Abstract
Objective:
To determine the relationship between radiologically identifiable brain injuries and delayed brain development as reflected by brain metabolic and microstructural integrity.
Methods:
Term newborns with congenital heart disease (CHD) (120 preoperatively and 104 postoperatively) were studied with MRI to determine brain injury severity (BIS), microstructure reflected by fractional anisotropy (FA) and average diffusivity (Dav), and metabolism reflected by N-acetylaspartate (NAA)/choline (Cho) and lactate/Cho. Brain development is characterized by increasing NAA/Cho and white matter FA, and by decreasing Dav and lactate/Cho.
Results:
Newly acquired brain injury was common (41% preoperative, 30% postoperative). Lower white matter FA (p = 0.005) and lower NAA/Cho (p = 0.01) were associated with increasing preoperative BIS. Higher neonatal illness severity scores (p = 0.03), lower preoperative oxygen saturation (p = 0.002), hypotension (p < 0.001), and septostomy (p = 0.002) were also predictive of higher preoperative BIS. Preoperative FA, Dav, and NAA/Cho did not predict new postoperative BIS. Increasing preoperative BIS predicted higher postoperative Dav (p = 0.002) and lactate/Cho (p = 0.008). Within the postoperative scan, new brain injuries were associated with lower white matter FA (p = 0.04). Postoperative BIS (new lesions) was associated with lower postoperative systolic (p = 0.03) and mean (p = 0.05) blood pressures.
Conclusions:
Brain injuries in newborns with CHD are strongly related to abnormalities of brain microstructural and metabolic brain development, especially preoperatively. Both newly acquired preoperative and postoperative brain injuries are related to potentially modifiable clinical risk factors.
Children with congenital heart disease (CHD) remain at risk for long-term neurodevelopmental deficits despite advances in perioperative and intraoperative care.1–7 While the burden of neurodevelopmental deficits relates to patient-specific factors,8 preoperative neurologic findings also predict subsequent impairments.9
Using MRI, newly acquired brain injuries are commonly detected neuroradiologically on MRI before and after surgery in term newborns with CHD.10–13 These injuries consist predominantly of white matter injury (WMI), a pattern of injury commonly diagnosed in premature newborns.11,12,14–17 Neuroimaging measures consistent with delayed brain development are increasingly recognized in the term CHD population, even prior to surgery18,19 and in utero.20
A complex interplay between neonatal brain injury and abnormal brain development, reflected in impaired microstructure and metabolism, is increasingly appreciated.21 In the premature newborn, WMI precedes more widespread abnormalities in brain development.22,23 Similarly, in newborns with CHD, preoperative brain injury precedes impaired development of the corticospinal tracts, even when the injuries did not directly involve this pathway on conventional MRI.24 Brain immaturity has not been consistently identified as a risk factor for acquired brain injury before or after surgery.14,25 In a prospective cohort of term newborns with CHD studied preoperatively and postoperatively with MRI to characterize brain injury severity (BIS) as well as microstructural and metabolic brain integrity, we hypothesize that 1) impaired preoperative brain development would be associated with increasing BIS preoperatively and postoperatively, and 2) preoperative BIS would predict less mature brain microstructure and metabolism postoperatively.
METHODS
Patients.
Between September 2001 and December 2009, we enrolled term neonates (>36 weeks' gestation) with CHD born or transferred to the University of California San Francisco Benioff Children's Hospital (UCSF) or British Columbia Children's Hospital, University of British Columbia (UBC) in a dual-center prospective cohort study. Thirty-nine infants were inborn, including 34 with a prenatal diagnosis of CHD; 6 additional outborn infants were prenatally diagnosed. Subgroups of this cohort have been previously reported.10,12,13,19,26 Both hospitals are regional tertiary level cardiac referral centers. Infants were included if heart surgery was planned within the first 3 months of life. They were excluded if a congenital infection or genetic malformation syndrome was suspected. Those infants diagnosed subsequently with a genetic disorder were also excluded (2 infants with 22q11, 1 infant with a translocation, and 1 infant with Williams syndrome). During the study period, 120 newborns were studied with preoperative scans (UCSF n = 84; UBC n = 36). An additional 6 infants were excluded from this analysis as they were too unstable for preoperative MRI (n = 3) or had excessive motion artifact on preoperative MRI (n = 3). Clinical data were prospectively collected from the medical records at both sites (until the postoperative MRI).10,12,13,19,26 Anesthetic and cardiopulmonary bypass (CPB) management of the 2 cohorts has been previously described10; see e-Methods on the Neurology® Web site at www.neurology.org.
Standard protocol approvals, registrations, and patient consents.
The research ethics board at each site approved the study protocol. Parents of enrolled newborns provided written informed consent.
MRI studies.
