Abstract
There is wide interpatient variability in drug response and toxicity to standard doses of most anticancer medications. Genetic polymorphisms in genes encoding metabolic enzymes, receptors and drug transporters targeted by anticancer medications are often found, in part, to be responsible for the observed variability. Approximately 80% of all sequence variations residing in genes is in the form of single nucleotide polymorphisms or SNPs. The location of SNPs can be in the protein coding sequence, regulatory regions or at exon-intron boundaries of genes. Adverse drug reactions resulting from these sequence variations are due to changes in the activity of the encoded protein (in many instances the protein is non-functional) or perturbations in the level of gene expression. The goal of pharmacogenetic testing is to identify genetic polymorphisms that predispose patients to an adverse drug reaction, thereby allowing the health care provider to make informed decisions pertaining to the type of drug, dosage and dosage scheduling to be administered.
Keywords: pharmacogenetics, single nucleotide polymorphisms, genetic variation, thiopurine S-methyltransferase, thymidylate synthase, dihydropyrimidine dehydrogenase, ATP-binding cassette transporters, multidrug resistance-associated protein, cancer health disparities
Introduction
As an anticancer drug is administered to a patient, it is absorbed and distributed to its site of action. Along the way, the drug can be metabolized by the liver (e.g. prodrug to active drug, active drug to inactive metabolites), translocated from one body compartment to another, bound to targets such as receptors, transporters or enzymes in the tumor itself, and ultimately excreted from the body. Clinically relevant genetic variations can occur in any of the genes involved in these processes. This review will focus on some key pharmacogenetic examples in order to emphasize the role of inherited polymorphisms in the form of SNPs on the pharmacokinetics and pharmacodynamics of anticancer drugs. The underlying message for the reader is that inherited genetic variations affecting anticancer drug response are fairly common occurrences across the patient population and that pharmacogenetic testing for some drugs is now available in the clinical setting, with more likely to follow as next generation sequencing of the human genome further defines genetic variants [1] and technology platforms continue to develop for pharmacogenetic/pharmacogenomic diagnostics [2].
Functional consequences of polymorphisms in genes: an overview
What are single nucleotide polymorphisms?
A single nucleotide polymorphism (SNP) is a DNA sequence variation that occurs when a single nucleotide position in the genome differs between two individuals (A, T, C or G represent possible alleles). Typically one of the alleles (e.g. C) will be the predominant allele, being found in the majority of the population, while the other allele (e.g. T) will represent the minor variant. SNPs represent one type of sequence polymorphism comprising ~78% of all genetic variations in the human genome, while the remaining 22% of sequence variants are of the type known as insertions, deletions, inversions, copy number variants and segmental duplications [3]. It has been estimated that the number of SNPs in the human genome is ~10–30 million [4, 5], and the number of protein coding genes in the human genome has been predicted to range from only ~20,000 to ~35,000 [6, 7]. Consequently, SNPs occur every 100 to 300 nucleotides along the 3 billion nucleotide human genome, approximately 10% of the SNPs can have more than 2 possible alleles, and 1 in every 1200 nucleotides of a gene may be different in any two individuals [4, 5, 8].
Classification of SNPs
SNPs can be classified more or less into 4 major categories, namely those found: i) in the protein coding sequence of genes, ii) in the regulatory regions of genes (e.g. promoter region, 5′-untranslated region, 3′-untranslated region, intronic sequences), iii) at exon-intron boundaries of genes, and iv) in the intergenic regions (intervening genomic segments separating genes). The consequences of these sequence variants can range from no measurable effects on protein function, to changes in the structure and function of the encoded proteins, to perturbations in the level of gene expression. In the latter two scenarios, such SNPs are candidates for drug response-modifying alleles and the inheritance of these alleles by patients receiving standard doses of drug therapy can lead to an adverse drug reaction or chemotherapy failure.
Synonymous and non-synonymous SNPs
SNPs residing in the coding sequence of a gene may not necessarily change the amino acid sequence of the encoded protein, especially when the SNP occurs at the third position of the triplet codon due to the degeneracy of the genetic code (Figure 1A). A SNP in which two or more alleles result in the same protein sequence is termed synonymous (also called a silent “mutation”). If the alleles for a particular SNP leads to different amino acid residue substitutions in the polypeptide, they are referred to as non-synonymous which can be further subdivided into missense or nonsense changes (Figure 1A). A missense change results in the coding of a different amino acid (missense changes are sometimes referred to as conservative or non-conservative), while a nonsense change inserts a premature stop codon into the gene. In either case, the translated protein may be rendered inactive or with markedly reduced activity. Non-synonymous changes (via mutation or SNP) appear to account for more than half of the known genetic variations associated with human inherited diseases [9]. Accordingly, numerous computational approaches have been initiated for the purposes of modeling proteins containing non-synonymous SNPs in the hopes of predicting if these sequence variants alter the three dimensional structure and function of the protein [10–13].
Figure 1. Functional consequences of SNPs.
(A) SNPs residing in the protein coding sequence of a gene can lead to synonymous or non-synonymous changes. The latter modifies the amino acid sequence (missense) of a protein or results in early termination of protein translation (nonsense), both potentially producing a non-functional protein. (B) SNPs in the promoter or regulatory enhancer regions of a gene can lead to changes in transcriptional activity. In the human tumor necrosis factor (TNF) gene promoter, the predominant G allele at nucleotide position -376 does not bind the OCT-1 transcription factor. The minor A allele at position -376 creates a de novo binding site for the OCT-1 TF, leading to increased transcriptional activity. Lines represent introns, boxes are exons. Exons and introns are not drawn to scale.
Regulatory SNPs
SNPs located in the regulatory regions of genes can have consequences on the regulation of gene expression through effects on transcription factor (TF) binding [14]. Transcriptional regulatory domains (cis-acting elements) residing in the promoter or intronic region of genes are short sequences (typically ~6–20 bases) which serve as the binding sites for TFs. SNPs that change the recognition site can potentially increase or decrease TF binding efficiency, leading to temporal and spatial alterations in gene expression and/or changes in the level of gene expression. Alternatively, SNPs in the promoter region can impart new TF binding properties, resulting in a gain-of-function. For example, the minor “A” allele of a SNP found in the promoter of the tumor necrosis factor gene creates a de novo binding site for the OCT-1 TF (Figure 1B), leading to increased transcriptional activity [15]. By comparison, the same promoter containing the predominant “G” allele does not bind OCT-1 [15]. Finally, another gene regulatory region that can be affected by SNPs is the 5′- or 3′-untranslated region [14]. These regions are located on either end of the transcribed mRNA molecule and play a role in the post-transcriptional regulation of the mRNA either by translational repression or changes in mRNA stability. The post-transcriptional control is mediated by the binding of regulatory factors (proteins or short 19–21 nucleotide long non-coding RNA molecules known as microRNAs) onto sequence motifs residing in the untranslated region of the mRNA [16, 17]. SNPs targeting these motifs in the 3′-untranslated region have been associated with changes in mRNA stability resulting from alterations in regulatory protein [18] or microRNA binding characteristics [19].
