Abstract
Objective:
The study goal was to assess the benefits and potential limitations in the use of ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles in the MRI diagnosis of CNS inflammatory diseases and primary CNS lymphoma.
Methods:
Twenty patients with presumptive or known CNS lesions underwent MRI study. Eighteen patients received both gadolinium-based contrast agents (GBCAs) and 1 of 2 USPIO contrast agents (ferumoxytol and ferumoxtran-10) 24 hours apart, which allowed direct comparative analysis. The remaining 2 patients had only USPIO-enhanced MRI because of a renal contraindication to GBCA. Conventional T1- and T2-weighted MRI were acquired before and after contrast administration in all patients, and perfusion MRI for relative cerebral blood volume (rCBV) assessment was obtained in all 9 patients receiving ferumoxytol.
Results:
USPIO-enhanced MRI showed an equal number of enhancing brain lesions in 9 of 18 patients (50%), more enhancing lesions in 2 of 18 patients (11%), and fewer enhancing lesions in 3 of 18 patients (17%) compared with GBCA-enhanced MRI. Four of 18 patients (22%) showed no MRI enhancement. Dynamic susceptibility-weighted contrast-enhanced perfusion MRI using ferumoxytol showed low rCBV (ratio <1.0) in 3 cases of demyelination or inflammation, modestly elevated rCBV in 5 cases of CNS lymphoma or lymphoproliferative disorder (range: 1.3–4.1), and no measurable disease in one case.
Conclusions:
This study showed that USPIO-enhanced brain MRI can be useful in the diagnosis of CNS inflammatory disorders and lymphoma, and is also useful for patients with renal compromise at risk of nephrogenic systemic fibrosis who are unable to receive GBCA.
CNS lymphoma (CNSL) presents a diagnostic challenge.1–9 Brain MRI typically reveals a single nodule or multiple nodules in the deep parenchyma that enhance with IV contrast.5,9–11 Unfortunately, CNSL mimics other diseases on routine neuroimaging, including CNS metastasis of systemic cancer, glioma, and demyelination, often leading to aggressive surgical approaches and/or delay in tissue diagnosis and treatment.12–14 Obtaining a tissue diagnosis in the least-invasive manner possible is critically important because CNSL is typically chemosensitive, infiltrative, and often in a deep location within the brain parenchyma.15,16
Use of a gadolinium-based contrast agent (GBCA) enables contrast-enhanced T1-weighted MRI as the GBCA permeates an incompetent blood-brain barrier. Unlike GBCA, ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles act as blood pool agents immediately after administration, but they are later taken up by inflammatory cells (e.g., macrophages), acting as an intracellular contrast agent, with abnormal parenchymal enhancement that peaks 24 hours after administration.17 In this study, we assessed both the benefits and limitations regarding the use of USPIO nanoparticles as contrast agents for anatomical and perfusion MRI in the diagnosis of CNS inflammatory diseases and primary CNSL. We propose that these agents may improve MRI visualization of CNSL and CNS inflammatory disease and help to establish the most informative target for biopsy. Unlike GBCA, USPIO may also be used safely in patients with renal failure.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was registered with and approved by the Oregon Health & Science University Institutional Review Board (eIRB no. 1562). The study was sponsored by the NIH, and all participants provided written informed consent.
Study population.
Twenty patients with CNSL or CNS inflammatory disease were enrolled as part of an ongoing prospective study. Between November 2002 and May 2011, eligible patients with suspected CNS tumors, demyelinating disease, or any CNS lesion with an inflammatory component underwent MRI after USPIO administration. Diagnoses were confirmed histologically (n = 19) or by characteristic clinical and radiographic findings and subsequent improvement on surveillance (n = 1). Exclusion criteria have been reported previously.18,19 Patients who were HIV positive receiving combination antiretroviral therapy were ineligible because of the potential for pharmacokinetic interactions with USPIO agents.
MRI examination.
All MRI scans were performed on a 3-tesla whole-body MRI system (TIM TRIO; Siemens, Erlangen, Germany) with a body radiofrequency coil transmitter and a 12-channel matrix head coil signal receiver. Specific MRI acquisition parameters are provided in table e-1 on the Neurology® Web site at www.neurology.org. The imaging protocol consisted of 3 consecutive days of MRI scans. On the first day, T1- and T2-weighted pre- and postcontrast sequences were acquired using gadoteridol gadolinium (III) chelate (ProHance; Bracco Diagnostics Inc., Princeton, NJ), with the exception of 2 subjects with renal insufficiency (estimated glomerular filtration rate <30 mL/min/1.73 m2) who did not receive gadoteridol and instead underwent only a 2-day MRI protocol with USPIO as the contrast agent. Gadoteridol was injected as an IV bolus at a dose of 0.1 mmol/kg body weight. On the following day, the same MRI sequences were obtained using ferumoxytol (n = 9) (AMAG Pharmaceuticals, Inc., Cambridge, MA) or ferumoxtran-10 (n = 11) (AMAG Pharmaceuticals, Inc.). Ferumoxtran-10 preceded ferumoxytol, with similar MRI properties. Manufacturing of ferumoxytran-10 was discontinued when ferumoxytol became available. Ferumoxtran-10 (2.6 mg/kg) was diluted in 90 mL of saline and infused at 4 mL/min. All contrast dosages have been used in previous human and preclinical studies.