Of the 120 newborns with CHD scanned preoperatively at a median of 5 days (range 1–43), 104 (87%) underwent a postoperative scan at a median of 20 days (range 3–61) after birth. Of the 16 newborns without postoperative scans, 10 died before the postoperative study was possible, 5 had permanent pacemakers implanted, and 1 had an MRI degraded by considerable motion artifact. The median number of days between surgery and postoperative MRI was 10 (interquartile range 8–15, range 0–64 days), with only one newborn scanned postoperatively within 48 hours of surgery. Newborns were scanned at each center using a specialized MRI-compatible isolette including a dedicated neonatal head coil. Study methods, including brain imaging, were consistent across the duration of the study (see e-Methods for detailed MRI methods).27 Neonates at UCSF were sedated with pentobarbital or morphine sulfate. At UBC, newborns were scanned in sleep without pharmacologic sedation.10
Two neuroradiologists (UCSF: A.J.B.; UBC: K.P.) blinded to all clinical information except age scored each conventional MRI scan for acquired injury and developmental brain abnormalities; high reliability has been documented previously.10 Postoperative brain injuries described in this study are limited to newly acquired lesions not evident on the preoperative scan. Brain injury was characterized as stroke, WMI, intraventricular hemorrhage (IVH), or global hypoxic ischemic injury (HI).10,12,19,26 We summarized the severity of the overall brain injuries on each scan as follows: 0 = normal, 1 = minimal injury (minimal WMI and IVH grade 1/228), 2 = stroke (all stroke), and 3 = moderate–severe injury (moderate and severe WMI, IVH 3,28 or global HI injury). The newborn received the highest score for the lesions identified at each scan. This categorization was necessary 1) to avoid comparing newborns with one pattern of injury to a reference group that included “normal” newborns as well as those with other injury patterns (e.g., comparing newborns with WMI to a reference group that included newborns with normal MRI and those with stroke or IVH), and 2) some newborns had multiple injuries on a single scan (see e-Methods).
Diffusion tensor imaging and 3D magnetic resonance spectroscopic imaging analysis.
Diffusion tensor imaging (DTI) provides a sensitive measure of regional brain microstructure and characterizes the 3D spatial distribution of water diffusion. With increasing maturity of the brain, average diffusivity (Dav) decreases by developing neuronal and glial cell membranes that restrict water diffusion.23,29,30 Fractional anisotropy (FA), a measure of the directionality of water diffusion, increases with white matter maturation, particularly with the maturation of the oligodendrocyte lineage and early events of myelination.23,31 Dav and FA were calculated from 7 regions of interest bilaterally using prespecified anatomic references.19 Correct region of interest placements were confirmed by neuroradiologists (A.J.B., K.P.). Magnetic resonance spectroscopic imaging (MRSI) is used to assess metabolic measures of brain development: N-acetylaspartate (NAA) and lactate.19 NAA, an acetylated amino acid found in high concentrations in neurons, increases with advancing cerebral maturity and decreases with neuronal injury.32 Lactate is elevated with disturbances in cerebral energy substrate delivery and oxidative metabolism.33 NAA/choline and lactate/choline were analyzed from the same brain regions as those used for DTI, as described previously.19
Data analysis.
Clinical variables between the 2 centers were compared using the 2-sample Wilcoxon rank-sum (Mann-Whitney) test (continuous data) and the Fisher exact test or χ2 test (categorical variables) as appropriate using Stata 9 (Stata Corporation, College Station, TX). Significance level was defined at a level of <0.05.
We used R for generalized least-squares models to examine the associations between the brain injury score and Dav, FA, NAA/choline, and lactate/choline, accounting for each subject's 2 scans and multiple regions of interest.34 In these models we adjusted for center, gestational age at MRI, and sex. In addition, regression analyses for the postoperative brain injury score included a term for the preoperative injury score. Given the differences in the distribution of congenital heart diagnoses across centers, and because study subjects were imaged in different MRI scanners at each center, an interaction term for site (UCSF or UBC) by region of interest was included in all analyses of the MRI data. The interaction term for site by region of interest allows for the MRSI and DTI values in each region to vary by site. A log-transformed outcome variable was used in all regressions to examine percent differences in the quantitative MRI measures. Using comparable models, we examined the association of select clinical variables, previously identified as risk factors for brain injury, with BIS: resuscitation score, Score for Neonatal Acute Physiology–Perinatal Extension (SNAP-PE), presence of preoperative hypotension, preoperative lowest saturation level (arterial), balloon atrial septostomy, bypass type, deep hypothermic circulatory arrest time, postoperative lowest mean, diastolic and systolic blood pressure, and days of inotropic support.
RESULTS
Study subjects and clinical condition.
