Abstract
Objective:
The present study characterizes the relationship between report of stroke symptoms (SS) or TIA and incident cognitive impairment in the large biracial cohort of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study.
Methods:
The REGARDS Study is a population-based, biracial, longitudinal cohort study that has enrolled 30,239 participants from the United States. Exclusion of those with baseline cognitive impairment, stroke before enrollment, or incomplete data resulted in a sample size of 23,830. Participants reported SS/TIA on the Questionnaire for Verifying Stroke-free Status at baseline and every 6 months during follow-up. Incident cognitive impairment was detected using the Six-item Screener, which was administered annually.
Results:
Logistic regression found significant association between report of SS/TIA and subsequent incident cognitive impairment. Among white participants, the odds ratio for incident cognitive impairment was 2.08 (95% confidence interval: 1.81, 2.39) for those reporting at least one SS/TIA compared with those reporting no SS/TIA. Among black participants, the odds ratio was 1.66 (95% confidence interval: 1.45, 1.89) using the same modeling. The magnitude of impact was largest among those with fewer traditional stroke risk factors, particularly among white participants.
Conclusions:
Report of SS/TIA showed a strong association with incident cognitive impairment and supports the use of the Questionnaire for Verifying Stroke-free Status as a quick, low-cost instrument to screen for people at increased risk of cognitive decline.
“Silent infarcts,” defined as vascular injury evident on neuroimaging without a clinical history of stroke, are evident in 8% to 20% of people older than 55 years.1,2 Population-based imaging studies have documented that increasing burden of white matter changes has a strong association with incident cognitive impairment.1,2 Direct application of these findings to large epidemiologic studies or to clinical practice is limited because of the cost and inconvenience associated with brain MRI.
In the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study, self-report of stroke symptoms on the Questionnaire for Verifying Stroke-free Status (QVSS) in the absence of diagnosed stroke was conceptualized as a potential marker of silent infarcts and brain ischemia. Previous work from the REGARDS Study found that stroke symptoms were reported by 18% of participants at baseline,3 and persons reporting stroke symptoms (SS) at baseline were 24% more likely to be cognitively impaired.4 Since publication of this earlier work, longitudinal data assessing cognitive function have become available, allowing investigation of the relationship between report of stroke symptoms and subsequent cognitive decline. Diagnosis of TIA implies that medical care was sought for reported transient neurologic symptoms. To minimize biases caused by access to medical care, we grouped reports of stroke symptoms and TIA to form a joint predictor variable. Thus, we hypothesized that report of stroke symptoms in the absence of clinical stroke is associated with higher risk of incident cognitive impairment, similar to the effect demonstrated in studies using an MRI-based definition of silent infarct.
METHODS
Sample.
The REGARDS Study is a national, population-based, longitudinal cohort study designed to investigate factors associated with the excess stroke mortality observed among African Americans and residents of the southeastern Stroke Belt region. The design has been previously reported.5 Briefly, recruitment of the cohort began in January 2003 and was completed in October 2007. The study oversampled the Stroke Belt/Buckle, with 21% of the cohort recruited from the Buckle of the Stroke Belt (coastal plain region of NC, SC, and GA), 35% from the Stroke Belt states (remainder of NC, SC, and GA, plus AL, MS, TN, AR, and LA), and the remaining 44% from the other 40 contiguous states. Participants were selected from commercially available lists of residents, using a combination of mail notification and telephone contact.
Standard protocol approvals, registrations, and participant consents.
Study procedures were reviewed and approved by the institutional review boards at the collaborating institutions. All subjects provided written informed consent to participate in the study.
Procedures.
Baseline data, including demographics, cardiovascular risk factor history, report of stroke symptoms, stroke, and TIA were collected via telephone interview at baseline. After the telephone interview, an in-home examination was used to gather physical measures including blood pressure, blood and urine samples, ECG, and medication inventory. Incident stroke was ascertained via telephone follow-up every 6 months using the QVSS and verified by medical record review and adjudication by a panel of neurologist stroke experts. The questions comprising the QVSS are included in the figure.
Figure. Stroke symptoms/TIA questionnaire items13.

Demographic data.
Sex, education (categorized as less than high school, high school graduate, some college, or college graduate and above), race (black or white), income (<$20k, $20k–$34k, $35k–$74k, ≥$75k, or refused), and Framingham Stroke Risk Profile (FSRP) were extracted from the REGARDS database for statistical modeling.
Measures.
