Abstract
Therapeutic hypothermia (TH) is a novel technique for improving the likelihood of survival with good neurologic outcome after cardiopulmonary arrest. While commercial temperature management systems (TMS) are intended to facilitate cooling of the body during TH, their operation also involves body exposure to heat. We describe the case of a 72-year-old female postarrest patient who underwent TH using a commercial water-circulating TMS and concurrent continuous renal replacement therapy. The patient developed bullous lesions on the thigh and torso suspected to constitute a scald burn injury from the TMS. Clinicians must be aware of this important adverse event when providing TH, especially in the setting of concurrent hemodialysis therapy.
Introduction
An important recommended treatment for comatose survivors of cardiac arrest is therapeutic hypothermia (TH), which may limit cerebral edema, cellular oxygen consumption, neurotoxicity, and resultant brain injury (Bernard et al., 2002; Hypothermia after Cardiac Arrest Study, 2002; Polderman, 2008; Peberdy et al., 2010). An additional postarrest treatment recommendation is the prevention of hyperthermia after the completion of TH (Polderman, 2008; Peberdy et al., 2010; Leary et al., 2012). Many commercial temperature management systems (TMS) exist to facilitate targeted temperature management of postarrest patients, including designs that circulate cool and/or warm water through external blankets or adhesive pads. While clinicians are often vigilant for cutaneous “cold” burns (frostbite), little attention is paid to the potential for scald burns resulting from use of these devices. We describe the case of a postarrest patient who developed bullous skin lesions suspected to be due to a scald burn injury from TMS use.
Case Report
A 72-year-old African-American woman was admitted from the emergency department to a regular hospital ward for an acute heart failure exacerbation. Her prior medical history included congestive heart failure, type two diabetes mellitus, hypertension, coronary artery disease (including a coronary artery bypass graft), right below-knee-amputation, and stage IV chronic kidney disease. Pertinent laboratory abnormalities on hospital admission included serum creatinine 2.9 mg/dL, serum magnesium 2.9 mg/dL, potassium 5.2 mEq/L, Troponin I 0.029, and beta-natriuretic peptide 899 pg/mL. Initial inpatient management focused on fluid and blood pressure optimization using hydralazine, isosorbide, and spironolactone.
On hospital day 2, the patient sustained a witnessed pulseless electrical activity cardiac arrest. The medical emergency team responded and provided resuscitative care. During the resuscitation, bedside rapid laboratory tests revealed a potassium level >8.0 mEq/L. Resuscitation interventions included chest compressions, endotracheal intubation, insertion of a femoral central venous line, and administration of epinephrine, calcium chloride, sodium bicarbonate, insulin, and dextrose 50%. Restoration of pulses occurred after 8 minutes of cardiopulmonary resuscitation. Post-resuscitation serum potassium was 6.1 mEq/L, and creatinine was 3.8 mg/dL.
The patient received continued postarrest care in the intensive care unit. Because of persistent coma after 30 minutes, the intensive care unit team decided to initiate TH. Clinicians provided TH using a commercial TMS (Arctic Sun®; Bard Medical, Inc., Louisville, CO). This device controls body temperature by circulating cool or warm water through adhesive cooling pads applied to the torso and thighs. The team placed additional ice packs on the patient's torso. The patient achieved TH goal temperature of 33°C in approximately 4.5 hours.
Because of volume overload, hyperkalemia, and oliguric acute kidney injury in the setting of chronic kidney disease, the consulting nephrologists initiated concurrent continuous renal replacement therapy (CRRT) at approximately 5 hours after reaching goal TH temperature. The external blood warmer on the CRRT system (Prismaflex® System; Gambro Inc., Lakewood, CO) was initially set at 35°C with subsequent adjustment up to 39°C. The patient underwent 24 hours of TH with slow rewarming at 0.25°C/hour. After completion of TH, per customary institutional practice, TMS use was continued for hyperthermia suppression. CRRT was continued with its warmer set at 39°C.