The cohort of 120 term newborns with CHD comprised 71 with transposition of the great arteries (TGA), 36 with single ventricle physiology (SVP), and 13 (UBC n = 4, UCSF n = 9) with other cardiac lesions (aortic coarctation [n = 6], pulmonary atresia [n = 3], tetralogy of Fallot [n = 2], truncus arteriosus [n = 1], and total anomalous pulmonary venous connection [n = 1]). Patient and clinical characteristics of the newborns at each center for the newborns with TGA and SVP are presented in tables e-1 and e-2 in e-Methods. Apart from a few practice pattern variations across centers (e.g., lower rate of cesarean delivery at UBC), differences in clinical variables were minor. The burden of brain injury did not differ across site on either preoperative or postoperative scans (stroke: preoperative p = 0.39, postoperative p = 0.090; WMI: p = 0.17, p = 0.28; IVH: p = 0.42, p = 0.72; BIS: p = 0.51, p = 0.07).
Brain injury severity and its association with brain development.
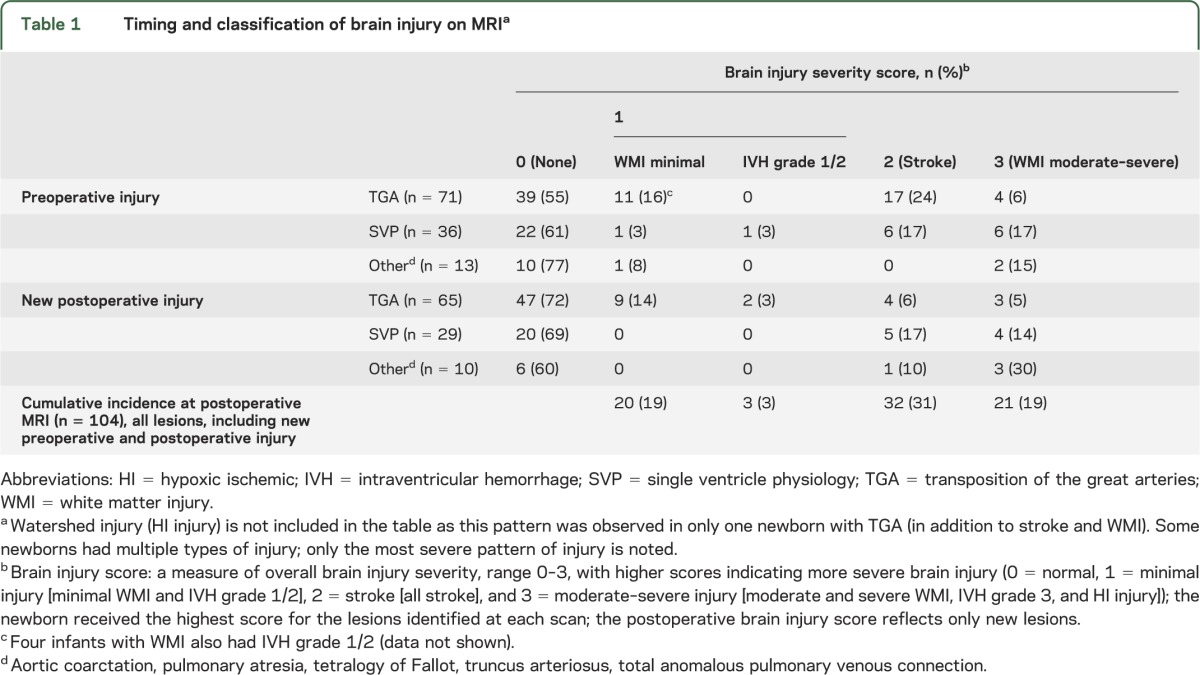
Newly acquired brain injury was common on the preoperative and postoperative MRI scans, with a predominance of stroke and WMI (table 1). Only one infant at UBC had a watershed pattern of injury before heart surgery, characteristic of global hypoxia-ischemia in the term newborn. Grade 3 or 4 IVH was not diagnosed in this cohort. The severity of newly acquired brain injuries did not differ on the preoperative and postoperative scans (p = 0.28).
Table 1.
Timing and classification of brain injury on MRIa
Cross-sectional analyses examining brain injury severity and brain development.
In preoperative cross-sectional analyses, we examined the association of preoperative BIS with preoperative FA, Dav, NAA/choline, and lactate/choline. An increasing preoperative brain injury score was associated with lower white matter FA (ratio was 3.0% lower for each point increase in injury score, p = 0.005) and lower NAA/choline (1.2% lower for each point increase in injury score, p = 0.01) (table 2). In similar postoperative cross-sectional analyses, an increasing severity of newly acquired postoperative brain injuries was associated with lower postoperative white matter FA (0.9% lower for each point increase in injury score, p = 0.04), but not other metrics of brain development (table 3).
Table 2.
