Abstract
The dimerization of the G protein-coupled receptors for endothelin-1 (ET-1), endothelin A receptor (ETA) and endolethin B receptor (ETB), is well established. However, the signaling consequences of the homodimerization and heterodimerization of ETA and ETB is not well understood. Here, we demonstrate that peptides derived from the C-termini of these receptors regulate the signaling capacity of ET-1. The C-termini of the ETA and ETB receptors are believed to consist of three α-helices, which may serve as points of interaction between the receptors. The third α-helix in the C-terminus is of particular interest because of its amphipathic nature. In a cell line expressing only the ETA receptor, expression of residues Y430–S442, representing the third helix of the ETB C-terminus, leads to a dramatic increase in the signaling induced by ET-1. In contrast, in a cell line containing only ETB, Y430–S442 has an antagonistic effect, slightly reducing the ET-1 induced signal. Computational docking results suggest that the α-helical ETB-derived peptide binds to the second and third intracellular loops of the ETA receptor consistent with the alteration of its signaling capacity. Our results described here provide important insight into ETA/ ETB receptor interactions and possibly a new approach to regulate specific G protein-coupled receptor signal transmission.
Keywords: G protein-coupled receptor, endothelin receptors, receptor C-terminus, receptor heterodimerization, ERK signaling
Introduction
Endothelin-1 (ET-1) is a vasoactive peptide signaling through two G protein-coupled receptor (GPCR) isoforms, endothelin A and endothelin B. In the arterial endothelium, the ETB receptor is the primary isoform expressed [1]. In the smooth muscle of the artery, both receptors are co-expressed [2]. In the endothelium, ETB acts to produce vasorelaxing factors such as nitric oxide and prostacyclin [3]. In the smooth muscle, the exact function of the ETB has not been resolved. Both receptors signal via tyrosine/serine kinase cascades such as the RAF/MEK/MAPK pathway [2,4].
Due to their vasoconstrictive function, the ETA/ETB receptors are targets for therapy in vascular diseases. In fact, nonselective, dual receptor blockers such as bosentan are used in the treatment of pulmonary arterial hypertension [5–7]. Although it is clear that ETA/ETB antagonists have clinical value in reversing some symptoms of pulmonary arterial hypertension, it is less clear whether blocking the entire signaling actions of both receptors is necessary and most likely not physiologically beneficial to the disease. Additionally, the nonselective antagonists have undesirable side effects such as induction of liver enzyme abnormalities and retention of fluid [8,9].
For a number of years we have been characterizing the roles of the cytoplasmic domains in the signaling of the GPCRs for the vasoactive peptide ligands endothelin, bradykinin, and angiotensin [10–18]. Using receptor chimera and site-directed mutations, we have shown that the cytoplasmic domains of the receptors are highly interactive and that these interactions are very sequence sensitive. For example, mutations/changes in the second intracellular loop (IC2) that suppress signaling can be overcome (signaling restored) by changes in the third intracellular loop or C-terminus. Using the structural features from NMR and molecular modeling we have developed structure–activity relationships for the interactions between the cytoplasmic domains. The possibility of interactions among the cytoplasmic regions is further enhanced by the dimerization, both homodimerization and heterodimerization of the receptors. There is strong evidence that the ETA and ETB receptors form dimers [19–21].
Here, we demonstrate that specific segments of the C-termini of the ETA and ETB receptors can alter the signaling capacity of the other receptor subtype. These inter-receptor interactions of the cytoplasmic domains may afford a novel target for regulation of ET-1-initiated signaling.
Methods
Homology Modeling
Structural models of the C-termini of the ETA and ETB receptors were generated using the program MODELLER [22,23]. The C-terminus of the rat bradykinin-2 (BK2) receptor, previously characterized by high-resolution NMR [17], was used as a template. The sequence homology of the C-termini of the receptors is high, with a sequence similarity of 53% for ETA/BK2 and 61% for ETB/BK2.
