Abstract
Objective:
To characterize the rate of cognitive decline during the premotor phase of essential tremor (ET) in comparison to prevalent ET cases and controls.
Methods:
In this population-based, prospective study of people aged 65 years and older (Neurological Disorders in Central Spain), a 37-item version of the Mini-Mental State Examination was administered at 2 visits (baseline and follow-up, approximately 3 years later). We compared the rate of cognitive decline in 3 groups: prevalent ET cases (i.e., participants diagnosed with ET at baseline and at follow-up), “premotor” ET cases (i.e., participants diagnosed with incident ET at follow-up, but not at baseline), and controls (i.e., participants not diagnosed with ET at baseline or follow-up).
Results:
The 2,375 participants included 135 prevalent ET cases, 56 premotor ET cases, and 2,184 controls. During the follow-up period of 3.4 ± 0.5 years (mean ± SD), the 37-item version of the Mini-Mental State Examination declined by 0.7 ± 3.3 points (0.2 ± 1.0 points/year) in prevalent ET cases, 1.1 ± 3.5 points (0.3 ± 1.0 points/year) in premotor ET cases, and 0.1 ± 3.9 points (0.0 ± 1.2 points/year) in controls (p = 0.014). The difference between premotor ET cases and controls was significant (p = 0.046), as was the difference between prevalent ET cases and controls (p = 0.027).
Conclusions:
In this prospective cohort, cognitive test scores in premotor and prevalent ET cases declined at a faster rate than in elders without this disease. A decline in global cognitive function may occur in a premotor phase of ET.
Mild cognitive deficits have been reported to occur in patients with essential tremor (ET) in many independent studies,1–8 including a population-based study of largely treatment-naive ET patients (Neurological Disorders in Central Spain [NEDICES] study).8 These studies suggest that a frontosubcortical-type dysfunction occurs in some patients with ET.9–14 In the NEDICES study, lower cognitive test scores were associated with more reported functional difficulty, indicating a clinical-functional correlate.15 The NEDICES study provided evidence that cognitive deficits in ET are not static, and appear to be progressing at a faster rate than in elders without this disease.16 There could be a “premotor” stage of ET, as there is in other adult-onset and late-life motor disorders. However, population-based studies that prospectively assess whether cognitive dysfunction appears in premotor ET (i.e., those subjects who were free of ET at baseline but who developed ET during the follow-up) have not been conducted.
We hypothesized that the cognitive deficits in premotor and prevalent ET would not be static, but would deteriorate, and that this deterioration would occur at a rate above that observed in similarly aged controls. Using data from the NEDICES study, we evaluated ET cases (participants diagnosed with ET at baseline and at follow-up), “premotor” ET cases (participants diagnosed with incident ET at follow-up, but not at baseline), and controls (participants not diagnosed with ET at baseline or follow-up) at 2 time points separated by approximately 3 years.
METHODS
Study population.
We derived the data for these analyses from the NEDICES study.17–28 We have previously published details regarding the study population and sampling methods.17–28 The study population was composed of subjects aged 65 years and older living in 3 well-defined geographical areas in central Spain (Las Margaritas, Lista, and Arévalo).
Standard protocol approvals, registrations, and patient consents.
Investigators obtained ethics approval from the Human Research Ethics Committee of the University Hospitals 12 de Octubre and La Princesa (Madrid). All enrollees signed written informed consent.
Evaluation.
Each participant received either a face-to-face evaluation (at baseline, 1994–1995, and at follow-up, 1997–1998) or a questionnaire (mailed to participants who were unavailable for face-to-face interviews). During the face-to-face interview, we collected data on demographics, current medications, medical conditions, depressive symptoms (the question, “Do you suffer from depression?”), and lifestyle. The interview included one screening question for ET (“Have you ever suffered from tremor of the head, hands, or legs that has lasted longer than several days?”).25,26 This question was a Spanish adaptation of that used by the Italian Longitudinal Study on Aging Working Group.29 To assess the performance of this screening question, we selected and contacted a random sample of approximately 4% of those who had screened negative.17 Of 205 subjects who were contacted, we successfully scheduled 183 for a neurologic examination by a senior neurologist who routinely evaluates patients with movement disorders (J. Olazarán [see http://www.ciberned.es/estudio-nedices]) as described.25,26 The diagnostic criteria for ET were similar to those used in the Sicilian study30 (see below), and none (0%) of the 183 subjects were diagnosed with ET.17
Subjects who screened positive for ET underwent a neurologic examination,25,26 which comprised a general neurologic examination and the motor portion of the Unified Parkinson’s Disease Rating Scale.31 During this examination, we asked participants to perform manual tasks to assess postural and kinetic tremors. For subjects who could not be examined, we obtained medical records from general practitioners, from in-patient hospitalizations, and from neurologic specialists (if they had visited one). In addition, we reviewed death certificate diagnoses for each screened subject who had died before neurologic examination.
