive to both low and high concentration reagents. To bypass this requirement, multi-color detection can be performed in a similar manner as has been reported for both capillary17,18 and microfluidic DNA sequencing,19 microfluidic genetic mutation detection,20 microfluidic flow cytometry,21 microfluidic water-monitoring22 or microfluidic discontinuous polyacrylamide gel immunoassays.23 By measuring insulin in a separate detection channel, the gain of that detector can be independently adjusted allowing simultaneous detection of both low and high concentration peptides. An added advantage of the multi-color format is more facile quantification of the immunoassays because it is difficult to separate all the antibody-bound peaks in free solution electrophoresis since the antibodies, which are all IgG, dominate the mobilities. Previously, multi-analyte offline CE competitive immunoassays using two-color detection have been developed for simultaneous measurement of insulin and glucagon content in groups of islets,24 as well as insulin, glucagon, and IAPP secretion with static incubation,25 but have not been applied to monitoring secretion profiles from islets.
This report describes the adaptation of the multianalyte, two-color detection scheme for online competitive immunoassays on a microfluidic device to measure simultaneous secretion profiles of insulin and IAPP. Islet perfusion with media containing 3, 11, or 20 mM glucose was accomplished by a gravity-driven, flow-splitting system. Reagents for the immunoassays were mixed online with islet-secreted analytes, allowed to react for ~2-min at 37 °C in an 8.15-cm mixing channel, then injected in 0.3-s plugs into a 1.5-cm separation channel every 11.75-s. Secretory responses to glucose stimulation were observed for both insulin and IAPP from groups of 4–10 islets. With optimization of other peptide assays, this method will be applicable to the study of the secretion dynamics of multiple analytes from islets of Langerhans.
Materials and methods
Chemicals and reagents
Potassium phosphate dibasic, potassium chloride, sodium chloride, sodium carbonate (Na2CO3), sodium bicarbonate (NaHCO3), sodium phosphate monobasic (NaH2PO4), bovine insulin, and Tween-20 were purchased from Sigma–Aldrich (Saint Louis, MO). Ethylenediaminetetraacetic acid (EDTA), bovine serum albumin (BSA), sodium hydroxide (NaOH), and ammonium hydroxide (NH4OH) were from EMD Chemicals (San Diego, CA). Nitric acid (HNO3), hydrofluoric acid (HF), and hydrogen peroxide (H2O2) were from Macron Fine Chemicals, and sulfuric acid (H2SO4) was from J.T.Baker (Avantor Performance Materials, Center Valley, PA). A monoclonal Ab to human insulin C-terminal (AbIns) was obtained from Meridian Life Science, Inc (Saco, ME). Mouse IAPP and a rabbit anti-rat IAPP antiserum IgG fraction (AbIAPP) were from Bachem Americas, Inc (Torrance, CA). Fluorescein isothiocyanate labeled-IAPP (IAPP*) was synthesized by Biomatik, Corp (Wilmington, DE) using the mouse amino acid sequence. Cy5 monofunctional N-hydroxysuccinimide ester was obtained from GE Healthcare Bio-Sciences (Piscataway, NJ). All islet isolation materials were purchased from Sigma–Aldrich unless otherwise stated. All solutions were prepared using ultrapure deionized water (NANOpure® Diamond TM deionization system, Barnstead International, Dubuque, IA).
Cy5 labeling and purification of Cy5-insulin
Bovine insulin was labeled with Cy5 according to the manufacturer’s instruction with purification and characterization as previously described.25 Briefly, 1 mg mL−1 insulin dissolved in 50 mM NaHCO3, pH 9.3, was added to a Cy5 dye vial and reacted for 3.5 hours at room temperature in the dark. Cy5-labeled insulin (Ins*) was separated using a PD-10 desalting column (GE Healthcare Bio-Sciences) and each 500 µL fraction collected was quantified by UV–Vis absorbance using the molar extinction coefficient of insulin (ε = 5530 M−1 cm−1 at 280 nm) and Cy5 (ε = 250,000 M−1 cm−1 at 650 nm). The three most concentrated fractions were purified by HPLC and each peak was collected and tested for its reactivity to AbIns by CE. The most reactive peak fractions were pooled, lyophilized, and reconstituted in PBS buffer for quantification by UV-Vis absorbance.
