Abstract
We have developed a protocol to produce large quantities of high purity myristoylated and nonmyristoylated Neuronal Calcium Sensor 1 (NCS-1) protein. NCS-1 is a member of the neuronal calcium sensor (NCS) family and plays an important role in modulating G-protein signaling and exocytosis pathways in cells. Many of these functions are calcium dependent and require NCS-1 to be modified with an N-terminal myristoyl moiety. In our system, a C-terminally 6X His-tagged variant of NCS-1 was co-expressed with yeast N-myristoyltransferase (NMT) in ZYP-5052 auto-induction media supplemented with sodium myristate (100 – 200 µM). With optimized growth conditions and a high capacity metal affinity purification scheme, > 50 mg of homogenous myristoylated NCS-1 is obtained from 1 L of culture in a single step. The properties of the C-terminally tagged NCS-1 variants are indistinguishable from those reported for untagged NCS-1. Using this system, we have also isolated and characterized mutant NCS-1 proteins that have attenuated (NCS-1 E120Q) and abrogated (NCS-1 ΔEF) ability to bind calcium. The large quantities of NCS-1 proteins isolated from small culture volumes of auto-inducible media will provide the necessary reagents for further biochemical and structural characterization. The affinity tag at the C-terminus of the protein provides a suitable reagent for easily identifying binding partners of the various NCS-1 constructs. Additionally, this method could be used to produce other recombinant proteins of the NCS family, and may be extended to express and isolate myristoylated variants of other proteins.
Introduction
Neuronal Calcium Sensor-1 (NCS-1), an acidic, highly helical protein (MW 22 kDa), is the human ortholog of frequenin, a protein involved in regulating synaptic neurotransmission in Drosophila [1]. NCS-1 is a member of the neuronal calcium sensor (NCS) family, a group of evolutionarily related proteins that interact with G-protein-coupled receptors and their partner kinases in a calcium dependent manner [2–3]. This family includes recoverin, neurocalcin, hippocalcin, GCAPs, and KChIPs, which are expressed predominantly in neuronal tissues. Calcium binding is critical for the interaction of NCS-1 with its protein substrates. For example, NCS-1 interacts in a calcium dependent fashion with G-protein coupled receptor kinase 2 to mediate the desensitization of the D2 dopamine receptor [4]. Furthermore, adrenal chromaffin cells expressing the NCS-1 variant E120Q, which has reduced affinity for calcium, showed an attenuated ability to respond to opioid inhibition of calcium currents [5]. As NCS-1 is involved in a variety of cellular pathways including G-protein signaling cascades and exocytosis, a more detailed investigation is necessary to determine the importance of calcium binding to the role NCS-1 in these critical cell signaling pathways.
Conserved structural features within the NCS family include the presence of multiple high affinity calcium binding EF-hand domains and an N-terminal myristoylation consensus sequence [6–10]. In NCS-1, there are four potential EF-hand signatures; however, the N-terminal EF-hand (EF-1) is nonfunctional due to a substitution of proline at critical position, which interrupts the helix-loop-helix motif. The functional EF-hand motifs lie within residues 66–94 (EF-2), 102–130 (EF-3), and 147–175 (EF-4). In the three functional EF-hands of NCS-1, conserved glutamic acidic residues at positions 84, 120, and 168 provide a bidentate ligand necessary to coordinate calcium [11]. Binding of calcium at these three sites induces conformational changes in the protein structure that expose a hydrophobic pocket that has been proposed to switch the protein to its active state [12–13]. The calcium binding properties of NCS-1 are incompletely characterized, however, and many details of the structural changes that accompany binding also remain unclear.
A further feature of NCS-1 that complicates its characterization is a myristoyl moiety that is covalently linked to the N-terminal glycine of the protein. N-terminal myristoylation of NCS-1 is thought to be important for protein structure, cellular localization [14], and function [15]. Myristoylation mediates structural changes in NCS-1 that increase its stability and affect the unfolding pathway of the protein [16]. Furthermore, the addition of the N-terminal myristoyl moiety alters the affinity of NCS-1 for calcium; myristoylated and unmodified NCS-1 show markedly different calcium binding profiles and stoichiometries [6, 17]. The mechanism through which myristoylation causes these larger structural effects is not currently understood.
Here we present a strategy that adapts an auto-inducible growth media [18] to produce and isolate milligram quantities of essentially homogenous myristoylated and non-myristoylated C-terminally 6X His-tagged NCS-1 in E. coli. Protein produced in this optimized system is indistinguishable from the untagged protein in a wide range of in vitro and in vivo assays of NCS-1 structure and function. Thus these proteins will be valuable reagents to study NCS-1 function and interactions. Employing this strategy, we have also produced, isolated and characterized variants of NCS-1 that have reduced (NCS-1 E120Q) and abolished (NCS-1 ΔEF) capability to bind calcium. Furthermore, this strategy may be useful for the expression and purification of other NCS proteins and for the production of myristoylated proteins in general.
Materials and Methods
Expression of myristoylated and non-myristoylated NCS-1 in E. coli
Myristoylated and nonmyristoylated NCS-1 constructs were expressed in E. coli BL21(DE3) (Novagen) using the ZYP-5052 auto-induction media [18]. The coding region for human NCS-1 was subcloned into the NdeI and XhoI restriction sites of T7-based expression plasmid pET23-a(+) to produce protein with a C-terminal 6X His-tag. Wild type NCS-1 or NCS-1 variant plasmids were transformed into competent BL21(DE3) cells either alone, or co-transformed with plasmid pBB131 (kindly provided by Dr. J Gordon, Washington University, St. Louis, MO) containing yeast N-myristoyltransferase (NMT). Expression of both NCS-1 and NMT is under control of an inducible lactose promoter. The co-expression of NCS-1 and yeast NMT in E. coli has been documented previously [19].
Nonmyristoylated NCS-1 was produced by growing cells containing the appropriate pET23-a(+) plasmid at 37°C in ZYP-5052 media [18] until the cultures reached saturation (16 to 20 hours), and bacteria were then harvested by centrifugation. Cultures typically reached an optical density of 9 – 11 absorbance units. Myristoylated NCS-1 was produced by growing cells transformed with plasmids expressing NCS-1 and NMT at 37°C in ZYP-5052 media supplemented with sodium myristate salt (NaMyr) (Sigma) at the indicated concentration. Three to five hours after the cultures reached saturation they were harvested by centrifugation. Cell pellets obtained from both growth regimens were stored at –20°C prior to lysis.
Bacterial cell lysis and purification of NCS-1 proteins
Bacterial cell pellets (wet weight 0.6 – 0.7 grams per 50 mL of total culture volume) were resuspended in 10 mL of primary-amine free Bugbuster (Novagen) containing 30,000 units rLysozyme (Novagen) and 100 units Benzonase Nuclease (Novagen). The resulting lysate was agitated on an orbital shaker at room temperature for 15 minutes, and then centrifuged for 45 minutes at 21,000 rcf in a refrigerated centrifuge (A14 rotor, Beckman-Coulter).