Diagnostic criteria for ET were similar to those used in the Sicilian study.30 The first criterion was action tremor of the head, limbs, or voice without any other recognizable cause. Second, the tremor had to be of gradual onset (i.e., slow and progressive) and 1) present for at least 1 year, or 2) accompanied by a family history of the same disorder (at least one reportedly affected first-degree relative). Third, on an Archimedes spiral, tremor severity had to be moderate or greater (rating ≥2 according to the Washington Heights–Inwood Genetic Study of ET Rating Scale).32 Subjects with tremor related to Parkinson disease, medications, or other known causes of tremor were not diagnosed with ET. We diagnosed Parkinson disease and other forms of parkinsonism when at least 2 cardinal signs were present on the Unified Parkinson’s Disease Rating Scale.31 The diagnosis of dementia was made by consensus of 2 neurologists, who applied the DSM-IV criteria.27,28
We administered a 37-item Mini-Mental State Examination (37-MMSE) to evaluate global cognitive performance in the cohort.8,27,28,33–37 The 37-MMSE included all of the standard MMSE items as well as 3 additional items: 1) an attention task, i.e., “say 1, 3, 5, 7, 9 backward”; 2) a visual order, i.e., a man raising his arms; and 3) a simple construction task, i.e., copying 2 overlapping circles.8,27,28,33–37 We grouped cognitive domain tasks into several subscores (orientation [0–10 points], memory [0–6 points], attention and calculation [0–10 points], language tasks [0–9 points], and construction/copying [0–2 points]).8,27,28,33–37
Final selection of participants.
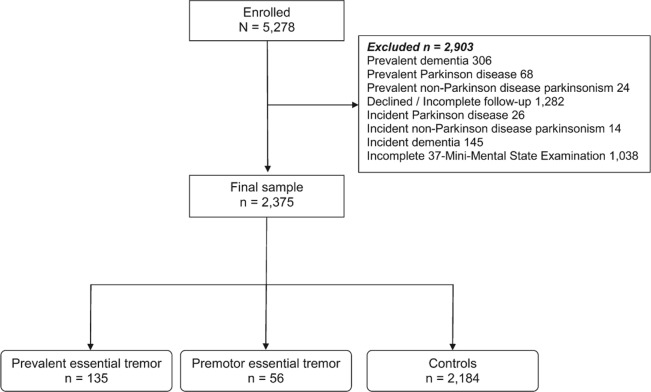
Of the 5,278 participants evaluated at baseline, we excluded 2,903 with incomplete data, dementia, or other neurodegenerative conditions (figure).
Figure. Flow chart of the study.
The final sample of 2,375 participants included 135 cases of prevalent ET, 56 premotor ET cases, and 2,184 controls (figure). The final sample of 2,375 participants was similar to the excluded 2,903 participants in terms of sex (1,346 [56.7%] vs 1,694 [58.4%] women, χ2 = 1.51, p = 0.22), but they were more educated (242 [10.2%] vs 469 [16.4%] illiterate, χ2 = 45.2, p < 0.001) and, on average, 3.4 years younger (72.4 ± 5.8 vs 75.8 ± 7.4 years, t = −18.1, p < 0.001).
Statistical analyses.
We performed analyses in SPSS version 20.0 (IBM Corp., Armonk, NY). None of the continuous variables were normally distributed (Kolmogorov-Smirnov, p < 0.001), even after log-transformation. Therefore, we compared baseline characteristic scores using a nonparametric approach including Kruskal-Wallis tests, Mann-Whitney tests, and Spearman ρ (rs). Linear regression analyses were not possible because the change in 37-MMSE score was also not normally distributed. Therefore, to initially assess the effects of possible confounders, we divided the main factors that could affect cognition (age, sex, educational level, presence/absence of depressive symptoms or use of antidepressant medication, use of medications with CNS effects, and geographical area) into categories, and we performed stratified analyses to compare the change in the 37-MMSE score across groups in each stratum. Because of the loss of power in these analyses, we did not report p values; rather, the aim was to determine whether the magnitude of the case-control difference persisted after stratification.
In additional analyses, we divided change in 37-MMSE score into tertiles (lower tertile ≥2-point improvement in score, upper tertile ≥2-point decline in score), comparing cases and controls regarding their distribution within these tertiles (χ2 test). In addition, we performed multivariate logistic regression analyses, thereby allowing us to assess, for a second time, the same confounders. In these models, the dependent variable was the upper tertile of 37-MMSE score change (reference = lower tertile) and the main independent variable was case-control status.