The Six-item Screener (SIS) was used as a global measure of cognitive status. The SIS assesses 3-item recall and orientation to year, month, and day of the week and has been validated against clinical diagnoses of dementia and mild cognitive impairment.6 Scores range from 0 to 6; scores <5 indicate cognitive impairment with sensitivity of 74.2 and specificity of 80.2 in community-based samples.6 The SIS has been used to document cognitive impairment in older patients seen in emergency departments7 and older depressed patients in a large randomized controlled trial.8 SIS scores are related to self-reported stroke symptoms and health behaviors,4 cardiovascular risk factors,9–11 and kidney dysfunction.12 Incident cognitive impairment was defined as decline from an initial SIS score of 5 or 6 to the most recent follow-up score of 4 or less.
SS/TIA were assessed using the QVSS13 at baseline and at 6-month intervals thereafter. Participants were categorized into those having at least one SS/TIA before their most recent SIS assessment vs those not having any SS/TIA during baseline or at any follow-up assessment.
Statistical analyses.
Associations between categorical variables and presence of at least one SS/TIA report were determined by χ2 tests, and the association between the continuous variable (FSRP) and SS/TIA status was determined by t test. A series of incremental logistic regression models were constructed to evaluate whether SS/TIA were associated with the outcome of incident cognitive impairment at the time of last SIS (model 1: unadjusted; model 2: adjusted for race, sex, education, income, and SIS test version; and model 3: adjusted for the model 2 factors as well as the FSRP). Statistical interactions between each of the covariates and the presence of any SS/TIA were evaluated at the α = 0.1 level. To identify the contribution of TIA relative to the other SS, and the contribution of baseline symptoms relative to more recent symptoms, secondary analyses included 1) evaluating only the 6 SS as outcomes and excluding those with prior TIA, and 2) evaluating only SS that occurred during the follow-up period and excluding those with baseline symptoms. For the purpose of comparing the magnitude of the contribution of each of the FSRP and SS/TIA to subsequent cognitive impairment, models with FSRP as predictor (without the SS/TIA variable) were fitted, and were adjusted using the same covariates as models assessing SS/TIA. A priori statistical tests were performed at 0.05 for main effects and 0.10 for assessments of interaction.
RESULTS