Fabrication of microfluidic chips
The perfusion channels and electroosmotic channels were etched in two different glass layers by conventional photolithography and wet-etching as described previously.26 Briefly, borofloat photomask blanks (Telic Co., Valencia, CA) with a layer of AZ1500 positive photoresist on a chrome layer were exposed with 18 mW cm−1 collimated UV radiation for 12-s through a patterned photomask (Digidat, Inc, Pasadena CA). The exposed photoresist was removed with AZ 400K Developer (AZ Electronic Materials Corp., Sommerville, NJ), and the underlying chrome was developed in a chrome etchant solution (CR-7S, Cyantek Corp., Fremont, CA). The exposed glass was etched in a 5:1:3 (v:v:v) mixture of H2O:HNO3:HF to 5-µm depth for the electroosmotic layer, or 35-µm depth for the perfusion layer. Channel dimensions were verified using a P-15 stylus profilometer (KLA-Tencor, Milpitas, CA). The access holes were drilled with a 500-µm diameter drill bit for the islet chamber and a 1.1-mm drill bit for all others on the perfusion layer, then the remaining photoresist and chrome were removed. The etched pieces were cleaned for 30-min in a 3:1 (v:v) solution of H2SO4:H2O2, and subsequently placed in a 5:1:1 (v:v:v) solution of H2O:NH4OH:H2O2 heated to 60 °C for another 30-min. The cleaned slides were rinsed, aligned, and bonded at 640 °C for 8-h. Microfluidic reservoirs (IDEX, Oak Harbor, WA) were applied to the devices above the drilled access holes.
Isolation of islets of Langerhans and culture
Pancreatic islets were obtained from 20–40 g male mice as previously described.27 Briefly, mice were sacrificed by cervical dislocation and collagenase P (Roche Diagnostics GmbH, Mannheim, Germany) (0.83 mg mL−1) was injected into the pancreas through the main pancreatic duct. The pancreas was collected and incubated in 10-mL of collagenase solution at 37 °C. A centrifuge and a 100-µm nylon filter (VWR, Radnor, PA) were used to separate islets from tissues. After isolation, the islets were incubated at 37 °C, 5% CO2 in RPMI 1640 media (Mediatech, Inc, Manassas, VA) containing 10% calf serum, 100 units mL−1 penicillin, 100 µg mL−1 streptomycin, and 10 µg mL−1 gentamicin. The islets were used within 5 days after isolation. Prior to experiments, islets were washed briefly (1 – 2-min) in pre-warmed RPMI media containing 3 mM glucose and 25 mM HEPES (Fisher, Pittsburgh, PA) and loaded into the islet chamber in this solution. The temperature of the device was controlled by two thermofoil heaters (Omega Engineering, Inc., Stamford, CT), with one situated below the perfusion channels and the other below the mixing channel. The heaters were powered by an 18-V, 500-mA power supply, that maintained a temperature of 37 °C as measured by a thermocouple placed near the islet reservoir.
Sample preparation
Antibodies (150 nM AbIns and 900 nM AbIAPP) or labeled antigens (150 nM Ins* and 60 nM IAPP*) were prepared in sample buffer composed of 20 mM NaH2PO4, 1 mM EDTA, pH 7.4, with 1 mg mL−1 BSA, and 0.1% (w/v) Tween 20. Peptide standards (0–750 nM unlabeled insulin and 0–75 nM IAPP) were dissolved in RPMI 1640 media supplemented with 25 mM HEPES and 3 mM glucose made to pH 7.4 with NaOH. Separation buffer was 20 mM Na2CO3, 20 mM NaHCO3, pH 9.0. Calibration curves and islet secretion profiles were obtained using the above solutions.