RESULTS
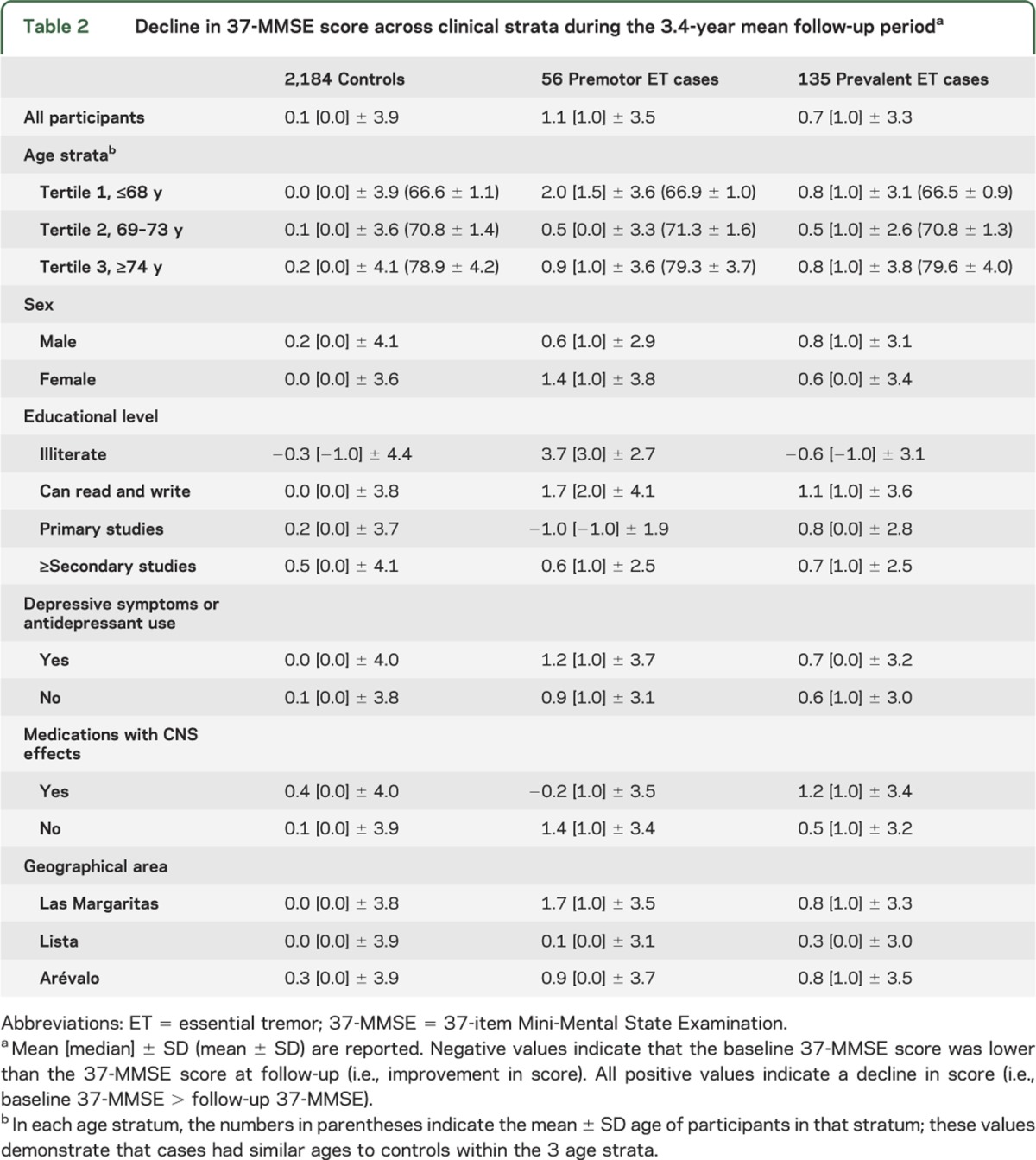
The final sample of 2,375 participants (mean ± SD age = 72.4 ± 5.8 years) comprised 135 prevalent ET cases, 56 premotor ET cases, and 2,184 controls. The mean follow-up was 3.4 ± 0.5 years (median = 3.3 years), which was similar in all groups (Kruskal-Wallis test, p = 0.253). Premotor and ET cases were marginally older than controls (table 1), and a higher proportion of premotor and ET cases were illiterate and lived in Las Margaritas, a predominantly working-class area (table 1). In addition, a higher proportion of ET cases was taking medications with CNS effects and reported more depressive symptoms than controls, but in other respects, cases and controls were similar (table 1). The baseline 37-MMSE score was lowest in prevalent ET cases and highest in controls (table 1), with significant differences in the prevalent ET cases vs controls comparison (Mann-Whitney test, p = 0.020), but not in the premotor ET cases vs controls comparison (Mann-Whitney test, p = 0.33). During the 3-year follow-up period, the 37-MMSE declined by 0.7 ± 3.3 points in prevalent ET cases (median = 1 point), 1.1 ± 3.5 points in premotor ET cases (median = 1 point), and 0.1 ± 3.9 points in controls (median = 0 points) (Kruskal-Wallis test, p = 0.014) (table 2). The difference between prevalent ET cases and controls was significant (Mann-Whitney test, p = 0.027); similarly, the difference between premotor ET cases and controls was significant (Mann-Whitney test, p = 0.046); however, the difference between premotor ET cases and prevalent ET cases was not significant (Mann-Whitney test, p = 0.563).