Patients who received ferumoxytol also underwent dynamic susceptibility-weighted contrast-enhanced (DSC) perfusion MRI (n = 9) because ferumoxytol can be administered via rapid IV bolus injection. The 11 patients who received ferumoxtran-10 were unable to have perfusion-weighted imaging (PWI) since this contrast agent cannot be given via rapid IV bolus because of the risk of anaphylactoid reactions. The total ferumoxytol dose (510 mg) was diluted in 17 mL of saline. First, 75 mg of ferumoxytol was administered as an IV bolus during DSC-MRI acquisition. Then, patients received the remainder of the dose (435 mg) over 15 minutes. After USPIO administration, all patients were monitored closely for 2 hours. On the third day, at least 22 hours after USPIO agent administration, T1- and T2-weighted imaging sequences were repeated to detect delayed USPIO-induced signal changes. All patients were followed for 1 month to note possible adverse reactions.
Image analysis.
All images from each patient were evaluated in a matched-pair fashion by the same neuroradiologist. Image assessment consisted of the following steps: 1) quantification of the total number of enhancing brain lesions on the T1-weighted gadoteridol-enhanced scan compared with the noncontrast T1-weighted scan; 2) quantification of brain lesions on T1- and T2-weighted scans 24 hours after USPIO administration compared with noncontrast T1- and T2-weighted scans; and 3) evaluation of postcontrast USPIO and GBCA scans for leptomeningeal disease.
All first-pass DSC-MRI data were processed using Lupe (Lund University Perfusion Evaluation, Sweden) perfusion image analysis software and the simple singular value decomposition–based calculation method as described by Ostergaard et al.,20 uncorrected for contrast leakage. Arterial input function was determined from the middle cerebral artery contralateral to the enhancing lesion. Color-coded relative cerebral blood volume (rCBV) maps were created on a voxel-wise basis. Normal white matter within the contralateral hemisphere was used as the internal reference standard; rCBV values were calculated by dividing the maximal rCBV in the enhancing lesion by that of contralateral normal-appearing white matter.
RESULTS
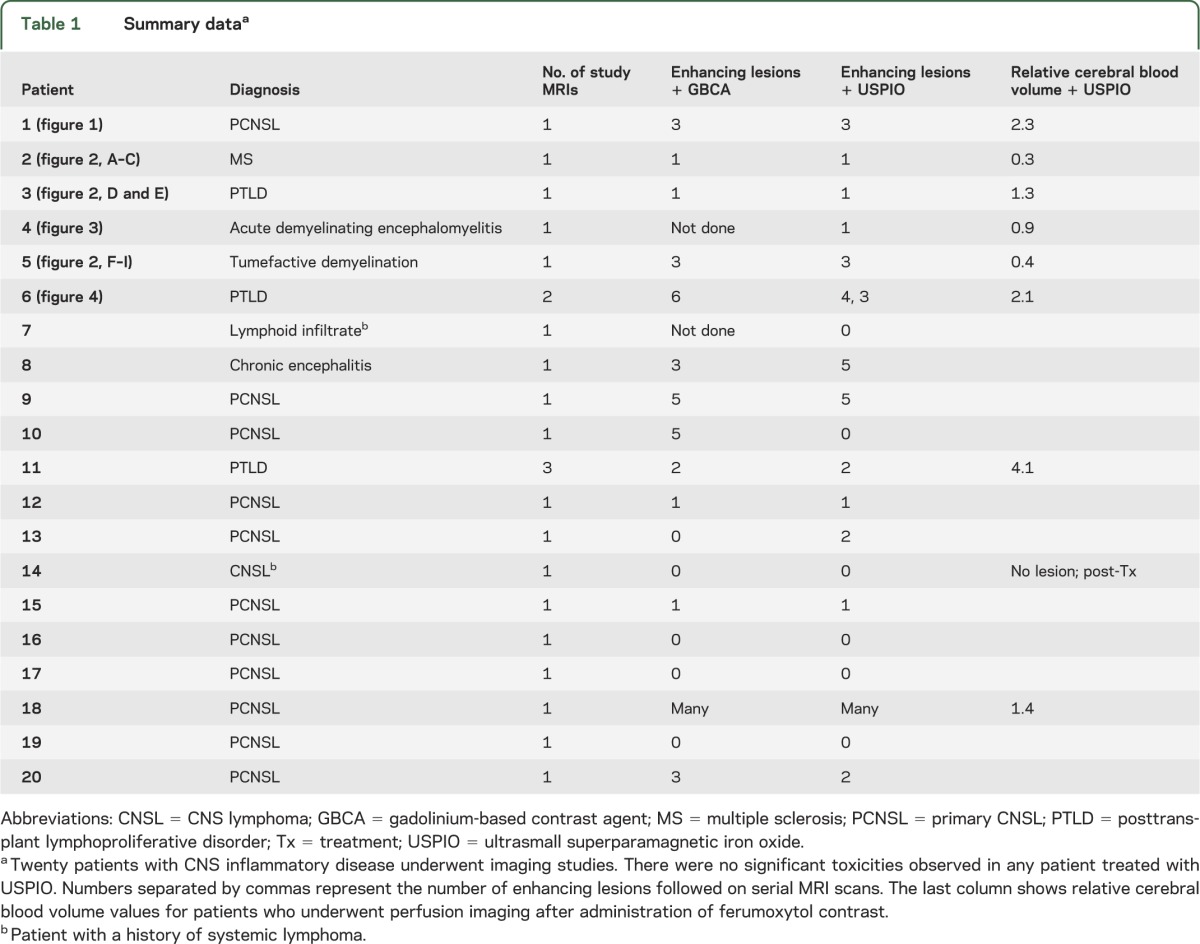