Detection and microfluidic chip operation
All electrophoresis experiments were performed on the stage of a Nikon Eclipse TS-100F inverted fluorescence microscope (Nikon Instruments, Inc., Melville NY). The two-color detection system consisted of the 488-nm line from a 50-mW solid state laser (Melles Griot, Carlsbad, CA) and the 635-nm line from a 25-mW laser (Radius, Coherent, Santa Clara, CA) directed into the microscope via a 6-ft bifurcated, randomized 38-fiber bundle with a 25.4-mm collimating lens (CeramOptec, East Longmeadow, NY), onto a 500/646-nm dichroic beamsplitter (Semrock, Rochester, NY), and through a 40×, 0.6 numerical aperture objective (Nikon Instruments, Inc., Melville NY). Emission light was collected with the same objective and passed through a 538/685-nm dual band pass filter (Semrock, Rochester, NY) and a spatial filter. The light was then split by a 570-nm long pass dichroic beamsplitter (Chroma, Bellows Falls, VT) into two channels. The green detection channel held a 550 ± 40-nm band pass filter while the red detection channel had a 675 ± 25-nm band pass filter (Omega Filters, Brattleboro, VT) in front of independent photomultiplier tubes (PMT) (Photon Technology International, Inc., Birmingham, NJ). Instrument control and data collection were performed using software written in LabView (National Instruments, Austin, TX). The voltage scheme used for injection and separation was based on a previously described method.28 Sample injection time was 0.3-s applied at 11.75-s intervals.
Microfluidic devices were conditioned daily with 1 M NaOH, deionized water, and experimental buffers each for at least 10-min before experiments, and cleaned with deionized water after experiments. During calibration or islet experiments, 90-µL of the appropriate solution was placed in the immunoassay reagents, gate, and waste reservoirs and covered with plastic caps with access holes for platinum electrodes. The perfusion inlet reservoirs were connected by fingertight fittings (IDEX, Oak Harbor, WA) with 40-cm lengths of Tygon tubing (0.06" o.d., 0.02" i.d., Cole-Parmer, Vernon Hills, IL) to 2-mL vials that contained perfusion media; the Tygon tubing was flushed by siphon with deionized water followed by the perfusion media prior to microfluidic chip connection.
To perfuse the islet chamber with 3 mM glucose, the vial with 3 mM glucose media was placed 4-cm higher than the microfluidic device while the vial with 11 or 20 mM glucose media was placed 0.5-cm higher. To change the perfusion media delivered to the islets, the height of perfusion media vials was exchanged.
Data analysis
Electropherograms were analyzed using an automated program.29 Calibration points were measured by perfusing different concentrations of insulin and IAPP standards in RPMI to the islet reservoir and measuring the peak heights of the bound (B) and free (F) labeled reagents. Calibration curves were produced by fitting a weighted four parameter logistic function to these bound-to-free (B/F) ratios. Detection limits were calculated by using the calibration curve to determine the concentration of insulin that would decrease the B/F ratio of a blank solution by an amount greater than three times the standard deviation of the B/F ratio of a blank solution. For islet monitoring, concentrations of islet-secreted insulin and IAPP were quantified with the four parameter logistic fit, converted to mass, and normalized to the perfusion flow rate and number of islets. In all figures and throughout the remainder of the text, the concentration of immunoassay reagents will be referred to the detected concentration in the separation channel, which accounts for dilution that occurred in the mixing channel.
Results and discussion
Insulin and IAPP are released in parallel from secretory granules within the β-cells of islets of Langerhans. There is some evidence that release of IAPP can be dissociated from insulin in obese and insulin resistant states in humans.30,31 IAPP and insulin are enzymatically cleared at different rates, so plasma concentration ratios do not necessarily reflect the relationship between IAPP and insulin upon secretion.32 Complex autocrine and paracrine interactions between insulin and IAPP, as well as independently regulated release, have also been described.33 The ability to simultaneously monitor insulin and IAPP secretion is of significance because while their secretion is coupled, their metabolic functions are not as clearly linked. Having a method to rapidly monitor their secretion dynamics would help delineate these functions. We have previously demonstrated a method for simultaneous quantitation of multiple peptides from static incubation of groups of islets using capillary electrophoresis competitive immunoassays.24,25 In this report, we apply the two-color detection scheme to microfluidic separations of immunoassay products with 12-s sampling frequency to measure secretion profiles of insulin and IAPP from groups of 4–10 islets.
Characterization of the microfluidic system
We previously developed a multi-analyte CE immunoassay method for simultaneous measurement of insulin, glucagon, and IAPP release from batches of islets of Langerhans.24,25 Migration of this method to a microfluidic system was required to monitor dynamic changes in secretion profiles; however, several challenges had to be overcome before this microfluidic system was robust.