SNPs affecting alternative splicing
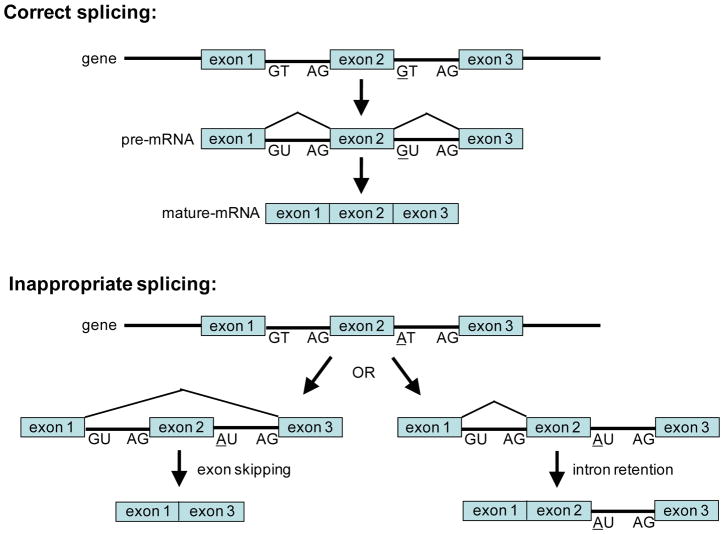
In addition to modifying the sequence of the encoded protein and affecting gene expression, SNPs can drastically alter the splicing pattern of mRNAs. A typical human pre-mRNA molecule contains an average of 8 exons with internal exons averaging 145 nucleotides in length, and between each exon is an intervening intron that averages >10 times the size of an exon [20]. Alternative splicing of a pre-mRNA molecule is a fundamental mechanism allowing cells to produce structurally and functional distinct proteins from a single gene by catalyzing a ‘cut-and-paste’ reaction that removes introns and joins together different combinations of exons. The end result is the formation of different mature alternatively spliced mRNAs. It has been estimated that ~60–70% of the genes in the human genome have the capacity to generate alternative mRNA species by the mechanism of alternative splicing [20, 21], and that ~80% of the alternatively spliced mRNAs results in changes in the amino acid sequence of the encoded protein [22], providing proteomic diversity to our genome (see review [23]). The control of alternative splicing involves the splicing machinery (comprised of small nuclear ribonucleoproteins and >100 proteins) that recognizes sequence regulatory domains found in the exonic and intronic splicing enhancers, exonic and intronic splicing silencers, the 5′ terminus of an intron defined by an GT dinucleotide motif (splice donor site), and the 3′ splice site end of an intron consisting of an A nucleotide branch point followed by a pyrimidine-rich track and the conserved AG dinucleotide splice acceptor site [24]. SNPs appearing in any of these regulatory domains can result in a disruption of normal splicing leading to the generation of abnormal mRNA species due to exon skipping, exon inclusion or intron retention (Figure 2) [23]. Consequently, the abnormal mRNA species may encode, for example, a truncated protein or significantly altered protein sequence which in either case will likely be non-functional. In the sections below, several examples are provided detailing how SNPs can affect gene products targeted by anticancer drugs, leading to an adverse drug reaction in patients that have inherited the detrimental allele.
Figure 2. SNP leading to inappropriate mRNA splicing.
Alternative splicing of a gene generates variable mRNAs, a process by which functionally diverse protein isoforms can be expressed in the cell. The 5′- and 3′-ends of the intronic regions (lines) of genes contain conserved GT and AG dinucleotide motifs serving as splice donor and acceptor sites, respectively (top panel; correct splicing). A SNP (G-to-A transition) occurring in the GT splice donor site of an intron following exon 2 leads to inappropriate slicing (bottom panel; inappropriate splicing). The consequences of inappropriate splicing can be skipping of exon 2 or retention of the intron containing the A allele SNP. In both cases, the encoded protein is likely to be non-functional. Lines are introns, boxes are exons.
Polymorphisms in the thiopurine S-methyltransferase gene
The thiopurine prodrugs 6-mercaptopurine (6-MP) and azathioprine are clinically employed in the treatment of acute lymphoblastic leukemia and as an immunosuppressant, respectively [25, 26]. Both of these agents undergo enzymatic conversion via the purine salvage pathway to the active antimetabolite 2′-deoxy-6-thioguanosine 5′-triphosphate, which is incorporated into DNA resulting in target cell cytotoxicity. Conversely, the thiopurine prodrugs can be inactivated through methylation by the enzyme thiopurine S-methyltransferase (TPMT). Any disturbance in the metabolic balance between prodrug activation and inactivation, such as a reduction in TPMT activity, can lead to life-threatening bone marrow toxicity and myelosuppression.
The activity of TPMT is influenced by the presence of SNPs where there exist at least 24 TPMT alleles, but only a handful of which have known clinically relevant effects [27, 28]. Large inter-individual variability across the patient population can be observed in terms of the rate of thiopurine methylation and inactivation. Alleles for low- and high-activity TPMT are inherited in an autosomal co-dominant fashion, resulting in a trimodal distribution within the Caucasian population consisting of 89% possessing high (normal) enzyme activity, 11% with intermediate activity and 0.3% with low activity [29–31].
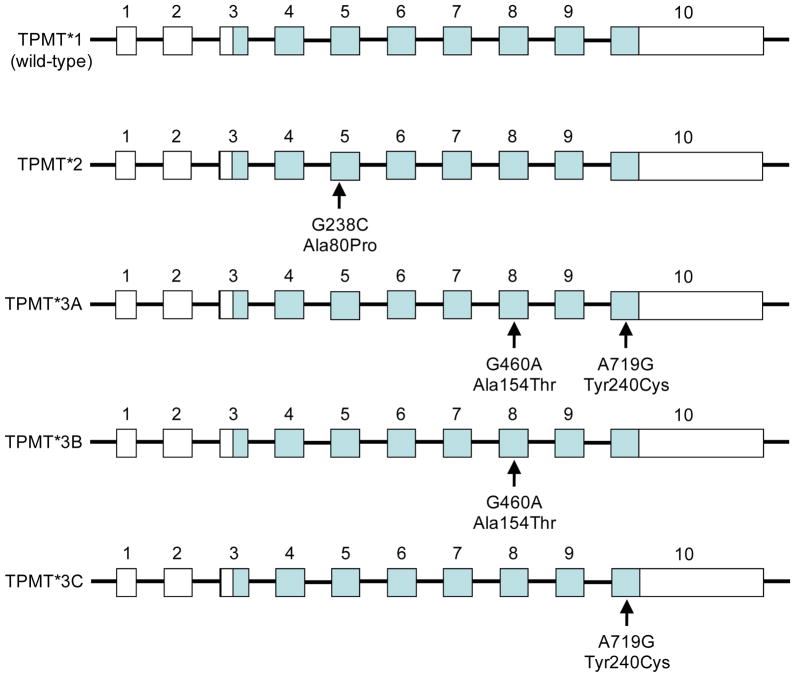
The wild-type (predominant) allele is designated as TPMT*1 and exhibits full enzymatic activity while the variant alleles (TPMT*2 – TPMT*24) have been associated with slightly to drastically reduced activities relative to the predominant allele [28]. Variant alleles TPMT*2 (G238C; Ala80Pro), TPMT*3A (G460A/A719G; Ala154 hr/Tyr240Cys), TPMT*3B (G460A; la154 hr) and TPMT*3C (A719G; Tyr240Cys) involve non-synonymous coding polymorphisms that result in alterations in the sequence of the encoded protein (Figure 3), and account for >80% of individuals with low TPMT activity [27, 29]. Patients homozygous for the TPMT*3A allele have negligible TPMT activity and consequently are at increased risk for life threatening myelosuppression upon treatment with standard doses of thiopurines [32, 33]. To circumvent the severe hematopoietic toxicity, TPMT activity-deficient patients can be treated with one-tenth of the conventional dose. Patients with TPMT*3B have a 9-fold reduction and those with TPMT*3C display a <2-fold reduction in enzyme activity compared to TPMT*1-expressing individuals [34, 35].
