Abstract
Objective:
In children with newly diagnosed childhood absence epilepsy (CAE), determine pretreatment EEG features and their associations with baseline neuropsychological function and short-term treatment outcome.
Methods:
In a multicenter, randomized clinical trial, patients with CAE underwent a pretreatment, 1-hour video-EEG and neuropsychological testing with freedom-from-failure and seizure-freedom (SF) outcome assessed at the 16- to 20-week visit.
Results:
Detailed evaluation of the pretreatment EEG was possible for 99.8% of participants (445/446). Median time to first seizure was 6.0 minutes (range 0–59 minutes), median number of seizures was 5 (range 1–60), and median seizure duration was 10.8 seconds (range 3.3–77.6 seconds). Median duration of shortest seizure per EEG was 7.5 seconds (range 3.0–77.6 seconds). Seizure frequency was not associated with baseline measures of attention, executive function, or treatment outcome. Presence of a seizure lasting ≥20 seconds was noted in 29% of subjects (129/440); these children had higher median omissions T score on the Conners Continuous Performance Test (56.3 vs 51.6, p = 0.01). Patients with a shortest seizure of longer duration were more likely to demonstrate treatment success by both freedom-from-failure (p = 0.02) and SF (p = 0.005) criteria, even after controlling for age, treatment group, and number of seizures, with good predictive value (area under the curve 78% for SF).
Conclusions:
CAE is reliably and quickly confirmed by EEG. Occurrence of a seizure ≥20 seconds, but not overall seizure frequency, was associated with differential baseline measures of attention. Patients whose shortest pretreatment EEG seizure was longer in duration were more likely to achieve SF, regardless of treatment.
Childhood absence epilepsy (CAE) accounts for 10% to 17% of childhood epilepsy1,2 and usually presents between 4 and 8 years. Its classic pretreatment EEG pattern consists of an approximate 3-Hz bilateral, synchronous, symmetric spike and slow wave discharge, with seizures often activated by hyperventilation (HV).3,4 Treatment response to initial monotherapy is suboptimal, with slightly more than half of children achieving complete seizure control with acceptable treatment-associated side effects.5,6
Although evidence exists for associations between CAE and deficits in attention,5–9 relationships among pretreatment EEG, baseline neuropsychological functioning, and response to initial therapy are unknown. The completion of a double-blinded, randomized clinical trial of 446 children with newly diagnosed CAE provides a unique opportunity to characterize pretreatment EEG features and determine associations with baseline measures of attention and executive function, and initial treatment outcome.
METHODS
Subject population.
Subject eligibility criteria have been previously reported in detail.5,6 The study's key inclusion criteria were i) clinical diagnosis of CAE, ii) age 2.5 to 13 years at study entry, and iii) an EEG demonstrating 2.7- to 5-Hz generalized spike wave (GSW) with normal background and at least one GSW burst lasting ≥3 seconds.
Study design.
The underlying clinical trial design, randomization, baseline neuropsychological testing battery/scoring, and outcome assessment have been previously described in detail.5,6 The study's baseline visit included a 1-hour video-EEG and an age-specific battery of neuropsychological testing. The neuropsychological tests utilized in this study were the Conners Continuous Performance Test (CPT-II for patients aged 6 years and older and K-CPT for patients younger than 6 years) for attention and the Wisconsin Card Sorting Test (WCST) for executive function. Subjects were randomized to ethosuximide (ETX), lamotrigine (LTG), or valproic acid (VPA).
EEG.
Before randomization and treatment, patients underwent a standardized, 1-hour video-EEG protocol. The protocol involved a 5-minute waking EEG baseline; first HV trial of 3 to 4 minutes; photic stimulation at 2 to 20 Hz; second HV trial if no electroclinical seizures were recorded during the first HV trial; and additional wakefulness for 1 hour total. Sleep was not recorded. For study eligibility, these pretreatment EEGs were reviewed first by local investigators and re-reviewed by a central EEG reader (D.D.). Disagreements regarding study eligibility were resolved by the study's EEG core (D.D., E.M., S.M., Y.S., J.L.P., and S.S.).
A normal EEG background was defined as evidence of a posterior dominant rhythm between 8 and 13 Hz during relaxed wakefulness. For younger patients, at least some epochs of an 8-Hz posterior dominant rhythm were required for inclusion. All bursts of GSW lasting 3 seconds or longer, with or without clinical signs, were considered to be seizures. Seizure onset was defined as the time of the first spike of a well-formed GSW complex, and seizure offset was defined as the end of the slow wave after the last spike. Seizures interrupted by at least 1 second of preseizure baseline EEG (either baseline wakefulness or baseline HV) were considered as distinct seizures; an ongoing seizure interrupted by less than 1 second of nonseizure activity was considered to be one continuous seizure. GSW bursts lasting less than 3 seconds were not considered to be seizures.
Study outcomes and sample size.
