Abstract
Objective:
We investigated the evolution of new multiple sclerosis (MS) lesions over time using frequency shifts of the magnetic resonance (MR) signal.
Methods:
Twenty patients with relapsing-remitting MS were serially scanned for 6 months at 1-month intervals. Maps of MR frequency shifts were acquired using susceptibility-weighted imaging. New lesions were identified by enhancement with gadolinium (Gd).
Results:
Forty new lesions were identified as areas of signal increase on Gd-enhanced scans. Up to 3 months before lesion appearance, the frequency in areas of future Gd enhancement was not detectably different from the frequency in normal-appearing white matter. Rapid increase in MR frequency was observed between 1 month before and 1 month after Gd enhancement. Two months postenhancement and later, the frequency stabilized and remained at a constantly increased level.
Conclusions:
These findings suggest that an increase in MR frequency does not simply reflect blood-brain barrier disruption or edema; rather, it reflects a change of tissue architecture as a consequence of new lesion formation. The data demonstrate that the MR frequency of focal MS lesions is increased before the lesions appear on conventional MRI. Unlike many other advanced imaging techniques, the images for frequency mapping can be rapidly acquired at high spatial resolution and standardized on most clinical scanners.
Multiple sclerosis (MS) is characterized by focal lesions with episodic inflammation resulting in demyelination of axons, formation of an astrocytic scar, progressive neurodegeneration, remyelination, and axonal injury to a highly variable degree.1 MRI is often used in the diagnosis and follow-up of MS.2-5 Recently, the phase images of gradient-echo magnetic resonance (MR) scans were shown to exhibit exquisite contrast between different brain structures and between healthy and diseased tissue.6 Using a lighthouse as an analogy, the MRI signal's magnitude corresponds to the brightness of the lighthouse beam and its phase to the beam's direction. Just as the lighthouse beam has a certain direction, the MR signal has a certain phase. Phase images can easily be converted into maps of MR frequency shifts. MR frequency is influenced by tissue microstructure and myelin content,7 making it a sensitive probe for the assessment of white matter (WM) integrity. Tissue changes, such as those occurring in MS lesions, can be monitored using frequency shift maps at spatial resolutions not achievable with other MR methods. In this study, we examined the MR frequency shifts in new MS lesions. We hypothesized that MS lesion formation leads to an increase in resonance frequency. Over 6 months, we performed monthly measurements of MR frequency in new lesions, as well as in normal-appearing WM (NAWM), diffusely abnormal WM (DAWM), persistent T1-hypointense lesions, and cortical gray matter (GM).
METHODS
Standard protocol approvals, registrations, and patient consents.
The protocol was approved by the Clinical Research Ethics Board of the University of British Columbia, and all subjects gave written informed consent in accord with the Declaration of Helsinki.
Subjects.
Twenty subjects (table) with relapsing-remitting MS were scanned 7 times over 6 months at 1-month intervals, referred to as scans 0 to 6; herein, the term “month” is used to refer to scan times before (months −6 to −1), at (month 0), and after (months 1–6) gadolinium (Gd) enhancement. Data were acquired between November 2007 and March 2009. Demographic and clinical data are summarized in the table. Subject 10 was treated with natalizumab. Age- and sex-matched healthy controls received scans 0 and 6.
Table.
Demographic and clinical data and the number of enhancing lesions in the 20 patientsa
Data acquisition.
Data were acquired on a Philips Achieva 3.0T system (Philips Healthcare, Andover, MA) using an 8-channel SENSE head coil. Fluid-attenuated inversion recovery (FLAIR) images were acquired for lesion detection (28 slices, slice thickness = 5 mm, field of view [FOV] = 240 × 191 × 140 mm3, matrix = 256 × 203, repetition time [TR]/echo time [TE]/inversion time = 11,000/125/2,800 milliseconds). Susceptibility-weighted data were acquired with a flow-compensated 3-dimensional gradient-echo method (FOV = 240 × 166 × 64 mm3, acquisition matrix = 480 × 231 × 32, acquisition voxel size 0.5 × 0.7 × 2 mm3, TR/TE = 40/20 milliseconds, flip angle = 19°, slice oversampling factor = 1.28, acquisition time = 4.55 minutes).8 Then, Gd-enhanced, T1-weighted images were acquired (28 slices, slice thickness = 5 mm, FOV = 240 × 191 × 140 mm3, matrix = 256 × 163, TR/TE = 800/10 milliseconds, 5 minutes after the injection of Gd [0.1 mmol/kg body weight]). Image slices of all scans were parallel to the subcallosal line, with the center slice of the transverse MR phase experiment located just superior to the ventricles.
Data processing.
The susceptibility-weighted images were reconstructed to a matrix of 560 × 560 × 64, resulting in a voxel size of 0.43 × 0.43 × 1 mm3. Magnitude and phase images were saved for further off-line processing. The phase images ϕ were unwrapped and high-pass filtered using 2-dimensional homodyne filtering9 and converted to maps of frequency shifts Δω relative to the Larmor frequency at 3 T (Δω [ppm] = ϕ/[TE × ω0]; ω0 is the frequency at B0). Venograms created by multiplying the magnitude images with a mask computed from the filtered phase images10 were used for image registration because of their good image contrast compared with magnitude or phase. The transformations computed for the venograms were then applied to the corresponding magnitude and phase images. All phase, FLAIR, and Gd-DTPA–enhanced T1-weighted images were registered to the venogram10 images of the phase scan of the first acquisition using FSL's FLIRT tool11 (12 degrees of freedom; search angle [−45; 45] for phase and [−90; 90] for T1 and FLAIR registration, trilinear interpolation, cost function = correlation ratio).