Cross-sectional analyses of the preoperative and postoperative brain injury and its association with MRSI and DTI measures of brain development before and after heart surgery, adjusted for center, gestational age at MRI, sex, and region of interest
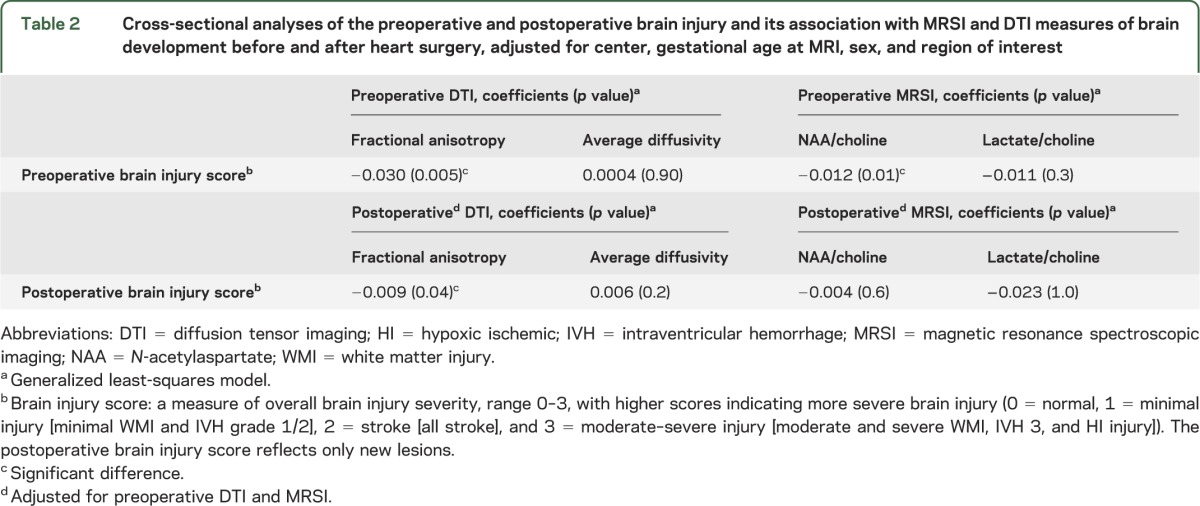
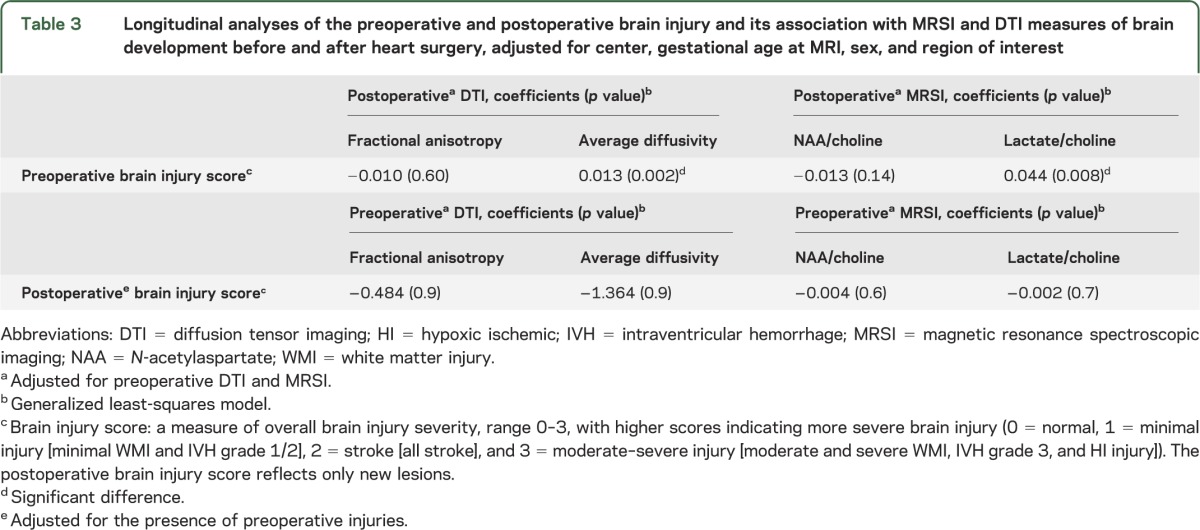
Table 3.
Longitudinal analyses of the preoperative and postoperative brain injury and its association with MRSI and DTI measures of brain development before and after heart surgery, adjusted for center, gestational age at MRI, sex, and region of interest
Longitudinal analyses examining brain injury severity and brain development.
In the preoperative longitudinal analyses, we examined whether preoperative BIS predicted postoperative DTI and MRSI values. An increasing preoperative brain injury score was associated with higher postoperative Dav (p = 0.002) (figure) and higher lactate/choline (p = 0.008). In the postoperative longitudinal analyses, we examined whether postoperative BIS was preceded by differences in preoperative DTI and MRSI values. Postoperative BIS was not predicted by preoperative DTI and MRSI measures (table 2).