Figure 3. Polymorphisms in the thiopurine S-methyltransferase gene.
The predominant allele TPMT*1 encodes an enzyme with full activity. Minor alleles TPMT*2, TPMT*3A, TPMT*3B and TPMT*3C contain one or two non-synonymous coding SNPs leading to changes in amino acid sequence of thiopurine S-methyltransferase. The encoded proteins derived from these minor alleles have significantly reduced to no enzyme activity. Lines represent introns, closed boxes are the translated regions, and open boxes are the untranslated regions of the gene. Exons and introns are not drawn to scale.
The molecular basis for the reduced TPMT activity associated with variants TPMT*2 and TPMT*3A appears to be a consequence of enhanced proteolytic degradation of the proteins encoded by these variant alleles [36]. Moreover, the degradative process is facilitated by the chaperone proteins heat shock protein 70 and heat shock protein 90, which have been demonstrated to preferentially bind and target the TPMT*3A protein to the ubiquitin-proteasome [37]. By comparison, the wild-type protein encoded by TPMT*1 is much less physically associated with the heat shock proteins [37].
Based on compelling pharmacogenetic evidence over the past decades, the FDA has included genotyping information in the drug label for 6-MP and recommends the usage of genotyping to guide dosing [38, 39]. The implementation of TPMT genotyping allows oncologists to be informed of patient risk for developing chemotherapy-associated toxicities with thiopurine treatment. Thus, genotype-tailored dosing for the individual patient holds the promise of minimizing adverse drug reactions.
5-flourouracil treatment and polymorphisms of the thymidylate synthase and dihydropyrimidine dehydrogenase genes
The pyrimidine analog 5-flourouracil (5-FU) is an antimetabolite used as an adjuvant or in the palliative treatment of a number of tumors, including pancreatic, head and neck, and breast cancers. This antimetabolite represents a mainstay as a chemotherapeutic drug for colorectal carcinomas, which is one of the leading causes of cancer mortality in the U.S. with approximately 100,000 new cases and >50,000 deaths each year [40]. 5-FU is readily transported into tumor cells (and normal cells) where it undergoes anabolic conversion into a number of active cancer-killing species [41]. One of the active metabolites, 5-flouro-2′-deoxyuridine-5′-monophosphate (FdUMP), in combination with 5,10-methylenetetrahydrofolate forms a stable ternary complex with the enzyme thymidylate synthase [42]. The ensuing suicide complex inhibits normal synthesis of 2′-deoxythymidine-5′-monophosphate (dTMP), resulting in an imbalance of deoxynucleotides, DNA damage and tumor cell cytotoxicity [41]. Thymidylate synthase is ordinarily responsible for the reductive methylation of the pyrimidine nucleotide 2′-deoxyuridine-5′-monophosphate (dUMP) to produce dTMP, with the methyl group coming from the methyl donor 5,10-methylenetetrahydrofolate.
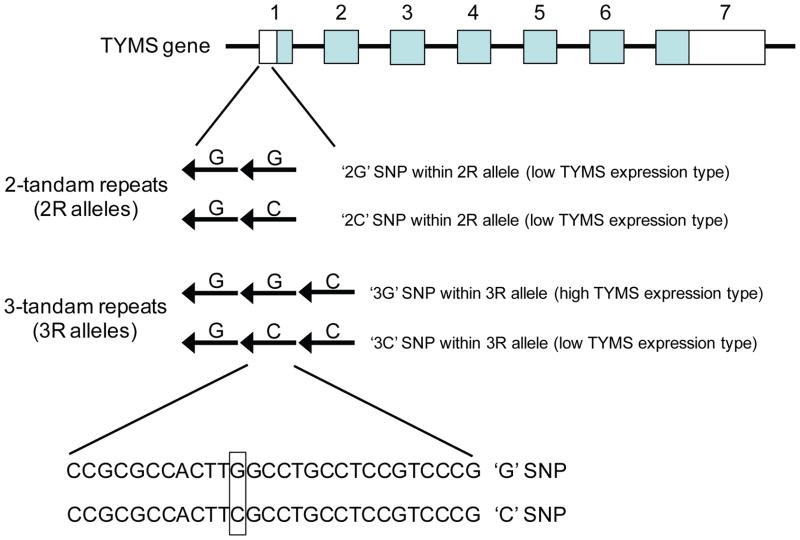
There is a growing view that alterations in thymidylate synthase levels may be associated with 5-FU resistance in cancer cells [43]. The 5′UTR of the human thymidylate synthase gene contains a variable number of tandem repeats (VNTR) polymorphism comprising of two (2R allele), three (3R allele) or more 28-base pair repeated sequences [44–46] (Figure 4). Early in vitro and clinical studies demonstrated that the expression of reporter gene constructs containing the 3R allele was 2 times higher than constructs containing the 2R allele [45], and the 3R allele was associated with higher thymidylate synthase mRNA and protein levels in colorectal/gastrointestinal cancers [47, 48]. From a treatment standpoint, colorectal cancer patients homozygous for the 3R genotype have a poorer antitumor response to 5-FU [47, 49–51]. It should be noted that the relationship between 2R/3R genotype and 5-FU response outcome appears to extend beyond colorectal carcinomas and includes for example acute lymphoblastic leukemia [52]. More recent studies indicate that the prediction of 5-FU response outcome may be more nuanced than simply genotyping a patient for the 2R and 3R alleles by polymerase chain reaction. The appearance of a G/C polymorphism in the 3R VNTR appears to stratify even further the response outcome of 5-FU-treated colorectal cancer patients (Figure 4), with the “3G” SNP in the 3R locus being associated with high thymidylate synthase levels and poor chemotherapy response and the “3C” SNP associated with low thymidylate synthase levels and a more positive treatment outcome [53, 54]. Taken together, evidence is compelling for the routine implementation of thymidylate synthase genotyping to identify patients who are likely to benefit from 5-FU treatment. At that the present time, however, the FDA has yet to recommend genotyping of the thymidylate synthase gene for patients scheduled to receive 5-FU treatment [38].
Figure 4. 3R and 2R alleles in the thymidylate synthase gene.
The 5′-untranslated region of exon 1 of the thymidylate synthase (TYMS) gene contains a 28-base pair repeat polymorphism (variable number of tandem repeats), mainly in the form of two repeats (2R) or three repeats (3R). Contained within the second repeat is a C/G SNP, producing the following alleles: 2R/2G, 2R/2C, 3R/3G and 3R/3C. Colorectal cancer patients genotyped as having one or two copies of the 3R/3G allele exhibit a poor response to 5-FU treatment. Lines represent introns, closed boxes are the translated regions, and open boxes are the untranslated regions of the gene. Arrows represent the 28-base pair repeats found in the 5′-untranslated region. Exons and introns are not drawn to scale.