Regions of interest.
Focal Gd-enhancing lesions were analyzed. For enhancing spheres with a nonenhancing core (so-called ring-enhancing lesions), the enhancing volume and the nonenhancing core were analyzed separately. The month of enhancement was defined as month 0. If enhancement was present in more than 1 month, the first month of enhancement was defined as month 0. Enhancing lesions appearing as focal hyperintensities with well-defined boundaries on the FLAIR images before month 0, so-called re-enhancing lesions, were excluded from the analysis to capture only new tissue damage. Enhancing lesions in areas that appeared as a diffusely hyperintense background with ill-defined boundaries before the month of enhancement were not excluded from the analysis. Regions of interest (ROIs) encompassing enhancing MS lesions were defined manually on one frequency map calculated from the last serial scan and the ROIs were projected onto all other months. For each lesion, the quality of image registration was evaluated visually and it was also confirmed that no ROI was larger than the lesion in any of the up to 7 scans. Representative ROIs were also drawn on the frequency maps in NAWM, DAWM, herein defined as nonfocal WM hyperintensities on FLAIR images with intensity intermediate between focal lesions and NAWM, in the cortical GM, and in lesion areas that appeared as T1 hypointensity for the duration of the study (black holes; 3–5 regions per subject). In healthy controls, regions equivalent to NAWM and DAWM were drawn in the same brain areas as in the subjects and are referred to as NWM (normal WM) and WM(D) (normal WM that corresponds to areas of DAWM in patients), respectively. Visible veins were not included in the regions to minimize the contribution from deoxygenated venous blood. For each ROI, the average frequency shift was computed.
Statistical analysis.
A linear mixed-effect model was implemented in R (lme4,12 languageR13) to incorporate the variables of different numbers of lesions and patients at each scan as well as a label for different tissue types. Sex, disease duration, age, and Expanded Disability Status Scale score were included as fixed-effect variables in the model.
RESULTS
Nine subjects had Gd-enhancing lesions on at least one time point. A total of 43 lesions enhanced in scans 0 to 6 during the course of the study. Three T2 lesions preexisted as focal areas of hyperintensity in FLAIR before Gd enhancement and were excluded, leaving 40 lesions for analysis. Nine Gd-enhancing lesions detected on scan 0 provided the longest follow-up at month 6, but there were no prelesion images available for analysis. At scans 0, 1, 2, 3, 4, 5, and 6, the numbers of enhancing lesions detected were 9, 7, 10, 4, 4, 4, and 2, respectively.
Nonenhancing tissue.
The MR frequency of cortical GM, persistent T1 black holes, DAWM, NAWM, NWM, and WM(D) remained constant over the duration of 6 months (figure 1A). The average frequency shift was 0.0041 ± 0.0002 ppm in cortical GM, 0.0023 ± 0.0002 ppm in persistent T1 hypointensities, −0.0002 ± 0.00004 ppm in DAWM, −0.00096 ± 0.00005 ppm in NAWM, −0.00049 ± 0.00004 ppm in NWM, and −0.00021 ± 0.00007 ppm in WM(D). The frequency of each tissue type remained constant over time, i.e., no significant differences were found between scans (p > 0.2). The frequency shifts in persistent T1 hypointensities ranged from −0.0005 to 0.008 ppm between lesions (figure 1B). Whereas there was no significant difference between DAWM and WM(D), differences between all other tissues were significant for all months (p < 0.01).
Figure 1. Frequency shifts in NAWM, DAWM, T1 hypointensities, cortical GM, NWM, and WM(D).