Figure. Association between preoperative brain injury score and average diffusivity before and after bypass surgery.
Box plots of average diffusivity before and after bypass surgery are presented by the preoperative brain injury score. This figure illustrates the decrease in average diffusivity (in mm2/sec × 10−6, adjusted for postmenstrual age) between the 2 time points, before and after surgery. Average diffusivity is expected to decrease over time. Note the blunted decrease in average diffusivity in neonates with brain injury, particularly when moderate–severe. MR = magnetic resonance.
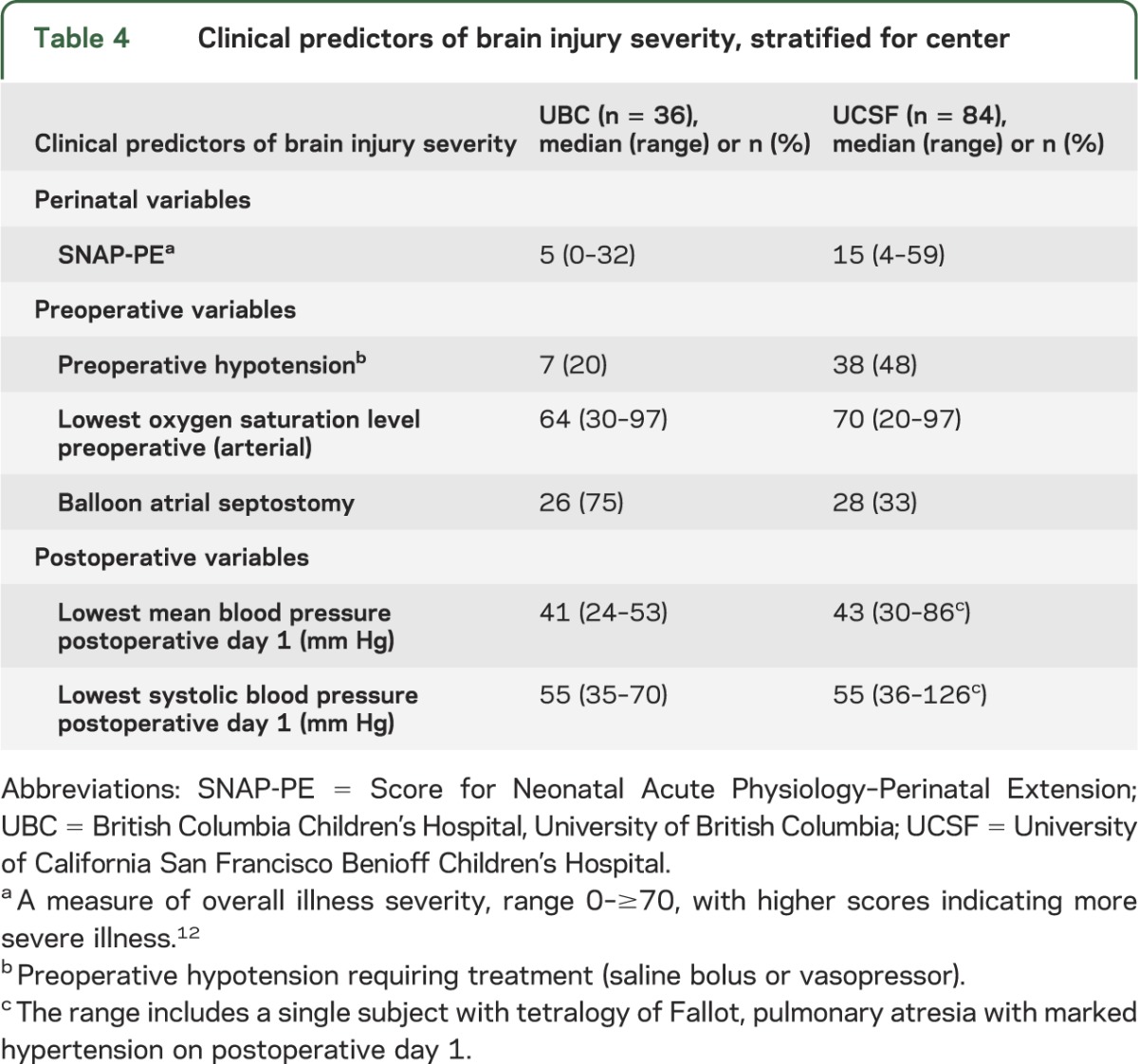
Clinical predictors of brain injury severity.
Clinical predictors of BIS are detailed in table 4. Preoperative BIS was predicted by SNAP-PE (p = 0.03), lower preoperative oxygen saturation (p = 0.002), the presence of preoperative hypotension (p < 0.001), and balloon atrial septostomy (p = 0.002). BIS was lower in newborns with prenatal diagnosis of CHD, but there was no association (p = 0.43). In newborns with TGA, preoperative stroke was highly related to balloon atrial septostomy (p = 0.001) but not site (p = 0.78). The severity of postoperative brain injuries was associated with postoperative lowest mean (p = 0.05) and systolic blood pressure (p = 0.03). The severity of postoperative brain injuries was not predicted by CPB type (p = 0.11), even after stratifying by CHD type (p = 0.20).