Soluble NCS-1 was purified by nickel-nitriloacetic acid (NiNTA) affinity chromatography. Clarified cell lysate was applied to a column containing 4.0 mL of freshly charged Ni Sepharose resin (GE Healthcare Lifesciences) equilibrated in buffer containing 2 mM CaCl2, 40 mM imidazole, 20 mM HEPES, 100 mM NaCl at pH 7.5. The flow rate remained constant throughout at 275 cm/hr. After application of cell lysate, the resin was washed with equilibration buffer until the UV absorbance of the eluent was less than 0.05 AU, typically three to five column volumes. Nonmyristoylated NCS-1 was eluted from the column in a linear imidazole gradient (40 mM – 500 mM imidazole). For purification of myristoylated NCS-1, the resin was washed with 5 column volumes of a buffer containing 115 mM imidazole prior to eluting the target protein in a linear imidazole gradient (115 – 500 mM). Protein enriched fractions were identified by UV absorption at 280 nm. Approximate protein levels were determined using Origin™ (OriginLab) graphical analysis software for peak integration. The UV extinction coefficient of NCS-1 was determined to be 22060 M−1cm−1 at 278 nm by the Edelhoch method as previously described [20].
In order to obtain protein of optimal purity, peak fractions were pooled and concentrated in an Amicon Ultra centrifugal concentrating device (Millipore) with a 10 kDa cut-off membrane, then subjected to gel filtration chromatography using a Superdex 75 10/300 GL gel filtration column (GE Healthcare Lifesciences), equilibrated in buffer composed of 1 mM DTT, 2 mM EGTA, 20 mM HEPES, 100 mM NaCl at pH 7.5. Flow rate remained constant at 32 cm/hr. Homogenous fractions containing NCS-1 proteins commonly eluted between 59 mL and 68 mL, and were pooled then subjected to buffer exchange into a final buffer containing 1 mM DTT, 20 mM HEPES, 100 mM NaCl at pH 7.5. In order to remove any trace amounts of calcium from the final buffer, the buffer solution was treated with Chelex 100 (BioRad) as per manufacturer’s instructions.
Calcium Shift SDS-PAGE Assay and Western Blotting
Electrophoretic mobility of purified NCS-1 proteins was assayed using SDS-PAGE with Western blotting for detection. Calcium-free NCS-1 was incubated with either 5 mM EGTA or 5 mM CaCl2 and resolved by 15% SDS-PAGE. Proteins were visualized by colloidal Comassie Brilliant Blue or by Western blot analysis. For Western blot analysis, proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (0.45 mm, PALL Life Sciences) and probed with chicken anti-frequenin (NCS-1) polyclonal antibody (1:14000 dilution, Rockland Immunochemicals). Membranes were then incubated with a horseradish peroxidase conjugated secondary antibody (1:14000 dilution, Jackson Immuno-Research). Immuno-reactive proteins were visualized using enhanced chemiluminescence (ECL plus; GE Healthcare).
Reverse phase high performance liquid chromatography (RP-HPLC) analysis of purified NCS-1
The relative level of myristoylated NCS-1 was determined by RP-HPLC with diode array based UV absorbance detection. Separation of myristoylated and non-myristoylated species was achieved on an analytical scale using a 4.6× 250mm C4 column with 5 µm bead and 300 Å pore sizes (JT Baker). The column was equilibrated in 10 column volumes of a solvent composed of 40% acetonitrile (ACN): 59.9% water (H2O): 0.1% trifluoroacetic acid (TFA) at a flow rate of 150 cm/hr. 20 µL of sample was loaded on the column and washed with an additional 2 column volumes of equilibration solvent. Bound NCS-1 was eluted in a linear ACN gradient (40%–60%). Signals corresponding to NCS-1 were detected by sample absorbance at wavelengths of 280 nm and 211 nm. Peak detection and area calculation were performed by 32Karat chromatography software version 7.0 (Beckman-Coulter).
Matrix assisted light desorption ionization – time of flight mass spectroscopy (MALDI-TOF)
10 µL aliquots from each RP-HPLC peak were collected and subjected to MALDI-TOF analysis on an Applied Biosystems Voyager System Mass Spectrometer. The aliquots were thoroughly mixed with 1 µL of a matrix solution containing ∼ 40 mM 3,5-Dimethoxy-4-hydroxycinnamic acid in a 1:2 ACN: H2O solution. Mass peaks were collected over 200 laser pulses and analyzed against an external reference standard composed of bovine serum albumin.
Circular dichroism (CD) spectroscopy
Far-UV (185 nm − 250 nm) CD spectra measurements were recorded on a Jasco J-710 Spectropolarimeter at 25° C using a 0.2 mm path length cylindrical cuvette (QS Hellma). NCS-1 proteins were analyzed at a final concentration of 1 mg/mL then dialyzed into 50 mM Tris-HCl, 100 mM NaCl at pH 7.5 incubated with either 2.5 mM EGTA or 5 mM CaCl2. Spectra for each sample were accumulated at 0.2 nm data intervals at a scan rate of 25 nm / min. For each sample, 3 scans were collected and averaged before subtracting the baseline buffer signal.
Ratiometric fluorescent calcium binding assay
The ability of each NCS-1 variant to bind calcium was monitored using a competition assay with the ratiometric fluorescence calcium chelator, indo-1 (Molecular Probes). For the described solution conditions, the Kd of indo-1 was determined to be 500 − 800 nM. 10µM indo-1 was dissolved in buffer containing 20 µM CaCl2, 1 mM DTT, 20 mM HEPES, 100 mM NaCl, and 1% v/v DMSO at pH 7.5. NCS-1 variants were introduced into this solution at varying concentrations. Fluorescence spectra were collected using an ISS PC-1 fluorometer with sample excitation at 370 nm (bandwith 8 nm). Sample fluorescence emission was monitored from 390 nm to 510 nm (bandwidth 8 nm). The fluorescence data is reported as the ratio of fluorescence emission intensity at 401 nm and 472 nm. In order to monitor the interaction of NCS-1 variants with the indo-1, fluorescence anisotropy values were recorded at the beginning and end of each titration.
Solubility and critical micelle concentration of sodium myristate salt (NaMyr)
The critical micelle concentration (CMC) of NaMyr in ZYP-5052 auto-induction media was determined by multi-angle laser light scattering (MALLS) using a Wyatt Technologies DAWN HELEOS® instrument. Increasing amounts of NaMyr were dissolved in ZYP-5052 auto-induction media and equilibrated to 37°C in a water bath prior to MALLS analysis. Temperature equilibrated solutions were degassed before circulation through the MALLS detector. HPLC grade toluene was used as a standard to convert machine voltages to Raleigh ratios. In order to minimize the MALLS contribution of dust particles, solutions were prefiltered with a Durapore 0.1 µm filter sheet (Millipore) as well as in-line filtered with an Anopore 0.1 µm filter membrane (Whatman).