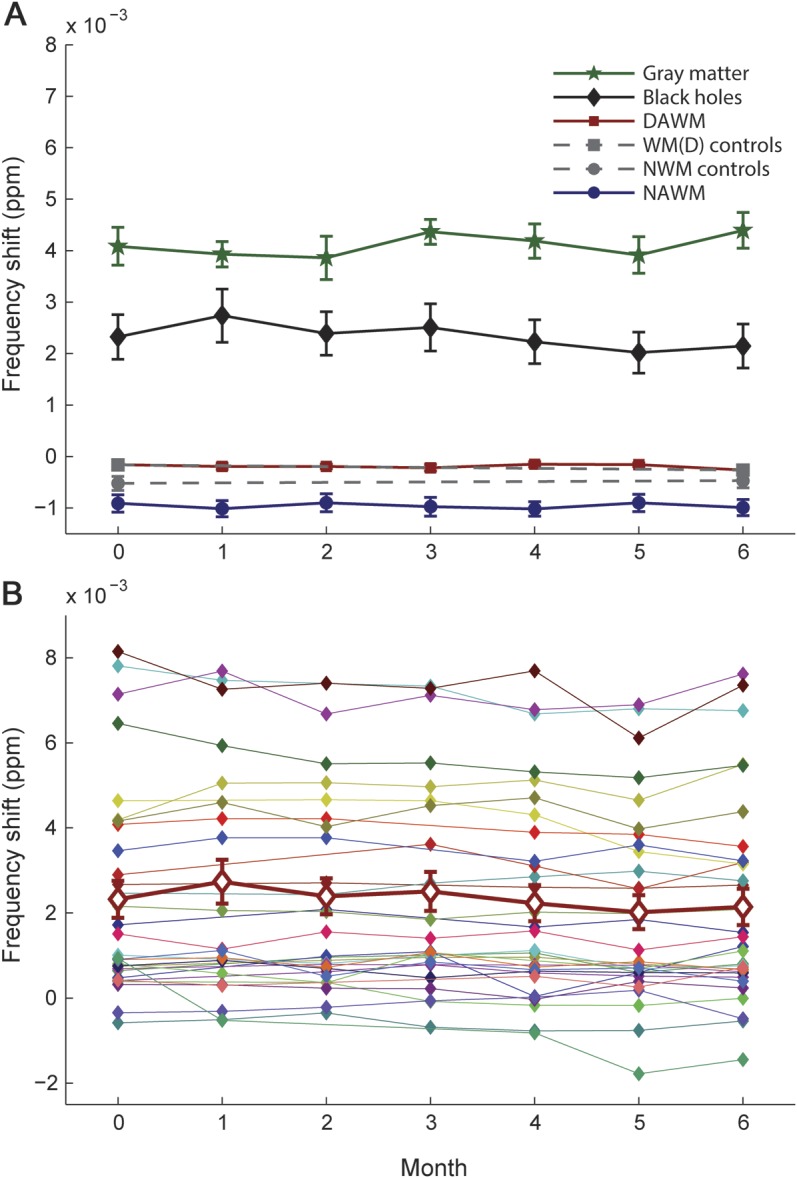
(A) No significant changes in frequency over time were observed within tissue types. Note that the error bars for DAWM (red squares) as well as for the corresponding control regions WM(D) (gray squares) were smaller than the symbols. The differences between all tissues were significant for all months (p < 0.01). NWM (gray circles) in controls was significantly (p < 0.001) different from NAWM (blue circles), whereas frequency shifts in DAWM were not significantly different from frequency shifts in the corresponding regions in healthy controls. (B) Temporal behavior of all individual black holes that were included in the analysis. All black holes showed a constant frequency shift over the time course of the study, but the frequency shifts of individual black holes varied strongly (−0.002 to 0.008 ppm). The thick line (red diamonds) represents the average over all black holes. DAWM = diffusely abnormal white matter; GM = gray matter; NAWM = normal-appearing white matter; NWM = normal white matter; WM(D) = normal white matter corresponding to areas of DAWM in patients.
Enhancing lesions.
Figure 2 shows a representative enhancing lesion. Before enhancement, this particular lesion was not visible on the frequency map. At the time of enhancement, the frequency was slightly increased compared with the surrounding tissue and increased further during the first 2 months after enhancement. The average time course of frequency shifts computed from all 40 enhancing lesions (figure 3A) exhibited 3 important features. First, beginning at month −3, the MR frequency in MS lesions became significantly increased compared with the MR frequency in NAWM (p < 0.001). Second, a rapid increase (p < 0.001) in frequency happened in lesion tissue during the 8 weeks between 1 month before enhancement and 1 month after enhancement first appeared. Third, the frequency in lesions remained increased at approximately 3.5 × 10−3 ppm during months 2 to 6. Post hoc analysis of the intervals from months −6 to −1, months −1 to 2, and months 2 to 6 using a piecewise linear function showed a significant increase only between month −1 and 2. The changes in frequency in lesions from months −6 to −1 and months 2 to 6 can be described by positive and negative slopes, respectively. However, these trends did not reach significance. Three of the 40 lesions were ring-enhancing lesions (figure 3B). The core of these lesions followed the behavior shown in figure 3A, whereas the enhancing part did not exhibit the characteristic increase in frequency. Figure 4 shows the evolution of frequency shifts for all lesions. Some lesions exhibited a behavior quite different from the general trend shown in figure 3A, such as an increase in frequency more than 3 months before Gd enhancement, a decrease in frequency during the months before enhancement, or no change in frequency during the first 2 months after enhancement. Enhancing lesions that evolved into T1 hypointensities at month 5 and later (solid lines in figure 4) exhibited the typical behavior shown in figure 3A.
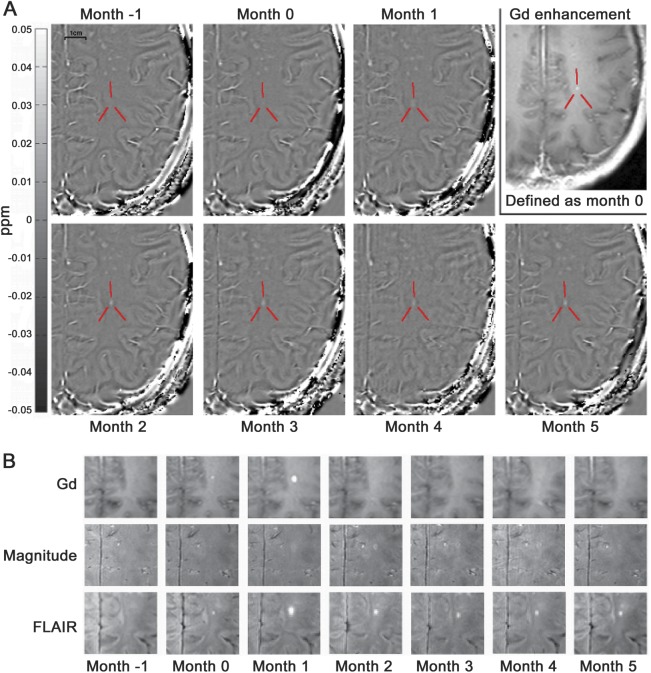
Figure 2. Serial MRI of an MS lesion using frequency shift mapping and conventional MRI.