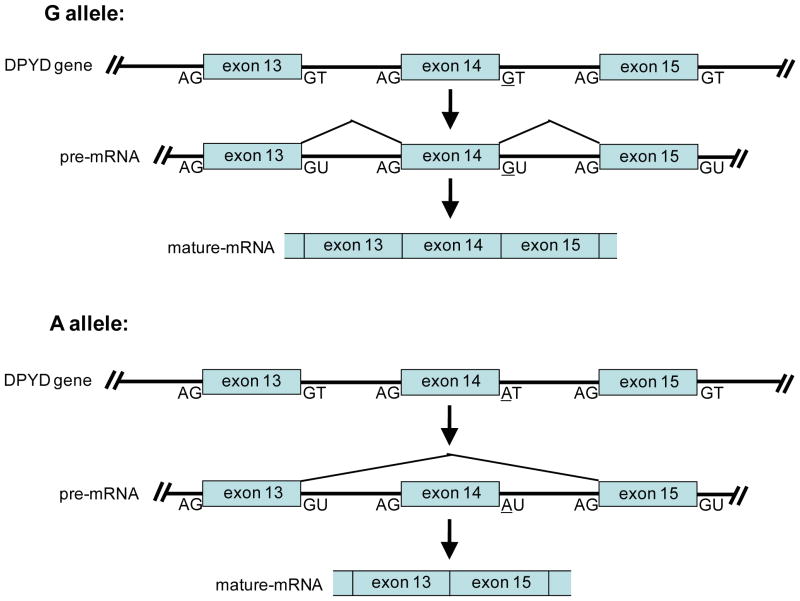
The bioavailability of 5-FU in patients is poor as the liver enzyme dihydropyrimidine dehydrogenase rapidly degrades up to ~80% of the administered antimetabolite to dihydrofluorouracil [55, 56]. Cancer patients with a deficiency in dihydropyrimidine dehydrogenase are at risk of developing life-threatening neurotoxcity and myelosuppression due to excessive anabolic conversion of undegraded 5-FU to active metabolites. In ~25–50% of the patients suffering from severe 5-FU toxicity, the associated dihydropyrimidine dehydrogenase deficiency can be traced back to an inherited SNP (G-to-A transition) in the invariant GT splice donor site contained in an intron that flanks exon 14, leading to exon skipping and an inactive encoded enzyme [57–61] (Figure 5). It has been estimated that ~3% of the population are heterozygous and 0.1% are homozygous for the inactivating A allele [61–63]. Additional polymorphisms and/or mutations potentially associated with reduced dihydropyrimidine dehydrogenase activity have been identified [60, 64]. To date, more than 40 variants are known to exist, including frameshift mutations, nonsense mutations, intronic mutations and 27 non-synonymous SNPs or mutations [60, 64]. Given the prevalence of the above pharmacogenetic data, the FDA has recommended genotyping of the dihydropyrimidine dehydrogenase gene in patients receiving 5-FU treatment [38].
Figure 5. Polymorphism in the dihydropyrimidine dehydrogenase gene.
The dihydropyrimidine dehydrogenase (DPYD) gene contains a G/A SNP in the splice donor site of an intron flanking the 3′-end of exon 14. Individuals inheriting the A allele have inappropriate splicing of the DPYD gene, leading to the skipping of exon 14 and the translation of a nonfunctional enzyme. Cancer patients with a deficiency in DPYD cannot properly degrade standard doses of 5-FU and are at risk of developing life-threatening neurotoxcity and myelosuppression. Lines represent introns, boxes are exons. Exons and introns are not drawn to scale.
Polymorphisms in drug transporters
Cancer cells exposed to anticancer agents have the capacity to develop resistance by employing a number of mechanisms such as decreased drug importation, alterations in target protein, increased metabolism/detoxification of the drug, and increased drug efflux [65]. The ATP-binding cassette (ABC) transporter family plays a critical role in the regulation of drug absorption, distribution and excretion. At this time, there are at least 48 family members, subdivided into 7 subfamilies (ABCA, ABCB, ABCC, ABCD, ABCE, ABCF, ABCG), ranging in protein size from several hundred to several thousand amino acids [66]. The over-expression of specific members of the ABC transporter family in cancer cells has been associated with increased anticancer drug efflux resulting in multidrug resistance, a major obstacle for successful cancer chemotherapy [67–69]. Given that genetic polymorphisms in ABC transporter genes have the potential to alter protein function and/or expression, it is not surprising that numerous studies have been initiated in the search for sequence variants linked to drug responsiveness in patients.
So, do polymorphisms in ABC transporters modify patient response to anticancer agents? Taken as a whole, the majority of clinical studies to date has been relatively small and performed as single institutional studies as opposed to large-scale multi-institutional undertakings, and consequently lack sufficient statistical power to draw consistent conclusions. The clinical consequences of transporter sequence variations in a number of different cancers have been either inconclusive or contradictory and thus remain to be fully delineated [43, 70–76]. It is clear that future large-scale clinical investigations are warranted pertaining to genetic polymorphisms and ABC transporter genes. Nonetheless, there are noteworthy examples (see below) that merit additional scrutiny.
Multidrug resistance 1 (MDR1) gene
The MDR1 gene (also known as ABCB1) encodes P-glycoprotein and represents one of the most extensively studied anticancer drug resistance transporters in pharmacogenetics. For example, the ABCB1 variant C3435T has been associated with decreased drug efflux activity, and a favorable clinical outcome for locally advanced breast cancer patients on preoperative anthracycline antibiotics [77]. Of interest is the finding that the C-to-T transition in exon 26 does not change the amino acid sequence of P- glycoprotein (i.e. synonymous SNP), rather the T allele is linked to lower MDR1 gene expression in intestinal enterocytes compared with the C allele [78]. A similar positive clinical outcome associated with the C3435T variant has been reported for acute myeloid leukemic (AML) patients, where expression of the T allele was lower in patient AML blast cells compared to the corresponding C allele [79]. It remains to be definitively determined from these studies whether treatment outcome is a consequence of polymorphic transporters in the cancer cells themselves (pharmacodynamic effect), in the intestinal enterocytes mediating drug excretion (pharmacokinetic effect), or both.
Breast cancer resistance protein (BCRP) gene
The BCRP gene (also known as ABCG2) was first identified in a mitoxantrone-resistant breast cancer cell line that did not over-express either ABCB1 or ABCC1 [80]. In a later prospective pilot study, ovarian and small cell lung cancer patients with a non-synonymous C421A (Gln141Lys) variation of the BCRP gene were shown to have elevated blood levels of the orally administered anticancer drug topotecan, a topoisomerase I inhibitor [81]. The authors of the pilot study subsequently demonstrated in vitro that the A allele was associated with a 30% decrease in efflux transport of topotecan compared to the C allele, and speculated that the increased oral bioavailability of topotecan is a reflection of decreased intestinal excretion by the C421A variant [81]. In addition to the observed pharmacokinetic effects, it is noteworthy that the C421A variant has been linked to increased risk and poor survival of diffuse large B-cell lymphoma [82]. Clearly, additional larger-scale investigations are warranted to confirm these findings.
Conclusions and future directions
It has been estimated that over half of all adverse drug reactions can be traced to the expression of polymorphic genes. Anticancer drugs typically have a very narrow therapeutic index, and the use of these agents at maximal tolerated doses renders a subgroup of individuals at higher risk to life-threatening toxicities due to the inheritance of specific polymorphisms in genes encoding target proteins and drug metabolizing enzymes. This review has served to highlight select prototypical examples, namely polymorphisms in the: i) TPMT gene leading to thiopurine toxicity, ii) dihydropyrimidine dehydrogenase gene resulting in adverse reactions to 5-FU, and iii) thymidylate synthase gene giving rise to 5-FU treatment failure. Additionally, genetic variation in ABC transporter genes such as MDR1 and BCRP can affect cancer risk and clinical outcome to anticancer agents. A thorough characterization of all genetic polymorphisms in the human genome coupled with an understanding of their role in clinical endpoints should facilitate the rational selection of cancer chemotherapeutic drug(s) and dosage for the purposes of individualizing patient treatment regimens (i.e. personalized or targeted medicine).