The homology models for transmembrane helices of the ETA (EDNRA_human, P25101) and ETB (EDNRB_human, P24530) receptors were created using SWISS-MODEL [24–26]. The homology models were built using the following templates: the nociceptin receptor (chain B of PDB ID: 4EA3) for ETA, and the turkey beta1 adrenergic receptor (PDB ID: 4AMJ chain B) for ETB. The structural features of the C-termini were incorporated into the models by using the hydrophobic clustering or amphipathic nature of the helices as a guide. The first helix (the most N-terminal helix) was placed lying along the membrane surface, perpendicular to the orientation of the seven-transmembrane helices. The next helix (the central helix of the C-terminus) was likewise adjusted to place the hydrophobic amino acids projecting towards the membrane surface, whereas the third helix, highly charged with few hydrophobic residues, was placed randomly before the energy minimization steps.
Energy Minimization
The models of the ETA and ETB receptors were subjected to energy minimization and molecular dynamics to relax the homology structures. The NAMD program was used for the simulations and VMD used for viewing and graphic manipulation [27,28]. The energy minimization and MD simulations were carried out in a membrane environment by using the standard protocol developed for membrane protein simulations provided within NAMD [29]. The total simulation time was 1 ns with 2 fs/step at constant temperature at 300 K. These energy minimized protein structures were used for subsequent docking experiments.
Docking Experiment
Multiple blind docking experiments were carried out for the third helical peptide of ETB, E3B (Y430–S442; YDNFRSSNKYSSS) against the ETA and ETB receptors by using AutoDock 4.0 [30]. The search space (the grid box) for these dockings was limited to the intercellular face of the receptors. After searching with a large grid box encompassing the entire cytoplasmic face, each hit (a promising binding mode of the peptide) was further refined by reducing the grid to this location, thus allowing for finer grain searching. During all of the docking calculations, the peptide was assumed to maintain a helical structure.
Transfection
For this study we chose to concentrate on small peptide segments of the far C-terminal domains of the ETA and ETB receptors. Peptide E3A consists of amino acids A415–N427 from the C-terminus of ETA, and peptide E3B consists of amino acids Y430–S442 from the C-terminus of ETB. Each peptide was reverse translated into sense and antisense oligonucleotides with an added XbaI restriction site followed by a translation initiation sequence upstream of the start codon and XhoI restriction site downstream of the stop codon for cloning into the pcMIN zeo DNA expression vector. All pairs of oligonucleotides (i.e. sense and antisense for each C-terminal peptide) were annealed and ligated into pcMIN zeo vector doubly digested with XhoI and XbaI. Oligonucleotide sequences used in this study are:
E3A: CTAGAAGCTTACCATGCACAACACAGACCGGAGCAGCCATAAGGACAGCATGAACTGAC
cE3A: TCGAGTCAGTTCATGCTGTCCTTATGGCTGCTCCGGTCTGTGTTGTGCATGGTAAGCTT
E3B: CTAGAAGCTTACCATGTATGACAACTTCCGTTCCAGTAATAAATACAGCTCATCTTGAC
cE3B: TCGAGTCAAGATGAGCTGTATTTATTACTGGAACGGAAGTTGTCATACATGGTAAGCTT.
cDNAs encoding the ETA or ETB receptors were stably transfected previously into wild-type Chinese hamster ovary cells by using neomycin as the selecting agent (CHO) [2,31,32]. In this study the CHO-ETA or CHO-ETB expressing cells were then stably cotransfected with an additional pcMIN zeo bicistronic vector containing cDNA encoding peptides E3A or E3B and a zeocin resistance gene. CHO-ETA and CHO-ETB cotransfected with pcMINwithout an E3A or E3B peptide cDNA but containing a zeocin resistance gene were used as controls. To ensure expression of these genes, cells were maintained in the selection medium containing 0.5 mg/ml geneticin (Promega, Madison, WI) and 0.25 mg/ml zeocin (Invitrogen, Carlsbad, CA) during all experiments.