Table 4.
Clinical predictors of brain injury severity, stratified for center
DISCUSSION
In the present study, we found that impaired metrics of microstructural and metabolic brain development are associated with increasing BIS, especially preoperatively in term newborns with CHD. Additionally, preoperative brain injuries are associated with further abnormal brain microstructure and metabolism postoperatively, attesting to the longer-term impact of these injuries. Both preoperative and postoperative brain injuries are related to potentially modifiable clinical risk factors.
The predominance of WMI in this cohort of term newborns with CHD, a pattern of brain injury expected in the premature newborn, is consistent with previous findings.11,13–17,35,36 While WMI is identified in 23%–40% of preoperative scans,10,25 it has not yet been reported on fetal imaging.20,37 Within the preoperative cross-sectional analyses, lower FA and NAA/choline, consistent with delayed brain development, were associated with an increasing severity of brain injury. One explanation for this finding, as documented previously in this population and in the preterm neonate, is that focal brain injuries on MRI are associated with more widespread brain abnormalities detected with DTI and MRSI (see reference 21 for review). Yet abnormalities in brain development (decreased total brain volume and NAA) have been recently documented during the third trimester of gestation in the fetus with CHD without focal brain injury.20 Considering this delayed fetal brain development and the very short postnatal time period, it seems very likely that abnormal brain development measured after birth precedes the acquisition of preoperative brain injury. In contrast, abnormalities in preoperative brain development did not predict new postoperative injuries.
Longitudinal studies in the developing fetus and preterm newborn have described distinct patterns of change over time in quantitative MRI parameters. Theoretical understanding of the physical (i.e., water diffusion) and chemical (i.e., metabolite concentrations) processes influencing these parameters combined with experimental knowledge of certain brain cellular and molecular developmental events offer a simplistic paradigm for assessing brain development with diffusion and spectroscopic MRI. For certain neurodevelopmental processes (e.g., myelination), this concept is both logical and supported by animal studies combining imaging and brain histology. Acquired brain injuries may differentially influence quantitative MRI measurements based on severity (i.e., chronic mild vs acute severe), timing, and mechanism. While many neurodevelopmental processes are not measured by diffusion and spectroscopic imaging (e.g., specific axonal targeting, synapse formation, or maturation of glial/neuronal signaling pathways), this simple paradigm presents an opportunity to discern the complex relationships between brain development and acquired brain injury in a longitudinal study of newborns with CHD.
The relationship between brain immaturity and brain injury has been controversial in the literature and may relate to the different metrics of brain development applied in each study.14,25 Brain immaturity, as reflected in the Total Maturation Index, was previously associated with the risk of preoperative and postoperative brain injury. In our current study, we observed a relationship between preoperative brain immaturity and preoperative brain injury but not postoperative brain injury. These differences may be related to the method for measuring brain development. MRSI and DTI provide dynamic metrics of brain development that exhibit changes with acquired injury and with development in the timescale from the preoperative to postoperative periods.19,38 In contrast, the Total Maturation Index is a qualitative scoring system based on gross morphologic brain features (e.g., presence of fetal structures, gyral development, and myelination) that may not evolve as rapidly. Thus it is possible that the reported association of the Total Maturation Index with postoperative injuries may reflect the observed relationship with preoperative brain immaturity. While these brain metrics may also reflect different aspects of brain development, these modalities each suggest an approximate 4-week lag in brain development measured preoperatively.
The severity of preoperative brain injury was associated with higher postoperative Dav and lactate/choline. This is consistent with our earlier finding of abnormal corticospinal tract maturation from the preoperative to postoperative scans in newborns with CHD and preoperative brain injury,24 as well as with observations in the premature newborn with WMI.21 WMI and preoperative delayed brain maturity were not related in a previous study using diffusion imaging and simplified brain metrics.25 However, diffusion imaging in this study was limited to a subset of that cohort (n = 19), and simplified brain metrics may not be especially sensitive to the mild degree of WMI observed. Taken together, the accumulated data suggest that focal lesions (i.e., stroke and WMI) in the rapidly developing newborn brain have longer-term impacts not limited to the immediate vicinity of the original lesion evident on MRI.