This study's primary EEG outcomes were i) time to first seizure, ii) number of seizures, iii) seizure duration, iv) total seizure exposure, and v) the presence/absence of any seizure lasting ≥20 seconds. Time to first seizure was defined as the time from EEG start to the time of the first burst of a GSW discharge, with or without clinical signs, which lasted ≥3 seconds. Total seizure exposure was the percent of time spent in seizures per hour.
CPT was assessed using confidence index and omission T score. The confidence index reflects the level of confidence, between 0% and 100%, that the patient tested has produced a performance consistent with the presence of a clinically significant attention disorder. A confidence index of ≥0.60 reflects a clinically significant attention disorder. The T score is a standardized score for age of the number of omissions, i.e., not hitting the keyboard when required by the test on the screen.10 Baseline executive function of the patient was assessed using 3 measures from the WCST11: perseverative responses, trials to achieving the first category, and total categories achieved on the test.
The study's sample size of 446 was selected for the clinical trial (to detect a 20% difference in freedom-from-failure [FFF] rates between the 3 medications at the 16- to 20-week visit5).
Statistical analysis.
Data were summarized and analyzed at the patient level, rather than the seizure level. Therefore, duration of seizures was defined in 3 ways for each patient: the shortest seizure duration among all seizures on the 60-minute EEG, the longest duration, and the median duration. Because of the high skewness in the EEG characteristics, medians, minimum, maximum, and quartiles are used to describe the EEG variables except for the presence or absence of any seizure >20 seconds, which was a dichotomous variable. Spearman correlations between the EEG variables and the CPT and WCST variables were calculated. Exact χ2 tests were performed to test whether there is a relationship between patients who had at least one seizure of ≥20 seconds and WCST categories achieved and trials to first category percentile.
Logistic regression or χ2 was used to explore whether the EEG characteristics were predictive of treatment outcome, controlling for treatment group (ETX, VPA, LTG) and age groups (<6 years, ≥6 years; <9 years, ≥9 years). Single variable models were explored first, followed by best subsets models to identify the best-fitting multivariable model of pretreatment EEG features to predict FFF and SF at the 16- to 20-week visit. Odds ratios for FFF and SF were calculated.12 SF was not defined for patients who discontinued before the 16- to 20-week visit (mostly because of intolerable side effects), hence the analysis was restricted to only those patients who reached that visit. Significance was defined at p = 0.05. Analyses were performed using SAS software (SAS Institute, Cary, NC).
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review boards at all 32 sites and the study's coordinating center. Written parental consent and, when appropriate, child assent were obtained in all cases. An NIH-appointed Data and Safety Monitoring Board provided oversight for this study.
RESULTS
Patient population.
Among the 453 subjects considered by local sites to have an EEG that qualified for the study, 99% (450/453) were confirmed by central review. Of the 3 subjects deemed ineligible because of EEG criteria, 1 had a slow EEG background, 1 had all GSW bursts <3 seconds in duration, and 1 had bursts of generalized paroxysmal fast activity preceding all spike and wave discharges. In addition, 3 subjects were deemed ineligible for other medical reasons and one withdrew consent after randomization and before receiving any study drug. The final study cohort was 446 subjects. Detailed evaluation of the pretreatment EEG was possible for 99.8% of study subjects (445/446); one EEG was not subsequently evaluable for technical reasons.
Table 1 shows participant demographics and circumstances of the first EEG seizure. The standardized EEG protocol was followed in 91% of subjects (406/445). Of the 39 subjects who did not follow the EEG protocol, 36 subjects had delayed HV trials and 3 subjects were too young to cooperate with fixed periods of HV.
Table 1.