All REGARDS Study participants having 2 or more SIS assessments were considered for analysis. Of 30,239 REGARDS Study participants having baseline information and informed consent, as a first step in data management (in order of application of the exclusion criteria), 56 were excluded because of data anomalies, then 1,930 because of self-reported stroke at baseline, 1,923 because of no SIS assessment, then 137 because of no SIS assessment before incident stroke, then 2,103 because of cognitive impairment at baseline (SIS score ≤ 4), and then 260 because of only one SIS assessment. There were 515 participants with confirmed strokes during follow-up (reported strokes were adjudicated by study physicians using hospital records), and we censored any SIS assessments after the date of that stroke, resulting in 1,439 assessments among these participants being excluded. Thus, 23,830 participants (mean baseline age 64.2 years) were identified who had at least 2 cognitive function assessments (11,096 with 2–4, and 12,734 with >4).
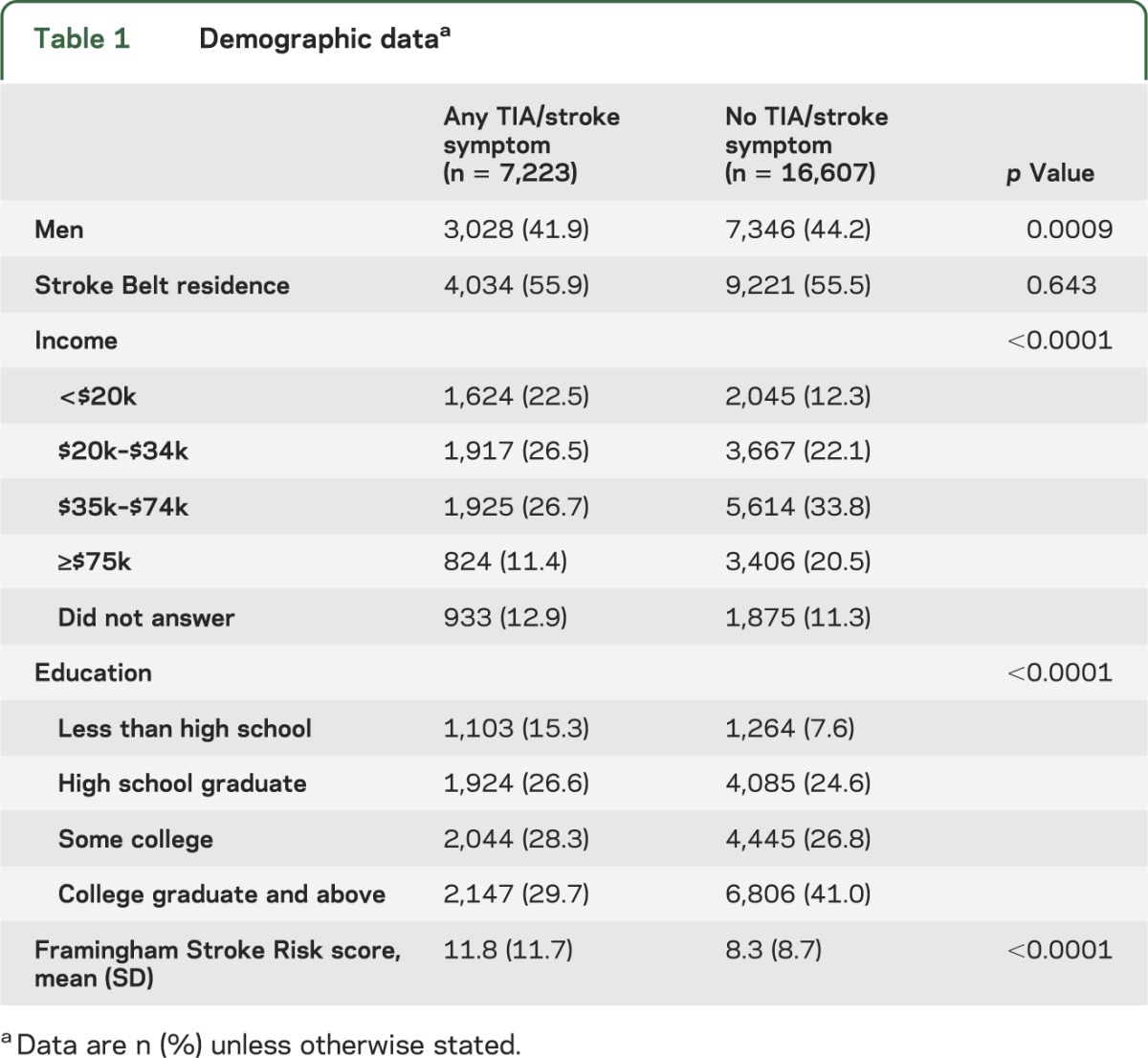
Women, participants with lower education or lower income, and those with higher FSRP score were more likely to have at least one self-reported SS or TIA (table 1).
Table 1.
Demographic dataa
Significant interactions between SS/TIA and race and SS/TIA and FSRP score were noted (full-model p values = 0.013 and 0.098, respectively), so the logistic regression results were stratified by race and selected percentiles of the FSRP distribution.
Table 2 shows that the association between any SS/TIA and incident cognitive impairment was significant for both black and white participants, but the magnitude of the association was larger in white participants. Among white participants, the odds of incident cognitive impairment were 2.08 times higher (95% confidence interval [CI]: 1.81, 2.39) for those reporting at least one SS/TIA compared with those reporting no SS/TIA in an unadjusted model. After adjustment for demographics and socioeconomic status (SES) factors, the odds ratio (OR) was attenuated slightly (1.85, 95% CI: 1.61, 2.13). Among black participants, the odds of incident cognitive impairment were 1.66 times higher (95% CI: 1.45, 1.89) for those reporting at least one SS/TIA compared with those reporting no SS/TIA in an unadjusted model. After adjustment for demographics and SES factors, the OR was attenuated slightly (1.41, 95% CI: 1.23, 1.62). Furthermore, the final model including FSRP showed that at lower stroke risk, the effect of SS/TIA was larger, particularly in white participants.
Table 2.
Results from logistic regression models of risk of incident cognitive impairment associated with report of SS/TIAa

Table 3 shows the SS results using only incident reports of SS (excluding baseline reports), in which we assessed the odds of incident cognitive impairment among those with at least one SS during the follow-up period. The ORs were much larger than for the results obtained for any SS/TIA including baseline; however, the interactions with race and FSRP were no longer significant.
Table 3.