Table 1.
Main baseline demographic and clinical variablesa
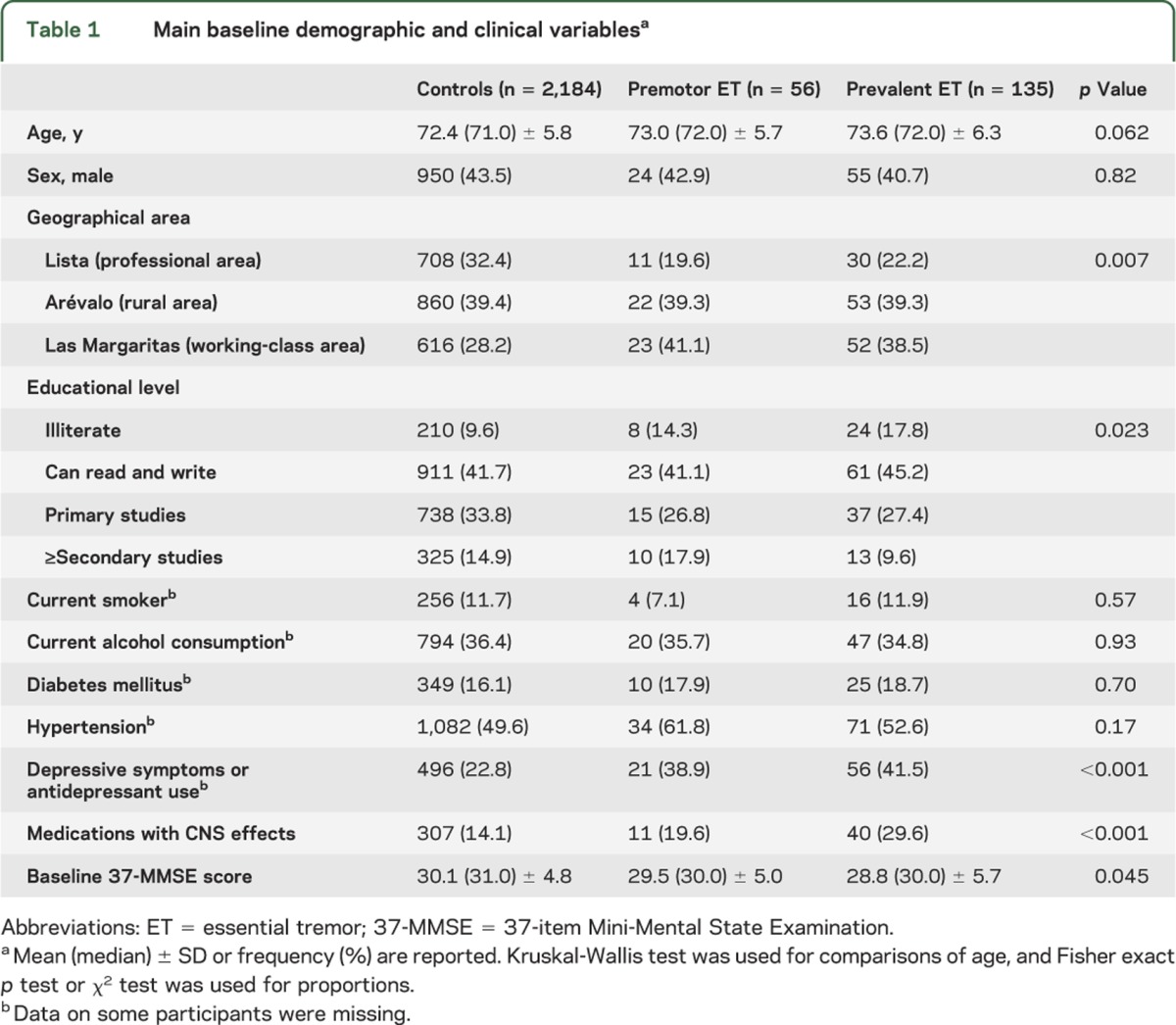
Table 2.