Participant demographics, treatment group, and circumstances of first GSW burst establishing eligibilitya
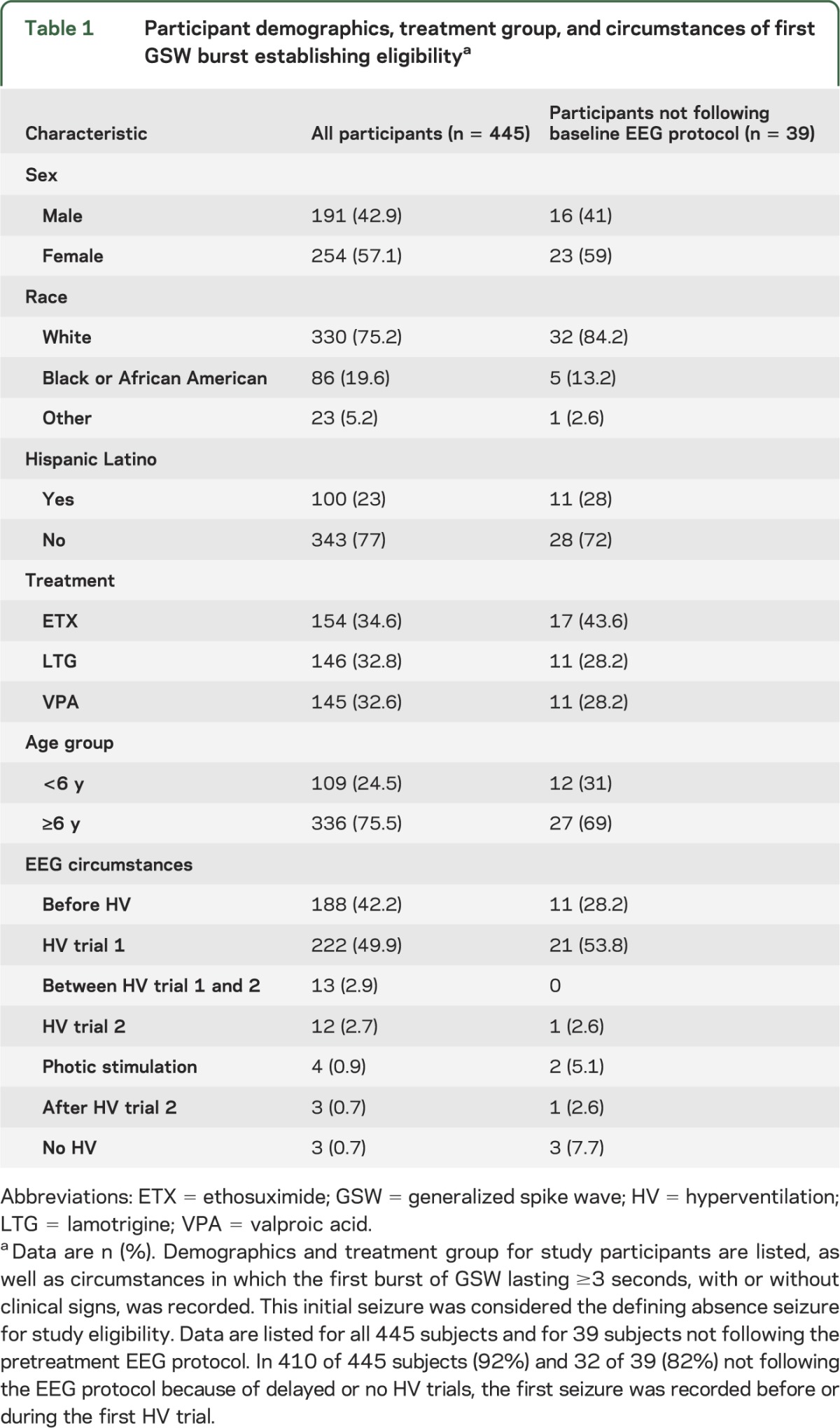
Time to first seizure.
The median time to first seizure was 6.0 minutes (range 0–58.9 minutes). In 427 of 445 subjects (94%), the first seizure was recorded within the first 30 minutes of pretreatment EEG. For the 36 subjects with delayed HV trials, time to first seizure was a mean of 17.24 minutes (range 1.0–58.9 minutes). Distributions for time to first seizure are shown in figure 1. For the 3 subjects too young to cooperate with formal HV trials, time to first seizure was 1 minute, 3.3 minutes, and 11.4 minutes of EEG recording.
Figure 1. EEG recording time to establish study eligibility (n = 445).
Time (in minutes) needed to record the first burst of generalized spike wave (GSW) lasting ≥3 seconds, with or without clinical signs, is shown on the horizontal axis. This initial seizure was considered the defining absence seizure for study eligibility. Median time to EEG confirmation of childhood absence epilepsy diagnosis was 5.96 minutes (range 0–58.9 minutes). In 427 of 445 subjects (94%), the first seizure was recorded within the first 30 minutes of pretreatment EEG.
Number and duration of seizures.