One of the primary differences in the previous CE method and the current method required incubating islets on the same system that the immunoassay reagents were mixed and separated. An imaging pyrometer was used to optimize the thermofoil positions to maintain the temperature of the microfluidic system upstream of the separation region at 37 °C while minimizing heating of the separation channel. The increased temperature promotes islet health and increases binding of the antibodies to the antigens11, but elevated temperatures increases the rates of Ab-Ag* dissociation34. Another change in the protocol was the use of smaller channel diameters, 5-µm, for the microfluidic device compared to the capillary (50-µm i.d.) used in the previous assay. These smaller channels reduced the current allowing for rapid separations and high sampling frequency. But, these small channels were more difficult to work with than the larger capillaries due to clogging. To minimize the occurrence of clogs, all solutions were filtered prior to their use and caps placed over the reservoirs to prevent dust from entering the channels during runs. NaOH that was used to condition the device at the beginning of the day was rinsed from the device with water to prevent precipitation of buffer components. And all conditioning steps were performed with electroosmotic flow because backpressure from the small channels was too high to use pressure-driven flow. Finally, achromatic optical components were used when possible to prevent chromatic aberration of the different wavelength lasers. Once these technical challenges had been overcome, the devices were reproducible and the method was robust enough that they could be used for several days without clogging or fouling.
Due to the presence of the islets on the microfluidic system, the ability to automate the rapid exchange of stimulants or provide a continual supply of fresh media for longer maintenance of the cells in the device was required. Several types of perfusion systems have been incorporated into microfluidic devices and used for islet-based research.13,16,35–37 The perfusion system developed here used gravity-driven flow through relatively large channels, with a flow-splitting feature adapted from a microchemostat design.38 The channel geometry of the device is shown in Figure 1A with Figures 1B and 1C detailing the flow-splitting feature used for switching the perfusion media. Hydrostatic pressure delivered stable flows of low and high glucose to the two inputs where they were split to either the perfusion waste channels or the islet chamber, depending on their relative flow rate. Modulating the height of the two vials shifted the flow rates of the two input streams and also the position of the desired flow. By changing the vial heights in an equal but opposite amount, the flow rate to the islet chamber remained constant and the step changes in perfusion media were fast, with no backflow, unwanted mixing, or pressure pulses. The flow rate to the islet chamber was measured to be 1.3 + 0.2 µL/min by perfusing FITC and Cy5 with 5-s required to deliver a new perfusion solution to the islet chamber (see Supporting Information, Figure S-1).
Figure 1. Channel design of the microfluidic device.
A. The perfusion channels, indicated by the heavy black lines, were 35-µm deep and thinner gray lines below the islet chamber indicate 5-µm deep channels. The Ag*, Ab and islet reservoirs were grounded and a high voltage of −4500 V was applied to the waste reservoir. B. The flow-splitting feature is shown when solution from the left perfusion solution (red) was introduced at a higher velocity than the right solution (green). The red solution pushed the green to waste while the red was split to both waste channels and the islet chamber. C. The heights of the perfusion vials were switched from those shown in part B and the green solution was delivered to the islet chamber while the red solution was directed toward waste. During islet experiments, different concentrations of glucose were used as the perfusion solutions.
EOF was used to sample secreted peptides from the islet chamber, the labeled antigen reservoir (Ag*), and the antibody reservoir (Ab) by grounding these reservoirs and applying high voltage to the waste reservoir. These reagents were directed into an 8.15-cm mixing channel where they mixed as they traveled to the injection cross of the separation channel. The times required for insulin and IAPP to travel the mixing channel were dependent on their electrophoretic mobilities and were measured by perfusing pulses of insulin and IAPP to the islet chamber while measuring the number of runs required for a change in the detected insulin and IAPP concentrations to be observed in the separation channel. These times varied slightly across devices but were approximately 2-min. More importantly, changes in the detected insulin concentrations always lagged changes in the detected IAPP concentrations by 3–5 runs due to the lower mobility of insulin. Within a single device, this lag was highly reproducible (< 1% RSD), indicating high reproducibility of relative flow rates. This lag in the detected insulin concentrations also altered the timing of the secretion profiles, which is discussed further below.