Detection of ERK Activity
Cells were seeded in 6-well cell culture plates until reaching confluence. At that point, they were serum starved for 30 min and then treated with or without 10 nM ET-1 for 5 min. This time span had previously been found to be optimal in detecting phosphorylated ERK [2,31]. Afterwards, cells were lysed using a 100 μl RIPA buffer [150 mM NaCl, 1.0% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0 (Sigma, St Louis, MO)] and a 1× complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN), and the total protein was isolated from each well. Total protein was quantified using a bicinchoninic assay. Equal amounts of protein were loaded and electrophoresed on sodium dodecyl sulfate polyacrylamide gel electrophoresis with a 4% stacking and 10% separating gel. Proteins were then transferred to a nitrocellulose membrane (BioRad, Hercules, CA) at 100 V and 4 °C for 1 h. The nitrocellulose membranes were blocked at room temperature for 1 h with 5% powdered milk in Tris-buffered saline with Tween 20 (TBS-T) (20 mM Tris, 150 mM NaCl, 0.1% Tween 20, pH 7.6) before incubating with primary rabbit antibody for pERK1/2 (Cell Signaling, Danvers, MA) overnight at 4 °C. Diluted 1 :1000 in a solution TBS-T with 5% bovine serum albumin. The membranes were washed in TBS-T followed by a 1-hour room temperature incubation in the corresponding anti-rabbit secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1 : 10 000 TBS-T. Membranes were washed again in TBS-T and developed in Amersham ECL chemiluminescent detection solution (GE Healthcare) for 1 min before exposure to X-ray film. Using Restore™ PLUS Western Blot Stripping Buffer (Thermo Scientific, Rockford, IL), the membranes were stripped and likewise probed for glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling, Danvers, MA) as a loading control. Films were scanned with an Epson Perfection 3170 scanner using an Epson Scan (version 1.22A) software. The images were then analyzed using the NIH ImageJ image analysis software to determine the intensity of each band. Relative ERK activity was measured by first normalizing all ERK bands with glyceraldehyde-3-phosphate dehydrogenase and then using that normalized ERK intensity value of E3A or E3B expressing cells divided by ERK band intensity values of empty vector control cells.
Results
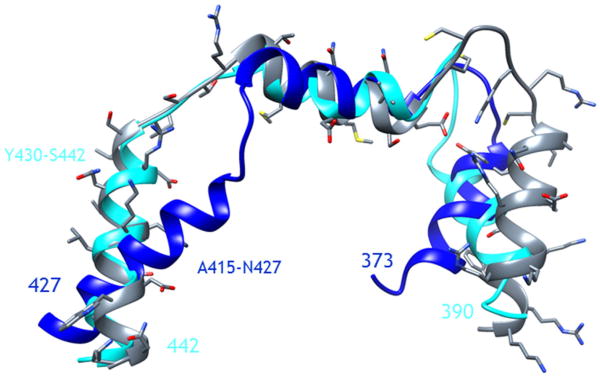
The models of the C-termini of the ETA and ETB receptors were developed using homology modeling and the structure of the C-terminus of the BK2 receptor, previously determined by high-resolution NMR [17]. The resulting structures consisting of three α-helices are shown in Figure 1. The first helix (the most N-terminal helix in the peptide) is commonly observed in GPCRs and is often referred to as the eighth helix. For both ETA and ETB, this helix is very amphipathic in nature and enriched with cysteines, which serve as possible sites of palmitoylation. In contrast, the second helix, consisting of S397–W407 and E410–K421 for ETA and ETB, respectively, has a more random pattern of hydrophobic and hydrophilic amino acids. The last helix of the C-termini of ETA and ETB, consisting of Q412–M426 and Y430–S442, respectively, are the longest of the three and consist largely of hydrophilic and charged amino acids.
Figure 1.
Superposition of the structure of the C-terminus of the BK2 receptor, as determined by NMR (gray), with the homology models of the C-termini of the endothelin A (dark blue) and endothelin B (light blue) receptors. The residues of the third α-helix of the C-termini of the endothelin A and endothelin B receptors are denoted.
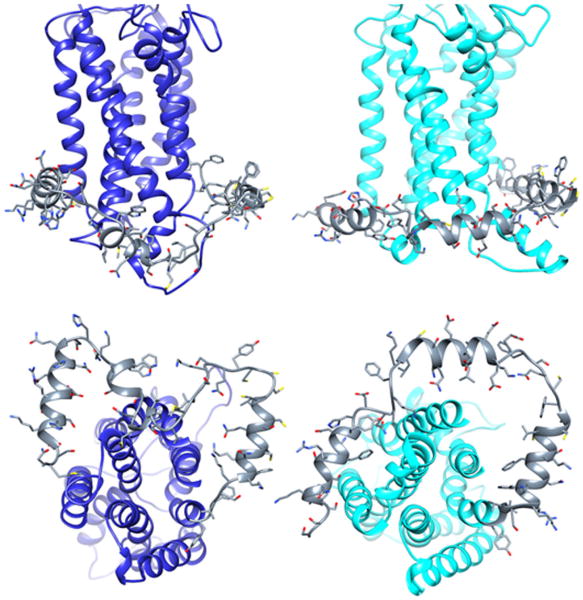
The homology models of the ETA and ETB receptors were generated using standard techniques are shown in Figure 2. During the energy minimization and MD simulations, carried out in a lipid environment, the helices maintain close contact with the membrane, particularly true for the first two helices of the C-termini. The third helix, which was placed randomly, also adopts close interactions with the membrane environment. It is interesting to note that for both receptors, the C-terminus is in close proximity to the IC2. We have previously demonstrated using site directed mutagenesis that with respect to signaling of the BK2 receptor, there is an interaction between tyrosine residues within the IC2 and C-terminus [10].