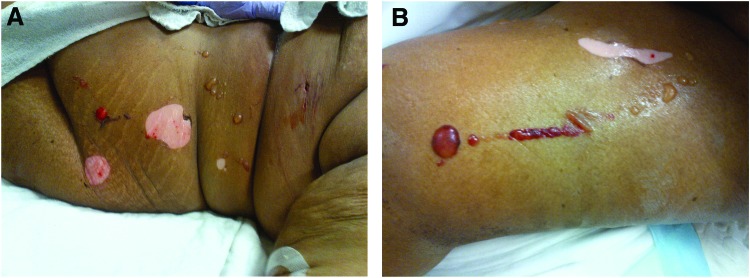
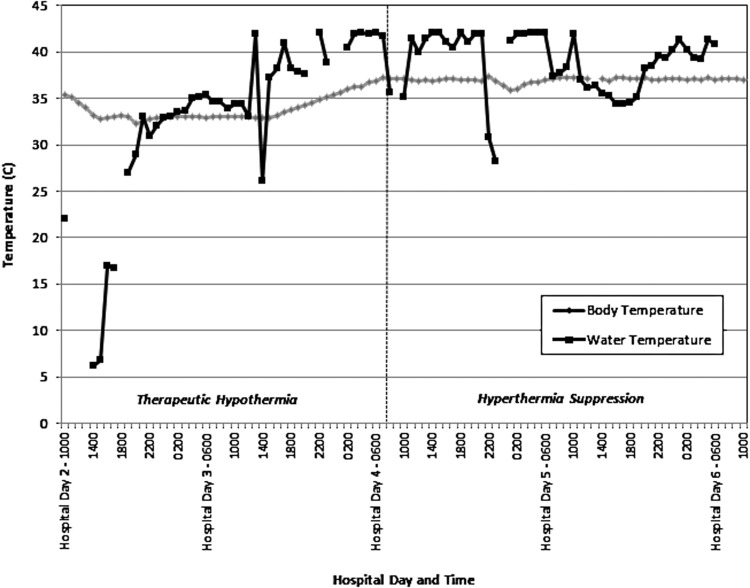
Four days postarrest (48 hours after completion of rewarming), the patient developed skin lesions under the left torso and left thigh cooling pads. Assessment revealed multiple bullae 1–4 cm in diameter with surrounding hyperemia (Fig. 1). The affected regions totaled approximately 1% of total body surface area. No other lesions were noted on the body, although hyperemic skin was observed under all cooling pad surfaces. Review of the TMS temperature logs indicated that the patient had been exposed to 42°C water in the cooling pads for sustained periods over the prior 48 hours. (Fig. 2). Nursing records indicated at least seven skin assessments during the prior 48 hours, without report of skin anomalies.
FIG. 1.
Bullae on left torso (A) and left thigh (B). Whitish areas reflect denuded skin where the bullae were removed. Color images available online at www.liebertpub.com/ther
FIG. 2.
Patient body temperature and circulating water temperature of temperature management system. Figure indicates phases of use for therapeutic hypothermia and hyperthermia suppression.
Cooling pad and TMS use were immediately discontinued. The cutaneous lesions were treated with conservative burn care measures and healed uneventfully. The patient received a tracheostomy for chronic ventilator management and was discharged to a long-term care facility on hospital day 31.
Discussion
We suspect that the bullous lesions sustained by the patient in this case may have been due to a scald burn injury from TMS use. This association is supported by several considerations including the documented prolonged exposure to hot circulating TMS water, the efficient temperature conduction properties of the TMS, and the absence of skin lesions on areas not covered by the cooling pads.
We do not believe that cooling pad misuse led to the observed lesions. The manufacturer recommends that the cooling pads should be removed or replaced when the adhesive is no longer adherent—usually 5 days. In this case, the bullous lesions were observed after only 4 days of use, and the pads did not show signs of undue wear. The manufacturer also recommends the performance of skin assessments every 4–6 hours. Nursing records in this case indicate at least seven normal skins assessments during the 48 hours prior to the identification of the bullous lesions.
Bullous lesions in the intensive care setting are often due to thermal etiologies. Bullous lesions may result from infections, drug reactions, allergic contact dermatitis, and friction blisters, among others (Badia et al., 1999; Agrawal et al., in press; Emre et al., in press). Friction and heat blisters are responsible for 16–29% of dermatologic manifestations in the ICU setting. Contact dermatitis, including bullous variations, is somewhat rarer, accounting for 2–15% of skin findings seen in the ICU (Gane, 1973; Badia et al., 1999; Agrawal et al., in press; Emre et al., in press). Bullous dermatitis has been reported as the result of thermal injury from other mechanisms (Vermeulen et al., 2000; Sokumbi et al., 2013).