Decline in 37-MMSE score across clinical strata during the 3.4-year mean follow-up perioda
In controls, we examined whether baseline 37-MMSE scores were associated with potential confounding variables. The 37-MMSE was correlated with age (rS = −0.227, p < 0.001), educational category (rS = 0.396, p < 0.001), geographical area (mean ± SD [median] = 29.0 ± 4.9 [30] in Las Margaritas vs 32.2 ± 4.0 [33] in Lista and 29.3 ± 4.8 [30] in Arévalo; Kruskal-Wallis test, p < 0.001), subjective depressive symptoms or antidepressant use (29.2 ± 4.8 [29] in those who responded “yes” vs 30.5 ± 4.7 [31] in those who responded “no”; Mann-Whitney test, p < 0.001), and sex 31.6 ± 4.5 [33] in men vs 29.1 ± 4.7 [29] in women; Mann-Whitney test, p < 0.001). However, the baseline 37-MMSE was not correlated with medications that could affect cognition (mean ± SD [median] = 29.9 ± 4.9 [31] in those taking a medication vs 30.2 ± 4.8 [31] in those not taking a medication; Mann-Whitney test, p = 0.44).
In stratified analyses, in nearly all strata, the decline in 37-MMSE score in both premotor and prevalent ET cases was higher than the decline in scores in controls (table 2), indicating that these variables were not likely to be a source of confounding.
We also assessed the cognitive decline per unit time (i.e., the rate of cognitive decline). The rate of cognitive decline was 0.0 ± 1.2 (median = 0.0) points/year for controls, 0.3 ± 1.0 (median = 0.3) points/year for premotor ET cases, and 0.2 ± 1.0 (median = 0.2) points/year for prevalent ET cases (Kruskal-Wallis test, p = 0.014). The difference between premotor ET cases and controls was significant (Mann-Whitney test, p = 0.039), as was the difference between prevalent ET cases and controls (Mann-Whitney test, p = 0.033); however, the difference between prevalent ET cases and premotor ET cases was not significant (Mann-Whitney test, p = 0.460).
Change in 37-MMSE score was stratified into tertiles: 50 (37.0%) prevalent ET cases, 24 (42.9%) premotor ET, and 691 (31.6%) controls were in the upper tertile; 29 (21.5%) prevalent ET, 11 (19.6%) premotor ET, and 686 (31.4%) controls were in the lower tertile (χ2 = 10.21, p = 0.037). In a logistic regression model, prevalent ET cases were 1.71 times more likely than controls to have a change in 37-MMSE score in the upper vs lower tertile (odds ratio [OR] = 1.71, 95% confidence interval [CI] = 1.07–2.74, p = 0.025), and premotor ET cases were 2.17 times more likely than controls to have a change in 37-MMSE score in the upper vs lower tertile (OR = 2.17, 95% CI = 1.05–4.46, p = 0.036). We further assessed the possible confounding effects of age, sex, geographical area, educational level, depressive symptoms or antidepressant use, and medications with CNS effects in a multivariate logistic regression model. In this model, prevalent ET cases were 1.67 times more likely than controls to have a change in 37-MMSE score in the upper vs lower tertile (ORprevalent ET = 1.67, 95% CI = 1.04–2.69, p = 0.035), and premotor ET cases were 2.38 times more likely than controls to have a change in 37-MMSE score in the upper vs lower tertile (ORpremotor ET = 2.38, 95% CI = 1.12–5.03, p = 0.023). Finally, we conducted a sensitivity analysis in which we only included confounders that were significant at a p value <0.005 (i.e., depressive symptoms or antidepressant use and medications with CNS effects), and the results were similar (ORprevalent ET = 1.68, 95% CI = 1.04–2.69, p = 0.033; and ORpremotor ET = 2.38, 95% CI = 1.13–5.02, p = 0.023).