There are a number of potential obstacles to be addressed in our pursuit of targeted medicine. The implementation of next generation sequencing platforms will be needed to expedite the identification of rare SNPs and grow the SNP database from the International HapMap Project [83]. Accordingly, our ability to precisely classify alleles linked to adverse drug reactions will be enhanced as a result of the additional genomic information. Moreover, technology platforms are under continual development in our quest for the ‘$1000 genome’ [84], and the lessons learned from these endeavors will enable pharmacogenetic and pharmacogenomic testing to be more reliable, accurate and accessible. Additional hurdles will need to be overcome such as greatly increasing the number of patients in pharmacogenetic studies in order to strengthen the statistical power of associating polymorphisms to variable drug responses in the clinical setting. This should be realized as sequencing costs for genotyping continue to descend. Another area that needs further investigation and emphasis is cancer health disparities. For example, African Americans have a higher incidence and/or mortality for breast, lung, prostate and colon cancers compared to non-Hispanic white women [85–89]. For at least some of these cancers, the disparities persist even after accounting for cultural, socioeconomic and access to health care differences [87, 89]. A major biological component for the disparities appears to be due in part to population-based polymorphisms residing in genes targeted by anticancer drugs [86, 90].
Acknowledgments
This work was supported by NIH grants CA120316, CA116937 and DK056108.
LITERATURE CITED
- 1.Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nature reviews. 2010;11:415–425. doi: 10.1038/nrg2779. [DOI] [PubMed] [Google Scholar]
- 2.Koch WH. Technology platforms for pharmacogenomic diagnostic assays. Nat Rev Drug Discov. 2004;3:749–761. doi: 10.1038/nrd1496. [DOI] [PubMed] [Google Scholar]
- 3.Levy S, Sutton G, Ng PC, Feuk L, Halpern AL, Walenz BP, Axelrod N, Huang J, Kirkness EF, Denisov G, Lin Y, MacDonald JR, Pang AW, Shago M, Stockwell TB, Tsiamouri A, Bafna V, Bansal V, Kravitz SA, Busam DA, Beeson KY, McIntosh TC, Remington KA, Abril JF, Gill J, Borman J, Rogers YH, Frazier ME, Scherer SW, Strausberg RL, Venter JC. The diploid genome sequence of an individual human. PLoS biology. 2007;5:e254. doi: 10.1371/journal.pbio.0050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, Leal SM, Pasternak S, Wheeler DA, Willis TD, Yu F, Yang H, Zeng C, Gao Y, Hu H, Hu W, Li C, Lin W, Liu S, Pan H, Tang X, Wang J, Wang W, Yu J, Zhang B, Zhang Q, Zhao H, Zhao H, Zhou J, Gabriel SB, Barry R, Blumenstiel B, Camargo A, Defelice M, Faggart M, Goyette M, Gupta S, Moore J, Nguyen H, Onofrio RC, Parkin M, Roy J, Stahl E, Winchester E, Ziaugra L, Altshuler D, Shen Y, Yao Z, Huang W, Chu X, He Y, Jin L, Liu Y, Shen Y, Sun W, Wang H, Wang Y, Wang Y, Xiong X, Xu L, Waye MM, Tsui SK, Xue H, Wong JT, Galver LM, Fan JB, Gunderson K, Murray SS, Oliphant AR, Chee MS, Montpetit A, Chagnon F, Ferretti V, Leboeuf M, Olivier JF, Phillips MS, Roumy S, Sallee C, Verner A, Hudson TJ, Kwok PY, Cai D, Koboldt DC, Miller RD, Pawlikowska L, Taillon-Miller P, Xiao M, Tsui LC, Mak W, Song YQ, Tam PK, Nakamura Y, Kawaguchi T, Kitamoto T, Morizono T, Nagashima A, Ohnishi Y, Sekine A, Tanaka T, Tsunoda T, Deloukas P, Bird CP, Delgado M, Dermitzakis ET, Gwilliam R, Hunt S, Morrison J, Powell D, Stranger BE, Whittaker P, Bentley DR, Daly MJ, de Bakker PI, Barrett J, Chretien YR, Maller J, McCarroll S, Patterson N, Pe’er I, Price A, Purcell S, Richter DJ, Sabeti P, Saxena R, Schaffner SF, Sham PC, Varilly P, Altshuler D, Stein LD, Krishnan L, Smith AV, Tello-Ruiz MK, Thorisson GA, Chakravarti A, Chen PE, Cutler DJ, Kashuk CS, Lin S, Abecasis GR, Guan W, Li Y, Munro HM, Qin ZS, Thomas DJ, McVean G, Auton A, Bottolo L, Cardin N, Eyheramendy S, Freeman C, Marchini J, Myers S, Spencer C, Stephens M, Donnelly P, Cardon LR, Clarke G, Evans DM, Morris AP, Weir BS, Tsunoda T, Mullikin JC, Sherry ST, Feolo M, Skol A, Zhang H, Zeng C, Zhao H, Matsuda I, Fukushima Y, Macer DR, Suda E, Rotimi CN, Adebamowo CA, Ajayi I, Aniagwu T, Marshall PA, Nkwodimmah C, Royal CD, Leppert MF, Dixon M, Peiffer A, Qiu R, Kent A, Kato K, Niikawa N, Adewole IF, Knoppers BM, Foster MW, Clayton EW, Watkin J, Gibbs RA, Belmont JW, Muzny D, Nazareth L, Sodergren E, Weinstock GM, Wheeler DA, Yakub I, Gabriel SB, Onofrio RC, Richter DJ, Ziaugra L, Birren BW, Daly MJ, Altshuler D, Wilson RK, Fulton LL, Rogers J, Burton J, Carter NP, Clee CM, Griffiths M, Jones MC, McLay K, Plumb RW, Ross MT, Sims SK, Willey DL, Chen Z, Han H, Kang L, Godbout M, Wallenburg JC, L’Archeveque P, Bellemare G, Saeki K, Wang H, An D, Fu H, Li Q, Wang Z, Wang R, Holden AL, Brooks LD, McEwen JE, Guyer MS, Wang VO, Peterson JL, Shi M, Spiegel J, Sung LM, Zacharia LF, Collins FS, Kennedy K, Jamieson R, Stewart J. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Consortium TIH. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 6.Claverie JM. Gene number. What if there are only 30,000 human genes? Science (New York, NY. 2001;291:1255–1257. doi: 10.1126/science.1058969. [DOI] [PubMed] [Google Scholar]
- 7.Stein LD. Human genome: end of the beginning. Nature. 2004;431:915–916. doi: 10.1038/431915a. [DOI] [PubMed] [Google Scholar]
- 8.Barbujani G, Colonna V. Human genome diversity: frequently asked questions. Trends Genet. 2010;26:285–295. doi: 10.1016/j.tig.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Krawczak M, Ball EV, Fenton I, Stenson PD, Abeysinghe S, Thomas N, Cooper DN. Human gene mutation database-a biomedical information and research resource. Human mutation. 2000;15:45–51. doi: 10.1002/(SICI)1098-1004(200001)15:1<45::AID-HUMU10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 10.Cavallo A, Martin AC. Mapping SNPs to protein sequence and structure data. Bioinformatics (Oxford, England) 2005;21:1443–1450. doi: 10.1093/bioinformatics/bti220. [DOI] [PubMed] [Google Scholar]