Large unilamellar lipid vesicle binding
The ability of myristoylated and nonmyristoylated NCS-1 to bind to large unilamellar lipid vesicles composed of palmitoyl oleoyl phosphatidyl choline (POPC) or palmitoyl oleoyl phosphatidyl serine (POPS) was assayed using intrinsic protein fluorescence, as described previously [17, 21]. POPC and POPS vesicles were prepared by extrusion through a 100 nm filter (Whatman). Intrinsic protein fluorescence was determined using a Safire2 (Tecan) microplate fluorometer with sample excitation at 278 nm (bandwith 10 nm). Sample fluorescence emission was monitored from 300 nm to 420 nm (bandwidth 5 nm). For each concentration of lipid, total fluorescence emission was corrected for light scattering. Data was plotted as a function of intrinsic protein fluorescence vs. concentration of lipid, and equilibrium dissociation constants were determined using Origin (OriginLab) curve fitting software to the following equation:
Where IntO is the observed relative intensity, IntB is the relative intensity of the bound species, Lt is the total concentration of NCS-1, Rt is the concentration of lipid, and Kd is the equilibrium dissociation constant.
D2 dopamine receptor/NCS-1 interaction
HEK-293 cells expressing FLAG-tagged D2-dopamine receptor were prepared and lysed as described previously [4]. Cell lysates were incubated with purified recombinant 6X His-tagged NCS-1 in PBS in the presence of 100 µM CaCl2 for 12 hours at 4°C. An anti-6x His epitope tag antibody (1:3000 dilution, Rockland) was added to the samples, and the samples were incubated with 30 µl of gamma bind sepharose resin beads (Amersham) for an additional 4 hours. Following three washes of PBS buffer, resin bound protein was eluted in SDS gel loading buffer. Proteins were separated by a 10% SDS-PAGE gel and transferred to a polyvinylidene fluoride (PVDF) membrane (0.45 mm, PALL Life Sciences). The membrane was probed with M2 anti-FLAG antibody (1:2000 dilution, Sigma). Membranes were then incubated with a horseradish peroxidase conjugated secondary antibody (1:14000 dilution, Jackson Immuno-Research). Immuno-reactive proteins were visualized using enhanced chemiluminescence (ECL plus ®; GE Healthcare).
Expression of 15N myristoylated NCS-1 and NMR Spectroscopy
To produce 15N myristoylated NCS-1 the ZYP-5052 media was replaced with minimal P-5052 autoinduction media as previously described [22]. 15N supplementation of the P-5052 media was achieved with the addition of 15NH4Cl obtained from Cambridge Isotopes. The remainder of the purification of 15N myristoylated NCS-1 was the same as described above.
Two-dimensional 1H–15N heteronuclear single quantum correlation (HSQC) experiments were collected at either 800 MHz on a Varian INOVA spectrometer or at 600 MHz on a Bruker Avance II spectrometer outfitted with a coldprobe at 298 K using WATERGATE for solvent suppression [23]. A uniformly 15N -labeled myristoylated NCS-1 sample was prepared by extensive buffer exchange into 50 mM Tris, 4mM CaCl2, 100 mM KCl, 5 mM DTT with a pH of 7.2 with no correction following the addition of 10% D2O. The protein concentration in the sample used for NMR experiments was 0.4 mM. HSQC spectra were collected at 256 scans per increment, 1920 (t2) × 80 (t1) complex points with acquisition times of 75 ms (1H) and 28.2 ms (15N). The carrier frequencies were centered on the chemical shift of water in 1H and in the center of the amide region at 118.0 ppm in 15N. NMR data processing was carried out using NMRPipe [24] and subsequently analyzed with NMRView [25]. Chemical shift assignments were obtained from the Biomagnetic Resonance Database [26] entry for myristoylated NCS-1 (entry 6942), and converted to NMRView xpk files using a publicly available script [27].
Results and Discussion
Purification and RP-HPLC / MALDI-TOF MS analysis of NCS-1 proteins
C-terminally 6X His-tagged NCS-1 constructs expressed in ZYP-5052 media resulted in high level accumulation of these proteins (up to 50% of total protein). The majority (>70%) of NCS-1 was found to be soluble in the BugBuster (Novagen) lysis solution, and was purified from the remainder of the soluble E. coli proteins by NiNTA affinity chromatography.
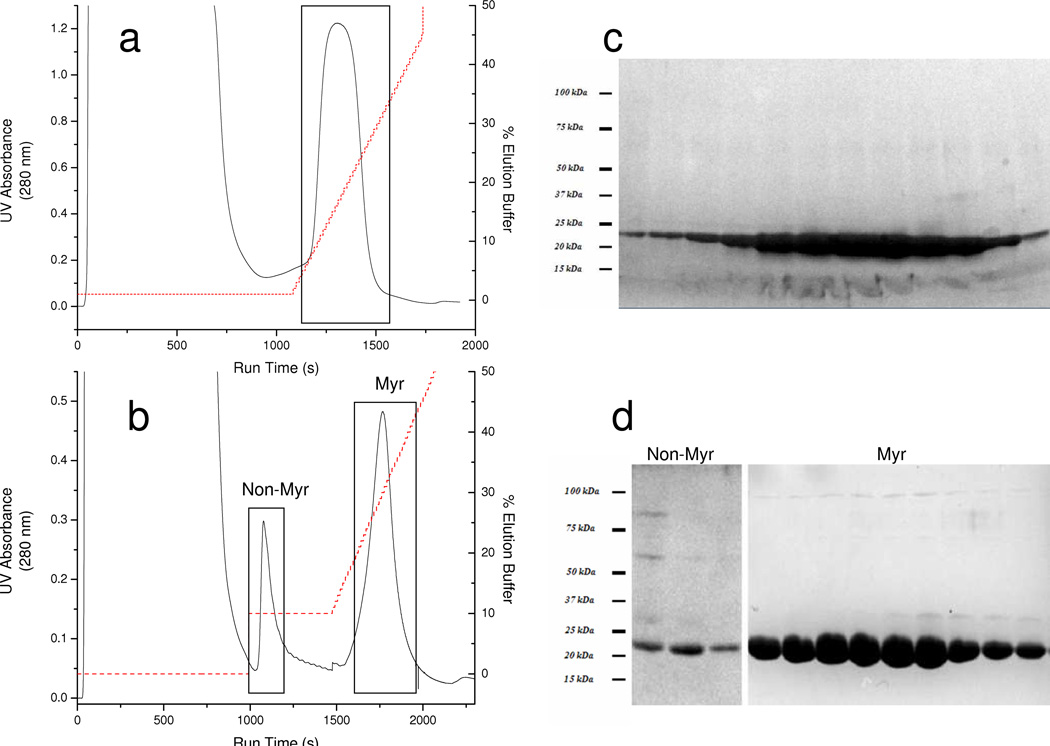
For NCS-1 variants lacking the N-terminal myristoyl moiety, virtually all of the NCS-1 bound to the resin and eluted in a single symmetric peak in a linear imidazole gradient. Nonmyristoylated wild-type, E120Q, and ΔEF NCS-1 proteins showed similar behavior, eluting early in the gradient, generally between 115 mM and 140 mM imidazole (Figure 1a). However, when the analogous NCS-1 constructs were co-expressed with NMT in NaMyr supplemented (0.5 mM) ZYP-5052 auto-induction media, the elution profile in the simple linear imidazole gradient was more complex, comprising at least two discrete peaks.