The summary data (table 1) show the comparative results of MRI with USPIO and with GBCA. Two patients with renal insufficiency were given only USPIO-based contrast because of the known risk of nephrogenic systemic fibrosis (NSF) with GBCA.17 Nine of 18 patients (50%) who received both contrast agents showed equal numbers of enhancing lesions on T1-weighted postcontrast images when comparing GBCA- with USPIO-based MRI contrast. Two of 18 patients (11%) who received USPIO-based contrast showed additional enhancement not visible with GBCA. Three of 18 patients (17%) demonstrated areas of enhancement when given GBCA that were not apparent on USPIO-enhanced scans. Four of 18 patients (22%) showed no enhancement with either agent.
Table 1.
Summary dataa
Postcontrast MRIs from 15 patients with CNS lymphoid malignancies, either posttransplant lymphoproliferative disorder (PTLD) or CNSL, were also reviewed for leptomeningeal disease. Two patients showed localized leptomeningeal enhancement on post-USPIO imaging around the primary tumor site, but only one of these showed comparable leptomeningeal enhancement with GBCA. We did not see diffuse leptomeningeal enhancement in any case.
Nine patients who received ferumoxytol were able to undergo perfusion MRI, and rCBV values were determined. Eight of those 9 patients had enhancing lesions amenable for rCBV calculation, whereas one posttherapy patient did not. The rCBV values in CNSL and PTLD were variable and ranged from 1.3 to 4.1, whereas demyelinating lesions demonstrated low rCBV values less than 1.0.
Several observations highlight the potential use of USPIO-based contrast studies.
Scenario 1: Improved targeting of surgical biopsy.
Case 1.
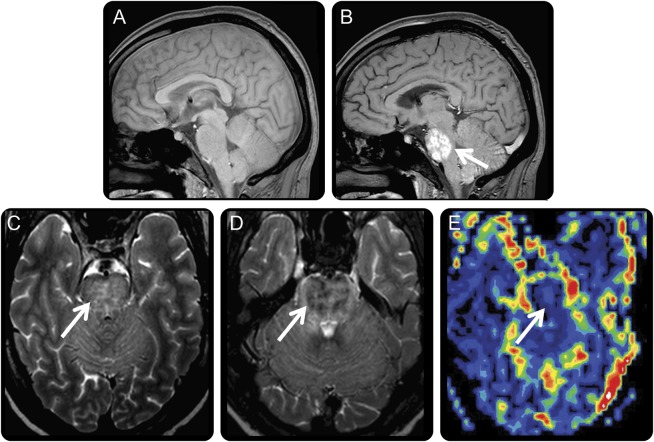
As an example of intense iron labeling using USPIO-based contrast compared with GBCA, we evaluated a 59-year-old woman who presented with progressive memory loss and confusion (table 1, patient 1). An initial MRI with GBCA showed multifocal, avidly enhancing, deep white matter lesions consistent with demyelinating disease or primary CNSL (figure 1B). Nondiagnostic cytologic studies prompted USPIO-based MRI. The T1-weighted images showed similar patterns of contrast enhancement between GBCA- and USPIO-enhanced images (figure 1, B and C). The T2-weighted images (figure 1, D and E) highlight key differences between USPIO-based contrast and GBCA. After USPIO administration, iron uptake by inflammatory cells appears profoundly hypointense in the centers of lesions (figure 1E) that are otherwise hyperintense on noncontrasted T2-weighted MRI (figure 1D). The patient underwent stereotactic needle biopsy of the right frontal T2-hypointense lesion seen on the postferumoxytol scan, which confirmed the suspected diagnosis of diffuse large B-cell lymphoma.