- 11.Jegga AG, Gowrisankar S, Chen J, Aronow BJ. PolyDoms: a whole genome database for the identification of non-synonymous coding SNPs with the potential to impact disease. Nucleic acids research. 2007;35:D700–706. doi: 10.1093/nar/gkl826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reumers J, Maurer-Stroh S, Schymkowitz J, Rousseau F. SNPeffect v2.0: a new step in investigating the molecular phenotypic effects of human non-synonymous SNPs. Bioinformatics (Oxford, England) 2006;22:2183–2185. doi: 10.1093/bioinformatics/btl348. [DOI] [PubMed] [Google Scholar]
- 13.Savas S, Kim DY, Ahmad MF, Shariff M, Ozcelik H. Identifying functional genetic variants in DNA repair pathway using protein conservation analysis. Cancer Epidemiol Biomarkers Prev. 2004;13:801–807. [PubMed] [Google Scholar]
- 14.Prokunina L, Alarcon-Riquelme ME. Regulatory SNPs in complex diseases: their identification and functional validation. Expert reviews in molecular medicine. 2004;6:1–15. doi: 10.1017/S1462399404007690. [DOI] [PubMed] [Google Scholar]
- 15.Knight JC, Udalova I, Hill AV, Greenwood BM, Peshu N, Marsh K, Kwiatkowski D. A polymorphism that affects OCT-1 binding to the TNF promoter region is associated with severe malaria. Nature genetics. 1999;22:145–150. doi: 10.1038/9649. [DOI] [PubMed] [Google Scholar]
- 16.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nature reviews. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 17.Keene JD. RNA regulons: coordination of post-transcriptional events. Nature reviews. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 18.Fritz DT, Jiang S, Xu J, Rogers MB. A polymorphism in a conserved posttranscriptional regulatory motif alters bone morphogenetic protein 2 (BMP2) RNA:protein interactions. Molecular endocrinology (Baltimore, Md. 2006;20:1574–1586. doi: 10.1210/me.2005-0469. [DOI] [PubMed] [Google Scholar]
- 19.Chin LJ, Ratner E, Leng S, Zhai R, Nallur S, Babar I, Muller RU, Straka E, Su L, Burki EA, Crowell RE, Patel R, Kulkarni T, Homer R, Zelterman D, Kidd KK, Zhu Y, Christiani DC, Belinsky SA, Slack FJ, Weidhaas JB. A SNP in a let-7 microRNA complementary site in the KRAS 3′ untranslated region increases non-small cell lung cancer risk. Cancer research. 2008;68:8535–8540. doi: 10.1158/0008-5472.CAN-08-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JM, Castle J, Garrett-Engele P, Kan Z, Loerch PM, Armour CD, Santos R, Schadt EE, Stoughton R, Shoemaker DD. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science (New York, NY. 2003;302:2141–2144. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- 22.Modrek B, Lee C. A genomic view of alternative splicing. Nature genetics. 2002;30:13–19. doi: 10.1038/ng0102-13. [DOI] [PubMed] [Google Scholar]
- 23.Faustino NA, Cooper TA. Pre-mRNA splicing and human disease. Genes & development. 2003;17:419–437. doi: 10.1101/gad.1048803. [DOI] [PubMed] [Google Scholar]
- 24.Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annual review of biochemistry. 2003;72:291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- 25.Estlin EJ. Continuing therapy for childhood acute lymphoblastic leukaemia: clinical and cellular pharmacology of methotrexate, 6-mercaptopurine and 6-thioguanine. Cancer treatment reviews. 2001;27:351–363. doi: 10.1053/ctrv.2002.0245. [DOI] [PubMed] [Google Scholar]
- 26.Lennard L. The clinical pharmacology of 6-mercaptopurine. European journal of clinical pharmacology. 1992;43:329–339. doi: 10.1007/BF02220605. [DOI] [PubMed] [Google Scholar]
- 27.Stanulla M, Schaeffeler E, Flohr T, Cario G, Schrauder A, Zimmermann M, Welte K, Ludwig WD, Bartram CR, Zanger UM, Eichelbaum M, Schrappe M, Schwab M. Thiopurine methyltransferase (TPMT) genotype and early treatment response to mercaptopurine in childhood acute lymphoblastic leukemia. Jama. 2005;293:1485–1489. doi: 10.1001/jama.293.12.1485. [DOI] [PubMed] [Google Scholar]
- 28.Ujiie S, Sasaki T, Mizugaki M, Ishikawa M, Hiratsuka M. Functional characterization of 23 allelic variants of thiopurine S-methyltransferase gene (TPMT*2 – *24) Pharmacogenetics and genomics. 2008;18:887–893. doi: 10.1097/FPC.0b013e3283097328. [DOI] [PubMed] [Google Scholar]
- 29.Otterness D, Szumlanski C, Lennard L, Klemetsdal B, Aarbakke J, Park-Hah JO, Iven H, Schmiegelow K, Branum E, O’Brien J, Weinshilboum R. Human thiopurine methyltransferase pharmacogenetics: gene sequence polymorphisms. Clinical pharmacology and therapeutics. 1997;62:60–73. doi: 10.1016/S0009-9236(97)90152-1. [DOI] [PubMed] [Google Scholar]
- 30.McLeod HL, Lin JS, Scott EP, Pui CH, Evans WE. Thiopurine methyltransferase activity in American white subjects and black subjects. Clinical pharmacology and therapeutics. 1994;55:15–20. doi: 10.1038/clpt.1994.4. [DOI] [PubMed] [Google Scholar]
- 31.Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. American journal of human genetics. 1980;32:651–662. [PMC free article] [PubMed] [Google Scholar]
- 32.Evans WE, Horner M, Chu YQ, Kalwinsky D, Roberts WM. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. The Journal of pediatrics. 1991;119:985–989. doi: 10.1016/s0022-3476(05)83063-x. [DOI] [PubMed] [Google Scholar]
- 33.Lennard L, Van Loon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clinical pharmacology and therapeutics. 1989;46:149–154. doi: 10.1038/clpt.1989.119. [DOI] [PubMed] [Google Scholar]
- 34.Corominas H, Domenech M, Gonzalez D, Diaz C, Roca M, Garcia-Gonzalez MA, Pena S, Baiget M. Allelic variants of the thiopurine S-methyltransferase deficiency in patients with ulcerative colitis and in healthy controls. The American journal of gastroenterology. 2000;95:2313–2317. doi: 10.1111/j.1572-0241.2000.02256.x. [DOI] [PubMed] [Google Scholar]
- 35.Tai HL, Krynetski EY, Yates CR, Loennechen T, Fessing MY, Krynetskaia NF, Evans WE. Thiopurine S-methyltransferase deficiency: two nucleotide transitions define the most prevalent mutant allele associated with loss of catalytic activity in Caucasians. American journal of human genetics. 1996;58:694–702. [PMC free article] [PubMed] [Google Scholar]
- 36.Tai HL, Krynetski EY, Schuetz EG, Yanishevski Y, Evans WE. Enhanced proteolysis of thiopurine S-methyltransferase (TPMT) encoded by mutant alleles in humans (TPMT*3A, TPMT*2): mechanisms for the genetic polymorphism of TPMT activity. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6444–6449. doi: 10.1073/pnas.94.12.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Sullivan W, Toft D, Weinshilboum R. Thiopurine S-methyltransferase pharmacogenetics: chaperone protein association and allozyme degradation. Pharmacogenetics. 2003;13:555–564. doi: 10.1097/01.fpc.0000054124.14659.99. [DOI] [PubMed] [Google Scholar]