Results from logistic regression models of risk of incident cognitive impairment and any SS at follow-upa

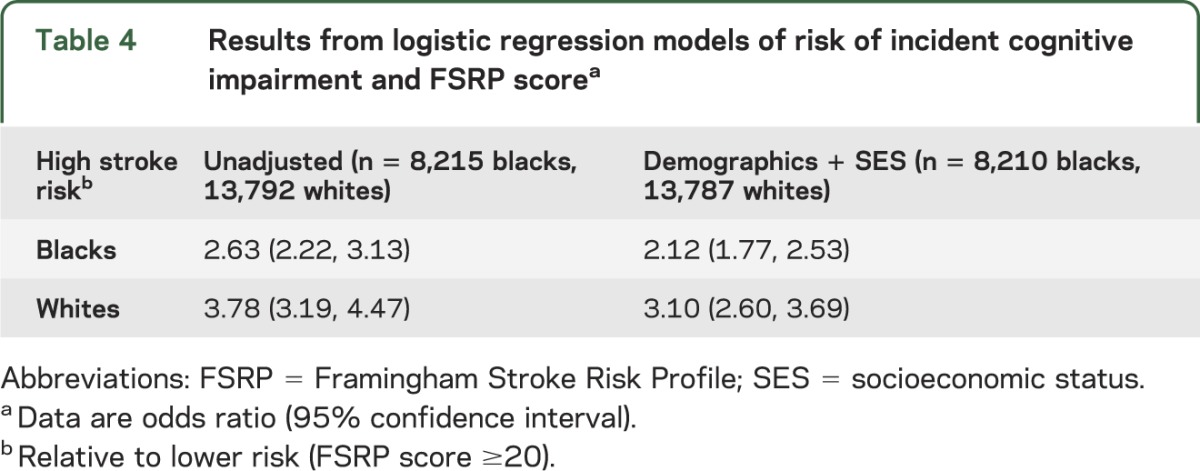
Table 4 shows the risk associated with high FSRP score (>20) after adjustment using the same covariates as previous models, but without including report of SS/TIA in the model. After adjustment for demographics and SES factors, the OR of incident cognitive impairment for those having a high FSRP score was 2.12 (95% CI: 1.77, 2.53) for black participants and 3.10 (95% CI: 2.60, 3.69) for white participants.
Table 4.
Results from logistic regression models of risk of incident cognitive impairment and FSRP scorea
DISCUSSION
In an initially stroke-free cohort and with censoring of incident stroke cases in the follow-up, report of SS/TIA was strongly related to increased risk of cognitive impairment. Interestingly, the increased risk associated with SS/TIA was larger in participants with lower FSRP score, suggesting that the QVSS assesses risk differently than the FSRP, possibly related to duration of exposure. The magnitude of the association was larger in white than black participants, suggesting differential sensitivity or exposure to this risk.
We hypothesized that there would be a higher incidence of cognitive impairment in participants experiencing stroke-like symptoms in the absence of clinical stroke, and that this difference would be mediated by the traditional stroke risk factors encapsulated in the FSRP. Our modeling suggests that the effect of SS/TIA differs by the risk level for stroke (as estimated by the FSRP, table 2), and for white subjects, the report of SS/TIA may reflect risk factors for cognitive impairment not encapsulated within the FSRP. Family history and environmental exposures are likely candidates because these are not represented in the FSRP. Alternatively, the SS/TIA report may account for duration or severity of exposure to the vascular risk factors already included in the FSRP, or may reflect racial differences in the appropriate weighting factors for the FSRP items.
It is increasingly clear that vascular risk factors have important roles for both vascular cognitive impairment and Alzheimer disease, and this may reflect pathologic processes in common to these 2 causes of dementia. Of participants in the Cardiovascular Health Study (older than 65 years), 28% had silent infarcts on MRI and substantially higher rates of impaired cognition,14 and these silent infarcts independently predicted risk of symptomatic stroke over a 4-year follow-up period.15 Similarly, in the Atherosclerosis Risk in Communities cohort, cross-sectional analyses identified increased risk of cognitive impairment in the context of white matter hyperintensities, ventricular and sulcal abnormalities, and silent infarcts.16 The effect size of high ventricular white matter change burden on the memory measure utilized in the Atherosclerosis Risk in Communities Study was an OR of 2.23, very comparable with the magnitude of effect of SS/TIA in an uncorrected model in our data. The Rotterdam Scan Study reported a hazard ratio for global cognitive decline of 2.26 associated with presence of “silent brain infarct” on brain MRI.17 The OR/hazard ratio associated with these MRI findings and the OR associated with report of SS/TIA are of nearly identical magnitude, suggesting that a simple questionnaire may augment predictive or risk stratification models using the FSRP with minimal added burden to researchers or to patients, an important consideration for other large epidemiologic studies in which the cost of sophisticated imaging is prohibitive.