Figure 1. MRIs of patient with primary CNSL.
MRIs from case 1. (A) T1-weighted MRI without contrast agent. (B) T1-weighted MRI immediately after administration of GBCA shows multifocal, enhancing, deep white matter lesions (arrows) suggesting primary CNSL. Note, however, that the relatively minimal mass effect for lesion extent and incomplete enhancing rims are more typical of demyelinating disease. (C) T1-weighted MRI 24 hours after administration of USPIO contrast shows more extensive enhancement because of phagocytic cellular uptake (arrows) than the GBCA-enhanced scan. (D) T2-weighted MRI without contrast shows multifocal confluent areas of T2 hyperintensity in white matter. (E) T2-weighted MRI 24 hours after ferumoxytol shows multiple areas of intense USPIO uptake (arrows). CNSL = CNS lymphoma; GBCA = gadolinium-based contrast agent; USPIO = ultrasmall superparamagnetic iron oxide.
Case 2.
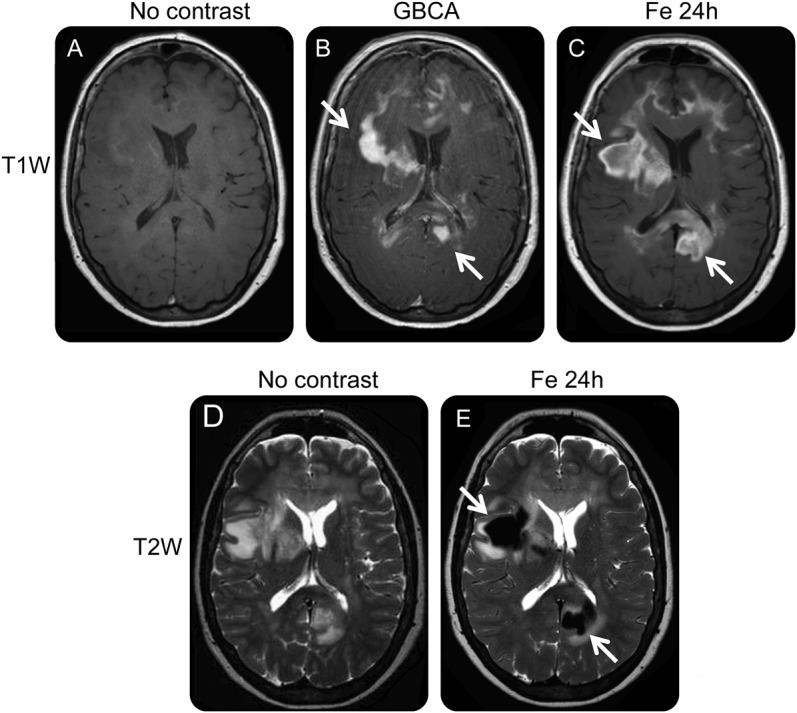
A 30-year-old woman presenting with several weeks of fatigue and confusion was referred with a provisional diagnosis of CNSL (table 1, patient 2). Neurologic examination showed gait instability. MRI showed an area of T2 signal abnormality and enhancement in the right deep frontal white matter and basal ganglia extending into the hypothalamus on post-GBCA T1-weighted images (figure 2, A and B). After nondiagnostic lumbar puncture, a stereotactic needle biopsy was performed, targeting the enhancement on the post-GBCA T1-weighted scan. Histologic evaluation showed only gliosis. The patient subsequently underwent USPIO-enhanced MRI. Ferumoxytol-enhanced T1-weighted images showed a similar enhancement pattern compared with GBCA. However, the USPIO images revealed a hypointense focus on T2-weighted images that was not visible on the GBCA-enhanced scan (figure 2C). She underwent repeat stereotactic needle biopsy targeting the area of T2-weighted hypointense signal, which revealed demyelination with intact axons, suggesting a diagnosis of multiple sclerosis.
Figure 2. USPIO imaging of CNS inflammation.
(A–C) MRIs from case 2. (A) T1-weighted MRI shows GBCA enhancement in the inferior right basal ganglia and hypothalamus (arrow). (B) T2-weighted MRI without contrast agent shows the first biopsy tract (arrow) targeting regions of GBCA enhancement on T1-weighted images. (C) T2-weighted MRI, 24 hours after administration of ferumoxytol, shows a markedly hypointense area suggesting the area of maximal iron uptake (arrow), which was the target of a second biopsy revealing demyelination. (D and E) MRIs from case 3, a patient with PTLD. (D) Pretreatment images: T1-weighted MRI (i) and T2-weighted MRI (ii) 24 hours after administration of ferumoxytol. Arrows show areas of intense iron uptake on both sequences. (E) Posttreatment images: T1-weighted MRI (i) and T2-weighted MRI (ii) 24 hours after administration of ferumoxytol show resolved enhancement and mass effect. (F–I) MRIs from case 4. (F) Precontrast axial T1-weighted MRI shows mild T1 hypointensity (arrow) and mass effect in the left occipital lobe. (G) Post-GBCA T1-weighted axial MRI shows patchy marginal nodular enhancement (arrow) raising concern for potential high-grade malignancy. (H) Precontrast T2-weighted axial MRI shows vasogenic edema consistent with high-grade glioma. (I) rCBV map obtained after administration of USPIO contrast shows low rCBV (arrow) in the lesion that would be atypical for high-grade glioma. Biopsy confirmed diagnosis of tumefactive demyelination. GBCA = gadolinium-based contrast agent; PTLD = posttransplant lymphoproliferative disorder; rCBV = relative cerebral blood volume; USPIO = ultrasmall superparamagnetic iron oxide.