Consistent with previous observations, preoperative and postoperative brain injuries are related to potentially modifiable clinical risk factors. In keeping with some previous studies,12,17,36 preoperative BIS was predicted by SNAP-PE, lower preoperative oxygen saturation, hypotension, and balloon atrial septostomy. These associations were significant for the entire cohort, even though some risk factors, such as balloon atrial septostomy, were specific to the subset of newborns with TGA. Postoperative lowest mean and systolic blood pressure were associated with the severity of brain injuries after bypass surgery.26 Similarly, a strong relationship between postoperative WMI and lowest diastolic blood pressure has been previously reported.16
A recent study performed on adolescents with corrected CHD in the neonatal period demonstrated that white matter abnormalities and volume loss were present on MRI scan at age 14 years.39 These long-term brain abnormalities were also associated with neurodevelopmental deficits at this age.39 Despite improvements in perioperative care, rates of long-term neurodevelopmental deficits in children with CHD have remained stable.2,5 Our findings point to complex relationships between perioperative clinical factors, abnormal brain development, and acquired brain injuries.
While a strength of this study is the dual-center design, providing more practice variability than a single-center cohort, the limited sample size among subgroups of CHD diagnoses prohibited our ability to contrast risk factors between these groups. Region of interest–based analysis of DTI and MRSI can be limited by reliable sampling of voxels between scans and risk of partial averaging. Future advances in MRI acquisition and analysis that allow for automatic segmentation and quantification of DTI and MRSI parameters from the preoperative to postoperative periods may also refine our ability to detect differences related to CHD subgroups and critical illness. Studies addressing the in utero interplay of brain development, brain injury, and clinical factors are under way so that novel brain protection strategies can be considered before birth.
Our findings suggest that in term newborns with CHD, delayed brain maturation is the substrate on which preoperative brain injuries, notably WMI, occur. In contrast, newly acquired postoperative brain injuries may be more strongly influenced by perioperative factors. Our findings point to the need for in utero strategies to promote optimal brain development, as well as the potential for clinical interventions targeting hemodycnamic stability and optimal oxygenation to reduce the burden of preoperative and postoperative brain injuries. The association of delayed brain maturation with the impaired long-term neurodevelopmental outcome in children with CHD undergoing heart surgery remains unclear and is the focus of ongoing studies as these newborns are followed through childhood.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the children and their parents who participated in this study.
GLOSSARY
- BIS
brain injury severity
- CHD
congenital heart disease
- CPB
cardiopulmonary bypass
- Dav
average diffusivity
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- HI
hypoxic ischemic
- IVH
intraventricular hemorrhage
- MRSI
magnetic resonance spectroscopic imaging
- NAA
N-acetylaspartate
- SNAP-PE
Score for Neonatal Acute Physiology–Perinatal Extension
- SVP
single ventricle physiology
- TGA
transposition of the great arteries
- UBC
British Columbia Children's Hospital University of British Columbia
- UCSF
University of California San Francisco Benioff Children's Hospital
- WMI
white matter injury
Footnotes
Editorial, page 204
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Anastasia Dimitropoulos: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis. Patrick S. McQuillen: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding. Viyeka Sethi: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, study supervision. Alisha Moosa: analysis or interpretation of data, acquisition of data. Vann Chau: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Duan Xu: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Rollin Brant: analysis or interpretation of data, statistical analysis. Anthony Azakie: study concept or design, study supervision. Andrew Campbell: study concept or design, acquisition of data, study supervision. A. James Barkovich: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data. Kenneth Poskitt: analysis or interpretation of data, acquisition of data. Steven P. Miller: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision, obtaining funding.
STUDY FUNDING
Supported by the Canadian Institutes of Health Research (MOP93780), the NIH (RO1 NS40117, R01NS063876, and P50 NS35902), a grant (5-M01-RR-01271) from the National Center for Research Resources, and the March of Dimes Foundation (5-FY05-1231, 6-FY2009-303), the American Heart Association (0365018Y), and the Larry L. Hillblom Foundation (2002/3E).