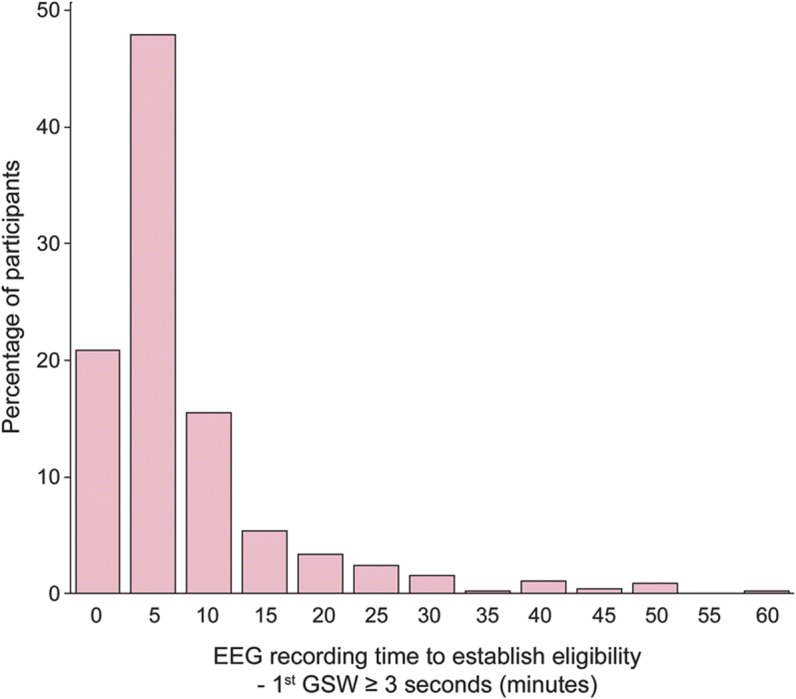
The EEG core reached consensus about seizure start and stop times in 98.8% of records (440/445). Among those 440 EEGs, the median number of seizures per 1-hour study EEG was 5 (range 1–60). The 25th percentile was 3 seizures and the 75th percentile was 8 seizures. The median seizure duration across the study EEGs was 10.8 seconds (range 3.3–77.6 seconds). The median duration of shortest seizure per EEG was 7.5 seconds (range 3–77.6 seconds), whereas the median longest seizure per EEG was 14.9 seconds (range 3.6–105.3 seconds). Box-and-whisker plots of shortest, longest, and median seizure duration are shown in figure 2. The median total seizure exposure was 1.5% (54 seconds), with a range of 0.1% (3.6 seconds) to 47.0% (28 minutes, 12 seconds). A seizure lasting ≥20 seconds was noted in 29% of subjects (129/440).
Figure 2. Pretreatment seizure duration (n = 440).
Box-and-whisker plots of seizure duration on pretreatment EEG are shown. The boundaries of the boxes are the 25th percentile and 75th percentile of the distributions of the shortest, median, and longest seizure duration per patient. The line within the box reflects the median of these durations and the “+” symbol reflects the mean. The whiskers extend 1.5 times the length of the box from the median to the applicable percentile (25th or 75th), beginning from the 25th or 75th percentile. The asterisks represent outliers that are beyond the end of the whiskers as defined here.
There was a moderate association between number of seizures and their duration (Spearman ρ = −0.52 and −0.33 for shortest and median duration, respectively). Patients with shorter burst durations tended to have more seizures. There was a positive correlation between the shortest seizure duration and longest seizure duration (Spearman ρ = 0.65), indicating some level of consistency within a patient regarding the duration of their seizures.
Other EEG features.
Occipital intermittent rhythmic delta activity was present in 21% of EEGs (93/440), whereas frontal intermittent rhythmic delta activity was present in 4.5% of EEGs (20/440). Focal sharp waves, distinct from fragments of GSW, were present in 2.5% of EEGs (11/440), and focal slowing was present in 0.7% of EEGs (3/440). A sample of 721 GSW bursts was reviewed for morphologic characteristics: 84% (604/721) of bursts contained single spike and wave discharges at onset, 3% (21/721) contained polyspike and wave discharges at onset, and consensus on spike morphology could not be reached with 13% of bursts (96/721). Because only 3% of bursts contained clear polyspikes and 13% of bursts contained unclear spike morphology (typically inconsistent morphology based on electrode location), morphology of GSW bursts was not used in predictive models, which were designed with variables readily generalizable to clinical practice.
Baseline EEG and neuropsychological data.
There were no significant correlations among the number of seizures, median seizure duration, or total seizure exposure and any attentional (CPT) or executive function (WCST) measures. However, subjects with at least one seizure ≥20 seconds had a higher median omissions T score on CPT compared with subjects whose seizures were all <20 seconds (56.3 vs 51.6, p = 0.01). These 2 subgroups (patients with and without a seizure ≥20 seconds) showed no difference between them for CPT confidence index, CPT commissions T score, WCST categories achieved, and WCST trials to first categories.
Baseline EEG and treatment outcome.
Of the 440 patients for whom baseline EEG characteristics are reported, 329 (75%) remained on treatment until the 16- to 20-week visit. Therefore, the outcome of SF vs not seizure-free, defined at the 16- to 20-week visit, was assessed only for these 329 patients. There were no differences in demographics, attention variables, or executive function variables at baseline between those who reached the 16- to 20-week visit and those who did not. Regarding EEG characteristics, those who discontinued had on average shorter burst durations. The mean shortest burst duration was 6.5 seconds on the discontinuing patients and 7.8 seconds on the patients who reached weeks 16–20 (p = 0.02). Results were similar for the median and longest burst durations (not shown).