- 38.Food and Drug Administration. [Accessed December 16, 2010];Pharmacogenetics. Table of Valid Genomic Biomarkers in the Context of Approved Drug Labels. http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/
- 39.Maitland ML, Vasisht K, Ratain MJ. TPMT, UGT1A1 and DPYD: genotyping to ensure safer cancer therapy? Trends in pharmacological sciences. 2006;27:432–437. doi: 10.1016/j.tips.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 41.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 42.Danenberg PV. Thymidylate synthetase - a target enzyme in cancer chemotherapy. Biochimica et biophysica acta. 1977;473:73–92. doi: 10.1016/0304-419x(77)90001-4. [DOI] [PubMed] [Google Scholar]
- 43.Relling MV, Dervieux T. Pharmacogenetics and cancer therapy. Nat Rev Cancer. 2001;1:99–108. doi: 10.1038/35101056. [DOI] [PubMed] [Google Scholar]
- 44.Kaneda S, Nalbantoglu J, Takeishi K, Shimizu K, Gotoh O, Seno T, Ayusawa D. Structural and functional analysis of the human thymidylate synthase gene. The Journal of biological chemistry. 1990;265:20277–20284. [PubMed] [Google Scholar]
- 45.Horie N, Aiba H, Oguro K, Hojo H, Takeishi K. Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5′-terminal regulatory region of the human gene for thymidylate synthase. Cell structure and function. 1995;20:191–197. doi: 10.1247/csf.20.191. [DOI] [PubMed] [Google Scholar]
- 46.Marsh S, Ameyaw MM, Githang’a J, Indalo A, Ofori-Adjei D, McLeod HL. Novel thymidylate synthase enhancer region alleles in African populations. Human mutation. 2000;16:528. doi: 10.1002/1098-1004(200012)16:6<528::AID-HUMU11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 47.Kawakami K, Omura K, Kanehira E, Watanabe Y. Polymorphic tandem repeats in the thymidylate synthase gene is associated with its protein expression in human gastrointestinal cancers. Anticancer research. 1999;19:3249–3252. [PubMed] [Google Scholar]
- 48.Pullarkat ST, Stoehlmacher J, Ghaderi V, Xiong YP, Ingles SA, Sherrod A, Warren R, Tsao-Wei D, Groshen S, Lenz HJ. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. The pharmacogenomics journal. 2001;1:65–70. doi: 10.1038/sj.tpj.6500012. [DOI] [PubMed] [Google Scholar]
- 49.Villafranca E, Okruzhnov Y, Dominguez MA, Garcia-Foncillas J, Azinovic I, Martinez E, Illarramendi JJ, Arias F, Martinez Monge R, Salgado E, Angeletti S, Brugarolas A. Polymorphisms of the repeated sequences in the enhancer region of the thymidylate synthase gene promoter may predict downstaging after preoperative chemoradiation in rectal cancer. J Clin Oncol. 2001;19:1779–1786. doi: 10.1200/JCO.2001.19.6.1779. [DOI] [PubMed] [Google Scholar]
- 50.Marsh S, McKay JA, Cassidy J, McLeod HL. Polymorphism in the thymidylate synthase promoter enhancer region in colorectal cancer. International journal of oncology. 2001;19:383–386. doi: 10.3892/ijo.19.2.383. [DOI] [PubMed] [Google Scholar]
- 51.Johnston PG, Lenz HJ, Leichman CG, Danenberg KD, Allegra CJ, Danenberg PV, Leichman L. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer research. 1995;55:1407–1412. [PubMed] [Google Scholar]
- 52.Krajinovic M, Costea I, Chiasson S. Polymorphism of the thymidylate synthase gene and outcome of acute lymphoblastic leukaemia. Lancet. 2002;359:1033–1034. doi: 10.1016/S0140-6736(02)08065-0. [DOI] [PubMed] [Google Scholar]
- 53.Kawakami K, Watanabe G. Identification and functional analysis of single nucleotide polymorphism in the tandem repeat sequence of thymidylate synthase gene. Cancer research. 2003;63:6004–6007. [PubMed] [Google Scholar]
- 54.Mandola MV, Stoehlmacher J, Muller-Weeks S, Cesarone G, Yu MC, Lenz HJ, Ladner RD. A novel single nucleotide polymorphism within the 5′ tandem repeat polymorphism of the thymidylate synthase gene abolishes USF-1 binding and alters transcriptional activity. Cancer research. 2003;63:2898–2904. [PubMed] [Google Scholar]
- 55.Diasio RB, Harris BE. Clinical pharmacology of 5-fluorouracil. Clinical pharmacokinetics. 1989;16:215–237. doi: 10.2165/00003088-198916040-00002. [DOI] [PubMed] [Google Scholar]
- 56.Heggie GD, Sommadossi JP, Cross DS, Huster WJ, Diasio RB. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer research. 1987;47:2203–2206. [PubMed] [Google Scholar]
- 57.Johnson MR, Hageboutros A, Wang K, High L, Smith JB, Diasio RB. Life-threatening toxicity in a dihydropyrimidine dehydrogenase-deficient patient after treatment with topical 5-fluorouracil. Clin Cancer Res. 1999;5:2006–2011. [PubMed] [Google Scholar]
- 58.Meinsma R, Fernandez-Salguero P, Van Kuilenburg AB, Van Gennip AH, Gonzalez FJ. Human polymorphism in drug metabolism: mutation in the dihydropyrimidine dehydrogenase gene results in exon skipping and thymine uracilurea. DNA and cell biology. 1995;14:1–6. doi: 10.1089/dna.1995.14.1. [DOI] [PubMed] [Google Scholar]
- 59.Wei X, McLeod HL, McMurrough J, Gonzalez FJ, Fernandez-Salguero P. Molecular basis of the human dihydropyrimidine dehydrogenase deficiency and 5-fluorouracil toxicity. The Journal of clinical investigation. 1996;98:610–615. doi: 10.1172/JCI118830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Kuilenburg AB. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer. 2004;40:939–950. doi: 10.1016/j.ejca.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 61.van Kuilenburg AB, Muller EW, Haasjes J, Meinsma R, Zoetekouw L, Waterham HR, Baas F, Richel DJ, van Gennip AH. Lethal outcome of a patient with a complete dihydropyrimidine dehydrogenase (DPD) deficiency after administration of 5-fluorouracil: frequency of the common IVS14+1G>A mutation causing DPD deficiency. Clin Cancer Res. 2001;7:1149–1153. [PubMed] [Google Scholar]
- 62.Etienne MC, Milano G, Renee N, Lagrange JL, Dassonville O, Thyss A, Schneider M, Francois E, Fleming R, Demard F. Population study of dihydropyrimidine dehydrogenase in cancer patients. Bulletin du cancer. 1995;82:705–710. [PubMed] [Google Scholar]
- 63.Gonzalez FJ, Fernandez-Salguero P. Diagnostic analysis, clinical importance and molecular basis of dihydropyrimidine dehydrogenase deficiency. Trends in pharmacological sciences. 1995;16:325–327. doi: 10.1016/s0165-6147(00)89065-3. [DOI] [PubMed] [Google Scholar]