DISCLOSURE
A. Dimitropoulos was supported by the Swiss National Science Foundation, grant 128212. P. McQuillen, V. Sethi, A. Moosa, V. Chau, D. Xu, R. Brant, A. Azakie, A. Campbell, A.J. Barkovich, and K. Poskitt report no disclosures. S. Miller is the Bloorview Children's Hospital Chair in Paediatric Neuroscience (from September 2012), and received support as a Tier 2 Canada Research Chair in Neonatal Neuroscience and a Michael Smith Foundation for Health Research Scholar award (to July 2012). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bellinger DC, Jonas RA, Rappaport LA, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med 1995;332:549–555 [DOI] [PubMed] [Google Scholar]
- 2.Bellinger DC, Wypij D, duDuplessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg 2003;126:1385–1396 [DOI] [PubMed] [Google Scholar]
- 3.Goldberg CS, Bove EL, Devaney EJ, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg 2007;133:880–887 [DOI] [PubMed] [Google Scholar]
- 4.Hovels-Gurich HH, Konrad K, Wiesner M, et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child 2002;87:506–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karl TR, Hall S, Ford G, et al. Arterial switch with full-flow cardiopulmonary bypass and limited circulatory arrest: neurodevelopmental outcome. J Thorac Cardiovasc Surg 2004;127:213–222 [DOI] [PubMed] [Google Scholar]
- 6.Limperopoulos C, Majnemer A, Shevell MI, et al. Functional limitations in young children with congenital heart defects after cardiac surgery. Pediatrics 2001;108:1325–1331 [DOI] [PubMed] [Google Scholar]
- 7.Snookes SH, Gunn JK, Eldridge BJ, et al. A systematic review of motor and cognitive outcomes after early surgery for congenital heart disease. Pediatrics 2010;125:e818–827 [DOI] [PubMed] [Google Scholar]
- 8.Gaynor JW, Wernovsky G, Jarvik GP, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg 2007;133:1344–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. J Pediatr 2000;137:638–645 [DOI] [PubMed] [Google Scholar]
- 10.Block AJ, McQuillen PS, Chau V, et al. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J Thorac Cardiovasc Surg 2010;140:550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation 2002;106:I109–I114 [PubMed] [Google Scholar]
- 12.McQuillen PS, Hamrick SE, Perez MJ, et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation 2006;113:280–285 [DOI] [PubMed] [Google Scholar]
- 13.Miller SP, McQuillen PS, Vigneron DB, et al. Preoperative brain injury in newborns with transposition of the great arteries. Ann Thorac Surg 2004;77:1698–1706 [DOI] [PubMed] [Google Scholar]
- 14.Andropoulos DB, Hunter JV, Nelson DP, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg 2010;139:543–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beca J, Gunn J, Coleman L, et al. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J Am Coll Cardiol 2009;53:1807–1811 [DOI] [PubMed] [Google Scholar]
- 16.Galli KK, Zimmerman RA, Jarvik GP, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg 2004;127:692–704 [DOI] [PubMed] [Google Scholar]
- 17.Petit CJ, Rome JJ, Wernovsky G, et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation 2009;119:709–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg 2009;137:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med 2007;357:1928–1938 [DOI] [PubMed] [Google Scholar]
- 20.Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 2010;121:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci 2009;32:496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huppi PS, Murphy B, Maier SE, et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics 2001;107:455–460 [DOI] [PubMed] [Google Scholar]
- 23.Miller SP, Vigneron DB, Henry RG, et al. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imaging 2002;16:621–632 [DOI] [PubMed] [Google Scholar]
- 24.Partridge SC, Vigneron DB, Charlton NN, et al. Pyramidal tract maturation after brain injury in newborns with heart disease. Ann Neurol 2006;59:640–651 [DOI] [PubMed] [Google Scholar]
- 25.Ortinau C, Beca J, Lambeth J, et al. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. J Thorac Cardiovasc Surg 2012;143:1264–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McQuillen PS, Barkovich AJ, Hamrick SE, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke 2007;38:736–741 [DOI] [PubMed] [Google Scholar]
- 27.Dumoulin CLRK, Piel JE, Rossi CJ, Giaquinto RO, Watkins RD, et al. Magnetic resonance imaging compatible neonate incubator. Magn Reson Eng 2002;15:117–128 [Google Scholar]
- 28.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529–534 [DOI] [PubMed] [Google Scholar]
- 29.Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 2002;15:435–455 [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee P, Miller JH, Shimony JS, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiology 2002;23:1445–1456 [PMC free article] [PubMed] [Google Scholar]
- 31.Drobyshevsky A, Song SK, Gamkrelidze G, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci 2005;25:5988–5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Huppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 2002;48:949–958 [DOI] [PubMed] [Google Scholar]
- 33.Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 2004;305:99–103 [DOI] [PubMed] [Google Scholar]
- 34.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988;44:1049–1060 [PubMed] [Google Scholar]
- 35.Chen J, Zimmerman RA, Jarvik GP, et al. Perioperative stroke in infants undergoing open heart operations for congenital heart disease. Ann Thorac Surg 2009;88:823–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukherjee D, Lindsay M, Zhang Y, et al. Analysis of 8681 neonates with transposition of the great arteries: outcomes with and without Rashkind balloon atrial septostomy. Cardiol Young 2010;20:373–380 [DOI] [PubMed] [Google Scholar]
- 37.Berman JI, Hamrick SE, McQuillen PS, et al. Diffusion-weighted imaging in fetuses with severe congenital heart defects. AJNR. AJNR Am J Neuroradiol 2011;32:E21–E22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barkovich AJ, Miller SP, Bartha A, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol 2006;27:533–547 [PMC free article] [PubMed] [Google Scholar]
- 39.von Rhein M, Scheer I, Loenneker T, Huber R, Knirsch W, Latal B. Structural brain lesions in adolescents with congenital heart disease. J Pediatr 2011;158:984–989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