Figure 1.
NiNTA chromatographs and SDS-PAGE gels representative of the various elution profiles obtained from 150 mL growths of recombinant E. coli
(a) Chromatography profile of nonmyristolated NCS-1 construct eluted in a simple linear imidazole gradient. Detector response is saturated at full peak height (b) Chromatography profile of optimized gradient method for the resolution of myristoylated from nonmyristoylated NCS-1. For each chromatograph, UV absorbance (solid line) and gradient of elution buffer (dotted line) are presented as a function of run time.
(c) SDS-PAGE gel of fractions collected during the linear imidazole gradient eluting nonmyristoylated NCS-1. Equivolume gel samples were collected at regular intervals from within the boxed area of (a). (d) SDS-PAGE gel of fractions collected during the wash step and linear gradient during the purification of myristoylated NCS-1. Equivolume gel samples were collected at regular intervals from fractions in the Non-Myr box and Myr box and run on the labeled gel. SDS-PAGE gels were stained with SimplyBlue (Invitrogen) colloidal coomassie stain. A representative molecular weight ladder is depicted on the left.
To further characterize this complex peak profile, aliquots from fractions collected during the simple linear imidazole gradient were subjected to RP-HPLC and MALDI-TOF MS analysis. Protein containing fractions appearing early in the gradient chromatographed as single peak on RP-HPLC analysis, and had a molecular weight indicative of nonmyristoylated NCS-1 (Figure 2a). Fractions eluting in the middle of the gradient showed two discrete peaks, with masses corresponding to nonmyristoylated and myristoylated NCS-1, respectively (Figure 2b). Fractions from the end of the gradient exclusively contained myristoylated NCS-1 (Figure 2c). The RP-HPLC and MADLI-TOF MS characterization of the fractions eluted during the imidazole gradient is consistent with the myristoylated construct having a greater affinity for the NiNTA column than the nonmyristoylated species.
Figure 2.
RP-HPLC chromatographs representative of aliquots collected from fractions of myristoylated NCS-1 eluted in a simple linear imidazole gradient. Chromatographs are shown from protein containing fractions collected from the early (a.), intermediate (b), and late (c) portions of the gradient. RT denotes peak retention time and MW denotes the molecular weight of the center peak fraction ascertained by MALDI-TOF MS. UV absorbance at 280 nm (solid line) and % elution buffer (dotted line) are presented as a function of run time.
To obtain homogenous myristoylated NCS-1, we optimized the imidazole gradient to include a wash step to elute the nonmyristoylated protein. In this optimized gradient, nonmyristoylated NCS-1 was eluted in a 115 mM imidazole wash step, while myristoylated NCS-1 eluted as a single symmetric peak later during the imidazole gradient at concentrations greater than 150 mM (Figure 1b). The identity the protein eluting in the linear gradient was confirmed by MALDI-TOF analysis. The peak corresponding to nonmyristoylated NCS-1 was of minimal relative intensity after optimizing the media as outlined below.
To further optimize the yield of the myristoylated NCS-1 construct, we investigated the effects of increasing concentrations of NaMyr (0–1.6mM) in the ZYP-5052 media on the yield of recombinant protein (Supplemental figure 1a). In these experiments, optimal levels of substrate myristoylation and recovery of recombinant protein occurred at NaMyr concentrations between 100 – 200 µM. These concentrations were used in all subsequent experiments. It is interesting to note that the CMC of NaMyr in the ZYP-5052 autoinduction media, determined by change in light scattering intensity, occurs at 600 µM (Supplemental figure 1b). An abrupt drop in protein production similarly occurs at concentrations > 600 µM NaMyr (Supplemental figure 1a), suggesting the detergent micelles may have a negative impact on bacterial growth or recombinant protein expression.
SDS-PAGE demonstrates the very high purity of the proteins eluted during the NiNTA separation, as shown in Figures 1c–d, with identical bands migrating at the expected position for NCS-1 (20 kDa - 25 kDa). Further, all of the fractions reacted with an anti-frequenin antibody known to recognize wild-type NCS-1 (data not shown). The faint band appearing at ∼ 25 kDa is most likely due to a fraction of gel shifted calcium-free NCS-1 as discussed below. For protein of the highest purity, the pooled NCS-1 fractions were concentrated and subjected to gel filtration chromatography (GE Healthcare Lifesciences Superdex 75). For all constructs, material eluting in the peak fractions following this treatment appear to be essentially homogenous (>99% by SDS-PAGE). The gel permeation chromatography profile under these conditions (1 mM DTT, 2 mM EGTA, 20 mM HEPES, 100 mM NaCl at pH 7.5) showed a single symmetric peak with a retention time consistent with that of an NCS-1 monomer (data not shown).
The overall yield of the non-myristoylated forms of NCS-1, NCS-1 E120Q, and NCS-1 ΔEF expressed in pET23-a(+) and grown in ZYP-5052 media was typically 45–60 mg of purified protein per 250 mL of total culture volume. The yield for the myristoylated NCS-1 construct in the optimized ZYP-5052 auto-induction media (100 – 200 µM NaMyr) was commonly 20–25 mg of pure N-terminally modified NCS-1 (>95% by RP-HPLC) per 250 mL of total culture volume. The yield for uniformly 15N -labeled myristoylated NCS-1 produced using an autoinduction media suitable for isotopic replacement, P-5052 under the same optimized expression and purification conditions was 6–8 mg.
For both modified and unmodified NCS-1, the use of novel C-terminally tagged affinity constructs and the bacterial auto-induction system greatly facilitated the purification of large amounts of protein from fairly small culture volumes. The NiNTA affinity purification strategy provided high selectivity and high capacity for the target protein using standard chromatography techniques [28–29]. Resolution of myristoylated from nonmyristoylated constructs was accomplished using an optimized gradient method. Using this purification strategy milligram quantities of NCS-1 were isolated in a one-step purification to >90% homogeneity by SDS-PAGE.
Effect of the C-terminal 6X His-tag and EF-Hand point mutations on NCS-1 structure
To demonstrate that the 6X His-tag and the point mutations introduced into the various NCS-1 polypeptides did not disrupt the secondary structure of the protein, each NCS-1 variant was analyzed by far-UV CD. Spectra were collected in the presence of either 2.5 mM EGTA or 5 mM CaCl2 (Figure 3). The far-UV CD profile of each construct shows characteristic local minima at 208 nm and 220 nm, consistent with a protein of high α-helical character [29]. The degree of fractional helicity predicted by this analysis is in good agreement with the helical content observed in X-ray crystallography experiments [13]. Furthermore, the CD spectra for NCS-1 variants containing a C terminal 6X His-tag track with those previously reported for NCS-1 proteins not containing the 6X His-tag [16, 19], consistent with the affinity tag not grossly affecting the protein structure.