Calibration Curves
Representative electropherograms from two calibration points are shown in Figure 2. The signals from the red and green detection channels are shown in red and green, respectively, and correspond to the left and right y-axes. The peak heights in the two detection channels were similar because the detector gain for each of the channels was set independently to provide these responses. If both immunoassay antigens were labeled with the same fluorophore and separated in a single detection channel, the peaks from the insulin immunoassay reagents would preclude detection of the smaller peaks from the IAPP immunoassay. However, with the use of the two-color detection system, peaks from both immunoassays were quantifiable and no spectral crosstalk was observed.
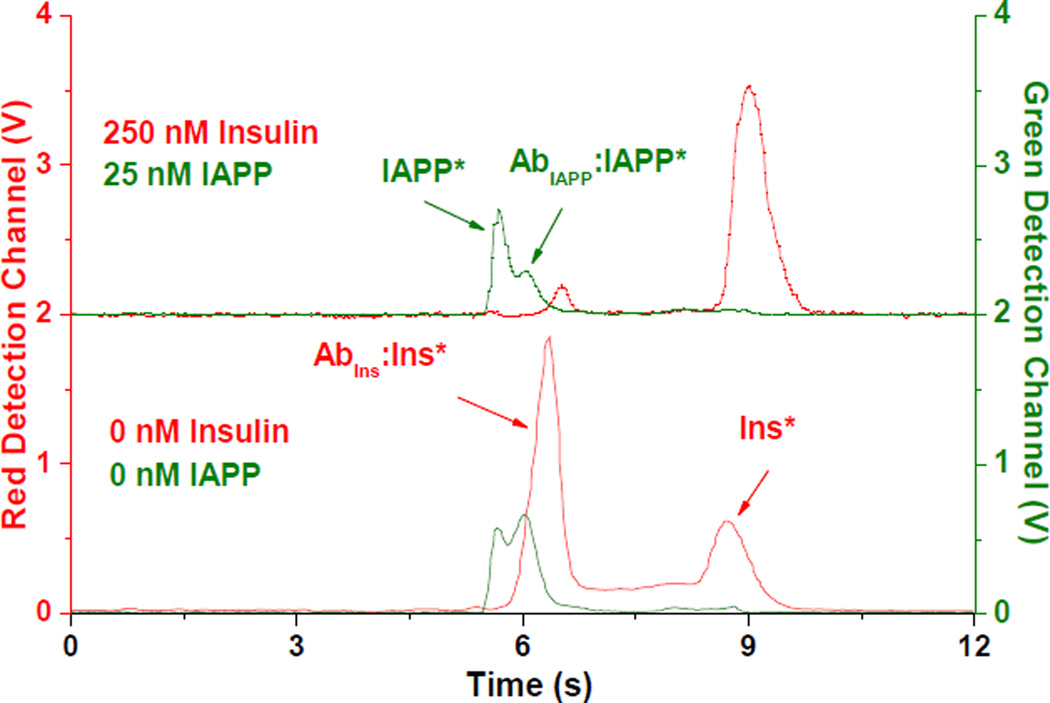
Figure 2. Representative electropherograms from IAPP and insulin immunoassays.
In both electropherograms, the immunoassay reagent concentrations were 50 nM AbIns, 300 nM AbIAPP, 50 nM Ins*, 20 nM IAPP*, and the concentrations of unlabeled insulin and IAPP are shown. The traces shown in red correspond to the red detection channel signal (left y-axis), and the traces shown in green correspond to the green detection channel signal (right y-axis). The top trace is offset for clarity.
The free IAPP* and bound AbIAPP:IAPP* peaks in the green detection channel were not as well resolved as the corresponding insulin immunoassay products in the red channel, similar to what we have observed using a capillary-based system. Use of a polyclonal anti-IAPP antibody likely contributed to the lower resolution and to the relatively small changes in B/F over the calibrated range of unlabeled IAPP analyte concentrations. Monoclonal antibodies were also tested, but none produced a reproducible bound peak in the electropherogram. To increase the resolution of the IAPP free and bound peaks, the injection time was varied. A 0.3-s injection time was determined to produce adequate resolution while maintaining a high B/F ratio in the absence of unlabeled IAPP. The sensitivity of the IAPP assay can be increased with longer injection times, but at the expense of resolution and assay quantitation.39
For all calibration points, B/F peak height ratios stabilized within 5-min with RSDs between 2–7% for 15 consecutive electropherograms (Figure 3). Calibration curves were used to quantify insulin and IAPP, with concentration ranges chosen for the amounts of insulin and IAPP expected to be released from groups of up to ten islets. We expected insulin to be released at a rate of approximately 40–400 pg islet−1 min−1 depending on glucose stimulation levels.12,14,15 The secretion rate of IAPP was expected to be ~10-fold lower than insulin.25 The concentration ratios of immunoassay reagents were therefore optimized for sensitivity in these ranges of secretion rates. For the calibration curves used to quantify islet secretion rates, the IAPP assay had an LOD of 1 nM, while the insulin assay was optimized for a higher detection limit and wider dynamic range, with a detection limit of 20 nM (Figure 4).
