Abstract
Objective:
We aimed to examine the association of APOE ε genotype with MRI markers of cerebrovascular disease (CVD): white matter hyperintensities, brain infarcts, and cerebral microbleeds.
Methods:
We performed a systematic review and meta-analysis of 42 cross-sectional or longitudinal studies identified in PubMed from 1966 to June 2012 (n = 29,965). This included unpublished data from 3 population-based studies: 3C-Dijon, Framingham Heart Study, and Sydney Memory and Ageing Study. When necessary, authors were contacted to provide effect estimates for the meta-analysis.
Results:
APOE ε4 carrier status and APOE ε44 genotype were associated with increasing white matter hyperintensity burden (sample size–weighted z score meta-analysis [meta]-p = 0.0034 and 0.0030) and presence of cerebral microbleeds (meta odds ratio [OR] = 1.24, 95% confidence interval [CI] [1.07, 1.43], p = 0.004, and 1.87 [1.26, 2.78], p = 0.002), especially lobar. APOE ε2 carrier status was associated with increasing white matter hyperintensity load (z score meta-p = 0.00053) and risk of brain infarct (meta OR = 1.41[1.09, 1.81], p = 0.008).
Conclusions:
APOE ε4 and APOE ε2 were associated with increasing burden in MRI markers for both hemorrhagic and ischemic CVD. While the association of APOE ε4 with an increased burden of CVD could be partly contributing to the relationship between APOE ε4 and AD, APOE ε2 was associated with MRI markers of CVD in the opposite direction compared to AD.
The ε4 allele of the APOE gene is a major risk factor for dementia and Alzheimer disease (AD).1–6 The association of APOE with cerebrovascular disease (CVD) is more controversial.7 APOE ε4 is a risk factor for cerebral amyloid angiopathy (CAA), a major determinant of intracerebral hemorrhage (ICH) in older individuals.1 Recent data from the International Stroke Genetics Consortium suggest an association of APOE ε4 with an increased risk of ICH, mostly lobar,8 and an association of the APOE ε2 allele with an increased risk and size of lobar ICH.8,9 Whether the epsilon polymorphism is also associated with an increased risk of ischemic stroke and MRI markers of CVD is unclear.7 MRI markers of CVD—white matter hyperintensities (WMH), brain infarcts (BI), and cerebral microbleeds (CMB)—are powerful predictors of stroke and dementia.10–13 They are highly prevalent in older community-dwelling persons,14–16 and can be assessed noninvasively and quantitatively in large population-based samples. Dissecting the relationship between APOE and MRI markers of CVD could provide important clues to the mechanisms underlying the association between APOE and risk of dementia. Indeed, although APOE is involved in modulating the metabolism and accumulation of amyloid β (Aβ), the precise mechanisms underlying its relationship with the risk of dementia are not fully understood.1 Whether the latter is also partly mediated by CVD, a powerful determinant of dementia risk, could be important for optimizing prevention strategies.
Our aim is to provide a systematic review and meta-analysis of studies evaluating the relationship of APOE ε genotype with MRI markers of CVD.
METHODS
Search strategy and selection criteria.
A research fellow and a neurologist identified references through searches of PubMed from 1966 to June 14, 2012, using predefined search terms. We also identified articles by reviewing reference lists of relevant articles and through searches of the authors' files. In addition, some studies (3C-Dijon,17 Framingham Heart Study [FHS],18,19 Sydney Memory and Ageing Study [Sydney-MAS]20) provided unpublished data for this review (methods e-1 on the Neurology® Web site at www.neurology.org).
We included studies testing the association of APOE genotype with at least one of the 3 MRI markers of CVD (WMH, BI, CMB), limited to those in adults.
The following were exclusion criteria: 1) evaluation of markers of CVD by CT scan only; 2) sample size <50 individuals; 3) studies evaluating separately the effect of each single nucleotide polymorphism comprising the APOE ε4 allele (methods e-1).
Data extraction.
We extracted the following data from the studies: sample size, study population (general population vs high-risk individuals), mean age, MRI characteristics and sequences, definition of the markers of CVD, measure of the association between APOE genotype and MRI markers, APOE genotypes used as reference, and adjustment variables when applicable. For measures of association between APOE genotype and MRI markers, we recorded odds ratios (ORs) for dichotomous MRI markers and standardized mean differences (measuring the difference in units of SD) or regression coefficients for continuous MRI markers; when none was available, raw numbers or mean values and SD by genotype group were used to compute OR or standardized mean differences, respectively. When none could be extracted, authors were contacted to provide those. If measures of association remained unavailable thereafter, qualitative results were reported. Two authors (S.D., S. Schilling) extracted the above information from each study, resolving any disagreement by discussion.
Variable definition.
The study population was defined as “general population” when the analysis was performed in a community-based setting or on participants described as “healthy individuals,” and as “high-risk population” when the study was conducted on persons selected for the presence of prevalent diseases such as cognitive impairment, stroke, depression, dementia, hypertension, or leukoaraiosis (or in populations enriched by design in persons with such prevalent disease). We excluded studies on white matter lesions occurring in inflammatory or neurodegenerative conditions and studies on WMH in monogenic cerebrovascular diseases (methods e-2).
In cross-sectional studies, WMH burden was studied as a dichotomized variable (presence vs absence or extensive vs low WMH burden), or as a continuous variable (grade from a semiquantitative visual scale or quantitatively measured volume). When computing ORs from raw numbers for WMH grades, we used the most severe grade vs all others. Some studies distinguished periventricular WMH (PVH) and deep WMH (DWMH). Whenever possible, we used results for global WMH, but when only PVH and DWMH were available, we used PVH in our primary meta-analysis and ran a secondary analysis using DWMH instead of PVH (methods e-2). In longitudinal studies, change in WMH grade or volume was retrieved. BI (or CMB) were defined by the presence of one or more BI (or CMB) vs none. Some studies distinguished CMB according to their location (lobar vs deep or infratentorial). In longitudinal studies, presence of incident CMB was evaluated.