(A) Frequency shift maps of an enhancing lesion (indicated by red lines) between 1 month before enhancement (month −1) and 5 months after enhancement (month 5), as well as the Gd-enhancing scan, which defines scan 1 as month 0 for this particular lesion. The lesion was barely visible at months −1 and 0 and became increasingly hyperintense during the months after Gd enhancement. (B) Gd-enhanced scan, with the magnitude corresponding to the phase/frequency and the FLAIR images at all months. Note that in this case, the strongest enhancement occurred at month 1. The scale bar in panel A corresponds to 1 cm. FLAIR = fluid-attenuated inversion recovery; Gd = gadolinium; MS = multiple sclerosis.
Figure 3. Frequency changes over time averaged over all 40 MS lesions, including ring-enhancing lesions.
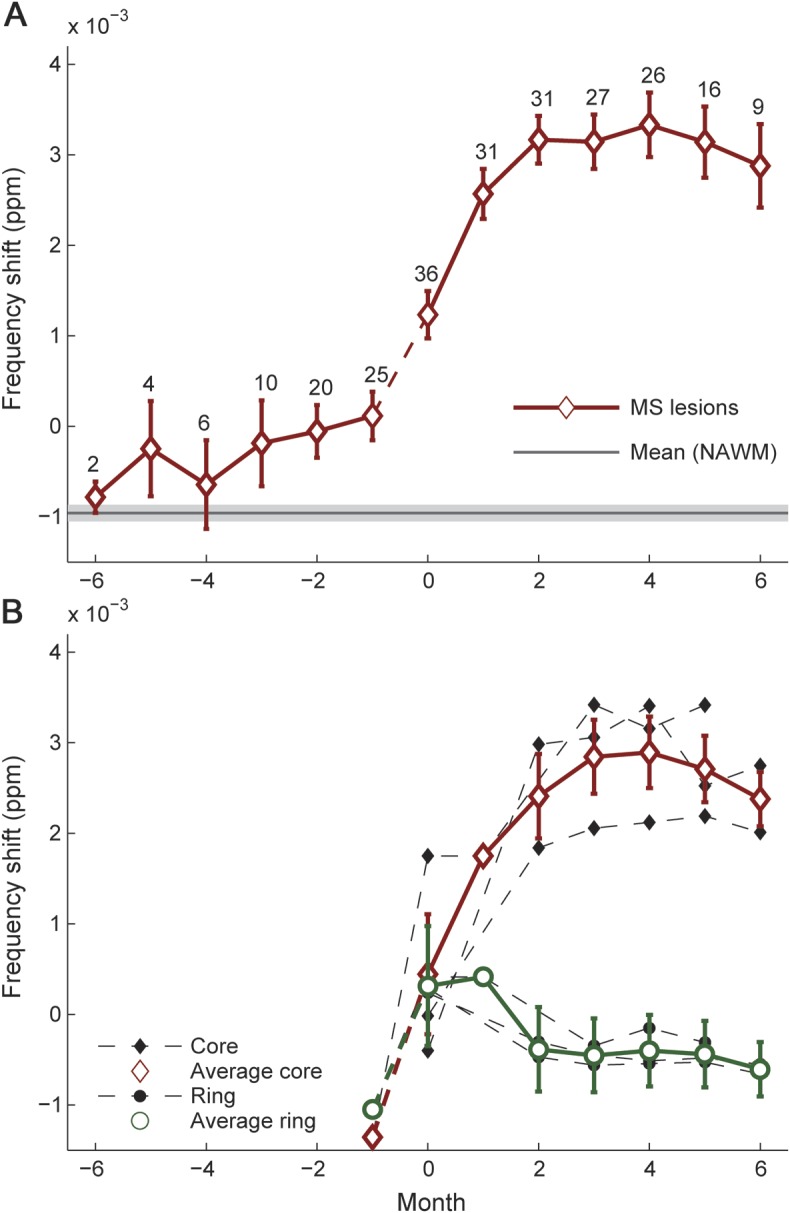
(A) Frequency shifts within Gd-enhancing MS lesions over time (average over 40 lesions, diamonds). The error bars indicate the SEM and the numbers at each time point are the number of lesions contributing to the respective data. Note that not all lesions were captured with frequency mapping at every month. For instance, 36 instead of 40 lesions contribute to month 0. The gray line and light gray shaded line indicate mean frequency shift and average standard error in NAWM, respectively. There is a pronounced increase in MR frequency during the 2 months around Gd enhancement. Because the events during the month before Gd enhancement may be rather abrupt and nonlinear, a dashed line was used for this time period. Significant differences between MS lesions and NAWM were found beginning 3 months before the lesion appears and for all subsequent months. The frequency shifts showed a significant positive slope between months −1 and 2 (p < 0.0001) and no significant slopes between months −6 and −1 and months 2 and 6. Month 0 and all subsequent months were significantly different from all time points before lesion onset. (B) Ring-enhancing lesions. The enhancing ring and the core of 3 ring-enhancing lesions were investigated separately. Whereas the cores exhibited a distinct frequency shift similar to focally enhancing lesions, no such evolution was observed for the enhancing ring. Note that the cores of the 3 ring-enhancing lesions were included in the average shown in panel A. Gd = gadolinium; MR = magnetic resonance; MS = multiple sclerosis; NAWM = normal-appearing white matter.