Figure 3. Series of electropherograms from insulin and IAPP immunoassays.
A series of 15 electropherograms is shown collected over 3-min from three calibration points. The concentrations of immunoassay reagents were the same as in Figure 2 and the concentrations of unlabeled insulin and IAPP are indicated. The data was separated into red and green detection channels for clarity and the B/F ratio of each electropherogram is shown in blue and plotted vs. the right y-axis. The RSD of the B/F value is shown above each set of electropherograms.
Figure 4. Calibration curves for insulin and IAPP.
The average B/F from 15 consecutive runs of insulin (red) and IAPP (green) are shown plotted against the concentration of unlabeled insulin (bottom x-axis) and IAPP (top x-axis). Error bars represent ±1 standard deviation and the curves are the best-fit lines corresponding to a weighted, 4-parameter logistic function.
Measurement of secretion from islets
As mentioned earlier, changes in insulin B/F lagged changes in IAPP B/F due to differences in their electrophoretic mobilities. This difference will cause peptides secreted from the islets at the same time to appear shifted in time at the detection point. As an example, Figure 5A shows 3 pulses of 10 nM IAPP and 100 nM insulin perfused into the islet chamber and the corresponding changes in the insulin (red line, left y-axis) and IAPP concentrations (green line, right y-axis). As seen, there is a noticeable lag of insulin with respect to IAPP. Within a single device, this lag was highly reproducible and was corrected by subtracting an appropriate number of runs, in this case 4, from the beginning of the insulin data resulting in the profile shown in Figure 5B. The number of runs to subtract was measured before or after each islet experiment while calibrating the device.
Figure 5. Alignment of insulin and IAPP pulses.
A. The blue line shows the timing of three 2.5-min pulses of insulin (100 nM) and IAPP (10 nM) perfused into the islet chamber. The corresponding detected insulin and IAPP concentrations are shown in red and green, respectively. B. The same data in A is shown after 4 runs were subtracted from the insulin profile.
To monitor insulin and IAPP secretion, islets were placed into the islet chamber and B/F measured as a function of time. Figure 6A shows the corrected secretion profiles of insulin (red) and IAPP (green) from 9 islets in the islet chamber in response to changing glucose concentrations (blue line, right y-axis). After 3-min of perfusion with 3 mM glucose, a step change to 20 mM was made, and detected insulin and IAPP concentrations rose sharply over the next 4–5-min. Insulin displayed both a longer rise time (~5-min) and greater change (~3-fold) during this glucose stimulation than did IAPP (~4-min and ~2-fold, respectively). After 10-min at high glucose, the glucose concentration was lowered to 3 mM and the islet secretion levels returned to basal levels within 10-min.
Figure 6. Simultaneous secretion profiles of insulin and IAPP.
A. Insulin (red) and IAPP (green) secretion profiles were measured by quantifying changes in B/F from 9 islets exposed to varying glucose concentrations (blue line, right y-axis). The insulin data was corrected by subtracting 5 points from the beginning of the data. B. Insulin and IAPP secretory profiles measured from a batch of 4 islets. The insulin data was corrected by subtracting 4 points from the beginning of the data.