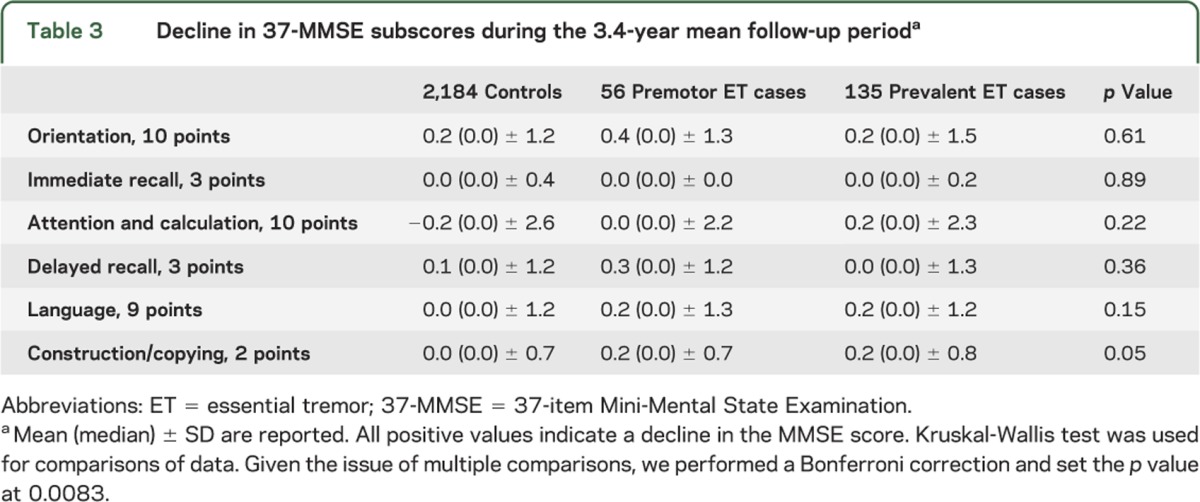
We compared changes in 37-MMSE subscores in cases and controls (table 3). Given the issue of multiple comparisons, we performed a Bonferroni correction and set the p value at 0.0083. None of the differences were significant, although there were several trends (for language, p = 0.15, and for construction/copying, p = 0.05).
Table 3.
Decline in 37-MMSE subscores during the 3.4-year mean follow-up perioda
Prevalent and premotor ET cases had lower median 37-MMSE scores than controls at baseline, suggesting that some of them may already have had mild cognitive deficits. In an additional analysis, we excluded all prevalent and premotor ET cases with baseline 37-MMSE scores that were below the mean baseline 37-MMSE score of controls (i.e., scores <31). In these analyses, the 37-MMSE declined by 1.3 ± 2.9 points (median = 1.0) in the 65 prevalent ET cases, 1.3 ± 3.3 points in the 27 premotor ET cases (median = 1.0 points), and 0.1 ± 3.9 points in the 2,184 controls (median = 0 points) (Kruskal-Wallis, p = 0.003).
Finally, we conducted sensitivity analyses to confirm the robustness of our findings. First, we excluded 31 premotor ET cases because these individuals had not been examined in person by a neurologist at baseline. The 37-MMSE declined by 0.7 ± 3.3 points (median = 1) in prevalent ET cases, 1.7 ± 3.6 points (median = 1) in the remaining 25 premotor ET cases, and 0.1 ± 3.9 points (median = 0) in controls (p = 0.010). The difference between these premotor ET cases and controls remained significant (p = 0.033), as was the difference between prevalent ET cases and controls (p = 0.027). Second, baseline handwriting samples from the 31 premotor ET cases and 31 age-matched controls were blindly reviewed by one of the authors (E.D.L.) and rated using Bain and Findley’s 10-point scale.38 None of the cases and controls had tremor that was in the ET range (all had Bain and Findley38 handwriting tremor scores ≤1, which are within the range of normal).38 Furthermore, each of the 31 was reinterviewed during the follow-up evaluation to establish that the onset of his or her tremor had been after the baseline assessment.
DISCUSSION
In the current prospective study of community-dwelling elders without dementia, we demonstrated that baseline cognitive test scores were lower in premotor ET cases than controls; moreover, during the 3-year follow-up period, these scores declined at a rate that was significantly faster in premotor ET cases than controls.
Premotor ET cases on average experienced a 1.1-point reduction in the 37-MMSE over 3 years. Although this reduction was significantly greater than that seen in controls, in absolute terms, it was a modest change.
For many years, ET was viewed as a monosymptomatic condition, characterized only by a kinetic arm tremor, but over the last 10 years, a plethora of previously unrecognized motor and nonmotor problems have emerged.39 A recent paradigm presents ET as a more complex clinical entity.39 Furthermore, the current and prior data focus on the possibility that nonmotor symptoms could precede the onset of classic motor manifestations. For example, in prospective analyses of the NEDICES study, baseline self-reported depression was associated with increased risk of incident ET.40 These prospective data suggest that the mood disorder in ET may be more than a secondary response to disease manifestations; this mood disorder may be a primary feature of the underlying disease. When these nonmotor problems present early and precede motor symptoms, we cautiously suggest that this should perhaps be regarded as evidence of premotor ET. Additional studies of such a premotor phase of ET are needed, particularly as they may be important for our understanding of when and where ET begins and how it evolves in these initial stages.