Figure 4. Condensed plot showing the temporal evolution of all enhancing multiple sclerosis lesions.
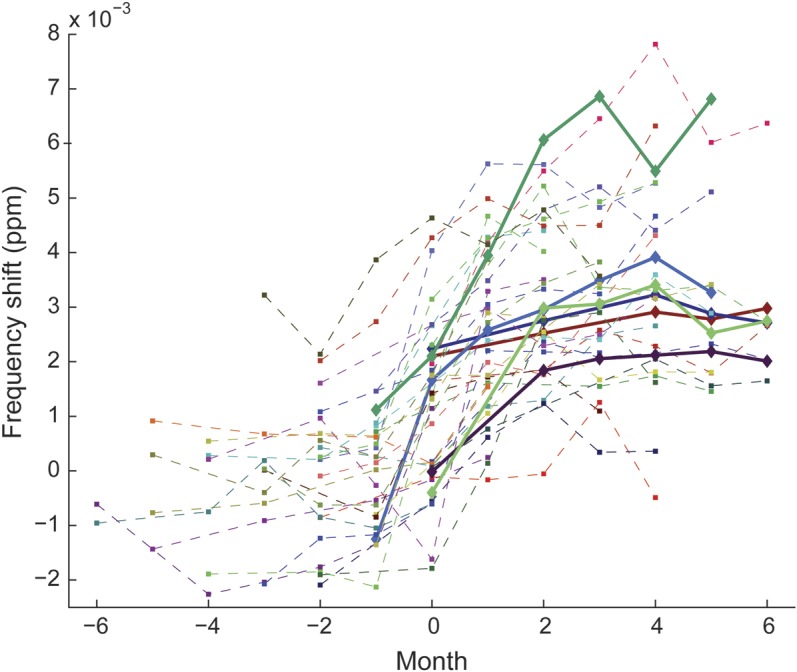
All lesions (focal and core of ring-enhancing lesions) contributing to the average in figure 3A are displayed. There is variability in frequency shifts between lesions. Note that some lesions reached frequency shift values up to 7 × 10−3 ppm, which is higher than the observed offset in cortical gray matter (figure 1A). Solid lines represent newly enhancing lesions that showed T1 hypointensity after 5 to 6 months.
DISCUSSION
Paramagnetic iron in damaged tissue has been proposed as a cause for MR frequency shifts in MS lesions.14–16 However, a study in 32 samples from 13 human subjects found no iron within lesions or areas surrounding the lesions.17 Also, there are no reports in the MR spectroscopy literature of an increase in spectral line width in MS lesions, which would be expected if iron was increased in MS lesions. However, a correspondence between MRI signs of increased iron (increased R2*, increased MR frequency) and activated microglia as well as increased iron content detected by Perls staining was found in the rims of leukocortical lesions18 and WM lesions,19 whereas other parts of lesions did not stain for iron.
More recent research showed that MR frequency is affected by tissue microstructure7; a change from anisotropic healthy WM into a structurally more isotropic lesion pattern increases the resonance frequency. Together with the axon, the wrapped myelin bilayers constitute an anisotropic structure. During demyelination, this structure becomes more isotropic as it is transformed into myelin debris and, if axonal degeneration occurs, also into axon debris. Myelin debris is known to persist for several months before it is completely removed by macrophages.20 MR frequency is affected by demyelination as seen in a recent cuprizone-fed mouse study,21 where demyelinated brain showed significantly reduced phase contrast between GM and WM. This is in agreement with work showing that the absence of myelin in the shiverer mouse leads to the disappearance of contrast between GM and WM.22 Further support for the role of myelin was provided by a study in human neonates, showing that GM-WM phase contrast is reduced in the absence of myelin.23 Recent work24 in rodents at high field, correlating histology and phase contrast, suggested that most of the GM-WM contrast originates from myelin. Interestingly, GM-WM phase contrast remained unchanged after iron extraction in that study. Finally, it was shown that even small tissue changes due to MS may lead to substantially increased frequency.25 These findings provide evidence that the magnetic properties of tissue structure and myelin have a significant influence on the MR frequency. The transition from healthy anisotropic WM to WM with reduced structural anisotropy due to the increase in tissue debris and the presence of various cells, such as macrophages, reactive astrocytes, and nonmacrophage inflammatory cells, and an expansion of the extracellular space would be sufficient to explain the frequency increase observed in MS lesions.