- 64.Ezzeldin H, Diasio R. Dihydropyrimidine dehydrogenase deficiency, a pharmacogenetic syndrome associated with potentially life-threatening toxicity following 5-fluorouracil administration. Clinical colorectal cancer. 2004;4:181–189. doi: 10.3816/ccc.2004.n.018. [DOI] [PubMed] [Google Scholar]
- 65.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 66.Dean M. [Accessed December 16 2010];NCBI Bookshelf: The Human ATP-Binding Cassette (ABC) Transporter Superfamily. 2002 http://www.ncbi.nlm.nih,gov/books/NBK31/
- 67.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. Journal of the National Cancer Institute. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 68.Sullivan GF, Yang JM, Vassil A, Yang J, Bash-Babula J, Hait WN. Regulation of expression of the multidrug resistance protein MRP1 by p53 in human prostate cancer cells. The Journal of clinical investigation. 2000;105:1261–1267. doi: 10.1172/JCI9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Norris MD, Bordow SB, Marshall GM, Haber PS, Cohn SL, Haber M. Expression of the gene for multidrug-resistance-associated protein and outcome in patients with neuroblastoma. The New England journal of medicine. 1996;334:231–238. doi: 10.1056/NEJM199601253340405. [DOI] [PubMed] [Google Scholar]
- 70.Bruggemann M, Trautmann H, Hoelzer D, Kneba M, Gokbuget N, Raff T. Multidrug resistance-associated protein 4 (MRP4) gene polymorphisms and treatment response in adult acute lymphoblastic leukemia. Blood. 2009;114:5400–5401. doi: 10.1182/blood-2009-09-243741. author reply 5401–5402. [DOI] [PubMed] [Google Scholar]
- 71.Ansari M, Sauty G, Labuda M, Gagne V, Laverdiere C, Moghrabi A, Sinnett D, Krajinovic M. Polymorphisms in multidrug resistance-associated protein gene 4 is associated with outcome in childhood acute lymphoblastic leukemia. Blood. 2009;114:1383–1386. doi: 10.1182/blood-2008-11-191098. [DOI] [PubMed] [Google Scholar]
- 72.Grimm C, Polterauer S, Zeillinger R, Tong D, Heinze G, Wolf A, Natter C, Reinthaller A, Hefler LA. Two multidrug-resistance (ABCB1) gene polymorphisms as prognostic parameters in women with ovarian cancer. Anticancer research. 2010;30:3487–3491. [PubMed] [Google Scholar]
- 73.Lang T, Schroth W, Brauch H, Schwab M. ABCC2 and clinical outcome of tamoxifen therapy. J Clin Oncol. 2010;28:e448. doi: 10.1200/JCO.2010.29.6665. author reply e449. [DOI] [PubMed] [Google Scholar]
- 74.Kiyotani K, Mushiroda T, Imamura CK, Hosono N, Tsunoda T, Kubo M, Tanigawara Y, Flockhart DA, Desta Z, Skaar TC, Aki F, Hirata K, Takatsuka Y, Okazaki M, Ohsumi S, Yamakawa T, Sasa M, Nakamura Y, Zembutsu H. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol. 2010;28:1287–1293. doi: 10.1200/JCO.2009.25.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van den Heuvel-Eibrink MM, Wiemer EA, de Boevere MJ, van der Holt B, Vossebeld PJ, Pieters R, Sonneveld P. MDR1 gene-related clonal selection and P-glycoprotein function and expression in relapsed or refractory acute myeloid leukemia. Blood. 2001;97:3605–3611. doi: 10.1182/blood.v97.11.3605. [DOI] [PubMed] [Google Scholar]
- 76.Marsh S, Paul J, King CR, Gifford G, McLeod HL, Brown R. Pharmacogenetic assessment of toxicity and outcome after platinum plus taxane chemotherapy in ovarian cancer: the Scottish Randomised Trial in Ovarian Cancer. J Clin Oncol. 2007;25:4528–4535. doi: 10.1200/JCO.2006.10.4752. [DOI] [PubMed] [Google Scholar]
- 77.Kafka A, Sauer G, Jaeger C, Grundmann R, Kreienberg R, Zeillinger R, Deissler H. Polymorphism C3435T of the MDR-1 gene predicts response to preoperative chemotherapy in locally advanced breast cancer. International journal of oncology. 2003;22:1117–1121. [PubMed] [Google Scholar]
- 78.Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Illmer T, Schuler US, Thiede C, Schwarz UI, Kim RB, Gotthard S, Freund D, Schakel U, Ehninger G, Schaich M. MDR1 gene polymorphisms affect therapy outcome in acute myeloid leukemia patients. Cancer research. 2002;62:4955–4962. [PubMed] [Google Scholar]
- 80.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sparreboom A, Loos WJ, Burger H, Sissung TM, Verweij J, Figg WD, Nooter K, Gelderblom H. Effect of ABCG2 genotype on the oral bioavailability of topotecan. Cancer biology & therapy. 2005;4:650–658. doi: 10.4161/cbt.4.6.1731. [DOI] [PubMed] [Google Scholar]
- 82.Hu LL, Wang XX, Chen X, Chang J, Li C, Zhang Y, Yang J, Jiang W, Zhuang SM. BCRP gene polymorphisms are associated with susceptibility and survival of diffuse large B-cell lymphoma. Carcinogenesis. 2007;28:1740–1744. doi: 10.1093/carcin/bgm113. [DOI] [PubMed] [Google Scholar]
- 83.Consortium IH. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 84.Podolak E. Sequencing’s new race. BioTechniques. 2010;48:105–111. doi: 10.2144/000113371. [DOI] [PubMed] [Google Scholar]
- 85.Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, Thun M. Trends in breast cancer by race and ethnicity: update 2006. CA: a cancer journal for clinicians. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 86.Abidoye O, Ferguson MK, Salgia R. Lung carcinoma in African Americans. Nature clinical practice. 2007;4:118–129. doi: 10.1038/ncponc0718. [DOI] [PubMed] [Google Scholar]
- 87.Hoffman RM, Gilliland FD, Eley JW, Harlan LC, Stephenson RA, Stanford JL, Albertson PC, Hamilton AS, Hunt WC, Potosky AL. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. Journal of the National Cancer Institute. 2001;93:388–395. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- 88.Thompson I, Tangen C, Tolcher A, Crawford E, Eisenberger M, Moinpour C. Association of African-American ethnic background with survival in men with metastatic prostate cancer. Journal of the National Cancer Institute. 2001;93:219–225. doi: 10.1093/jnci/93.3.219. [DOI] [PubMed] [Google Scholar]
- 89.Laiyemo AO, Doubeni C, Pinsky PF, Doria-Rose VP, Bresalier R, Lamerato LE, Crawford ED, Kvale P, Fouad M, Hickey T, Riley T, Weissfeld J, Schoen RE, Marcus PM, Prorok PC, Berg CD. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. Journal of the National Cancer Institute. 2010;102:538–546. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma BB, Hui EP, Mok TS. Population-based differences in treatment outcome following anticancer drug therapies. The lancet oncology. 2010;11:75–84. doi: 10.1016/S1470-2045(09)70160-3. [DOI] [PubMed] [Google Scholar]