Figure 3.
Far-UV Circular Dichroism spectra for NCS-1 constructs containing 2.5 mM EGTA (solid line) or 5 mM CaCl2 (dashed line). Ellipticity is expressed as a function of wavelength for (a) non-myristoylated NCS-1, (b) myristoylated NCS-1, (c) NCS-1 E120Q, and (d) NCS-1 ΔEF constructs.
Addition of 5 mM CaCl2 to samples containing myristoylated and nonmyristoylated wild-type NCS-1 resulted in decreased ellipticity at 208 and 222 nm, likely reflecting an increase in helical content or stabilization of helices already present in NCS-1 due to calcium binding (Figure 3a–b). Consistent with the loss of calcium binding capacity, a smaller decrease was observed with the E120Q NCS-1 mutant protein (Figure 3c). Although the integrity of the EF-3 domain is interrupted by the E120Q mutation, the EF-2 and EF-4 domains remain intact, and the mutant protein retains some affinity for calcium, as previously suggested [13]. These data also suggest the conformational change of NCS-1 that occurs in the presence of calcium involves additional regions of the protein outside of the EF-2 domain.
To completely disrupt calcium binding, the NCS-1 ΔEF construct was designed by replacing critical glutamic acid residues (E84Q, E120Q, E168Q) found in each of the three calcium binding EF-hand regions with glutamine. The CD spectra for NCS-1 ΔEF and those of the other NCS-1 variants collected in the presence of EGTA show identical profiles, suggesting that the introduced mutations do not drastically affect the apo structure of the mutant. As expected, incubation of NCS-1 ΔEF with calcium does not affect the CD spectra (Figure 3d), consistent with the amino acid substitutions in this construct markedly altering the calcium binding capacity of the protein.
To further evaluate the effect of the C-terminal affinity tag and the calcium binding mutations on NCS-1 structure, we performed an SDS-PAGE calcium shift assay. In the presence of CaCl2, both myristoylated and nonmyristoylated wild-type NCS-1 variants migrate slightly faster than the same proteins in the absence of calcium, corresponding to a 1–2 kDa increase in mobility on SDS-PAGE (Figure 4). This is consistent with the ability of the protein constructs to bind calcium [19, 31]. Consistent with our CD analysis, the NCS-1 E120Q variant also demonstrates an increase in electrophoretic mobility in the presence of calcium suggesting that it also can bind calcium. Moreover, the ΔEF variant showed no change in relative migration with calcium exposure, consistent with it retaining little or no ability to bind calcium.
Figure 4.
NCS-1 and its variants analyzed by 15% SDS-PAGE. Proteins were incubated with 5 mM CaCl2. From left to right: Lanes 1 & 2 – Myristoylated NCS-1, Lanes 3 & 4 – Nonmyristoylated NCS-1, Lanes 5& 6 – NCS1 E120Q, Lanes 7 & 8 NCS-1 ΔEF. A representative molecular weight ladder is depicted on the left.
Finally, to probe the effects of the 6X His-tag on the tertiary fold of the protein, a two-dimensional 1H–15N HSQC NMR experiment was performed on myristoylated NCS-1 in the presence and absence of calcium. In the presence of calcium, the spectra shows a dispersion of resonances along both frequency axes indicating the protein construct containing the 6X His-tag is properly folded and is of suitable quality for structural study by NMR based techniques (Figure 5). Comparison of this spectrum with a recently published HSQC spectrum of a myristoylated NCS-1 construct lacking a 6X His-tag [32] demonstrates a very similar cross-peak profile suggesting the proteins have a common tertiary structure. Furthermore, both spectra also demonstrate a shift in cross-peak profile following the addition of calcium. The HSQC collected for the tagged and untagged NCS-1 variant both show resonances increased in dispersion with the emergence of additional upfield resonances assigned to glycine residues present in calcium binding EF-hand regions (data not shown). These similarities suggest the 6X His-tag does not have a large effect on the structural changes induced by calcium binding to NCS-1.
Figure 5.
1H-15N HSQC spectrum recorded for 0.4 mM C-terminally 6X His-tag myristoylated NCS-1 in a buffer composed of 50 mM Tris, 4mM CaCl2, 100 mM KCl, 5 mM DTT with a pH of 7.2. Inset: Overlay of assignments for myristoylated NCS-1 on an area of the HSQC spectrum rich in glycine resonances. Assignments were transferred from BRMB entry 6942.
A more detailed comparison of the HSQC spectrum of the C-terminally tagged myristoylated NCS-1 construct with the published cross-peak assignments (BMRB entry 6942) is shown in Figure 5, inset. The concordance between the assigned and observed peaks is clearly evident, and reinforces the similarity in the tertiary structure of these two protein constructs. Furthermore, of the 11 assigned glycine residues in the untagged protein spectra, 10 residues showed a matching cross-peak within a distance of 0.16 ppm in the HSQC of the tagged variant, suggesting the tertiary fold of the protein is not overly disturbed by the presence of the C-terminal tag.
Interaction of 6X His-tagged NCS-1 with lipid and protein binding partners
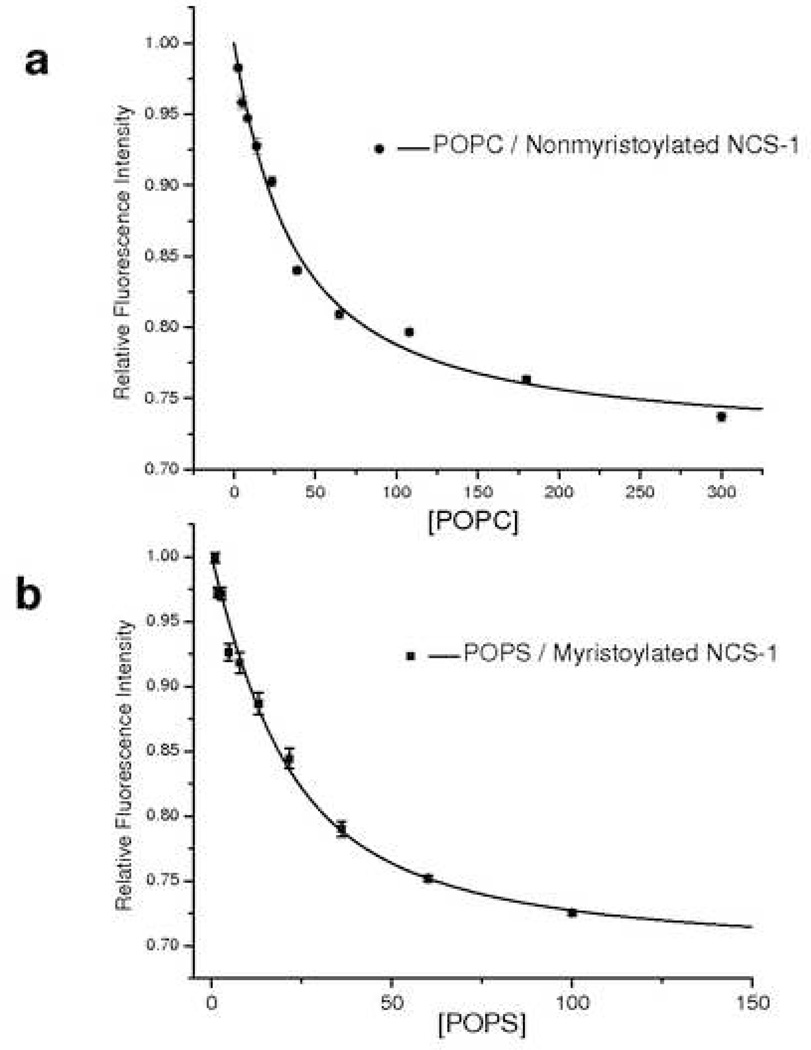
To demonstrate that the 6X His-tag does not interrupt the ability of NCS-1 to interact with lipid substrates, lipid binding to large unilamellar vesicles composed of either POPC or POPS was investigated. In vitro, NCS-1 and myristoylated NCS-1 have been shown to preferentially bind to POPC or POPS vesicles, respectively [17]. As demonstrated in Figure 6a, nonmyristoylated NCS-1 binds POPC vesicles with an apparent Kd of 31 ± 4 µM (R2 = 0.98755) as measured by the quenching of intrinsic protein fluorescence by increasing concentrations of lipid. Consistent with previously reported results, this interaction was observed in both the presence and absence of calcium. Presented in Figure 6b, myristoylated NCS-1 was found to interact with POPS vesicles in a calcium dependent manner with a Kd of 12 ± 3 µM (R2 = 0.98847). Both myristoylated NCS-1 and nonmyristoylated NCS-1 showed negligible fluorescence quenching in the presence of POPC and POPS respectively (data not shown). The ability of the constructs to preferentially bind to specific lipid vesicles is not perturbed by the presence of the 6X His-tag.