Our data also address the temporal link between SS/TIA and incident impairment, and analysis of only participants having reported SS/TIA during the follow-up period of the study strengthened the association between SS/TIA and incident impairment, reflected as a larger OR. This may reflect recall accuracy in the cohort reporting history of SS/TIA at baseline. Alternatively, this finding would support the hypothesis that experience of SS/TIA captures durations of exposure or degree of control of vascular risk factors—clinically relevant aspects not captured by simple arithmetic tabulation of risk factors such as the FSRP. Namely, participants having a remote history of SS/TIA may have experienced the event during a period of poorer risk factor control prompting improved subsequent medical management, whereas those having incident events during the observation period may have had a shorter window for the possibility of improved medical management. Finally, although at baseline a history of stroke symptoms was elicited, information on the timing of these events was not recorded. As such, the baseline stroke symptoms could have occurred in the distant past, and such events may not be as strongly related to incident cognitive impairment during our study as more recent symptoms because of improved control of risk factors in the time before study enrollment. This is in contrast to the events recorded during follow-up that have a better-defined temporal association with the incident cognitive impairment.
There are several limitations to our study. The QVSS includes questions related to common stroke symptoms; however, some items may be misunderstood by the subject, and other items (e.g., sensory changes) may be related to etiologies other than stroke (e.g., neuropathy). We do not have cerebral imaging of all subjects reporting stroke or TIA for adjudicated review, and subjects may not understand the difference between being told they had a “stroke,” “TIA,” or “mini-stroke.” The brief nature of the SIS (3 orientation items and 3 brief recall items) is a limitation of the current dataset. The SIS provides only a crude measure of incident cognitive impairment, and the relationships between self-reported stroke symptoms and incident cognitive impairment may be underestimated as a result. The SIS has demonstrated good sensitivity in the REGARDS Study, which enrolled a cohort having higher than average risk of vascular events by design. The applicability of our findings to populations with low risk of cerebrovascular events is uncertain.
We attempted to reduce the effect of differential access to health care and possible differences in the willingness to seek medical attention by grouping report of stroke-like symptoms and report of having experienced TIA. The QVSS13 seeks to elicit transient neurologic symptoms that suggest CNS involvement. Although it seems very likely that a person reporting these symptoms in an emergency room setting would be diagnosed with TIA, this is obviously not equivalent to a person having undergone in-person interview and examination with a physician (and possibly also having had neuroimaging or other diagnostic testing).
Our results demonstrate the power of a simple screening measure (inquiry of stroke-like symptoms) for identifying people at increased risk of incident cognitive impairment, in addition to the previously reported increased risk for stroke.18 Our findings suggest that elicitation of SS/TIA offers a simple, low-tech instrument that identifies people at higher risk of incident cognitive impairment that augments use of the FSRP. This demonstrates the feasibility of a “low-tech” assay to address neurologically important questions, with potential application for assessment in underserved regions with limited technologic resources. This report fits into the previous body of observations that emphasize the strong relationship among vascular risk factors, stroke risk, and risk for incident cognitive impairment. Our findings should be replicated in other epidemiologic cohorts to validate our findings in other populations and to directly compare the predictive value of the QVSS to MRI white matter changes. Furthermore, the performance of the QVSS should be evaluated vs a clinical assessment of incident dementia. Our study suggests the QVSS could provide a cost-effective tool for practicing physicians to identify patients at increased risk of developing dementia, hopefully providing an opportunity to reduce that risk.
ACKNOWLEDGMENT
The authors thank the other investigators, the staff, and the participants of the REGARDS Study for their contributions. A full list of participating investigators and institutions is available at www.regardsstudy.org.
GLOSSARY
- CI
confidence interval
- FSRP
Framingham Stroke Risk Profile
- OR
odds ratio
- QVSS
Questionnaire for Verifying Stroke-free Status
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
- SES
socioeconomic status
- SIS
Six-item Screener
- SS
stroke symptoms
AUTHOR CONTRIBUTIONS
Brendan J. Kelley, MD: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data. Leslie A. McClure, PhD: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, statistical analysis, obtaining funding. Abraham J. Letter, MS: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, statistical analysis. Virginia G. Wadley, PhD: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, obtaining funding. Frederick W. Unverzagt, PhD: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data. Brett M. Kissela, MD: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, obtaining funding. Dawn Kleindorfer, MD: drafting/revising the manuscript for content, including medical writing for content. George Howard, DrPH: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision or coordination, obtaining funding.