Gd enhancement is the radiologic sign of an active lesion as it signifies breakdown of the blood-brain barrier. The absence of Gd enhancement does not necessarily mean that there is no blood-brain barrier breakdown, because the signal increase caused by Gd may be below the detection threshold. One finding of the present study was that MR frequency increased rapidly at the time of enhancement, relative to one or more months prior. Prelesional tissue changes have been detected using magnetization transfer (MT).26 The underlying events that cause these imaging changes are unknown. However, we speculate that the increase in MR frequency is primarily attributable to demyelination, breakdown of tissue architecture, and the presence of macrophages rather than inflammation alone, because these frequency changes do not recover for at least 6 months after lesion development. This is longer than the time course for the normal resolution of inflammation-related edema. In contrast, MT ratio lesional changes often show partial recovery that typically starts 3 months after Gd enhancement. This in part reflects resolution of edema.27,28 In a serial study, 13 of 22 investigated Gd-enhancing lesions showed reduced MT ratio before enhancement.29 We believe that the early frequency increase is mostly attributable to demyelination and the presence of lymphocytes, monocytes, and macrophages rather than axonal loss, which occurs later due to prolonged demyelination. This theory of early demyelination can be further explored in vivo with advanced MRI techniques, such as multiecho T2 relaxation, that are myelin specific.30 Persistent demyelination and the presence of macrophages may in part explain the continued elevated resonance frequency after Gd enhancement resolves. However, axonal loss may also be a contributing factor. The variability between lesions (figure 4) suggests that frequency shifts have the sensitivity to capture lesion heterogeneity in terms of varying degrees of tissue damage that is otherwise missing from conventional MRI measures. Interestingly, in the 3 ring-enhancing lesions, only the nonenhancing core displayed positive frequency shifts, whereas the area of enhancement did not exhibit an increase in frequency. Future research is required to determine whether this behavior is a common feature of ring-enhancing lesions.
The MR signal's frequency remained constant over the duration of the study in NAWM, DAWM, persistent T1 hypointensities, and cortical GM, without significant changes over time in any of these tissue types (figure 1A). That the frequency was increased in DAWM compared with NAWM and NWM may be explained by subtle structural changes. However, the control regions in healthy subjects have the same frequency shift as the DAWM regions defined in patients. One possible explanation for this discrepancy is that DAWM is found predominantly in periventricular areas, whereas NAWM regions often had to be drawn in areas closer to cortical GM. Differences in fiber orientation between these brain structures could account for these frequency differences.7,31
The frequency in persistent T1 hypointensities shows more variability between individual lesions and over time than NAWM and DAWM, which is expected from the heterogeneity of MS lesions (figure 1). In particular, some persistent T1 hypointensities may be transient black holes showing variable signs of demyelination, edema, or remyelination as well as variable degrees of axonal loss.25,32,33 Moreover, phase contrast in lesions with extreme tissue destruction may disappear, which could explain the frequency shifts close to zero in several of the persistent T1 hypointensities (figure 1B).25 This interpretation is in agreement with the fact that the frequency of cortical GM exceeds the average frequency of WM lesions later than 2 months after Gd enhancement, although GM has a smaller magnetic susceptibility of 3.50 × 10−3 ppm compared with 5.57 × 10−3 ppm in WM.7 Only 6 enhancing lesions became persistent T1 hypointensities within the 6 months of the study. Although their pattern of frequency shifts follows the overall course displayed in figure 3A, the small number of lesions and the short duration after Gd enhancement do not allow for further conclusions.
This study has limitations. Lesions were only captured between 6 months pre-enhancement to 6 months postenhancement. The statistical power is lower at the beginning and end of the time interval (figure 3A). Following subjects over a longer period of time could show how long the offset in frequency seen 2 to 6 months after enhancement remains and how different lesions further evolve. Moreover, one would expect very old demyelinated lesion tissue, where all the macrophages are cleared, to show significant structural anisotropy, as it is a well-organized structure with nicely aligned axons (albeit without myelin sheaths) between which there are fibrillary astrocytes with elongated narrow processes. A larger subject population would allow for a differentiation between subgroups of lesions, such as lesions that show signs of remyelination and other features of lesion reversal, including resolution of edema and inflammation,34 vs those that evolve into chronic black holes with greater axonal loss, matrix destruction, and more extracellular fluid35 and that have a stronger correlation with disability than lesion volume on T2-weighted scans.36 Because Gd enhancement normally persists for approximately 3 to 4 weeks,37 a study with a higher temporal resolution of 2 weeks, for instance, would shed more light on the impact of the findings in this study. Not all subjects in the present study had new MS lesions and the results are more dominated by the subjects who had large numbers of enhancing lesions.
Frequency mapping using MR phase imaging has some advantages. A whole-brain scan can be acquired in less than 10 minutes with a spatial resolution of 0.5 × 0.7 × 2 mm3 (reconstructed to 0.43 × 0.43 × 1 mm3), which is 30 times better than that of typical diffusion tensor imaging acquisition, for instance. The MR phase has a considerably higher signal-to-noise ratio than the magnitude.38 However, the raw-phase data require postprocessing that needs to be standardized. Moreover, specificity of frequency images needs further validation. The investigation of the sources of phase contrast is an active area of research. Phase is quantitative, but the pathophysiologic significance is not yet fully understood. However, this lack of understanding does not preclude clinical research, such as how lesion load on frequency shift maps reflects the clinical status of a person with MS. Future research will show whether frequency shifts can detect global changes in WM or GM typical of MS within the first 2 years. Therefore, MR frequency shift mapping may complement more conventional imaging techniques for the investigation of MS.