Figure 6.
POPC / POPS vesicle binding measured as a function of relative intrinsic protein fluorescence as a function of lipid concentration. Fluorescence measurements were corrected for the light scattered by lipid vesicles. (a.) Binding curve representing POPC vesicles binding to nonmyristoylated NCS-1. (b.) Binding curve representing POPS vesicles binding to myristoylated NCS-1. Note the change in scale of the x axis. Error bars are smaller than sample points (< 1%) in some cases).
NCS-1 has been shown to interact with and modulate the function of the D2 dopamine receptor [4]. Pull-down assays using crude eukaryotic lysate expressing the D2 dopamine receptor serve to confirm the 6X His-tag does not perturb the interaction of NCS-1 with a known interacting protein, and to test the suitability of the 6X His-tagged construct as a reagent to identify binding partners. The result of such an assay is presented in Figure 7, demonstrating that purified recombinant NCS-1 is capable of binding the D2 dopamine receptor present in crude HEK-293 lysate. This observation is consistent with earlier reported results [4], showing the 6X His-tag has not abrogated the ability of NCS-1 to interact with a known binding partner. These results also demonstrate the purified construct may be suitable for screening for and describing the interactions with novel binding partners, either in single pulldown or in high-throughput assays. Furthermore, when transfected into HEK-293 cells stably expressing the D2 dopamine receptor, the 6X His-tagged NCS-1 variant can be isolated in a co-immunoprecipitation experiment (data not shown) demonstrating the 6X His-tagged variant shows a wild type phenotype in vivo.
Figure 7.
Pull down of the D2-long dopamine receptor (D2R) by interaction with recombinant NCS-1 analyzed by a 10% SDS-PAGE gel and visualized by Western blot. From left to right: Lane 1 – D2R cell lysate, Lane 2 – D2R cell lysate mock pulldown (no NCS-1 added), Lane 3 – D2R pulldown in the presence of NCS-1. A representative molecular weight ladder is depicted on the left.
Calcium binding of NCS-1 variants
We used a ratiometric fluorescence assay to monitor the relative affinity and capacity to bind calcium of each NCS-1 variant. This assay utilizes indo-1 as a fluorescent probe, which selectively binds free calcium resulting in a change in its fluorescence emission maximum of 401 nm to 472 nm as a function of increasing concentration of free calcium in solution [33]. The calcium binding capacity of the fluorescent indicator is nearly saturated at 401/472 ratio > 1.1. In this assay, NCS-1 variants that can bind calcium will compete with indo-1 for free calcium resulting in a decrease in the relative 401/472 ratio.
As shown in Figure 8, the high calcium affinity of myristoylated NCS-1 is demonstrated by the marked decrease in the ratio (401/472 nm) of fluorescence emission observed with the incubation of 4 µM of protein in a solution containing 10 µM of indo-1 dye and 20 µM CaCl2. Nonmyristoylated NCS-1 also competes with indo-1 for calcium in solution, although with a lower efficiency than myristoylated NCS-1, as a higher concentration (17 µM) of protein is required to produce an analogous decrease in the emission ratio. Furthermore, the NCS-1 E120Q protein is a considerably less efficient binder of calcium since a much higher protein concentration (110 µM) is required to decrease this ratio. These results are consistent with a number of previous studies of the calcium binding properties for these variants [5, 12–13, 17], and demonstrate the presence of the C-terminal 6X His-tag does not obscure calcium binding properties of the protein. Finally, even at concentrations of 220 µM the NCS-1 ΔEF construct shows no capacity to affect the fluorescent properties of indo-1 in the presence of calcium, indicating that under these conditions it does not appreciably bind calcium. The three mutations present in the ΔEF protein (E84Q, E120Q, and E168Q) have diminished the calcium binding capacity of the protein below levels directly measurable by this assay, and thus the ΔEF protein may be the most appropriate mimic of the apo state in cellular and biochemical assays.
Figure 8.
Ratio of fluorescence intensities at 401 nm / 472 nm of 10 µM indo−1. +Ca denotes the addition of 20 µM CaCl2. Fluorescence anisotropy values of emitted light remained constant during each competition experiment demonstrating there was no quantifiable interaction between the protein construct and dye. In a calcium free state, indo-1 has a characteristic fluorescence emission maximum at 401 nm (ex=370). When bound to calcium, the fluorescence emission maximum of the dye shifts to 472 nm. Comparison of the ratio of fluorescence emission signals at 401 nm and 472 nm allows for the control of artifacts common in fluorescence experiments including photo-bleaching and unequal dye distribution. A high 401/472 ratio is expected for indo-1 dye saturated with calcium. Concentrations and type of construct are provided on the bottom axis.
Additionally, this assay has been extended as discussed elsewhere [34] to probe equilibrium calcium binding constants for the myristoylated and nonmyristoylated 6X His-tag constructs. Calcium binding constants similar to those previously published [17, 32] have been observed (data not shown), demonstrating there is no change in the calcium binding behavior of these constructs.