While minor skin irritation has been reported from TH as well as the use of the Arctic Sun TMS, there are no prior reports of warming-related thermal injury. Haugk et al. (2007) evaluated the feasibility of Arctic Sun TMS cooling in 27 patients, finding no serious skin abnormalities. In a randomized trial including 34 uses of the Arctic Sun TMS, Heard et al. (2010) similarly reported no serious skin abnormalities related to TMS use. Jarrah et al. (2011) reported Arctic Sun TMS use on 69 patients, finding minor TMS-related skin injury in four (6%) cases; these included small skin tears and ecchymoses. In a single case report, Varon et al. (2008) described large desquamation of skin from removal of the TMS cooling pads; it is not clear from the case description if there was a thermal etiology for the injury. We note that in the presented case, there were no similar skin lesions in body areas not covered by the cooling pads, making systemic etiologies for the bullae less likely.
There are plausible pathophysiologic connections between prolonged exposure to hot TMS circulating water and a scald burn injury. The severity of a scald burn injury depends upon the water temperature and duration of exposure; burns may result from either brief high or prolonged lower temperature exposures (Moritz, 1947). In pathologic examinations of porcine burn models, Moritz observed second-degree scald burns from exposure to 50°C heat for as little as 2 minutes, and transepidermal necrosis from exposure to 45°C for 3 hours (Moritz, 1947). The patient in this case was exposed to 42°C water for almost 48 hours. Moritz noted that delayed vesication can occur after long duration low temperature exposure, potentially explaining why bullae were not noted earlier on this patient. English and Hemmerling (2008) showed that the heat transfer coefficient of the Arctic Sun pads is equivalent to water, and therefore heat transfer from this TMS is identical to immersion in water. Prior reports have highlighted burns caused by the intraoperative warming efforts including the use of warmed fluid bags and bottles and water-circulating mattresses (Cheney et al., 1994; Wankhede et al., 2007; Siddik-Sayyid et al., 2008).
We believe that the prolonged exposure to hot circulating water likely resulted from the unanticipated interaction between the TMS and CRRT. Although newer dialysis systems are equipped with a blood warmer, up to half of CRRT patients can experience significant drops in body temperature due to the extracorporeal radiant heat losses (Finkel and Podoll, 2009; Yagi et al., 1998). The commercial TMS in this case achieves targeted body temperatures by varying the temperature of circulating water in the adhesive pads from 4°C to 42°C in response to body temperature measurements. From our experience, heat losses from concurrent CRRT often trigger the TMS to raise circulating water to high temperatures. In an ideal situation, the TMS circulating water should reach extreme temperature ranges for only brief periods. However, in this case, the TMS water temperature increase was prolonged (almost 48 hours) because of the sustained CRRT heat losses.
Hemodialysis may be necessary in the postarrest state to treat volume overload, hyperkalemia, or drug toxicity. As highlighted by this case, concurrent operation of a TMS and CRRT can be complex. In our practice, we have developed three potential approaches to providing TH with concurrent CRRT:
Postpone hemodialysis/CRRT until completion of TH.
Operate the TMS and CRRT concurrently, with the CRRT blood warmer set to 35–37°C. Adjust the CRRT warmer to avoid extreme or fluctuating water temperatures on the TMS.
Discontinue TMS use. Manage TH using only the CRRT system.
Among other practice alterations, we no longer use the commercial TMS and CRRT concurrently during the hyperthermia suppression phase. We have reprogrammed the TMS control units to a maximum circulating water temperature of 40°C. We have also continued our standard TH practices of documenting body and TMS water temperature every 30 minutes and checking skin condition on a frequent basis.
In conclusion, we describe the case of a postarrest patient who developed bullous lesions believed to be due to scald burns from commercial TMS use. Clinicians must be aware of this important adverse event when providing TH, especially in the setting of concurrent hemodialysis therapy.
Author Disclosure Statement
No competing financial interests exist.