The next step change to 20 mM glucose induced a greater increase in secretion for both insulin and IAPP than the first step change increase, representing a 4-fold increase over basal for insulin and a 3-fold increase over basal for IAPP. This glucose concentration was held for a shorter time and then reduced to 11 mM glucose. Detected secretion levels decreased to about 6 pg islet−1 min−1 for IAPP and 80 pg islet−1 min−1 for insulin, values close to the maxima during the first stimulation. While perfused media continually flowed over the islets at 1.3 µL min−1, the solution from the islet chamber was sampled by electrophoresis at ~5 nL min−1. The remainder of the perfusate flowed into the 90 µL reservoir above the islet chamber, limiting the experimental time to ~45-min before the accumulated volume in the reservoir had to be removed. Connecting a slow siphon to the islet reservoir or a line to a withdrawing syringe pump would allow for longer uninterrupted monitoring, and could help prevent build up of the secretion products in the islet reservoir.13
Approximately 20 islet experiments were performed using between 4 and 11 islets for each experiment. Figure 6B details the corrected secretion profile measured from an experiment with 4 islets using an 11 mM stimulatory glucose level. Islet response times to the 11 mM glucose stimulation were slower compared to the response times with 20 mM step changes, and the detected insulin and IAPP secretory rates were approximately 2- and 3-fold lower, respectively. In other studies, insulin secretion could be detected from single islets at levels close to the LOD, but IAPP was below the LOD at all glucose concentrations tested using less than 4 islets. This is not surprising considering the immunoassays were optimized for detecting secretion from more islets.
CONCLUSION
The system presented here provides a method to measure secretory profiles of multiple peptides released from islets of Langerhans at a high sampling frequency while minimizing labor, time, and reagent costs. Although IAPP and insulin are shown here, this method is applicable to other islet-secreted peptides as well. The ability to monitor fast changes in islet secretory profiles is critical for understanding normal glucose homeostasis as well as to pinpoint defects that contribute to various metabolic diseases. Several features of this system can be improved, including adapting the perfusion system to deliver varying concentrations of glucose, and if necessary, addition of other detection channels for measurement of other peptides. Because multiple hormones are involved in glucose regulation, the development of techniques to measure rapid changes in secretory patterns of several peptides will be a valuable tool in islet biology research.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by a grant from the National Institutes of Health (R01 DK080714).
REFERENCES
- 1.Thorens B. Int. J. Obes. (Lond) 2008;32(Suppl 6):S62–S71. doi: 10.1038/ijo.2008.208. [DOI] [PubMed] [Google Scholar]
- 2.Young AA, Gedulin BR, Rink TJ. Metab. Clin. Exp. 1996;45:1–3. doi: 10.1016/s0026-0495(96)90192-4. [DOI] [PubMed] [Google Scholar]
- 3.Cluck MW, Chan CY, Adrian TE. Pancreas. 2005;30:1–14. [PubMed] [Google Scholar]
- 4.Kahn SE, Andrikopoulos S, Verchere CB. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 5.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. Diabetes. 1999;48:491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 6.Ahren B. Curr. Mol. Med. 2005;5:275–286. doi: 10.2174/1566524053766004. [DOI] [PubMed] [Google Scholar]
- 7.Chevenne D, Trivin F, Porquet D. Diabetes Metab. 1999;25:459–476. [PubMed] [Google Scholar]
- 8.Hellman B, Salehi A, Gylfe E, Dansk H, Grapengiesser E. Endocrinology. 2009;150:5334–5340. doi: 10.1210/en.2009-0600. [DOI] [PubMed] [Google Scholar]
- 9.Schultz NM, Kennedy RT. Anal. Chem. 1993;65:3161–3165. [Google Scholar]
- 10.Tao L, Aspinwall CA, Kennedy RT. Electrophoresis. 1998;19:403–408. doi: 10.1002/elps.1150190307. [DOI] [PubMed] [Google Scholar]