Conclusion
We have demonstrated that the bacterial auto-induction system developed by Studier can be supplemented to produce large amounts of soluble recombinant NCS-1. Inclusion of a C-terminal 6X His-tag allowed for the efficient recovery of the various NCS-1 constructs through the use of NiNTA chromatography. The introduction of this affinity tag did not affect the secondary structure, calcium binding ability, or the calcium induced structural changes of the protein, demonstrating that our expression and purification produces protein suitable for biochemical or structural studies. Additionally, when supplementing the ZYP-5052 auto-induction media with a suitable substrate (100 – 200 µM NaMyr), bacteria co-transformed with yeast NMT also express myristoylated NCS-1 in high yields. Recovery of myristoylated NCS-1 is enhanced by the differential affinity of myristoylated and nonmyristoylated NCS-1 for the NiNTA resin. This allowed for single step recovery of essentially homogenous myristoylated NCS-1. We also describe the purification and characterization of NCS-1 variants with impaired (E120Q) or abrogated (ΔEF) calcium binding. The introduced mutations do not grossly affect the apo structure of the protein, making these constructs ideal for studying the role of calcium binding on NCS-1 structure and function. Furthermore the presence of the 6X His-tag does not overtly affect the tertiary fold of myristoylated NCS-1 or the ability of the protein construct to undergo calcium induced structural changes. The presence of the C-terminal 6X His-tag may aid in the process of screening for or characterizing the interaction of the various NCS-1 constructs with its binding partners.
The availability of large quantities of homogenous myristoylated NCS-1 and calcium binding variants NCS-1 E120Q and NCS-1 ΔEF will allow for further characterization of the structural and functional features of NCS-1. Additionally, along with its many roles in NCS proteins, the myristoylation modification has been demonstrated to play a significant role in a number of important protein driven and larger pathological processes [35–36]. With a suitable method of detection of target protein myristoylation, it should be possible to extend this purification system to produce large quantities of myristoylated variants of other proteins.
Supplementary Material
(a) Total NCS-1 production (solid line) and % Myristoylation (dashed line) as a function of NaMyr concentration. 50 mL auto-induction cultures were grown in increasing amounts of NaMyr substrate. A large decrease in recombinant NCS-1 production occurs at concentrations of substrate greater than the CMC.
(b) Scattered light at 90° as a function of sodium myristate (NaMyr) concentration in 37°C ZYP-5052 media. The large increase in scatter intensity between data points at 400 µM and 600 µM indicates the CMC. Some error bars are not visible as they are smaller than sample points.
Acknowledgments
The authors are indebted to Maria Bewley, Shorena Nadaraia-Hoke, and Brian Tash for their assistance in reading the manuscript, Ira Ropson for assistance collecting the CD spectra and analyzing NMR data, and Kathleen Griffin for general technical assistance. We also thank Joe Bednarczyk of the Molecular Genetics Core Facility, Penn State College of Medicine, for DNA sequencing. Purchase and maintenance of the 600 MHz Bruker Avance II spectrometer was provided by NIH instrument grant S210RR021172 to JMF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pongs O, Lindemeier J, Zhu XR, Theil T, Engelkamp D, Krah-Jentgens I, Lambrecht HG, Koch KW, Schwemer J, Rivosecchi R, et al. “Frequenin--a novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system.”. Neuron. 1993;11(1):15–28. doi: 10.1016/0896-6273(93)90267-u. [DOI] [PubMed] [Google Scholar]
- 2.Nef S, Fiumelli H, de Castro E, Raes MB, Nef P. “Identification of neuronal calcium sensor (NCS-1) possibly involved in the regulation of receptor phosphorylation.”. J Recept Signal Transduct Res. 1995;15(1–4):365–378. doi: 10.3109/10799899509045227. [DOI] [PubMed] [Google Scholar]
- 3.Sallese M, Iacovelli L, Cumashi A, Capobianco L, Cuomo L, De Blasi A. “Regulation of G protein-coupled receptor kinase subtypes by calcium sensor proteins.”. Biochim Biophys Acta. 2000;1498(2–3):112–121. doi: 10.1016/s0167-4889(00)00088-4. [DOI] [PubMed] [Google Scholar]
- 4.Kabbani N, Negyessy L, Lin R, Goldman-Rakic P, Levenson R. “Interaction with neuronal calcium sensor NCS-1 mediates desensitization of the D2 dopamine receptor”. J Neurosci. 2002;22(19):8476–8486. doi: 10.1523/JNEUROSCI.22-19-08476.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss JL, Archer DA, Burgoyne RD. “Neuronal Ca2+ sensor-1/frequenin functions in an autocrine pathway regulating Ca2+ channels in bovine adrenal chromaffin cells.”. J Biol Chem. 2000;275(51):40082–40087. doi: 10.1074/jbc.M008603200. [DOI] [PubMed] [Google Scholar]
- 6.Burgoyne RD, Weiss JL. “The neuronal calcium sensor family of Ca2+-binding proteins.”. Biochem. J. 2001;353(1–12) [PMC free article] [PubMed] [Google Scholar]
- 7.Ames JB, Hendricks KB, Strahl T, Huttner IG, Hamasaki N, Thorner J. “Structure and calcium-binding properties of Frq1, a novel calcium sensor in the yeast Saccharomyces cerevisiae.”. Biochemistry. 2000;39(40):12149–12161. doi: 10.1021/bi0012890. [DOI] [PubMed] [Google Scholar]
- 8.Flaherty KM, Zozulya S, Stryer L, McKay DB. “Three-dimensional structure of recoverin, a calcium sensor in vision.”. Cell. 1993;75(4):709–716. doi: 10.1016/0092-8674(93)90491-8. [DOI] [PubMed] [Google Scholar]
- 9.Stephen R, Palczewski K, Sousa MC. “The crystal structure of GCAP3 suggests molecular mechanism of GCAP-linked cone dystrophies.”. J Mol Biol. 2006;359(2):266–275. doi: 10.1016/j.jmb.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scannevin RH, Wang K, Jow F, Megules J, Kopsco DC, Edris W, Carroll KC, Lü Q, Xu W, Xu Z, Katz AH, Olland S, Lin L, Taylor M, Stahl M, Malakian K, Somers W, Mosyak L, Bowlby MR, Chanda P, Rhodes KJ. “Two N-terminal domains of Kv4 K(+) channels regulate binding to and modulation by KChIP1.”. Neuron. 2004;41(4):587–598. doi: 10.1016/s0896-6273(04)00049-2. [DOI] [PubMed] [Google Scholar]
- 11.Gifford JL, Walsh MP, Vogel HJ. “Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs.”. Biochem J. 2007;405(2):199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 12.Cox JA, Drussel I, Comte M, Nef S, Nef P, Lenz SE, Gundelfinger ED. “Cation binding and conformational changes in VILIP and NCS-1, two neuron-specific calcium-binding proteins.”. J. Biol. Chem. 1994;269:32807–32814. [PubMed] [Google Scholar]