Figure 2.
Molecular models of the endothelin A (left) and endothelin B (right) receptors incorporating the structural features of the C-termini after energy minimization and molecular dynamics. The C-termini are colored as gray ribbons with side chains displayed.
To decipher the signaling capacity of each receptor individually, we developed CHO cell lines stably expressing only ETA or ETB and, in a separate procedure, also stably expressing the E3A and E3B C-terminal motifs. We then tested for the ability of these third helical segments (E3A and E3B) to alter ET-1-activated receptor signaling. The results are shown in Figure 3A and B. In CHO-ETA, the expression of E3A reduced ET-1-induced ERK activity, whereas E3B greatly increased ERK activity compared with cells expressing an empty vector (Figure 3A). This opposing action was also seen in CHO-ETB where E3A increased ET-1-induced ERK activity and E3B reduced ERK activity compared with cells expressing an empty vector (Figure 3B).
Figure 3.
The relative cellular presence of pERK after stimulation with endothelin-1 of stably transfected CHO cells expressing the (A) endothelin A (CHO-ETA); or (B) endothelin B (CHO-ETB) receptors and either E3A or E3B C-terminal peptides. The level of pERK is shown relative to the control CHO-ETA or CHO-ETB cells, transfected with an empty vector (Emp Vec) not expressing the peptides. All tested cell cultures were treated with endothelin-1 at 10 nM (a maximal stimulatory dose) for 5 min (optimum incubation time). Blots are representative of three independent experiments. Bar graphs represent the mean value of relative ERK activation compared with the control (empty vector) cells. Error bars represent the standard deviation. Data were evaluated for statistical significance by use of a student's t-test. * = P < 0.05 versus Emp Vec.
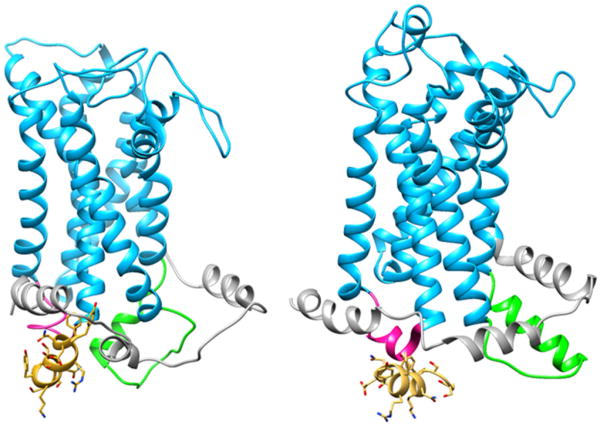
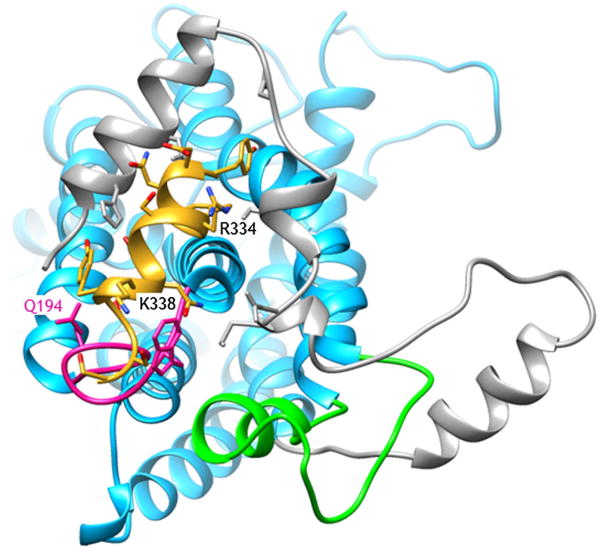
Given the significant alteration of ETA signaling induced by E3B, we searched for possible modes of binding of this 13-residue long peptide, assumed to be α-helical, to the cytoplasmic face of the receptor. The energy-minimized and MD-relaxed homology models were used for a series of docking simulations that targeted different regions within the cytoplasmic face of the receptors. The lowest energy complex structure for E3B binding to the ETA receptor is shown in Figure 4. There are several interactions between the E3B peptide and the cytoplasmic face of the ETA receptor (Figure 5), particularly involving the first and second helical regions of the C-terminus of ETA. There are hydrogen bonds and Coulombic interactions involving one face of the E3B helix (N432, S436, N437, and S440) with corresponding residues of the C-terminus and IC2 of ETA. In contrast, there is little interaction of the E3B with the ETB receptor (Figure 4). Despite extensive docking calculations with various grid search boxes and energy minimization calculations, a similarly stable complex was not observed.