Table e-1 details study quality criteria, including sample size, whether reference group and adjustment variables were specified, whether allele frequencies were reported for APOE ε4 and APOE ε2, type of WMH quantification, WMH measure used for analysis, and whether methods for differentiating BI from dilated perivascular spaces were specified.
Statistical analyses.
Meta-analysis was performed when at least 3 studies were available for the same outcome. Associations of WMH, BI, and CMB with the following genotype groups were summarized: APOE ε4 carriers (ε4+), homozygous APOE ε4 carriers (ε44), and APOE ε2 carriers (ε2+) (methods e-3). The reference group was APOE ε4 noncarriers (APOE ε4−) and APOE ε2 noncarriers (APOE ε2−) in most studies (some excluded APOE ε24 carriers), and APOE ε33 in the others. When several adjustment models were used, we included the least adjusted one. For quantitative measures of WMH burden, we used models accounting for total intracranial volume whenever available. We also ran secondary analyses using the most adjusted data. When both continuous and dichotomous measurements of WMH burden were available, we used the continuous data.
For BI and CMB, we calculated pooled ORs using an inverse variance–weighted meta-analysis. A fixed-effects meta-analysis was used in the absence of heterogeneity and a random-effects model if heterogeneity between studies was significant (heterogeneity p value <0.05 or I2 >50%). For WMH burden, we combined results from all studies using a sample size–weighted z score–based meta-analysis. This approach accounts for the fact that WMH burden was measured on different scales and analyzed either quantitatively or qualitatively across studies (methods e-3). We also calculated pooled ORs or standardized mean differences separately for studies using dichotomous or continuous measures of WMH burden, using an inverse variance–weighted meta-analysis. In secondary analyses, we ran a sample size–weighted z score–based meta-analysis for associations with continuous measures of WMH burden, as studies used different quantitative or semiquantitative measurements, and performed separate meta-analyses in the general population and in high-risk populations.
We applied a Bonferroni correction for multiple testing, accounting for the 3 tested phenotypes (WMH, BI, and CMB), yielding a significance threshold of p < 0.0166, although this may be conservative given the strong correlation between WMH, BI, and CMB.12,21,22
Meta-analysis was performed using the Cochrane RevMan (version 5.1) software (http://www.cc-ims.net/RevMan/current.htm) for inverse variance–weighted meta-analyses and R (http://cran.r-project.org) for sample size–weighted z score–based meta-analyses. Graphs were obtained using the rmeta package in R (http://cran.r-project.org/web/packages/rmeta/index.html).
RESULTS
The initial literature search identified 237 articles, of which 44 met the inclusion criteria. We excluded 5 articles because they overlapped with another larger study and added unpublished data from 3 studies (3C-Dijon, FHS, and Sydney-MAS). Hence, the total number of articles included in this systematic review was 42, comprising 29,965 subjects.
APOE ε4 and MRI markers of CVD.
APOE ε4 and WMH.
APOE ε4+ and WMH.
When combining the 27 available studies (n = 18,309, 14,491 in the general population and 3,818 in high-risk populations, tables e-2 and e-3)2,3,5,11,13,21,23–41 (FHS, Sydney-MAS, unpublished data) in a sample size–weighted z score–based meta-analysis, APOE ε4+ was significantly associated with increasing WMH burden: pz score = 0.0034.
A meta-analysis restricted to studies using continuous WMH burden and providing standardized mean differences (n = 8,917, 8,405 in the general population and 512 in high-risk populations)2,3,11,24,26,30,33,35,36,38,39 (Sydney-MAS, unpublished data) yielded a borderline nominal association of APOE ε4+ with increasing WMH burden: pooled standardized mean difference = 0.047 (95% confidence interval 0.0006, 0.094) (p = 0.05) (pz score = 0.0631) (figure 1A). When adding studies using continuous WMH but providing regression coefficients (n = 4,004)5,40,41 (FHS, unpublished data), in a sample size–weighted z score–based meta-analysis, the association was pz score = 0.0028.
Figure 1. Meta-analysis of studies testing the association between APOE ε4+ and white matter hyperintensities burden and between APOE ε44 and white matter hyperintensities burden.
(A) Meta-analysis of studies testing the association between APOE ε4+ and white matter hyperintensities (WMH) burden: fixed-effects inverse variance–weighted meta-analysis for studies on APOE ε4+ and continuous WMH (providing standardized mean differences) (heterogeneity test: I² = 24%, p = 0.20) (i); secondary meta-analyses in the general population (heterogeneity test: I² = 39%, p = 0.11) and in high-risk populations (heterogeneity test: I² = 0%, p = 0.63) also using a fixed-effects inverse variance–weighted meta-analysis. Fixed-effects inverse variance–weighted meta-analysis for APOE ε4+ and dichotomized WMH (heterogeneity test: I² = 0%, p = 0.65) (ii); secondary meta-analyses in the general population (heterogeneity test: I² = 0%, p = 0.46) and in high-risk populations (heterogeneity test: I² = 0%, p = 0.74) also using a fixed-effects inverse variance–weighted meta-analysis. Secondary analyses using random-effects meta-analyses yielded similar results (figure e-4). Additive model: additive genetic model relating the number of copies of the ε4 allele to WMH volume. (B) Meta-analysis of studies testing the association between APOE ε44 and WMH burden. Fixed-effects inverse variance–weighted meta-analysis on WMH burden studied as a continuous (heterogeneity test: I² = 8%, p = 0.36) (i) and as a dichotomous variable (heterogeneity test: I² = 0%, p = 0.93) (ii). For secondary analyses using a random-effects model, see figure e-5. APOE ε4+ = APOE ε4 carrier status; FHS = Framingham Heart Study; HTN = hypertension; OR = odds ratio; p = p value from inverse variance–weighted meta-analysis; p z score = p value from sample size–weighted z score–based meta-analysis; SMD = standardized mean deviation; Sydney MAS = Sydney Memory and Ageing Study.