Scenario 2: Avoidance of NSF.
NSF is an idiopathic and potentially fatal complication that may occur after gadolinium administration in patients with renal insufficiency. The proposed mechanism for development of such fibrosis involves decreased renal clearance of GBCA resulting in deposition in the skin and other organs. The end result is multiorgan fibrosis causing skin contractures, intense pruritus, and potential cardiac and respiratory failure.17 The risk of NSF led the US Food and Drug Administration to recommend that no GBCA be given to any patient with an estimated glomerular filtration rate less than 30 mL/min/1.73 m2, or to any renal transplant patient—a policy that is strictly adhered to by most practicing radiologists and adopted by the American College of Radiology.21 Therefore, USPIOs, which are already used as iron replacement in dialysis patients, are an attractive alternative to GBCAs in such patients.
Case 3.
To avoid the potential consequences of NSF, we enrolled a 67-year-old renal transplant patient with suspected CNS PTLD in the USPIO-based MRI protocol (table 1, patient 3). Images acquired before treatment (figure 2D) showed a left-frontal enhancing mass consistent with PTLD, which was confirmed with biopsy. She had previously undergone MRI with GBCA (at another institution, despite her history of renal transplant) and this showed a similar appearance; however, the subsequent use of USPIO-based contrast enabled her to have follow-up without the risk of NSF. USPIO-based MRI, repeated 1 year after the biopsy (figure 2E), showed an excellent response to monotherapy with rituximab.
Scenario 3: Correlating cerebral blood volume and CNS inflammation.
Case 4.
A 23-year-old woman presented with a right homonymous hemianopsia and a rapidly expanding left occipital lesion on standard MRI with GBCA (table 1, patient 5; figure 2G). A craniotomy was planned for resection of the lesion. Before surgery, she was evaluated with USPIO-based perfusion imaging and this showed low blood volume (rCBV = 0.4; figure 2I). Because the GBCA-based imaging suggested that the enhancing mass was high-grade neoplasm but low blood volume was noted upon perfusion imaging, the decision was made to instead perform a needle biopsy. The biopsy revealed destruction of myelin with intact axons, and CSF studies showed oligoclonal bands with an increased immunoglobulin G synthesis rate. The neurology team then managed her tumefactive demyelination.
Case 5.
A 30-year-old man with end-stage renal disease due to uncontrolled type 1 diabetes (serum creatinine = 3.0 mg/dL) presented with episodic headaches and altered mental status, but he was neurologically intact at presentation (table 1, patient 4). Noncontrast MRI revealed an expansile lesion in the pons. GBCA MRI was contraindicated because of the risk of NSF. He underwent imaging before and 24 hours after the administration of ferumoxytol (figure 3, A and B); USPIO contrast images showed intense USPIO uptake in the pons, which correlated with hyperintense T2-weighted signal abnormality on precontrast imaging (figure 3C). T2-weighted images after USPIO contrast showed hypointense foci (figure 3D). USPIO-based perfusion MRI demonstrated low blood volume (rCBV = 0.9) (figure 3E), which we interpreted to favor non-neoplastic pathology. Rather than proceed with a potentially morbid biopsy, follow-up MRI at 6 weeks and 6 months were obtained, and showed that the lesion had regressed. A presumptive diagnosis of acute demyelinating encephalomyelitis was made by neurologic consultants.
Figure 3. MRIs of patient with headaches and altered mental status unable to receive GBCA because of renal failure.
MRIs from case 5. (A) Precontrast T1 imaging shows mild pontine expansion and slightly decreased T1 signal throughout. (B) T1-weighted sagittal MRI 24 hours post-USPIO shows intense iron uptake in the pons (arrow). (C) An axial T2-weighted precontrast image shows patchy nonspecific increased T2 signal within the pons (arrow). (D) An image 24 hours post-USPIO shows patchy hypointensities in the pons (arrow) correlating with iron uptake. (E) USPIO-based DSC perfusion imaging shows low rCBV centrally within the pons (arrow) corresponding to the area of enhancing abnormality that is similar to normal-appearing white matter. DSC = dynamic susceptibility-weighted contrast-enhanced; GBCA = gadolinium-based contrast agent; rCBV = relative cerebral blood volume; USPIO = ultrasmall superparamagnetic iron oxide.