References
- Agrawal P. Peter JV. George R. Dermatological manifestations and relationship to outcomes of patients admitted to a medical intensive care unit: a study from a tertiary care hospital in India. Postgrad Med J. 2013 doi: 10.1136/postgradmedj-2012-131610. in press. [DOI] [PubMed] [Google Scholar]
- Badia M. Trujillano J. Gasco E. Casanova JM. Alvarez M. Leon M. Skin lesions in the ICU. Intensive Care Med. 1999;25:1271–1276. doi: 10.1007/s001340051056. [DOI] [PubMed] [Google Scholar]
- Bernard SA. Gray TW. Buist MD. Jones BM. Silvester W. Gutteridge G. Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Cheney FW. Posner KL. Caplan RA. Gild WM. Burns from warming devices in anesthesia—a closed claims analysis. Anesthesiology. 1994;80:806–810. doi: 10.1097/00000542-199404000-00012. [DOI] [PubMed] [Google Scholar]
- Emre S. Emre C. Akoglu G. Demirseren DD. Metin A. Evaluation of dermatological consultations of patients treated in intensive care unit. Dermatology. 2013 doi: 10.1159/000346939. in press. [DOI] [PubMed] [Google Scholar]
- English MJ. Hemmerling TM. Heat transfer coefficient: Medivance Arctic Sun Temperature Management System vs. water immersion. Eur J Anaesthesiol. 2008;25:531–537. doi: 10.1017/S0265021508003931. [DOI] [PubMed] [Google Scholar]
- Finkel KW. Podoll AS. Complications of continuous renal replacement therapy. Seminars in Dialysis. 2009;22:155–159. doi: 10.1111/j.1525-139X.2008.00550.x. [DOI] [PubMed] [Google Scholar]
- Gane NF. A guide to bullous lesions of the skin. J Clin Pathol. 1973;26:235–237. doi: 10.1136/jcp.26.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugk M. Sterz F. Grassberger M. Uray T. Kliegel A. Janata A. Richling N. Herkner H. Laggner AN. Feasibility and efficacy of a new non-invasive surface cooling device in post-resuscitation intensive care medicine. Resuscitation. 2007;75:76–81. doi: 10.1016/j.resuscitation.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Heard KJ. Peberdy MA. Sayre MR. Sanders A. Geocadin RG. Dixon SR. Larabee TM. Hiller K. Fiorello A. Paradis NA, et al. A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation. 2010;81:9–14. doi: 10.1016/j.resuscitation.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hypothermia after Cardiac Arrest Study. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Jarrah S. Dziodzio J. Lord C. Fraser GL. Lucas L. Riker RR. Seder DB. Surface cooling after cardiac arrest: effectiveness, skin safety, and adverse events in routine clinical practice. Neurocrit Care. 2011;14:382–388. doi: 10.1007/s12028-011-9506-y. [DOI] [PubMed] [Google Scholar]
- Leary M. Grossestreuer AV. Iannacone S. Gonzalez M. Shofer FS. Povey C. Wendell G. Archer SE. Gaieski DF. Abella BS. Pyrexia and neurologic outcomes after therapeutic hypothermia for cardiac arrest. Resuscitation. 2012 doi: 10.1016/j.resuscitation.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Moritz AR. Studies of thermal injury. American Journal of Pathology. 1947;23:915–941. [PMC free article] [PubMed] [Google Scholar]
- Peberdy MA. Callaway CW. Neumar RW. Geocadin RG. Zimmerman JL. Donnino M. Gabrielli A. Silvers SM. Zaritsky AL. Merchant R, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S768–786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- Siddik-Sayyid SM. Abdallah FW. Dahrouj GB. Thermal burns in three neonates associated with intraoperative use of Bair Hugger warming devices. Pediatric Anesthesia. 2008;18:337–339. doi: 10.1111/j.1460-9592.2008.02474.x. [DOI] [PubMed] [Google Scholar]
- Sokumbi O. Comfere NI. McEvoy MT. Peters MS. Bullous dermatitis artefacta. Am J Dermatopathol. 2013;35:110–112. doi: 10.1097/DAD.0b013e31825dd246. [DOI] [PubMed] [Google Scholar]
- Varon J. Acosta P. Wintz R. Mendoza N. Unusual side effect from hydrogel pads during therapeutic hypothermia. Resuscitation. 2008;78:248–249. doi: 10.1016/j.resuscitation.2008.03.223. [DOI] [PubMed] [Google Scholar]
- Vermeulen C. Janier M. Panse I. Daniel F. [Localized bullous pemphigoid induced by thermal burn] Ann Dermatol Venereol. 2000;127:720–722. [PubMed] [Google Scholar]
- Wankhede AG. Dongre AP. Sariya DR. Burns caused by fan heater used for managing post-operative hypothermia in a premature neonate. J Forensic Leg Med. 2007;14:289–292. doi: 10.1016/j.jcfm.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Yagi N. Leblanc M. Sakai K. Wright EJ. Paganini EP. Cooling effect of continuous renal replacement therapy in critically ill patients. Am J Kidney Dis. 1998;32:1023–1030. doi: 10.1016/s0272-6386(98)70078-2. [DOI] [PubMed] [Google Scholar]