Analyzing studies using dichotomized WMH burden assessment (n = 5,282, 3,627 in the general population and 1,655 in high-risk populations)21,23,25,27–29,31,32,34,37 revealed no significant association of APOE ε4+ with large WMH burden: pooled OR = 1.07 (0.93, 1.22) (p = 0.34) (pz score = 0.39) (figure 1A). Results were similar after adding one study using a 3-class variable for WMH with ordinal logistic regression (n = 106, pz score = 0.44).13
Four small studies (n = 420 in high-risk populations) could not be included in this meta-analysise21,e22,e25,e28: one found a significant association between APOE ε4+ and WMH.e22
APOE ε4+ and WMH progression.
Two studies (n = 2,304 in the general population) did not report any significant association between APOE ε4+ and WMH progression (table e-4).e32,e33
APOE ε44 and WMH.
When meta-analyzing 12 studies (n = 7,494, 6,254 in the general population, 1,240 in high-risk populations, tables e-2 and e-3)3,11,13,21,28,30,31,37–39 (Sydney-MAS, FHS, unpublished data) in a sample size–weighted meta-analysis, APOE ε44 was significantly associated with increasing WMH burden: pz score = 0.0030.
Combining studies using continuous WMH and providing standardized mean differences (n = 2,447, 2,119 in the general population, 328 in high-risk populations)3,11,30,38,39 (Sydney-MAS, unpublished data) yielded a significant association between APOE ε44 and increasing WMH burden: pooled standardized mean difference = 0.41 (0.17, 0.65) (p = 0.0009) (pz score = 0.0016) (figure 1B). When adding one study providing regression coefficients (n = 1,574) (FHS, unpublished data), the association was still nominally significant: pz score = 0.032.
Studies examining WMH as a dichotomous variable (n = 3,399, 2,487 in the general population, 912 in high-risk populations)21,28,31,37 showed a nominally significant association of APOE ε44 with increasing WMH burden: pooled OR = 1.63 (1.004, 2.64) (p = 0.048) (pz score = 0.046) (figure 1B). Adding one study using a 3-class WMH variable with ordinal logistic regression (n = 74) did not modify this association (pz score = 0.039).13
One study (n = 56 in high-risk populations) could not be included in this meta-analysis and reported a significant association between APOE ε44 and increasing WMH.e22
APOE ε44 and WMH progression.
One longitudinal study from the general population reported a significant association of APOE ε44 with worsening WMH burden (table e-4).3
APOE ε4 and BI.
APOE ε4+ and BI.
In a meta-analysis including 7 studies (n = 8,223, 8,005 in the general population, 218 in high-risk populations)4,11,24,34,37 (3C-Dijon, FHS, unpublished data), APOE ε4+ was not associated with BI: pooled OR = 1.03 (0.90, 1.18) (p = 0.67) (figure e-1, table e-5). One study (n = 82 in high-risk populations) could not be included in this meta-analysis and did not report any association between APOE ε4+ and BI.32
APOE ε44 and BI.
APOE ε44 was not associated with BI in a meta-analysis including 4 studies (n = 6,029, 5,956 in the general population, 73 in high-risk populations)11,37 (3C-Dijon, FHS, unpublished data): pooled OR = 0.86 (0.29, 2.52) (p = 0.79) (figure e-2, table e-5).
We did not identify longitudinal studies assessing the relationship between APOE genotype and incident BI.
APOE ε4 and CMB.
APOE ε4+ and CMB.
Meta-analyzing 9 studies (n = 6,698, 5,387 in the general population and 1,311 in high-risk populations, tables e-6 and e-7)11,12,21,22,34,42–44 (FHS, unpublished data) yielded a significant association of APOE ε4+ with an increased risk of CMB: pooled OR = 1.24 (1.07, 1.43) (p = 0.004) (figure 2A), which was significant for lobar (n = 3,689, pooled OR = 1.34 [1.09, 1.64], p = 0.006) but not deep CMB (n = 3,777, pooled OR = 1.15 [0.89, 1.49], p = 0.29).12,21,43,44 Of note, results were significant in the general population but not in the smaller subset of studies performed in high-risk populations, comprising participants with diverse preexisting conditions (ICH, cerebral ischemia, hypertension, AD, other neurologic disorders).
Figure 2. Meta-analysis of studies testing the association between APOE ε4+ and cerebral microbleeds and between APOE ε44 and cerebral microbleeds.

(A) Meta-analysis of studies testing the association between APOE ε4+ and cerebral microbleeds (CMB). Fixed-effects inverse variance–weighted meta-analysis (heterogeneity test: I² = 0%, p = 0.88); secondary meta-analysis in the general population (heterogeneity test: I² = 0%, p = 0.45) and in high-risk population (heterogeneity test: I² = 0%, p = 0.96) also using a fixed-effects inverse variance–weighted meta-analysis. For secondary analysis using a random-effects model, see figure e-7. (B) Meta-analysis of studies testing the association between APOE ε44 and CMB. Fixed-effects inverse variance–weighted meta-analysis (heterogeneity test: I² = 11%, p = 0.34). For secondary analysis using a random-effects model, see figure e-7. APOE ε4+ = APOE ε4 carrier status; FHS = Framingham Heart Study; OR = odds ratio.
APOE ε44 and CMB.
APOE ε44 was significantly associated with an increased risk of CMB in a meta-analysis combining 6 studies (n = 5,158, 4,798 in the general population, 360 in high-risk populations, tables e-6 and e-7)11,12,21,22,42 (FHS, unpublished data): pooled OR = 1.87 (1.26, 2.78) (p = 0.002) (figure 2B).