This study had limitations. First, we used a 2-phase procedure to screen for ET. Therefore, it is likely that some premotor cases were not properly diagnosed with ET at baseline because they screened negatively. However, in a prior pilot study, none (0%) of the 183 participants who had screened negatively for any of the neurologic disorders tested in the NEDICES study were diagnosed with ET,17 indicating that use of the screening question was likely to yield few false negatives. Furthermore, we conducted additional analyses in which we excluded 31 premotor cases that were not evaluated at baseline on direct examination and found similar results. Second, the 37-MMSE is a relatively abbreviated screening tool for dementia. The use of more detailed neuropsychological test batteries would enable future investigators to study these changes in greater detail. A final limitation of the study was that there were no available data on tremor severity. Hence, we were not able to comment on any relationships between tremor severity, or change in tremor severity, and change in cognitive scores.
This study also had several strengths. First, the study was population-based, allowing us to assess a group of patients with relatively mild ET unselected for medical treatment or surgery. Second, we conducted the assessments prospectively in a standardized manner. Third, we compared cases with a large sample of several thousand controls. Fourth, we adjusted for the potential confounding effects of a number of important factors.
Using a prospective, population-based design, we demonstrated that cognitive test scores in “premotor” ET cases declined at an accelerated rate compared with controls. This study provides further evidence that cognitive deficits in ET are not static. Further studies are required to confirm these results, expressly with premotor prospective data and eventually with a pathologic/biomarker correlate.
GLOSSARY
- CI
confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- ET
essential tremor
- 37-MMSE
37-item Mini-Mental State Examination
- NEDICES
Neurological Disorders in Central Spain
- OR
odds ratio
AUTHOR CONTRIBUTIONS
J. Benito-León and E.D. Louis collaborated in the conception, organization, and execution of the research project, the statistical analysis design, the writing of the manuscript first draft, and the review and critique of the manuscript. Á. Sánchez-Ferro and F. Bermejo-Pareja collaborated in the conception, organization, and execution of the research project, and the review and critique of the manuscript.
STUDY FUNDING
Additional information about collaborators and detailed funding of the NEDICES study can be found on the Web (http://www.ciberned.es/estudio-nedices). The Spanish Health Research Agency and the Spanish Office of Science and Technology supported the NEDICES study. Drs. Benito-León and Bermejo-Pareja are supported by grant R01 NS039422 from the NIH, Bethesda, MD. Dr. Sánchez-Ferro is supported by a grant “Contrato de Investigación Río Hortega” from Instituto de Salud Carlos III, Spain. Dr. Elan D. Louis has received research support from the NIH, Bethesda, MD: National Institute of Neurological Disorders and Stroke grants R01 NS042859 (principal investigator), R01 NS39422 (principal investigator), T32 NS07153-24 (principal investigator), R01 NS073872 (principal investigator), R21 NS077094 (coinvestigator), and R01 NS36630 (coinvestigator). Dr. Louis has also received research support from the Parkinson's Disease Foundation (principal investigator).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Gasparini M, Bonifati V, Fabrizio E, et al. Frontal lobe dysfunction in essential tremor: a preliminary study. J Neurol 2001;248:399–402 [DOI] [PubMed] [Google Scholar]
- 2.Lombardi WJ, Woolston DJ, Roberts JW, Gross RE. Cognitive deficits in patients with essential tremor. Neurology 2001;57:785–790 [DOI] [PubMed] [Google Scholar]
- 3.Troster AI, Woods SP, Fields JA, et al. Neuropsychological deficits in essential tremor: an expression of cerebello-thalamo-cortical pathophysiology? Eur J Neurol 2002;9:143–151 [DOI] [PubMed] [Google Scholar]
- 4.Lacritz LH, Dewey R, Jr, Giller C, Cullum CM. Cognitive functioning in individuals with “benign” essential tremor. J Int Neuropsychol Soc 2002;8:125–129 [DOI] [PubMed] [Google Scholar]