Supplementary Material
ACKNOWLEDGMENT
The authors sincerely thank all of the volunteers and their families, the UBC MRI Research Centre, the MRI technologists, Yinshan Zhao for advice on statistics (MS/MRI Research Group, University of British Columbia), and Joel Oger (Division of Neurology, University of British Columbia) for valuable comments and for help with subject recruitment.
GLOSSARY
- DAWM
diffusely abnormal white matter
- FLAIR
fluid-attenuated inversion recovery
- FOV
field of view
- Gd
gadolinium
- GM
gray matter
- MR
magnetic resonance
- MS
multiple sclerosis
- MT
magnetization transfer
- NAWM
normal-appearing white matter
- NWM
normal white matter
- ROI
region of interest
- TE
echo time
- TR
repetition time
- WM
white matter
- WM(D)
normal WM that corresponds to areas of DAWM in patients
Footnotes
AUTHOR CONTRIBUTIONS
Vanessa Wiggermann: data analysis, interpretation of results, statistical analysis, manuscript writing. Enedino Hernández Torres: data processing, data analysis, manuscript editing. Irene Vavasour: study design, data acquisition, revising manuscript. G.R. Wayne Moore: revising manuscript. Cornelia Laule: study coordination, study design, manuscript editing. Alex MacKay: involved in obtaining funding for project, study design, reviewing the manuscript. David Li: study design, interpretation of results, drafting/revising of manuscript. Anthony Traboulsee: recruitment of subjects, review and interpretation of results, development/revising of manuscript. Alexander Rauscher: concept, study design, design of data acquisition and data analysis, supervision of data analysis, interpretation of results, development and writing of manuscript.
STUDY FUNDING
This work was supported by the Multiple Sclerosis Society of Canada, the Natural Sciences and Engineering Research Council of Canada (402039-2011), the Deutscher Akademischer Austauschdienst (CA-EN-1265), and the CIHR New Investigator Award Program (261306). A.R. is recipient of a Canadian Institutes of Health Research New Investigator award. C.L. is the recipient of the Women Against MS End MS Research and Training Network Transitional Career Development Award from the MS Society of Canada. G.R.W.M. is supported by the Multiple Sclerosis Society of Canada. We acknowledge the continued research support at our site by Philips Healthcare.
DISCLOSURE
V. Wiggermann, E. Hernández Torres, I.M. Vavasour, G.R.W. Moore, C. Laule, A.L. MacKay, and D.K.B. Li report no disclosures. A. Traboulsee has received grant support from Roche Pharmaceuticals, Bayer Pharmaceuticals, and honoraria from Teva Innovation, Biogen Idec, EMD Serono, Sanofi-Genzyme, Chugai, and Roche Pharmaceuticals. A. Rauscher reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lassmann H. The pathologic substrate of magnetic resonance alterations in multiple sclerosis. Neuroimaging Clin N Am 2008;18:563–576 [DOI] [PubMed] [Google Scholar]
- 2.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 2001;50:121–127 [DOI] [PubMed] [Google Scholar]
- 3.Newcombe J, Hawkins CP, Henderson CL, et al. Histopathology of multiple sclerosis lesions detected by magnetic resonance imaging in unfixed postmortem central nervous system tissue. Brain 1991;114:1013–1023 [DOI] [PubMed] [Google Scholar]
- 4.Ormerod IE, Miller DH, McDonald WI, et al. The role of NMR imaging in the assessment of multiple sclerosis and isolated neurological lesions: a quantitative study. Brain 1987;110:1579–1616 [DOI] [PubMed] [Google Scholar]
- 5.Stewart WA, Alvord EC, Jr, Hruby S, Hall LD, Paty DW. Magnetic resonance imaging of experimental allergic encephalomyelitis in primates. Brain 1991;114:1069–1096 [DOI] [PubMed] [Google Scholar]
- 6.Rauscher A, Sedlacik J, Barth M, Mentzel HJ, Reichenbach JR. Magnetic susceptibility weighted MR phase imaging of the human brain. AJNR Am J Neuroradiol 2005;26:736–742 [PMC free article] [PubMed] [Google Scholar]
- 7.He X, Yablonskiy DA. Biophysical mechanisms of phase contrast in gradient echo MRI. Proc Natl Acad Sci USA 2009;106:13558–13563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichenbach JR, Haacke EM. High-resolution BOLD venographic imaging: a window into brain function. NMR Biomed 2001;14:453–467 [DOI] [PubMed] [Google Scholar]
- 9.Noll DC, Nishimura DG, Macovski A. Homodyne detection in magnetic resonance imaging. IEEE Trans Med Imaging 1991;10:154–163 [DOI] [PubMed] [Google Scholar]
- 10.Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology 1997;204:272–277 [DOI] [PubMed] [Google Scholar]
- 11.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001;5:143–156 [DOI] [PubMed] [Google Scholar]
- 12.Bates D, Maechler M. Lme4: linear mixed-effects models using S4 classes. R package version 0.999999-0; 2012
- 13.Baayen RH. LanguageR: data sets and functions with ‘Analyzing Linguistic Data: A Practical Introduction to Statistics.’ R package version 1.4; 2011