Scenario 4: Potential limitations in imaging after USPIO administration—CNSL and venous thromboembolism.
Case 6.
A 63-year-old woman underwent USPIO-based MRI as part of evaluation of brainstem PTLD (table 1, patient 6). T2-weighted images 24 hours after USPIO administration showed a markedly hypointense signal suggesting intense iron uptake by lymphoid cells (figure 4B) and the diagnosis was confirmed with biopsy. The patient tolerated PTLD treatment well and was neurologically stable. One month after treatment, she developed a deep vein thrombosis; MRI (without contrast) was performed before initiation of anticoagulation. T2-weighted MRI showed focal hypointensity (figure 4C) concerning for brainstem hemorrhage. This resulted in placement of an IV filter instead of anticoagulation therapy. However, it is important to recognize that acute brainstem hemorrhage was highly unlikely given the patient's stable neurologic examination. The findings were thought to represent residual USPIO contrast, given relatively diminished similar pattern of signal abnormality to prior ferumoxytol-enhanced MRI with overall decreased mass effect. Noncontrast head CT performed within 24 hours confirmed no hyperdensity in the brainstem to suggest recent hemorrhage. Surveillance MRI scans (without contrast) at 3 months showed continued decrease in T2 signal hypointensity (figure 4D).
Figure 4. T2-weighted MRIs of patient with PTLD showing prolonged duration of iron labeling.

MRIs from case 6. (A) Pretreatment image without contrast shows increased T2 signal abnormality and mild mass effect in the pons and right cerebellum. (B) T2-weighted image 24 hours after initial ferumoxytol imaging shows intense iron uptake (arrow). Reprinted with permission from the American Journal of Roentgenology.19 (C) After 1 month, the patient underwent noncontrast MRI after diagnosis of deep vein thrombosis. T2 hypointense areas (arrow) were initially misinterpreted as asymptomatic brainstem hemorrhage but likely represented residual iron-labeled inflammatory cells because a noncontrast CT showed no hyperdensity in the brainstem (not shown). (D) Punctate T2 hypointense signal remained (arrow), but was diminished 3 months after ferumoxytol administration. PTLD = posttransplant lymphoproliferative disorder.
DISCUSSION
USPIO-enhanced MRI offers a number of potential advantages over conventional GBCA-enhanced MRI in the diagnostic workup of CNSL and inflammatory intracranial neoplastic mimics. First, USPIO-enhanced MRI may offer different or additional areas of enhancement that may offer improved surgical targeting opportunities. This may be particularly true for T2-weighted enhanced scans with USPIO, which may offer an alternative sampling site for biopsy to conventional postcontrast T1-weighted scans with GBCA. Second, ferumoxytol-based DSC PWI may offer discrimination of inflammatory diseases such as demyelination from neoplastic disorders. Third, USPIO can be safely used in patients with renal failure who cannot receive GBCA.
Based on our prior work, immediate post-USPIO MRI shows intravascular, not abnormal parenchymal enhancement.22–24 The second day of imaging at 24 hours is a relatively intracellular-weighted (and/or interstitial-weighted) rather than intravascular-weighted MRI given that ferumoxytol has an intravascular half-life of approximately 14 to 20 hours.25 This proved helpful in one patient in this series who underwent a second diagnostic biopsy in which a different surgical target was selected based on the T2-weighted USPIO-enhanced MRI.
Preclinical studies in animal models have shown via histologic analysis that hypointense foci on delayed (24 hours) T2-weighted MRI in the brain correspond with iron staining of phagocytic cells (such as macrophages), and efforts to confirm these findings in human biopsy specimens are ongoing.25,26 Abnormal parenchymal enhancement with GBCA, by contrast, is due to increased vascular permeability from blood-brain barrier breakdown.25 Future studies are needed to confirm the localization of ferumoxytol in human tissues. Determining whether localization is primarily within inflammatory phagocytic cells (intracellular) or interstitial (due to slower extravascular leakage compared with GBCA) remains an ongoing project.
DSC-based perfusion studies in this series were based only on ferumoxytol because it can be given via IV bolus injection without the risk of anaphylactoid reactions due to mast cell degranulation that were relatively common with earlier iron oxide nanoparticle preparations such as ferumoxtran-10. In the literature, DSC-based PWI of CNSL has shown variable results using GBCA, with values overlapping with respect to malignant glioma in published studies.14 Our results are consistent with reported findings in which demyelinating or inflammatory lesions show low rCBV27 whereas CNSL demonstrates modestly elevated rCBV values that do not permit reliable differentiation from high-grade glioma.12 Our experience with ferumoxytol-based DSC perfusion in malignant gliomas has shown higher reliability than GBCA-based DSC PWI. USPIO-based DSC perfusion requires neither expensive leakage correlation software algorithms nor preloading with additional doses of USPIO, as is necessary with GBCA.24 Our limited number of PWIs in CNSL cases in this series do not allow generalization, but will be a subject of future study.