Figure 4.
The low energy conformations from molecular docking of E3B to the endothelin A (left) and endothelin B (right) receptors. The C-terminus, second intracellular loop (IC2) and third intracellular loop (IC3) of the receptors are color-coded in gray, pink, and light green, respectively.
Figure 5.
Expanded view (from cytoplasmic face) of the interaction between the cytoplasmic domain of the endothelin A receptor and the E3B peptide. The endothelin receptor is blue, with the C-terminus, the second intracellular loop(IC2), and the third intracellular loop (IC3) color-coded in gray, pink, and light green, respectively. The E3B peptide is colored in yellow, with the side chains colored by heteroatom. Some of the key residues (Q194, R334, and K338) discussed in the text are highlighted.
Discussion
We previously demonstrated interplay among the various cytoplasmic domains of the GPCRs for the vasoactive peptide bradykinin and angiotensin [12–17]. Modifications within the intracellular loop that were deleterious to receptor signaling could be overcome with corresponding modifications within the other regions within the cytoplasmic domains [17]. These results in sum demonstrated that the three intracellular loops and C-terminus together function as signaling motifs with partially overlapping and interactive roles.
Recently, we showed that exposure of the ETB receptor to a cell permeable peptide consisting of the third intracellular loop of the ETB receptor either increased or decreased ETA/ETB signaling [31]. These differential actions by the peptide were puzzling. The C-terminus of the ETA/ETB receptors was then chosen for study to obtain a better understanding of this phenomenon. Specific regions of the C-termini were selected on the basis of putative sites of posttranslational modifications and predicted structural features. We generated models of the structure of the C-termini of the receptors on the basis of the structure of the sequence-related C-terminus of the BK2 receptor [17]. On the basis of these structural features, we focused our efforts on the third (most C-terminal) α-helix of the C-termini (E3A and E3B). This helix is highly charged and does not display the amphipathic nature of the other two helices. We hypothesize that the first and second helix would associate with the membrane and therefore less likely to form multiple or high-affinity interactions with the cytoplasmic face of the receptors.
To empirically demonstrate these interactions, the amino acid sequence of the E3A and E3B helices were stably expressed in CHO cells transfected with either ETA or ETB receptors. CHO cells do not natively express either receptor [31]. Here we examined the effect of these signaling motifs on both homoreceptor and heteroreceptor downstream signaling. The biological action of these peptides in combination with the activation of the receptors by ET-1 proved dramatic. There is a clear interaction between the E3B peptide and the ETA receptor; an interaction resulting in a 22-fold enhancement of ERK phosphorylation. Additionally, the ETB peptide combined with the ETB receptor and the ETA peptide combined with the ETA receptor decreased ET-1 activation of ERK. These results illustrate heterologous ET-1 receptor activation and homologous receptor interference.