APOE ε4 and incident CMB.
One study (n = 654 in the general population)e41 reported a significant association of APOE ε44 but not APOE ε4+ with incident lobar CMB (table e-8). Another study (n = 197 in high-risk populations) did not report any association between APOE ε4+ and incident CMB (table e-8).e42
APOE ε2 and MRI markers of CVD.
APOE ε2 and WMH.
APOE ε2+ and WMH.
In a sample size–weighted meta-analysis including 9 studies (n = 6,669, 5,282 in the general population, 1,387 in high-risk populations, table e-9)21,23,31,34,35,39 (3C-Dijon, FHS, Sydney-MAS, unpublished data), APOE ε2+ was significantly associated with increasing WMH burden: pz score = 0.00053.
Combining studies using WMH as a continuous variable (n = 2,708, all general population)35,39 (3C-Dijon, Sydney-MAS, unpublished data) showed a nonsignificant trend of association between APOE ε2+ and WMH burden: pooled standardized mean difference = 0.03 (−0.01, 0.07) (p = 0.20) (pz score = 0.07) (figure 3A). This was similar when adding one study using continuous WMH but providing regression coefficients (n = 2,353) (FHS, unpublished data), pz score = 0.09. In a meta-analysis of 4 studies examining WMH as a dichotomous variable (n = 1,608, 221 in the general population, 1,387 in high-risk populations),21,23,31,34 APOE ε2+ was significantly associated with increasing WMH burden: pooled OR = 1.80 (1.35, 2.42) (p = 0.00008) (pz score = 0.00011) (figure 3A).
Figure 3. Meta-analysis of studies testing the association between APOE ε2+ and white matter hyperintensities burden and between APOE ε2+ and brain infarcts.
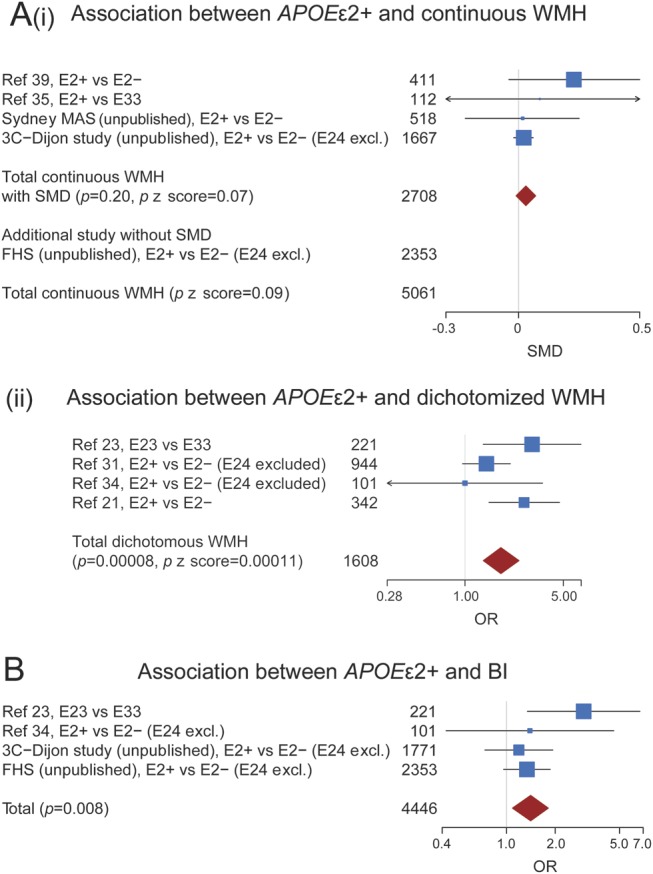
(A) Meta-analysis of studies testing the association between APOE ε2+ and white matter hyperintensities (WMH) burden. Fixed-effects inverse variance–weighted meta-analysis for studies on APOE ε2+ and continuous WMH (providing standardized mean differences) (heterogeneity test: I² = 41%, p = 0.17) (i); all studies are from the general population. Fixed-effects inverse variance–weighted meta-analysis for APOE ε2+ and dichotomized WMH (heterogeneity test: I² = 45%, p = 0.14) (ii). For secondary analysis using a random-effects model, see figure e-8. (B) Meta-analysis of studies testing the association between APOE ε2+ and brain infarcts (BI). Fixed-effects inverse variance–weighted meta-analysis (heterogeneity test: I² = 25%, p = 0.26). For secondary analysis using random-effects model, see figure e-9. APOE ε2+ = APOE ε2 carrier status; FHS = Framingham Heart Study; OR = odds ratio; p = p value from inverse variance–weighted meta-analysis; p z score = p value from sample size–weighted z score–based meta-analysis; SMD = standardized mean difference; Sydney MAS = Sydney Memory and Ageing Study.
APOE ε2 and BI.
APOE ε2+ and BI.
In a meta-analysis including 4 studies (n = 4,446, 4,345 in the general population, 101 in high-risk populations, table e-10)23,34 (3C-Dijon, FHS, unpublished data), APOE ε2+ was significantly associated with BI: pooled OR = 1.41 (1.09, 1.81) (p = 0.008) (figure 3B).
APOE ε2 and CMB.
APOE ε2+ and CMB.
In a meta-analysis including 7 studies (n = 5,389, 4,508 in the general population, 881 in high-risk populations, figure e-3, table e-11)12,21,34,42–44 (FHS, unpublished data), APOE ε2+ was not associated with CMB (pooled OR = 1.09 [0.89, 1.32], p = 0.41), lobar CMB (n = 3,003, pooled OR = 1.19 [0.91, 1.55], p = 0.20), or deep CMB (n = 3,228, pooled OR = 1.03 [0.70, 1.52], p = 0.87).12,21,43,44
APOE ε2+ and incident CMB.