- 13.Bourne Y, Dannenberg J, Pollmann V, Marchot P, Pongs Immunochemical localization and crystal structure of human frequenin (neuronal calcium sensor 1) J. Biol. Chem. 2001;276(11):949–955. doi: 10.1074/jbc.M009373200. [DOI] [PubMed] [Google Scholar]
- 14.O'Callaghan DW, Ivings L, Weiss JL, Ashby MC, Tepikin AV, Burgoyne RD. "Differential use of myristoyl groups on neuronal calcium sensor proteins as a determinant of spatio-temporal aspects of Ca2+ signal transduction.". J Biol Chem. 2002;277(16):14227–14237. doi: 10.1074/jbc.M111750200. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Várnai P, Tuymetova G, Balla A, Tóth ZE, Oker-Blom C, Roder J, Jeromin A, Balla T. "Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells.". J Biol Chem. 2001;276(43):40183–40189. doi: 10.1074/jbc.M104048200. [DOI] [PubMed] [Google Scholar]
- 16.Muralidhar D, Jobby MK, Krishnan K, Annapurna V, Chary KV, Jeromin A, Sharma Y. “Equilibrium unfolding of neuronal calcium sensor-1: N-terminal myristoylation influences unfolding and reduces protein stiffening in the presence of calcium.”. J Biol Chem. 2005;280(16):15569–15578. doi: 10.1074/jbc.M414243200. [DOI] [PubMed] [Google Scholar]
- 17.Jeromin A, Muralidhar D, Parameswaran MN, Roder J, Fairwell T, Scarlata S, Dowal L, Mustafi SM, Chary KV, Sharma Y. "N-terminal myristoylation regulates calcium-induced conformational changes in neuronal calcium sensor-1.". J Biol Chem. 2004;279(26):27158–27167. doi: 10.1074/jbc.M312172200. [DOI] [PubMed] [Google Scholar]
- 18.Studier FW. "Protein production by auto-induction in high density shaking cultures.". Protein Expr Purif. 2005;41(1):207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Fisher JR, Sharma Y, Iuliano S, Piccioti RA, Krylov D, Hurley J, Roder J, Jeromin A. "Purification of myristoylated and nonmyristoylated neuronal calcium sensor-1 using single-step hydrophobic interaction chromatography.". Protein Expr Purif. 2000;20(1):66–72. doi: 10.1006/prep.2000.1298. [DOI] [PubMed] [Google Scholar]
- 20.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. "How to measure and predict the molar absorption coefficient of a protein.". Protein Sci. 1995;4(11):2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrlich LS, Fong S, Scarlata S, Zybarth G, Carter C. "Partitioning of HIV-1 Gag and Gag-related proteins to membranes.". Biochemistry. 1996;35(13):3933–3943. doi: 10.1021/bi952337x. [DOI] [PubMed] [Google Scholar]
- 22.Tyler RC, Sreenath HK, Singh S, Aceti DJ, Bingman CA, Markley JL, Fox BG. "Auto-induction medium for the production of [U-15N]- and [U-13C, U-15N]-labeled proteins for NMR screening and structure determination.". Protein Expr Purif. 2005;40(2):268–278. doi: 10.1016/j.pep.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Piotto M, Saudek V, Sklenár V. "Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions.". J Biomol NMR. 1992;2(6):661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- 24.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. "NMRPipe: a multidimensional spectral processing system based on UNIX pipes.". J Biomol NMR. 1995;6(3):277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 25.Johnson BA. "Using NMRView to visualize and analyze the NMR spectra of macromolecules". Methods Mol Biol. 2004;278:313–352. doi: 10.1385/1-59259-809-9:313. [DOI] [PubMed] [Google Scholar]
- 26.Ulrich EL, Akutsu H, Doreleijers JF, Harano Y, Ioannidis YE, Lin J, Livny M, Mading S, Maziuk D, Miller Z, Nakatani E, Schulte CF, Tolmie DE, Kent Wenger R, Yao H, Markley JL. "BioMagResBank.". Nucleic Acids Res. 2008;36:D402–D408. doi: 10.1093/nar/gkm957. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schekar SC. [Accessed: 01-15-08]; http://www.ccpn.ac.uk/ccpn/software/contributed-software/s-chandra-shekar-nmrview-scripts/introduction.
- 28.Arnold FH. "Metal-affinity separations: a new dimension in protein processing.". Biotechnology (N Y) 1991;9(2):151–156. doi: 10.1038/nbt0291-151. [DOI] [PubMed] [Google Scholar]
- 29.Porath J. "Immobilized metal ion affinity chromatography.". Protein Expr Purif. 1992;3(4):263–281. doi: 10.1016/1046-5928(92)90001-d. [DOI] [PubMed] [Google Scholar]
- 30.Brahms S, Brahms J. "Determination of protein secondary structure in solution by vacuum ultraviolet circular dichroism.". J Mol Biol. 1980;138(2):149–178. doi: 10.1016/0022-2836(80)90282-x. [DOI] [PubMed] [Google Scholar]
- 31.McFerran BW, Weiss JL, Burgoyne RD. "Neuronal Ca(2+) sensor 1. Characterization of the myristoylated protein, its cellular effects in permeabilized adrenal chromaffin cells, Ca(2+)-independent membrane association, and interaction with binding proteins, suggesting a role in rapid Ca(2+) signal transduction.". J Biol Chem. 1999;274(42):30258–30265. doi: 10.1074/jbc.274.42.30258. [DOI] [PubMed] [Google Scholar]
- 32.Aravind P, Chandra K, Reddy PP, Jeromin A, Chary KV, Sharma Y. “Regulatory and structural EF-hand motifs of neuronal calcium sensor-1: Mg 2+ modulates Ca 2+ binding, Ca 2+ -induced conformational changes, and equilibrium unfolding transitions.”. J Mol Biol. 2008;376(4):1100–1115. doi: 10.1016/j.jmb.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 33.Grynkiewicz G, Poenie M, Tsien RY. "A new generation of Ca2+ indicators with greatly improved fluorescence properties.". J Biol Chem. 1985;260(6):3440–3450. [PubMed] [Google Scholar]
- 34.Linse S. "Calcium binding to proteins studied via competition with chromophoric chelators". Methods Mol Biol. 2002;173:15–24. doi: 10.1385/1-59259-184-1:015. [DOI] [PubMed] [Google Scholar]
- 35.Sharma RK. "Potential role of N-myristoyltransferase in pathogenic conditions.". Can J Physiol Pharmacol. 2004;82(10):849–859. doi: 10.1139/y04-099. [DOI] [PubMed] [Google Scholar]
- 36.Boutin JA. "Myristoylation.". Cell Signal. 1997;9(1):15–35. doi: 10.1016/s0898-6568(96)00100-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Total NCS-1 production (solid line) and % Myristoylation (dashed line) as a function of NaMyr concentration. 50 mL auto-induction cultures were grown in increasing amounts of NaMyr substrate. A large decrease in recombinant NCS-1 production occurs at concentrations of substrate greater than the CMC.
(b) Scattered light at 90° as a function of sodium myristate (NaMyr) concentration in 37°C ZYP-5052 media. The large increase in scatter intensity between data points at 400 µM and 600 µM indicates the CMC. Some error bars are not visible as they are smaller than sample points.