For the molecular modeling efforts, carried out to examine possible interactions between the receptors and the C-terminus derived peptides, we concentrated on E3B as it produced the largest alteration in signaling. From the docking, E3B is found to associate with the cytoplasmic domain of the ETA receptor, binding between the helical domains of the C-terminus and the small IC2. Interestingly, no such binding mode was found for E3B with the ETB receptor. These findings suggest that E3B is competing with ETB for coupling to proteins in the signaling pathway and therefore antagonizing the ET-1 signaling (Figure 3). In contrast, the association of E3B with the cytoplasmic region of the ETA, leads to a significant enhancement of the ET-1-induced signaling. Of course, this hypothesis is based on the homology modeling of the receptors cytoplasmic domains and requires experimental verification using site-directed mutagenesis and examination of receptor chimera; experiments which are ongoing. Based on simple sequence comparison, there are a number of differences in the cytoplasmic faces of ETA and ETB. Although the IC2 are very similar in ETA and ETB, a glutamine (Gln194) is replaced with a lysine (ETA – >ETB), and in the E3B/ETA complex (Figure 5) Gln194 is forming hydrogen bonds with Ser440 and Ser442 of E3B. Similarly, the second helix of the C-terminus of ETB is more positively charged than that of ETA, and therefore is less conducive to E3B binding. In the complex (Figure 5), E3B has Arg334 and Lys338 projecting towards the second helix in the C-terminus.
Future experiments combining cell permeable copies of receptor signaling motifs and cDNA stable expression of these sequences could become a basic as well as clinical tool to pinpoint receptor signaling action.
Acknowledgments
Partial funding for this work was provided by the National Institutes of Health (HL-025776 and HL-025776-25S1 to P.P. and GM-54082 to D.F.M.). All simulations were carried out on the Discovery Linux Cluster at Dartmouth College.
Footnotes
Special issue devoted to contributions presented at the 13th Naples Workshop on Bioactive Peptides, June 7-10, 2012, Naples.
References
- 1.Takayanagi R, Kitazumi K, Takasaki C, Ohnaka K, Aimoto S, Tasaka K, Ohashi M, Nawata H. Presence of non-selective type of endothelin receptor on vascular endothelium and its linkage to vasodilation. FEBS Lett. 1991;282:103–6. doi: 10.1016/0014-5793(91)80454-b. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Taylor L, Rich C, Toselli P, Stone P, Green D, Warburton R, Hill N, Goldstein R, Polgar P. Transgenic expression of an altered angiotensin type I AT1 receptor resulting in marked modulation of vascular type I collagen. J Cell Physiol. 2012;227:2013–21. doi: 10.1002/jcp.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rich S, McLaughlin VV. Endothelin receptor blockers in cardiovascular disease. Circulation. 2003;108:2184–90. doi: 10.1161/01.CIR.0000094397.19932.78. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson D, Wackenfors A, Gustafsson L, Ugander M, Ingemansson R, Edvinsson L, Malmsjo M. PKC and MAPK signalling pathways regulate vascular endothelin receptor expression. Eur J Pharmacol. 2008;580:190–200. doi: 10.1016/j.ejphar.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 5.Channick RN, Sitbon O, Barst RJ, Manes A, Rubin LJ. Endothelin receptor antagonists in pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:62S–7S. doi: 10.1016/j.jacc.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R, Gomberg-Maitland M. Current therapeutics and practical management strategies for pulmonary arterial hypertension. Am Heart J. 2011;162:201–13. doi: 10.1016/j.ahj.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8:443–55. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–23. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 9.Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, McLaughlin V, Hill N, Tapson VF, Robbins IM, Zwicke D, Duncan B, Dixon RA, Frumkin LR. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–7. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 10.Prado GN, Mierke DF, Pellegrini M, Taylor L, Polgar P. Motif mutation of bradykinin B2 receptor second intracellular loop and proximal C terminus is critical for signal transduction, internalization, and resensitization. J Biol Chem. 1998;273:33548–55. doi: 10.1074/jbc.273.50.33548. [DOI] [PubMed] [Google Scholar]
- 11.Prado GN, Mierke DF, LeBlanc T, Manseau M, Taylor L, Yu J, Zhang R, Pal-Ghosh R, Polgar P. Role of hydroxyl containing residues in the intracellular region of rat bradykinin B(2) receptor in signal transduction, receptor internalization, and resensitization. J Cell Biochem. 2001;83:435–47. doi: 10.1002/jcb.1241. [DOI] [PubMed] [Google Scholar]