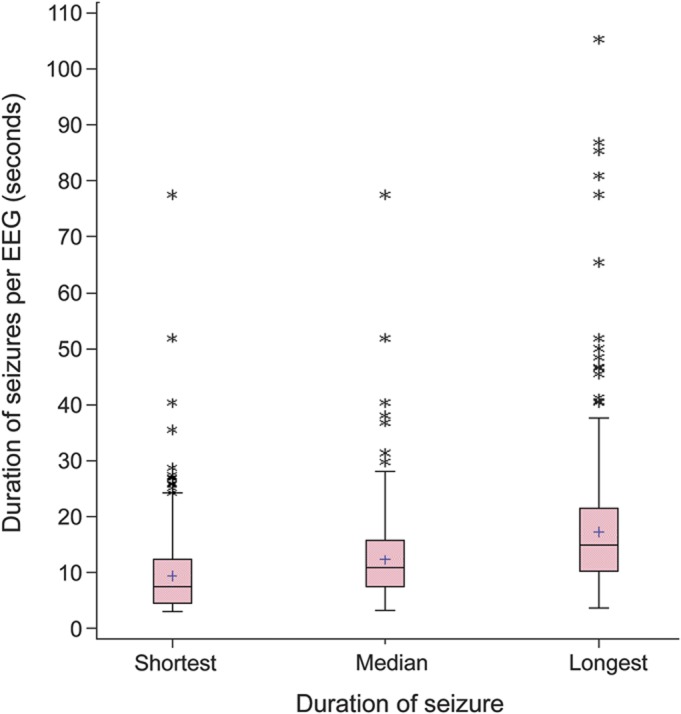
Table 2 shows single variable logistic regression models for the EEG characteristics as well as primary predictive variables, such as age group and treatment. Logistic regression modeling showed that subjects with a shortest seizure of longer duration on baseline EEG or a longer median duration of all seizures on baseline EEG were more likely to demonstrate treatment success by both FFF and SF criteria. Predictive models demonstrated that duration of shortest seizure on pretreatment EEG discriminated between patients who achieved FFF and SF at the 16- to 20-week visit, with an area under the curve of 67% for predicting FFF and 78% for predicting SF (figure 3). Tests of interaction, including with treatment group, did not change this relationship. A similar relationship to that observed with the shortest seizure on the baseline EEG existed using the median seizure duration as the predictor variable. Logistic regression revealed no significant associations among number of seizures, total seizure exposure, or duration of longest seizure and treatment outcome, assessed by either FFF or SF. No association was found between the presence of at least one seizure ≥20 seconds on baseline EEG and treatment outcomes.
Table 2.
Logistic regression predictive model of FFF and SF at 16- to 20-week visita
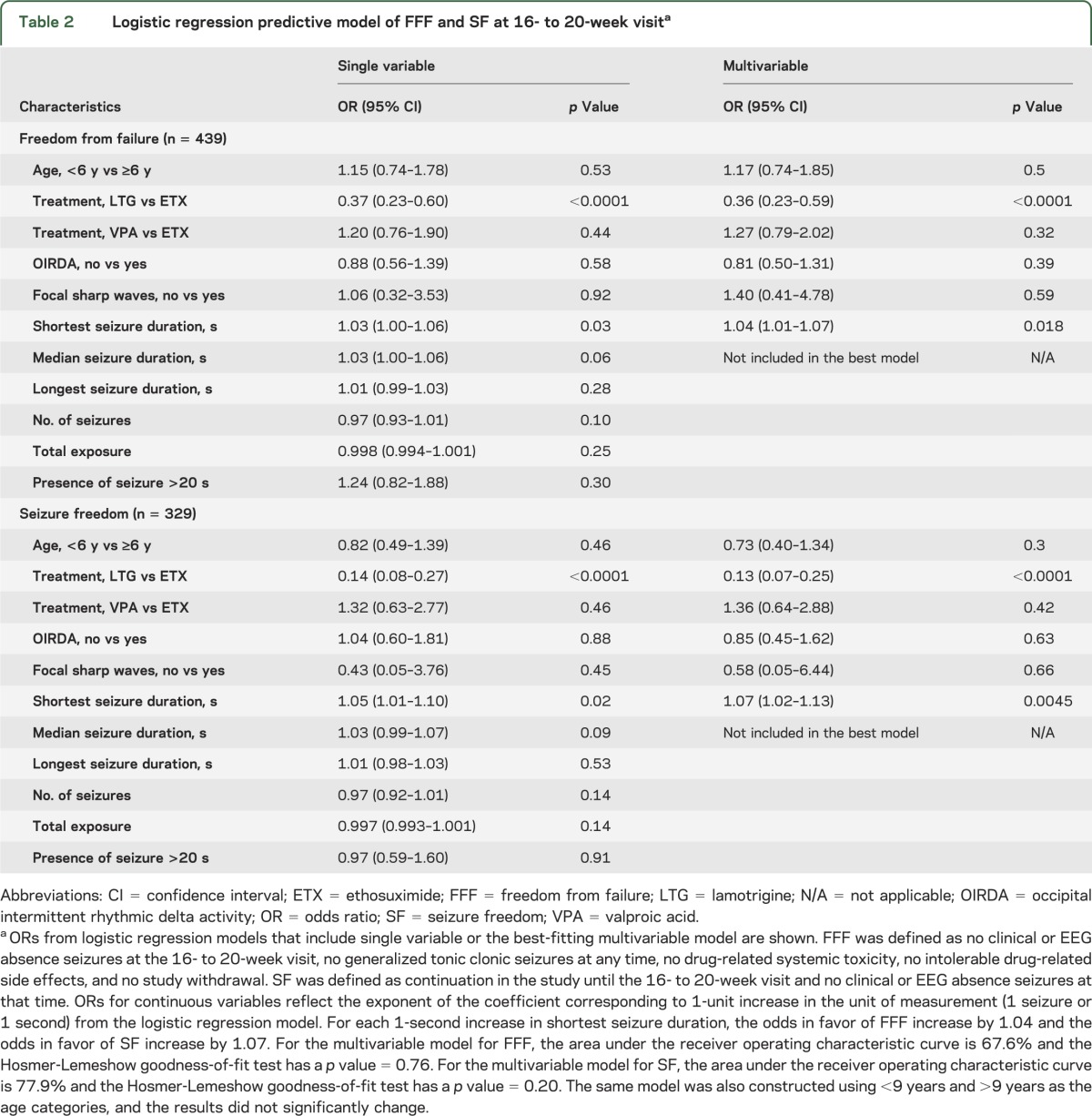
Figure 3. Predictive models of freedom-from-failure (FFF) (A) and seizure-freedom (SF) (B) outcomes at 16- to 20-week visit based on pretreatment EEG (n = 439 for FFF and n = 329 for SF).