- 14.Haacke EM, Makki M, Ge Y, et al. Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J Magn Reson Imaging 2009;29:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond KE, Metcalf M, Carvajal L, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 tesla with sensitivity to iron. Ann Neurol 2008;64:707–713 [DOI] [PubMed] [Google Scholar]
- 16.Khalil M, Enzinger C, Langkammer C, et al. Quantitative assessment of brain iron by R2* relaxometry in patients with clinically isolated syndrome and relapsing-remitting multiple sclerosis. Mult Scler 2009;15:1048–1054 [DOI] [PubMed] [Google Scholar]
- 17.Walton JC, Kaufmann JC. Iron deposits and multiple sclerosis. Arch Pathol Lab Med 1984;108:755–756 [PubMed] [Google Scholar]
- 18.Pitt D, Boster A, Pei W, et al. Imaging cortical lesion in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol 2010;67:812–818 [DOI] [PubMed] [Google Scholar]
- 19.Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 tesla. Brain 2011;134:3602–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278–285 [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Shmueli K, Kang BT, et al. The contribution of myelin to magnetic susceptibility weighted contrasts in high-field MRI of the brain. Neuroimage 2012;59:3967–3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Li W, Johnson GA, Wu B. High-field (9.4 T) MRI of brain dysmyelination by quantitative mapping of magnetic susceptibility. Neuroimage 2011;56:930–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong K, Ernst T, Buchthal S, Speck O, Anderson L, Chang L. Phase contrast imaging in neonates. Neuroimage 2011;55:1068–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lodygensky GA, Marques JP, Maddage R, et al. In vivo assessment of myelination by phase imaging at high magnetic field. Neuroimage 2012;59:1979–1987 [DOI] [PubMed] [Google Scholar]
- 25.Yablonskiy DA, Luo J, Sukstanskii AL, Iyer A, Cross AH. Biophysical mechanisms of MRI signal frequency contrast in multiple sclerosis. Proc Natl Acad Sci USA 2012;109:14212–14217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippi M, Rocca MA, Martino G, Horsfield MA, Comi G. Magnetization transfer changes in the normal appearing white matter precede the appearance of enhancing lesions in patients with multiple sclerosis. Ann Neurol 1998;43:809–814 [DOI] [PubMed] [Google Scholar]
- 27.van Waesberghe JH, van Walderveen MA, Castelijns JA, et al. Patterns of lesion development in multiple sclerosis: longitudinal observations with T1-weighted spin-echo and magnetization transfer MR. AJNR Am J Neuroradiol 1998;19:675–683 [PMC free article] [PubMed] [Google Scholar]
- 28.Dousset V, Gayou A, Brochet B, Caille JM. Early structural changes in acute MS lesions assessed by serial magnetization transfer studies. Neurology 1998;51:1150–1155 [DOI] [PubMed] [Google Scholar]
- 29.Laule C, Vavasour IM, Moore GRW, et al. Water content and myelin water fraction in multiple sclerosis: a T2 relaxation study. J Neurol 2004;251:284–293 [DOI] [PubMed] [Google Scholar]
- 30.MacKay AL, Whittall K, Adler J, Li D, Paty D, Graeb D. In vivo visualization of myelin water in brain by magnetic resonance. Magn Reson Med 1994;31:673–677 [DOI] [PubMed] [Google Scholar]
- 31.Denk C, Hernández Torres E, MacKay A, Rauscher A. The influence of white matter fibre orientation on MR signal phase and decay. NMR Biomed 2011;24:246–252 [DOI] [PubMed] [Google Scholar]
- 32.Sahraian MA, Radue EW, Haller S, Kappos L. Black holes in multiple sclerosis: definition, evolution, and clinical correlations. Acta Neurol Scand 2010;122:1–8 [DOI] [PubMed] [Google Scholar]
- 33.Brück W, Bitsch A, Kolenda H, Brück Y, Stiefel M, Lassmann H. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol 1997;42:783–793 [DOI] [PubMed] [Google Scholar]
- 34.Bitsch A, Kuhlmann T, Stadelmann C, Lassmann H, Lucchinetti C, Bruck W. A longitudinal MRI study of histopathologically defined hypointense multiple sclerosis lesions. Ann Neurol 2001;49:793–796 [DOI] [PubMed] [Google Scholar]
- 35.van Walderveen MA, Barkhof F, Pouwels PJ, van Schijndel RA, Polman CH, Castelijns JA. Neuronal damage in T1-hypointense multiple sclerosis lesions demonstrated in vivo using proton magnetic resonance spectroscopy. Ann Neurol 1999;46:79–87 [DOI] [PubMed] [Google Scholar]
- 36.Truyen L, van Waesberghe JH, van Walderveen MA, et al. Accumulation of hypointense lesions (“black holes”) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology 1996;47:1469–1476 [DOI] [PubMed] [Google Scholar]
- 37.Cotton F, Weiner HL, Jolesz FA, Guttmann CRG. MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology 2003;60:640–646 [DOI] [PubMed] [Google Scholar]
- 38.Duyn JH, van Gelderen P, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M. High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci USA 2007;104:11796–11801 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.