STUDY FUNDING
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, NIH, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the NIH. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. Dr. Kelley is the Bob and Sandy Heimann Endowed Chair for Alzheimer's Disease Education and Research at the University of Cincinnati.
DISCLOSURE
B. Kelley has served as a consultant to Lilly, and has received research support from Novartis and from NIH. L. McClure receives research support from Genzyme Corporation, NIH, and NASA. A. Letter since 2012 is a full-time biostatistician for W.L. Gore and Associates on clinical studies of aortic endoprostheses. V. Wadley receives grant funding from Genzyme Corporation, receives research support from NIH, and has served on an advisory board for Amgen, Inc. F. Unverzagt has served as a consultant to Eli Lilly and Company and UCB Biosciences, receives research support from NIH and Posit Science Inc., and holds stock in Eli Lilly, Inc. B. Kissela is a consultant and receives honoraria for speaking for Allergan, is an unpaid consultant with research support from NexStim, receives research support from NIH, and provides medicolegal reviews. D. Kleindorfer serves as a consultant for Genentech, and receives research support from the NIH and the CDC. G. Howard receives research support from NIH, and is on study advisory boards for Cerevast, LifeVest, and PhotoThera. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Knopman DS, Penman AD, Catellier DJ, et al. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology 2011;76:1879–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611–619 [DOI] [PubMed] [Google Scholar]
- 3.Howard VJ, McClure LA, Meschia JF, Pulley L, Orr SC, Friday GH. High prevalence of stroke symptoms among persons without a diagnosis of stroke or transient ischemic attack in a general population: the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Arch Intern Med 2006;166:1952–1958 [DOI] [PubMed] [Google Scholar]
- 4.Wadley VG, McClure LA, Howard VJ, et al. Cognitive status, stroke symptom reports, and modifiable risk factors among individuals with no diagnosis of stroke or transient ischemic attack in the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Stroke 2007;38:1143–1147 [DOI] [PubMed] [Google Scholar]
- 5.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–143 [DOI] [PubMed] [Google Scholar]
- 6.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item Screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002;40:771–781 [DOI] [PubMed] [Google Scholar]
- 7.Wilber ST, Lofgren SD, Mager TG, Blanda M, Gerson LW. An evaluation of two screening tools for cognitive impairment in older emergency department patients. Acad Emerg Med 2005;12:612–616 [DOI] [PubMed] [Google Scholar]
- 8.Steffens DC, Snowden M, Fan MY, Hendrie H, Katon WJ, Unutzer J. Cognitive impairment and depression outcomes in the IMPACT study. Am J Geriatr Psychiatry 2006;14:401–409 [DOI] [PubMed] [Google Scholar]
- 9.Unverzagt FW, McClure LA, Wadley VG, et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology 2011;77:1729–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsivgoulis G, Alexandrov AV, Wadley VG, et al. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology 2009;73:589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pullicino PM, Wadley VG, McClure LA, et al. Factors contributing to global cognitive impairment in heart failure: results from a population-based cohort. J Card Fail 2008;14:290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 2008;52:227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones WJ, Williams LS, Meschia JF. Validating the Questionnaire for Verifying Stroke-Free Status (QVSFS) by neurological history and examination. Stroke 2001;32:2232–2236 [DOI] [PubMed] [Google Scholar]
- 14.Price TR, Manolio TA, Kronmal RA, et al. ; CHS Collaborative Research Group Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults: the Cardiovascular Health Study. Stroke 1997;28:1158–1164 [DOI] [PubMed] [Google Scholar]
- 15.Bernick C, Kuller L, Dulberg C, et al. Silent MRI infarcts and the risk of future stroke: the Cardiovascular Health Study. Neurology 2001;57:1222–1229 [DOI] [PubMed] [Google Scholar]
- 16.Mosley TH, Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities Study. Neurology 2005;64:2056–2062 [DOI] [PubMed] [Google Scholar]
- 17.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2003;34:1126–1129 [DOI] [PubMed] [Google Scholar]
- 18.Kleindorfer D, Judd S, Howard VJ, et al. Self-reported stroke symptoms without a prior diagnosis of stroke or transient ischemic attack: a powerful new risk factor for stroke. Stroke 2011;42:3122–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]