One studye42 (n = 197 in high-risk populations) found a significant association between APOE ε2+ and incident CMB (table e-12), while another study did not (n = 563 in the general population)e41 (table e-12).
DISCUSSION
In this systematic review and meta-analysis comprising 42 studies on 29,965 participants, APOE ε4+ (mostly APOE ε44) and APOE ε2+ genotypes were associated with increasing WMH load, APOE ε4+ genotype was associated with an increased prevalence of CMB, especially lobar, and APOE ε2+ genotype was associated with a higher frequency of BI.
One previous systematic review had examined the association between APOE ε4 carrier status and WMH burden, including 24 studies on 8,546 individuals, reporting no significant association.45 An earlier qualitative review had reached a similar conclusion.46 However, previous reviews did not examine the association of APOE ε44 or APOE ε2 with WMH.45 Besides, since then, data from 12 additional studies on the association between APOE ε4 carrier status and WMH burden have become available, thus increasing power to detect associations.
Mechanisms underlying associations of APOE genotypes with WMH burden are unclear.47 CAA, a condition characterized by Aβ accumulation in the media of cerebral and leptomeningeal arterioles48 that is highly prevalent in aging populations,49 is one potential mediator. Indeed, although CAA primarily affects cortical arterioles, APOE ε4–enhanced thickening of the affected arterial wall by amyloid deposits causes reduction of the vessel lumen,48,50 potentially leading to reduced blood flow in distal perfusion zones of the white matter.51 Supporting this hypothesis, APOE ε4 was recently shown to be associated with occipital distribution of WMH, a location where CAA predominates.52 The vasculopathic abnormalities associated with the APOE ε2 allele in CAA patients,50 including fibrinoid necrosis and blood–brain barrier damage,53 might also affect small arteries in the white matter.54 ApoE also plays a key role in lipid metabolism, but it is unclear whether APOE isoforms differentially influence lipid metabolism in the brain,1 and the relationship between lipid levels and white matter disease is not well established.27 Inflammation may also contribute to the observed relation of APOE ε2 and APOE ε4 with increasing WMH burden.55
To date, no meta-analysis has evaluated the relationship between APOE ε polymorphism and MRI-defined BI, to our knowledge. The absence of a significant association between APOE ε4 and BI is in line with the lack of robust association between APOE ε4 and ischemic stroke found in a recent meta-analysis,7 although we may have been underpowered for this phenotype. Interestingly, we found a significant association between APOE ε2 and BI, consistent with the association between APOE ε2 and WMH burden, especially dichotomized extensive WMH; additional studies are needed to explore this relationship further, including analyses focused on lacunar brain infarcts, a more homogeneous entity reflecting primarily small-artery disease.
A systematic review recently addressed the relationship between the APOE ε polymorphism and CMB, including a meta-analysis on 7 studies.56 In line with our results, they found that APOE ε4 carriers had a significantly increased risk of having at least one CMB compared to APOE ε33 carriers,56 with a stronger association for lobar CMB. They did not find a significant relationship between APOE ε2 and CMB, regardless of their location. Their review did not consider recessive models, and we included 3 additional studies in our meta-analysis.
We observed a significant association of APOE ε4+ with CMB, which was even stronger for APOE ε44. The association of APOE ε4 with an increased risk of CMB, especially lobar, is consistent with recent data showing a significant relationship between APOE ε4 and lobar ICH.7,8 The association of APOE ε4 with intracerebral bleeding is thought to be primarily mediated by CAA,8 representing a major cause of ICH in older individuals.57 Histopathologic data suggest that APOE ε4 enhances Aβ deposition in the vessel wall.50 Although we did not identify any significant association of APOE ε2 with CMB, the sample size was smaller and for lobar CMB there was a trend for an association in the same direction as for APOE ε4. APOE ε2 has been associated with an increased risk of lobar ICH,8 and with larger hematoma volumes,9 possibly by increasing vessel wall damage caused by Aβ deposition,9 thus leading to increased vulnerability of vessels surrounding the hematoma.50 Hence ε2 carriers might be more predisposed to larger, symptomatic hematomas but not microhemorrhages.
This is the largest systematic review and meta-analysis on the association of the APOE ε polymorphism with several MRI markers of CVD. A substantial effort was made to obtain additional unpublished information by contacting authors. In contrast with previous reviews, we have studied concomitantly several markers of ischemic and hemorrhagic CVD. Moreover, we combined all studies on APOE and WMH, including studies using WMH as a continuous or dichotomous variable, by performing a sample size–weighted z score–based meta-analysis, thus increasing sample size and power to detect associations. In addition, we have summarized information on the effect of APOE ε2 and APOE ε44, whenever available.
We were limited by the fact that most studies provided effect estimates for APOE ε4 carriers vs noncarriers only, with varying reference groups. Many studies have used APOE ε4− and APOE ε2− as reference groups, rather than APOE ε33, which may have reduced our power to detect associations. Indeed, the fact that the relationship of APOE ε4 and APOE ε2 with MRI markers of CVD appears to be in the same direction, including APOE ε2 carriers in the reference group for APOE ε4 carriers, and vice versa, might have attenuated differences. Although we accounted for statistical heterogeneity by using a random-effects model when appropriate, there were differences between studies concerning MRI characteristics, definition of MRI markers, and adjustment variables. The detection of CMB is strongly correlated with magnetic strength58 and some studies included in this meta-analysis were performed on MRI scanners <1.5 T; this may have led to an underestimation of CMB prevalence and consequently reduced power to detect associations. Populations differed in terms of age distribution and vascular risk profile, which was partly accounted for in secondary meta-analyses performed separately in the general population or in groups at high risk of cerebrovascular disease (figures 1A, 2A and B, e-3, e-4, e-6, e-7, and e-10). Of note, most individuals included in the meta-analyses were community participants, and, whenever possible, we have shown that significant associations were maintained when restricting analyses to the general population. The vast majority of studies were performed in Caucasians; given the large differences in APOE ε allele distributions worldwide,8 our results may not be generalizable to other ethnic groups. Finally, as in all systematic reviews and meta-analyses of published data, we cannot exclude some degree of publication bias, although we have tried to minimize it by systematically adding unpublished results from 3 large population-based samples. Confirmation of our findings could be obtained in the future by prospective meta-analyses within large consortia using harmonized phenotypic criteria and analytical models.