Two subjects in this series underwent USPIO-enhanced MRI because they were unable to receive GBCA at our institution due to risk of NSF. This situation may be particularly problematic for renal transplant patients, who are at risk of both NSF and PTLD, given immunosuppression. Ferumoxytol-enhanced MRI offers a diagnostic opportunity without the attendant risks.
Limitations of USPIO-enhanced MRI include the following: 1) requirement for at least 2 consecutive days of imaging to obtain anatomical “enhanced” scans similar to GBCA-enhanced MRI, 2) ferumoxytol-based DSC perfusion MRI, similar to GBCA-based DSC perfusion MRI, remains unable to discriminate between malignant lymphoid and glial neoplasms, and 3) potential for USPIO enhancement to persist up to several months after injection may result in confusion for hemorrhage. However, increased use of USPIO-based imaging agents over time will surely result in awareness of this possibility. Likely because of this known uncommon effect, the package insert for ferumoxytol warns prescribers that the compound can “alter MRI studies” for up to 3 months.28 Nevertheless, the US federal government continues to fund multiple clinical studies investigating the MRI-based applications of ferumoxytol.29 This labeling unfortunately creates a perception that ferumoxytol administration is contraindicated when, in fact, an MRI may be desirable. Clinicians and radiologists should be made aware of any prior USPIO administration, however, in order to allow proper interpretation of MRI studies. Noncontrast head CT may also resolve any question of hemorrhage, as in the case presented here.
Our experience highlights the benefits and limitations of the use of USPIO-based MRI, which provides relevant information in CNS inflammation and neoplastic diseases.24,30 Our preliminary findings of USPIO-enhanced imaging in CNSL require further evaluation in studies involving systemic lymphoma. Importantly, USPIO imaging provides a safe alternative for MRI in patients with renal failure. Based on these findings, the US Food and Drug Administration label should encourage, not deter, further use of USPIO agents in the setting of MRI.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Aliana Culp for her assistance with word processing for the manuscript.
GLOSSARY
- CNSL
CNS lymphoma
- DSC
dynamic susceptibility-weighted contrast-enhanced
- GBCA
gadolinium-based contrast agent
- NSF
nephrogenic systemic fibrosis
- PCNSL
primary CNS lymphoma
- PTLD
posttransplant lymphoproliferative disorder
- PWI
perfusion-weighted imaging
- rCBV
relative cerebral blood volume
- USPIO
ultrasmall superparamagnetic iron oxide
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
B. Farrell and B. Hamilton: critical revision of the manuscript for important intellectual content, analysis and interpretation. E. Dósa, E. Rimely, M. Nasseri, and S. Gahramanov: analysis and interpretation, acquisition of data. C. Lacy: acquisition of data. E. Frenkel, N. Doolittle, and P. Jacobs: critical revision of the manuscript for important intellectual content. E. Neuwelt: critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
This work was supported by a Veterans Administration merit review grant and by NIH grants NS53468, NS44687, and CA137488, in part with federal funds from the National Cancer Institute, NIH, under contract no. HHSN261200800001E, and by the Walter S. and Lucienne Driskill Foundation to E.A.N.
DISCLOSURE
B. Farrell, B. Hamilton, E. Dósa, E. Rimely, M. Nasseri, S. Gahramanov, C. Lacy, E. Frenkel, N. Doolittle, and P. Jacobs report no disclosures. E. Neuwelt's studies involving ferumoxytol were entirely funded by Veterans Administration and NIH research grants, with some of the ferumoxytol USPIO nanoparticles donated by AMAG Pharmaceuticals. In light of the results from this study, and subsequent to completion of the manuscript, Oregon Health & Science University has received a sponsored research agreement from AMAG to conduct clinical trials of MRI with ferumoxytol. None of the authors has financial interest in this agent or in its developer, AMAG. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Abla O, Weitzman S, Blay JY, et al. Primary CNSL in children and adolescents: a descriptive analysis from the International Primary CNSL Collaborative Group (IPCG). Clin Cancer Res 2011;17:346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNSL. J Clin Oncol 2005;23:5034–5043 [DOI] [PubMed] [Google Scholar]
- 3.Blay JY. Primary cerebral non-Hodgkin lymphoma in non-immunocompromised subjects [in French]. Bull Cancer 1997;84:976–980 [PubMed] [Google Scholar]