- 11.Roper MG, Shackman JG, Dahlgren GM, Kennedy RT. Anal. Chem. 2003;75:4711–4717. doi: 10.1021/ac0346813. [DOI] [PubMed] [Google Scholar]
- 12.Shackman JG, Reid KR, Dugan CE, Kennedy RT. Anal. Bioanal. Chem. 2012;402:2797–2803. doi: 10.1007/s00216-012-5755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shackman JG, Dahlgren GM, Peters JL, Kennedy RT. Lab Chip. 2005;5:56–63. doi: 10.1039/b404974h. [DOI] [PubMed] [Google Scholar]
- 14.Reid KR, Kennedy RT. Anal. Chem. 2009;81:6837–6842. doi: 10.1021/ac901114k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dishinger JF, Kennedy RT. Anal. Chem. 2007;79:947–954. doi: 10.1021/ac061425s. [DOI] [PubMed] [Google Scholar]
- 16.Dishinger JF, Reid KR, Kennedy RT. Anal. Chem. 2009;81:3119–3127. doi: 10.1021/ac900109t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue G, Yeung ES. Electrophoresis. 2002;23:1490–1498. doi: 10.1002/1522-2683(200205)23:10<1490::AID-ELPS1490>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 18.Keithley RB, Rosenthal AS, Essaka DC, Tanaka H, Yoshimura Y, Palcic MM, Hindsgaul O, Dovichi NJ. Analyst. 2013;138:164–170. doi: 10.1039/c2an36286d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Shi Y, Ja WW, Mathies RA. Anal. Chem. 1999;71:566–573. doi: 10.1021/ac980783v. [DOI] [PubMed] [Google Scholar]
- 20.Kourkine IV, Hestekin CN, Buchholz BA, Barron AE. Anal. Chem. 2002;74:2565–2572. doi: 10.1021/ac020025b. [DOI] [PubMed] [Google Scholar]
- 21.Tung YC, Zhang M, Lin CT, Kurabayashi K, Skerlos SJ. Sensor. Actuat. B. 2004;98:356–367. [Google Scholar]
- 22.VanderNoot VA, Renzi RF, Mosier BP, Van de Vreugde JL, Shokair I, Haroldsen BL. Electrophoresis. 2010;31:2632–2640. doi: 10.1002/elps.201000052. [DOI] [PubMed] [Google Scholar]
- 23.Hou C, Herr AE. Anal. Chem. 2010;82:3343–3351. doi: 10.1021/ac100182j. [DOI] [PubMed] [Google Scholar]
- 24.Guillo C, Roper MG. Electrophoresis. 2008;29:410–416. doi: 10.1002/elps.200700399. [DOI] [PubMed] [Google Scholar]
- 25.Guillo C, Truong TM, Roper MG. J. Chromatogr. A. 2011;1218:4059–4064. doi: 10.1016/j.chroma.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker CA, Bulloch R, Roper MG. Anal. Bioanal. Chem. 2010;399:1473–1479. doi: 10.1007/s00216-010-4144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Roper MG. Anal. Chem. 2009;81:1162–1168. doi: 10.1021/ac802579z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson SC, Ermakov SV, Ramsey JM. Anal. Chem. 1999;71:3273–3276. doi: 10.1021/ac990059s. [DOI] [PubMed] [Google Scholar]
- 29.Shackman JG, Watson CJ, Kennedy RT. J. Chromatogr. A. 2004;1040:273–282. doi: 10.1016/j.chroma.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Sanke T, Hanabusa T, Nakano Y, Oki C, Okai K, Nishimura S, Kondo M, Nanjo K. Diabetologia. 1991;34:129–132. doi: 10.1007/BF00500385. [DOI] [PubMed] [Google Scholar]
- 31.Hanabusa T, Kubo K, Oki C, Nakano Y, Okai K, Sanke T, Nanjo K. Diabetes Res. Clin. Pract. 1992;15:89–96. doi: 10.1016/0168-8227(92)90073-z. [DOI] [PubMed] [Google Scholar]
- 32.Westermark P, Andersson A, Westermark GT. Physiol. Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson E. Int. J. Mol. Med. 1999;3:577–584. doi: 10.3892/ijmm.3.6.577. [DOI] [PubMed] [Google Scholar]
- 34.Mason DW, Williams AF. Biochem. J. 1980;187:1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sankar KS, Green BJ, Crocker AR, Verity JE, Altamentova SM, Rocheleau JV. PLoS One. 2011;6:e24904. doi: 10.1371/journal.pone.0024904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Easley CJ, Rocheleau JV, Head WS, Piston DW. Anal. Chem. 2009;81:9086–9095. doi: 10.1021/ac9017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godwin LA, Pilkerton ME, Deal KS, Wanders D, Judd RL, Easley CJ. Anal. Chem. 2011;83:7166–7172. doi: 10.1021/ac201598b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferry MS, Razinkov IA, Hasty J. Synthetic Biology, Part A. In: Voigt C, editor. Methods in Enzymology. Vol. 497. Academic Press; 2011. pp. 295–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lomasney AR, Guillo C, Sidebottom AM, Roper MG. Anal. Bioanal. Chem. 2009;394:313–319. doi: 10.1007/s00216-009-2622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.