- 12.Piserchio A, Prado GN, Zhang R, Yu J, Taylor L, Polgar P, Mierke DF. Structural insight into the role of the second intracellular loop of the bradykinin 2 receptor in signaling and internalization. Biopolymers. 2002;63:239–46. doi: 10.1002/bip.10072. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Prado GN, Taylor L, Piserchio A, Gupta A, Mierke DF, Polgar P. Global chimeric exchanges within the intracellular face of the bradykinin B2 receptor with corresponding angiotensin II type Ia receptor regions: generation of fully functional hybrids showing characteristic signaling of the AT1a receptor. J Cell Biochem. 2002;85:809–19. doi: 10.1002/jcb.10171. [DOI] [PubMed] [Google Scholar]
- 14.Prado GN, Taylor L, Zhou X, Ricupero D, Mierke DF, Polgar P. Mechanisms regulating the expression, self-maintenance, and signaling-function of the bradykinin B2 and B1 receptors. J Cell Physiol. 2002;193:275–86. doi: 10.1002/jcp.10175. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Liu B, Eramian D, Mierke D, Taylor L, Polgar P. K317, R319, and E320 within the proximal C-terminus of the bradykinin B2 receptor form a motif important for phospholipase C and phospholipase A2 but not connective tissue growth factor related signaling. J Cell Biochem. 2004;92:547–59. doi: 10.1002/jcb.20075. [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Polgar P, Lubinsky D, Gupta M, Wang L, Mierke D, Taylor L. Coulombic and hydrophobic interactions in the first intracellular loop are vital for bradykinin B2 receptor ligand binding and consequent signal transduction. Biochemistry. 2005;44:5295–306. doi: 10.1021/bi048288i. [DOI] [PubMed] [Google Scholar]
- 17.Piserchio A, Zelesky V, Yu J, Taylor L, Polgar P, Mierke DF. Bradykinin B2 receptor signaling: structural and functional characterization of the C-terminus. Biopolymers. 2005;80:367–73. doi: 10.1002/bip.20220. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Lubinsky D, Tsomaia N, Huang Z, Taylor L, Mierke D, Navarro J, Miraz O, Polgar P. Activation of ERK, JNK, Akt, and G-protein coupled signaling by hybrid angiotensin II AT1/bradykinin B2 receptors expressed in HEK-293 cells. J Cell Biochem. 2007;101:192–204. doi: 10.1002/jcb.21161. [DOI] [PubMed] [Google Scholar]
- 19.Gregan B, Schaefer M, Rosenthal W, Oksche A. Fluorescence resonance energy transfer analysis reveals the existence of endothelin-A and endothelin-B receptor homodimers. J Cardiovasc Pharmacol. 2004;44(1):S30–3. doi: 10.1097/01.fjc.0000166218.35168.79. [DOI] [PubMed] [Google Scholar]
- 20.Evans NJ, Walker JW. Endothelin receptor dimers evaluated by FRET, ligand binding, and calcium mobilization. Biophys J. 2008;95:483–92. doi: 10.1529/biophysj.107.119206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rapoport RM, Zuccarello M. Endothelin(A)-endothelin(B) receptor cross-talk and endothelin receptor binding. J Pharm Pharmacol. 2011;63:1373–7. doi: 10.1111/j.2042-7158.2011.01334.x. [DOI] [PubMed] [Google Scholar]
- 22.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 23.Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY, Pieper U, Sali A. Curr Protoc Bioinformatics. 1. Vol. 15. John Wiley & Sons, Inc.; 2006. Comparative protein structure modeling using Modeller; pp. 5.6.1–5.6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 25.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–5. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–23. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 27.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–8. 27–8. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 28.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten, K Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gumbart J, Wang Y, Aksimentiev A, Tajkhorshid E, Schulten K. Molecular dynamics simulations of proteins in lipid bilayers. Curr Opin Struct Biol. 2005;15:423–31. doi: 10.1016/j.sbi.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 And AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallum CO, Wilson JL, Rupasinghe C, Berg E, Yu J, Green DS, Taylor L, Mierke D, Polgar P. Enhancing and limiting endothelin-1 signaling with a cell-penetrating peptide mimicking the third intracellular loop of the ETB receptor. Chem Biol Drug Des. 2012;80:374–81. doi: 10.1111/j.1747-0285.2012.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson JL, Taylor L, Polgar P. Endothelin-1 activation of ETB receptors leads to a reduced cellular proliferative rate and an increased cellular footprint. Exp Cell Res. 2012;318:1125–33. doi: 10.1016/j.yexcr.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]