- 4.Cavaliere R, Petroni G, Lopes MB, Schiff D. Primary central nervous system post-transplantation lymphoproliferative disorder: an International Primary Central Nervous System Lymphoma Collaborative Group Report. Cancer 2010;116:863–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doolittle ND, Abrey LE, Shenkier TN, et al. Brain parenchyma involvement as isolated central nervous system relapse of systemic non-Hodgkin lymphoma: an International Primary CNSL Collaborative Group Report. Blood 2008;111:1085–1093 [DOI] [PubMed] [Google Scholar]
- 6.Fine HA, Mayer RJ. Primary central nervous system lymphoma. Ann Intern Med 1993;119:1093–1104 [DOI] [PubMed] [Google Scholar]
- 7.Herrlinger U, Schabet M, Clemens M, et al. Clinical presentation and therapeutic outcome in 26 patients with primary CNSL. Acta Neurol Scand 1998;97:257–264 [DOI] [PubMed] [Google Scholar]
- 8.Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg 1988;68:835–853 [DOI] [PubMed] [Google Scholar]
- 9.Rock JP, Cher L, Hochberg FH, Test AB. Central nervous system lymphomas in AIDS and non-AIDS patients. In: Youmans JR, editor. Neurological Surgery, 4th ed Philadelphia: WB Saunders; 1995 [Google Scholar]
- 10.Angelov L, Doolittle ND, Kraemer DF, et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNSL: a multi-institutional experience. J Clin Oncol 2009;27:3503–3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buhring U, Herrlinger U, Krings T, Thiex R, Weller M, Kuker W. MRI features of primary central nervous system lymphomas at presentation. Neurology 2001;57:393–396 [DOI] [PubMed] [Google Scholar]
- 12.Law M, Cha S, Knopp EA, Johnson G, Arnett J, Litt AW. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 2002;222:715–721 [DOI] [PubMed] [Google Scholar]
- 13.Lee IH, Kim ST, Kim HJ, Kim KH, Jeon P, Byun HS. Analysis of perfusion weighted image of CNSL. Eur J Radiol 2010;76:48–51 [DOI] [PubMed] [Google Scholar]
- 14.Tang YZ, Booth TC, Bhogal P, Malhotra A, Wilhelm T. Imaging of primary central nervous system lymphoma. Clin Radiol 2011;66:768–777 [DOI] [PubMed] [Google Scholar]
- 15.Morris PG, Abrey LE. Therapeutic challenges in primary CNSL. Lancet Neurol 2009;8:581–592 [DOI] [PubMed] [Google Scholar]
- 16.Soussain C, Ricard D, Fike JR, Mazeron JJ, Psimaras D, Delattre JY. CNS complications of radiotherapy and chemotherapy. Lancet 2009;374:1639–1651 [DOI] [PubMed] [Google Scholar]
- 17.Neuwelt EA, Hamilton BE, Varallyay CG, et al. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int 2009;75:465–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dosa E, Guillaume DJ, Haluska M, et al. Magnetic resonance imaging of intracranial tumors: intra-patient comparison of gadoteridol and ferumoxytol. Neuro Oncol 2011;13:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton BE, Nesbit GM, Dosa E, et al. Comparative analysis of ferumoxytol and gadoteridol enhancement using T1- and T2-weighted MRI in neuroimaging. AJR Am J Roentgenol 2011;197:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: experimental comparison and preliminary results. Magn Reson Med 1996;36:726–736 [DOI] [PubMed] [Google Scholar]
- 21.Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol 2007;188:1447–1474 [DOI] [PubMed] [Google Scholar]
- 22.Gahramanov S, Raslan AM, Muldoon LL, et al. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys 2011;79:514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gahramanov S, Muldoon LL, Li X, Neuwelt EA. Improved perfusion MR imaging assessment of intracerebral tumor blood volume and antiangiogenic therapy efficacy in a rat model with ferumoxytol. Radiology 2011;261:796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gahramanov S, Muldoon LL, Varallyay CG, et al. Pseudoprogression of glioblastoma after chemo- and radiation therapy: diagnosis by using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology 2013;266:842–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinstein JS, Varallyay CG, Dosa E, et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies: a review. J Cereb Blood Flow Metab 2010;30:15–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroeter M, Saleh A, Wiedermann D, Hoehn M, Jander S. Histochemical detection of ultrasmall superparamagnetic iron oxide (USPIO) contrast medium uptake in experimental brain ischemia. Magn Reson Med 2004;52:403–406 [DOI] [PubMed] [Google Scholar]
- 27.De Keyser J, Steen C, Mostert JP, Koch MW. Hypoperfusion of the cerebral white matter in multiple sclerosis: possible mechanisms and pathophysiological significance. J Cereb Blood Flow Metab 2008;28:1645–1651 [DOI] [PubMed] [Google Scholar]
- 28.Feraheme [package insert]. Lexington, MA: AMAG Pharmaceuticals, Inc.; 2012 [Google Scholar]
- 29.Neuwelt EA, Varallyay CG, Manninger S, et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery 2007;60:601–611 [DOI] [PubMed] [Google Scholar]
- 30.Tourdias T, Roggerone S, Filippi M, et al. Assessment of disease activity in multiple sclerosis phenotypes with combined gadolinium- and superparamagnetic iron oxide-enhanced MR imaging. Radiology 2012;264:225–233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.