APOE ε4 and APOE ε2 were associated with increasing burden in MRI markers for both hemorrhagic and ischemic CVD. WMH, CMB, and lacunar BI (the vast majority of BI) are prominent features of cerebral small-vessel disease59 and are strongly correlated in both community and hospital-based samples.12,21,22 It is therefore expected that they may share some genetic determinants.47 However, it is intriguing to note that APOE ε2 was associated with an increased burden of MRI-based CVD, while it has a protective effect on the occurrence of AD.60 Hence, while the association of APOE ε4 with an increased burden of CVD could be partly contributing to the relationship between APOE ε4 and AD, APOE ε2 was associated with MRI markers of CVD in the opposite direction compared to AD.
Future studies evaluating simultaneously in the same dataset the association of APOE with MRI markers of ischemic and hemorrhagic CVD could help elucidate whether these associations are independent or reflect the strong correlation between the phenotypes. Examining neuropathologic correlates of MRI-based CVD and their association with APOE genotypes could also help in understanding the pathophysiologic link between APOE and CVD. Finally, studies focusing on the influence of age and hypertension on the relationship between APOE and CVD could further contribute to deciphering the complex relationship of APOE with vascular brain aging.2,26
Supplementary Material
ACKNOWLEDGMENT
The authors thank the staff and the participants of the 3C-Dijon Study, the Framingham Heart Study, and the Sydney Memory and Ageing Study for their contributions; and Dr. David C. Steffens and Dr. Celia Hybels for providing detailed effect estimates for their published article.
GLOSSARY
- Aβ
amyloid β
- AD
Alzheimer disease
- BI
brain infarcts
- CAA
cerebral amyloid angiopathy
- CMB
cerebral microbleeds
- CVD
cerebrovascular disease
- DWMH
deep white matter hyperintensities
- FHS
Framingham Heart Study
- ICH
intracerebral hemorrhage
- OR
odds ratio
- PVH
periventricular white matter hyperintensities
- Sydney-MAS
Sydney Memory and Ageing Study
- WMH
white matter hyperintensities
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Mrs. Sabrina Schilling: acquisition of data, analysis and interpretation, statistical analysis, drafting/revising the manuscript. Dr. Anita L. DeStefano: acquisition of data, drafting/revising the manuscript. Dr. Perminder S. Sachdev: acquisition of data, drafting/revising the manuscript. Mr. Seung Hoan Choi: acquisition of data, drafting/revising the manuscript. Dr. Karen A. Mather: acquisition of data, drafting/revising the manuscript. Dr. Charles D. DeCarli: drafting/revising the manuscript. Dr. Wei Wen: acquisition of data, drafting/revising the manuscript. Dr. Peter Høgh: acquisition of data, drafting/revising the manuscript. Dr. Naftali Raz: acquisition of data, drafting/revising the manuscript. Dr. Rhoda Au: drafting/revising the manuscript. Dr. Alexa Beiser: drafting/revising the manuscript. Dr. Philip A. Wolf: drafting/revising the manuscript. Dr. José Rafael Romero: drafting/revising the manuscript. Dr. Yi-Cheng Zhu: drafting/revising the manuscript. Dr. Kathryn L. Lunetta: acquisition of data, drafting/revising the manuscript. Dr. Lindsay Farrer: drafting/revising the manuscript. Dr. Carole Dufouil: drafting/revising the manuscript. Dr. Lewis H. Kuller: acquisition of data, drafting/revising the manuscript. Dr. Bernard Mazoyer: drafting/revising the manuscript. Dr. Sudha Seshadri: acquisition of data, drafting/revising the manuscript. Dr. Christophe Tzourio: acquisition of data, drafting/revising the manuscript. Dr. Stéphanie Debette: study concept and design, analysis and interpretation, statistical analysis, study supervision or coordination, drafting/revising the manuscript.