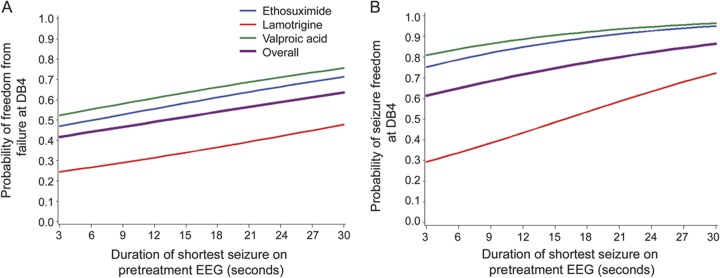
A shortest seizure of longer duration on pretreatment EEG predicted a higher likelihood of FFF and SF at 16- to 20-week visit (DB4), even after controlling for treatment group (p = 0.018, area under the curve [AUC] = 67.6% for FFF and p = 0.0045, AUC = 77.9% for SF). Overall denotes the model without controlling for treatment group. A similar relationship exists using the median duration of all seizures as the predictor variable, but shortest seizure duration is more directly applied in a clinical setting.
DISCUSSION
This study demonstrates that the frequency of pretreatment seizures in CAE, as measured by a 1-hour video-EEG, is not associated with baseline measures of attention and executive function or with treatment outcome. However, patients with seizure duration longer than 20 seconds did perform worse on baseline measures of attention by being more likely to commit errors of omission on attention testing. Interestingly, patients with a shortest seizure of longer duration (and longer median duration of all seizures) before treatment responded more favorably to treatment, even when including adjustments for age group, treatment group, and other factors. Placing the results in a clinical context, patients with consistently shorter seizures (<7.5 seconds) had a worse response to initial treatment (63% seizure-free at 16- to 20-week visit), whereas patients with longer seizures responded more favorably to initial treatment (74% seizure-free at 16- to 20-week visit), but were more likely to display subtle attentional problems if pretreatment seizures lasted longer than 20 seconds. These findings underscore the complex relationship between seizures and inattention in patients with CAE.
Because EEG was not recorded simultaneously during the neuropsychological testing, it is unknown whether the errors of omission occurred during absence seizures, whether such neuropsychological features are part of the interictal phenotype of CAE patients with a tendency toward longer seizures, or both. Features of inattention are seen in 35% of children with newly diagnosed CAE,5 but this study suggests that patients with pretreatment seizures longer than 20 seconds are at even greater risk for inattention. In contrast to some past reports,4 which found longer seizures to be uncommon in CAE, 30% of this CAE cohort had at least one pretreatment seizure longer than 20 seconds.
An explanation for the association of longer seizures with more favorable treatment response is not immediately clear. Longer duration of the shortest seizure (>7.5 seconds) and longer median duration of all seizures (>10.8 seconds) were associated with better treatment response, regardless of treatment group or age group. Of note, the duration of the single longest seizure per EEG was not associated with treatment response, indicating that this relationship is not explained by patients with rare prolonged seizures. Across other epilepsies, etiology rather than seizure duration is more consistently associated with response to treatment. Patients with CAE with shorter seizure duration may have different genetic etiologies than CAE patients with longer seizures, and this question merits further study.
Seizure circumstances and duration in this study were similar to previous reports of CAE. A study of 47 children with CAE found an average EEG seizure duration of 9.4 seconds, with 83% of seizures occurring during HV.13 Another study of 37 children with CAE reported that EEG seizure duration ranged from 3 to 40 seconds, with 3 patients having seizures longer than 20 seconds.14 The current study is the first to evaluate associations among ictal EEG features, baseline measures of attention and executive function, and treatment outcome.