STUDY FUNDING
The 3C study is conducted under a partnership agreement between the Institut National de la Santé et de la Recherche Médicale (INSERM), the Victor Segalen–Bordeaux II University, and the SanofiSynthélabo Company. The Fondation pour la Recherche Médicale funded the preparation and initiation of the study. The 3C Study is also supported by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, Haute Autorité de la Santé, Institut National de Prévention et d’Education pour la Santé, Conseils Régionaux of Bourgogne, Fondation de France, Ministry of Research–INSERM Program “Cohortes et collections de données biologiques,” Mutuelle Générale de l’Education Nationale, Institut de la Longévité, Conseil Général de la Côte d’Or. The Sydney Memory and Ageing study was supported by a National Health and Medical Research Council of Australia Program Grant (ID 350833). The Framingham Heart Study and the listed authors are supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01-HC-25195) and by grants from the National Institute of Neurological Disorders and Stroke (NS17950), the National Heart, Lung and Blood Association (HL93029, U01HL 096917), and the National Institute of Aging (AG08122, AG16495, AG033193, AG031287, K23 AG038444). Dr. S. Debette is a recipient of Chaire d’Excellence Junior Grant from the Agence Nationale de la Recherche (ANR). Dr. N. Raz is supported by a grant from the National Institute on Aging (R37 AG011230).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol 2011;10:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogh P, Garde E, Mortensen EL, Jorgensen OS, Krabbe K, Waldemar G. The apolipoprotein E epsilon4-allele and antihypertensive treatment are associated with increased risk of cerebral MRI white matter hyperintensities. Acta Neurol Scand 2007;115:248–253 [DOI] [PubMed] [Google Scholar]
- 3.Godin O, Tzourio C, Maillard P, Alperovitch A, Mazoyer B, Dufouil C. Apolipoprotein E genotype is related to progression of white matter lesion load. Stroke 2009;40:3186–3190 [DOI] [PubMed] [Google Scholar]
- 4.Schmidt H, Schmidt R, Fazekas F, et al. Apolipoprotein E e4 allele in the normal elderly: neuropsychologic and brain MRI correlates. Clin Genet 1996;50:293–299 [DOI] [PubMed] [Google Scholar]
- 5.Lunetta KL, Erlich PM, Cuenco KT, et al. Heritability of magnetic resonance imaging (MRI) traits in Alzheimer disease cases and their siblings in the MIRAGE study. Alzheimer Dis Assoc Disord 2007;21:85–91 [DOI] [PubMed] [Google Scholar]
- 6.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis: APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997;278:1349–1356 [PubMed] [Google Scholar]
- 7.Sudlow C, Martinez Gonzalez NA, Kim J, Clark C. Does apolipoprotein E genotype influence the risk of ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage? Systematic review and meta-analyses of 31 studies among 5961 cases and 17,965 controls. Stroke 2006;37:364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biffi A, Sonni A, Anderson CD, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol 2010;68:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biffi A, Anderson CD, Jagiella JM, et al. APOE genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: a genetic association study. Lancet Neurol 2011;10:702–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuur M, van Swieten JC, Schol-Gelok S, et al. Genetic risk factors for cerebral small-vessel disease in hypertensive patients from a genetically isolated population. J Neurol Neurosurg Psychiatry 2011;82:41–44 [DOI] [PubMed] [Google Scholar]
- 12.Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam Scan Study. Stroke 2010;41:S103–S106 [DOI] [PubMed] [Google Scholar]
- 13.Vuorinen M, Solomon A, Rovio S, et al. Changes in vascular risk factors from midlife to late life and white matter lesions: a 20-year follow-up study. Dement Geriatr Cogn Disord 2011;31:119–125 [DOI] [PubMed] [Google Scholar]
- 14.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611–619 [DOI] [PubMed] [Google Scholar]
- 15.Debette S, Bis JC, Fornage M, et al. Genome-wide association studies of MRI-defined brain infarcts: meta-analysis from the CHARGE Consortium. Stroke 2010;41:210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fornage M, Debette S, Bis JC, et al. Genome-wide association studies of cerebral white matter lesion burden: the CHARGE consortium. Ann Neurol 2011;69:928–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.3C Study Group Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology 2003;22:316–325 [DOI] [PubMed] [Google Scholar]
- 18.Debette S, Beiser A, DeCarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke 2010;41:600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeerakathil T, Wolf PA, Beiser A, et al. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke 2004;35:1831–1835 [DOI] [PubMed] [Google Scholar]
- 20.Sachdev PS, Brodaty H, Reppermund S, et al. The Sydney Memory and Ageing Study (MAS): methodology and baseline medical and neuropsychiatric characteristics of an elderly epidemiological non-demented cohort of Australians aged 70-90 years. Int Psychogeriatr 2010;22:1248–1264 [DOI] [PubMed] [Google Scholar]
- 21.Lemmens R, Gorner A, Schrooten M, Thijs V. Association of apolipoprotein E ε2 with white matter disease but not with microbleeds. Stroke 2007;38:1185–1188 [DOI] [PubMed] [Google Scholar]
- 22.Goos JD, Kester MI, Barkhof F, et al. Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke 2009;40:3455–3460 [DOI] [PubMed] [Google Scholar]
- 23.Schmidt R, Schmidt H, Fazekas F, et al. Apolipoprotein E polymorphism and silent microangiopathy-related cerebral damage: results of the Austrian Stroke Prevention Study. Stroke 1997;28:951–956 [DOI] [PubMed] [Google Scholar]
- 24.DeCarli C, Reed T, Miller BL, Wolf PA, Swan GE, Carmelli D. Impact of apolipoprotein E epsilon4 and vascular disease on brain morphology in men from the NHLBI twin study. Stroke 1999;30:1548–1553 [DOI] [PubMed] [Google Scholar]
- 25.Nebes RD, Vora IJ, Meltzer CC, et al. Relationship of deep white matter hyperintensities and apolipoprotein E genotype to depressive symptoms in older adults without clinical depression. Am J Psychiatry 2001;158:878–884 [DOI] [PubMed] [Google Scholar]
- 26.de Leeuw FE, Richard F, de Groot JC, et al. Interaction between hypertension, apoE, and cerebral white matter lesions. Stroke 2004;35:1057–1060 [DOI] [PubMed] [Google Scholar]
- 27.Crisby M, Bronge L, Wahlund LO. Low levels of high density lipoprotein increase the severity of cerebral white matter changes: implications for prevention and treatment of cerebrovascular diseases. Curr Alzheimer Res 2010;7:534–539 [DOI] [PubMed] [Google Scholar]
- 28.Hirono N, Yasuda M, Tanimukai S, Kitagaki H, Mori E. Effect of the apolipoprotein E epsilon4 allele on white matter hyperintensities in dementia. Stroke 2000;31:1263–1268 [DOI] [PubMed] [Google Scholar]
- 29.Sawada H, Udaka F, Izumi Y, et al. Cerebral white matter lesions are not associated with apoE genotype but with age and female sex in Alzheimer's disease. J Neurol Neurosurg Psychiatry 2000;68:653–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doody RS, Azher SN, Haykal HA, Dunn JK, Liao T, Schneider L. Does APO epsilon4 correlate with MRI changes in Alzheimer's disease? J Neurol Neurosurg Psychiatry 2000;69:668–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szolnoki Z, Somogyvari F, Kondacs A, et al. Specific APO E genotypes in combination with the ACE D/D or MTHFR 677TT mutation yield an independent genetic risk of leukoaraiosis. Acta Neurol Scand 2004;109:222–227 [DOI] [PubMed] [Google Scholar]
- 32.Bracco L, Piccini C, Moretti M, et al. Alzheimer's disease: role of size and location of white matter changes in determining cognitive deficits. Dement Geriatr Cogn Disord 2005;20:358–366 [DOI] [PubMed] [Google Scholar]
- 33.Wen HM, Baum L, Cheung WS, et al. Apolipoprotein E epsilon4 allele is associated with the volume of white matter changes in patients with lacunar infarcts. Eur J Neurol 2006;13:1216–1220 [DOI] [PubMed] [Google Scholar]
- 34.Seifert T, Lechner A, Flooh E, Schmidt H, Schmidt R, Fazekas F. Lack of association of lobar intracerebral hemorrhage with apolipoprotein E genotype in an unselected population. Cerebrovasc Dis 2006;21:266–270 [DOI] [PubMed] [Google Scholar]
- 35.Raz N, Yang Y, Dahle CL, Land S. Volume of white matter hyperintensities in healthy adults: contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim Biophys Acta 2012;1822:361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hafsteinsdottir SH, Eiriksdottir G, Sigurdsson S, et al. Brain tissue volumes by APOE genotype and leisure activity-the AGES-Reykjavik Study. Neurobiol Aging 2012;33:829.e1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuller LH, Shemanski L, Manolio T, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke 1998;29:388–398 [DOI] [PubMed] [Google Scholar]
- 38.Steffens DC, Trost WT, Payne ME, Hybels CF, MacFall JR. Apolipoprotein E genotype and subcortical vascular lesions in older depressed patients and control subjects. Biol Psychiatry 2003;54:674–681 [DOI] [PubMed] [Google Scholar]
- 39.Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol Aging 2009;30:946–956 [DOI] [PubMed] [Google Scholar]
- 40.He J, Iosif AM, Lee DY, et al. Brain structure and cerebrovascular risk in cognitively impaired patients: Shanghai Community Brain Health Initiative-pilot phase. Arch Neurol 2010;67:1231–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biffi A, Anderson CD, Desikan RS, et al. Genetic variation and neuroimaging measures in Alzheimer disease. Arch Neurol 2010;67:677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry 2008;79:1002–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim M, Bae HJ, Lee J, et al. APOE epsilon2/epsilon4 polymorphism and cerebral microbleeds on gradient-echo MRI. Neurology 2005;65:1474–1475 [DOI] [PubMed] [Google Scholar]
- 44.Cordonnier C, van der Flier WM, Sluimer JD, Leys D, Barkhof F, Scheltens P. Prevalence and severity of microbleeds in a memory clinic setting. Neurology 2006;66:1356–1360 [DOI] [PubMed] [Google Scholar]
- 45.Paternoster L, Chen W, Sudlow CL. Genetic determinants of white matter hyperintensities on brain scans: a systematic assessment of 19 candidate gene polymorphisms in 46 studies in 19,000 subjects. Stroke 2009;40:2020–2026 [DOI] [PubMed] [Google Scholar]
- 46.Cherbuin N, Leach LS, Christensen H, Anstey KJ. Neuroimaging and APOE genotype: a systematic qualitative review. Dement Geriatr Cogn Disord 2007;24:348–362 [DOI] [PubMed] [Google Scholar]
- 47.Rost NS, Greenberg SM, Rosand J. The genetic architecture of intracerebral hemorrhage. Stroke 2008;39:2166–2173 [DOI] [PubMed] [Google Scholar]
- 48.Greenberg SM. Cerebral amyloid angiopathy and vessel dysfunction. Cerebrovasc Dis 2002;13(suppl 2):42–47 [DOI] [PubMed] [Google Scholar]
- 49.Tanskanen M, Makela M, Myllykangas L, et al. Prevalence and severity of cerebral amyloid angiopathy: a population-based study on very elderly Finns (Vantaa 85+). Neuropathol Appl Neurobiol 2012;38:329–336 [DOI] [PubMed] [Google Scholar]
- 50.McCarron MO, Nicoll JA, Stewart J, et al. The apolipoprotein E epsilon2 allele and the pathological features in cerebral amyloid angiopathy-related hemorrhage. J Neuropathol Exp Neurol 1999;58:711–718 [DOI] [PubMed] [Google Scholar]
- 51.Smith EE, Nandigam KR, Chen YW, et al. MRI markers of small vessel disease in lobar and deep hemispheric intracerebral hemorrhage. Stroke 2010;41:1933–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu YC, Chabriat H, Godin O, et al. Distribution of white matter hyperintensity in cerebral hemorrhage and healthy aging. J Neurol 2012;259:530–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol 2011;37:75–93 [DOI] [PubMed] [Google Scholar]
- 54.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003;34:806–812 [DOI] [PubMed] [Google Scholar]
- 55.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology 2012;78:720–727 [DOI] [PubMed] [Google Scholar]
- 56.Maxwell SS, Jackson CA, Paternoster L, et al. Genetic associations with brain microbleeds: systematic review and meta-analyses. Neurology 2011;77:158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–539 [DOI] [PubMed] [Google Scholar]
- 58.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol 2009;8:643–653 [DOI] [PubMed] [Google Scholar]
- 60.Corder EH, Saunders AM, Risch NJ, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 1994;7:180–184 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