The current study also demonstrates that the diagnosis of CAE can be reliably made across many study sites and quickly confirmed by EEG. Although we used a 1-hour baseline EEG to confirm the diagnosis, only 6% of patients required more than 30 minutes of EEG to confirm the diagnosis. Consistent with the CAE phenotype, patients quickly had seizures either spontaneously or with HV, with 92% of patients having a seizure before or during the first trial of HV. Past studies of CAE have used EEGs or video-EEGs ranging from 20 minutes to 24 hours to confirm the diagnosis.15–18 In this study, a standardized 1-hour video-EEG protocol was practical and adequate to assess subject eligibility. This practice should be applied judiciously in clinical settings. The subjects randomized in this trial were carefully screened for clinical features associated with CAE. Some subjects had office-based HV trials demonstrating clinical absence seizures before the study EEG, and some subjects had prior routine EEGs demonstrating abnormalities consistent with CAE. These results do not imply that children evaluated for staring spells or other concerns for absence seizures can be evaluated solely with a routine EEG. Such children require a clinical evaluation and, if indicated, EEG testing with trials of HV. If a waking EEG baseline segment, 2 HV trials, and a trial of photic stimulation are performed within 30 minutes, additional EEG recording has a low diagnostic yield in this setting.
The superior effectiveness of ETX and VPA compared with LTG in controlling seizures without intolerable adverse events in patients with CAE has been shown at 16–20 weeks and 12 months of treatment.5,6 However, the VPA cohort experienced a higher rate of adverse events as well as significant negative effects on attentional measures not seen in the ETX cohort, indicating that ETX is the optimal initial empirical monotherapy for CAE.6 The current study indicates that CAE patients with pretreatment seizures lasting more than 20 seconds are at further increased risk for inattention, suggesting VPA should be used with particular caution as initial monotherapy in patients with longer-duration pretreatment seizures.
This study demonstrates that CAE is reliably and quickly confirmed by EEG but has interpatient variability in pretreatment seizure number and seizure duration. Patients with longer seizures at baseline are at greater risk for inattention but have more favorable initial treatment response. This information can be useful in counseling patients about the complex relationships among seizures, inattention, and treatment response in CAE. Further phenotypic and genetic studies of CAE are indicated, as well as long-term follow-up of this cohort.
Supplementary Material
GLOSSARY
- CAE
childhood absence epilepsy
- CPT
Continuous Performance Test
- ETX
ethosuximide
- FFF
freedom from failure
- GSW
generalized spike wave
- HV
hyperventilation
- LTG
lamotrigine
- SF
seizure freedom
- VPA
valproic acid
- WCST
Wisconsin Card Sorting Test
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and study supervision: D. Dlugos, S. Shinnar, A. Cnaan, S. Moshé, E. Mizrahi, D. Masur, Y. Sogawa, J.B. Le Pichon, C. Levine, D. Hirtz, P. Clark, P.C. Adamson, and T. Glauser. Statistical analysis: A. Cnaan and F. Hu.
STUDY FUNDING
Supported by the NIH (grants U01-NS045911and U01-NS045803).
DISCLOSURE
D. Dlugos is funded by NIH grants 1R01NS053998, 2U01NS045911, 1R01LM011124, and U01NS077276. He has also given expert testimony in medicolegal cases. S. Shinnar is funded by NIH grants NS R01-NS043209, 2U01-NS045911, NS 066929, U10NS077308, and P20 NS080185. He was previously funded by NIH grants NS 053998 and UL1 RR025759. He served on a DSMB for King Pharmaceuticals, has received personal compensation for serving on scientific advisory boards for Questcor, Sunovion, and Upsher-Smith, for consulting for Eisai, Questcor, and Neuronex, and speaker honoraria from Eisai and Questcor. He has also given expert testimony in medicolegal cases. A. Cnaan is funded by NIH grants 2U01-NS045911, UL1RR031988, P30HD040677, P50AR060836, R01AR061875, and R01HD058567, Department of Defense grant W81XWH-09-1-0592, and Department of Education grant H133B090001. She is also the statistician member of an Independent Data Monitoring Committee for GlaxoSmithKline (GSK). F. Hu is funded by NIH grants U01NS045911 and P50AR060836, Department of Defense grant W81XWH-09-1-0592, and Department of Education grant H133B090001. S. Moshé is funded by NIH grants NS43209, NS20253, and NS45911 and receives support from the Heffer and Segal Family Foundations. He received a consultant fee from Eisai and a speaker's fee and travel expenses from GSK. E. Mizrahi serves on the scientific advisory board of the Treatment of Neonatal Seizures with Medical Off-patent Research Study (European Union: EU FP7); receives research support from the US Department of Defense (award number:W81XWH-08-2-0149) and for sponsored research from NeuroPace, Inc.; received travel funds and honoraria from the University of Maryland, Baltimore, the Children's National Medical Center, Washington, DC, and the Texas Neurological Society, Austin; received travel funds from International Leagues Against Epilepsy, Pediatric Neurology Society of Argentina, Congress of the Union of European Neonatal and Perinatal Societies, and FLENI Institute of Argentina; and received honorarium from the American Academy of Neurology. D. Masur is funded by NIH grants R01-NS043209 and 2U01-NS045911. He has also given expert testimony in medicolegal cases. Y. Sogawa is funded by NIH grant NIH U01-NS045911. She has received support from the Susan Spencer Young Clinical Investigator Award from the American Epilepsy Society. She was an NSADA trainee under K12NS-048856. J.B. Le Pichon, C. Levine, and D. Hirtz report no disclosures. P. Clark is funded by NIH grants 2U01-NS045911 and U10-NS077311. She has received consulting and speaking fees from Eisai. P.C. Adamson is funded by NIH NCI grants U10CA098543, U10CA098543-10S1, and U10CA098543-10S2. T. Glauser is funded by NIH grants 2U01-NS045911, U10-NS077311, R01-NS053998, R01-NS062756, R01-NS043209, R01-LM011124, and R01-NS065840. He has received consulting fees from Supernus, Sunovion, Eisai, UCB, Lundbeck, and Questcor, and is on the speakers bureau of Eisai and Questcor. He also serves as an expert consultant for the US Department of Justice and has received compensation for work as an expert on medicolegal cases. He receives royalties from a patent license from AssureRx Health. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B. How well can epilepsy syndromes be identified at diagnosis? A reassessment 2 years after initial diagnosis. Epilepsia 2000;41:1269–1275 [DOI] [PubMed] [Google Scholar]
- 2.Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Epilepsia 2001;42:464–475 [DOI] [PubMed] [Google Scholar]
- 3.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30:389–399 [DOI] [PubMed] [Google Scholar]
- 4.Loiseau P, Duche B, Pedespan JM. Absence epilepsies. Epilepsia 1995;36:1182–1186 [DOI] [PubMed] [Google Scholar]
- 5.Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid and lamotrigine in childhood absence epilepsy. N Engl J Med 2010;362:790–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: initial monotherapy outcomes at 12 months. Epilepsia 2013;54:141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouma PA, Westendorp RG, van Dijk JG, Peters AC, Brouwer OF. The outcome of absence epilepsy: a meta-analysis. Neurology 1996;47:802–808 [DOI] [PubMed] [Google Scholar]
- 8.Wirrell EC, Camfield CS, Camfield PR, Dooley JM, Gordon KE, Smith B. Long-term psychosocial outcome in typical absence epilepsy: sometimes a wolf in sheeps’ clothing. Arch Pediatr Adolesc Med 1997;151:152–158 [DOI] [PubMed] [Google Scholar]
- 9.Pavone P, Bianchini R, Trifiletti RR, Incorpora G, Pavone A, Parano E. Neuropsychological assessment in children with absence epilepsy. Neurology 2001;56:1047–1051 [DOI] [PubMed] [Google Scholar]
- 10.Conners CK. Conners’ Continuous Performance Test II: Technical Guide and Software Manual. North Tonawanda, NY: Multi-Health Systems; 2002 [Google Scholar]
- 11.Heaton KR, Chelune GJ, Talley JL, Kay GG, Curtis G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources; 1993 [Google Scholar]
- 12.Rosner B. Fundamentals of Biostatistics, 4th ed Belmont, CA: Duxbury Press; 1995 [Google Scholar]
- 13.Sadleir LG, Farrell K, Smith S, Connolly MB, Scheffer IE. Electroclinical features of absence seizures in childhood absence epilepsy. Neurology 2006;67:413–418 [DOI] [PubMed] [Google Scholar]
- 14.Ma M, Zang Y, Yang Z, et al. Childhood absence epilepsy: electroclinical features and diagnostic criteria. Brain Dev 2011;33:114–119 [DOI] [PubMed] [Google Scholar]
- 15.Callaghan N, O’Hare J, O’Driscoll D, O’Neill B, Daly M. Comparative study of ethosuximide and sodium valproate in the treatment of typical absence seizures (petit mal). Dev Med Child Neurol 1982;24:830–836 [DOI] [PubMed] [Google Scholar]
- 16.Frank LM, Enlow T, Holmes GL, et al. Lamictal (lamotrigine) monotherapy for typical absence seizures in children. Epilepsia 1999;40:973–979 [DOI] [PubMed] [Google Scholar]
- 17.Holmes GL, Frank LM, Sheth RD, et al. Lamotrigine monotherapy for newly diagnosed typical absence seizures in children. Epilepsy Res 2008;82:124–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coppola G, Auricchio G, Federico R, Carotenuto M, Pascotto A. Lamotrigine versus valproic acid as first-line monotherapy in newly diagnosed typical absence seizures: an open-label, randomized, parallel group study. Epilepsia 2004;45:1049–1053 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