- 5.Higginson CI, Wheelock VL, Levine D, King DS, Pappas CT, Sigvardt KA. Cognitive deficits in essential tremor consistent with frontosubcortical dysfunction. J Clin Exp Neuropsychol 2008;30:760–765 [DOI] [PubMed] [Google Scholar]
- 6.Woods SP, Scott JC, Fields JA, Poquette A, Troster AI. Executive dysfunction and neuropsychiatric symptoms predict lower health status in essential tremor. Cogn Behav Neurol 2008;21:28–33 [DOI] [PubMed] [Google Scholar]
- 7.Passamonti L, Novellino F, Cerasa A, et al. Altered cortical-cerebellar circuits during verbal working memory in essential tremor. Brain 2011;134:2274–2286 [DOI] [PubMed] [Google Scholar]
- 8.Benito-León J, Louis ED, Bermejo-Pareja F; Neurological Disorders in Central Spain Study Group Population-based case-control study of cognitive function in essential tremor. Neurology 2006;66:69–74 [DOI] [PubMed] [Google Scholar]
- 9.Labiano-Fontcuberta A, Benito-León J. Essential tremor: update [in Spanish]. Med Clin 2013;140:128–133 [DOI] [PubMed] [Google Scholar]
- 10.Benito-León J, Louis ED. Update on essential tremor. Minerva Med 2011;102:417–439 [PubMed] [Google Scholar]
- 11.Benito-León J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol 2006;2:666–678 [DOI] [PubMed] [Google Scholar]
- 12.Labiano-Fontcuberta A, Benito-León J, Bermejo-Pareja F. Neuropsychiatric disturbances in essential tremor [in Spanish]. Med Clin 2012;138:171–176 [DOI] [PubMed] [Google Scholar]
- 13.Benito-León J, Louis ED. Clinical update: diagnosis and treatment of essential tremor. Lancet 2007;369:1152–1154 [DOI] [PubMed] [Google Scholar]
- 14.Bermejo-Pareja F. Essential tremor: a neurodegenerative disorder associated with cognitive defects? Nat Rev Neurol 2011;7:273–282 [DOI] [PubMed] [Google Scholar]
- 15.Louis ED, Benito-León J, Vega-Quiroga S, Bermejo-Pareja F. Cognitive and motor functional activity in non-demented community-dwelling essential tremor cases. J Neurol Neurosurg Psychiatry 2010;81:997–1001 [DOI] [PubMed] [Google Scholar]
- 16.Louis ED, Benito-León J, Vega-Quiroga S, Bermejo-Pareja F. Faster rate of cognitive decline in essential tremor cases than controls: a prospective study. Eur J Neurol 2010;17:1291–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermejo F, Gabriel R, Vega S, et al. Problems and issues with door-to-door, two-phase surveys: an illustration from central Spain. Neuroepidemiology 2001;20:225–231 [DOI] [PubMed] [Google Scholar]
- 18.Morales JM, Bermejo FP, Benito-León J, et al. Methods and demographic findings of the baseline survey of the NEDICES cohort: a door-to-door survey of neurological disorders in three communities from central Spain. Public Health 2004;118:426–433 [DOI] [PubMed] [Google Scholar]
- 19.Bermejo-Pareja F, Benito-León J, Vega QS, et al. The NEDICES cohort of the elderly: methodology and main neurological findings [in Spanish]. Rev Neurol 2008;46:416–423 [PubMed] [Google Scholar]
- 20.Vega S, Benito-León J, Bermejo-Pareja F, et al. Several factors influenced attrition in a population-based elderly cohort: neurological disorders in Central Spain Study. J Clin Epidemiol 2010;63:215–222 [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Guzman J, Bermejo-Pareja F, Benito-León J, et al. Prevalence of stroke and transient ischemic attack in three elderly populations of central Spain. Neuroepidemiology 2008;30:247–253 [DOI] [PubMed] [Google Scholar]
- 22.Martínez-Salio A, Benito-León J, Diaz-Guzman J, Bermejo-Pareja F. Cerebrovascular disease incidence in central Spain (NEDICES): a population-based prospective study. J Neurol Sci 2010;298:85–90 [DOI] [PubMed] [Google Scholar]
- 23.Benito-León J, Bermejo-Pareja F, Rodriguez J, et al. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov Disord 2003;18:267–274 [DOI] [PubMed] [Google Scholar]
- 24.Benito-León J, Bermejo-Pareja F, Morales-González JM, et al. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology 2004;62:734–741 [DOI] [PubMed] [Google Scholar]
- 25.Benito-León J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord 2003;18:389–394 [DOI] [PubMed] [Google Scholar]
- 26.Benito-León J, Bermejo-Pareja F, Louis ED; Neurological Disorders in Central Spain Study Group Incidence of essential tremor in three elderly populations of central Spain. Neurology 2005;64:1721–1725 [DOI] [PubMed] [Google Scholar]
- 27.Bermejo-Pareja F, Benito-León J, Vega S, et al. Consistency of clinical diagnosis of dementia in NEDICES: a population-based longitudinal study in Spain. J Geriatr Psychiatry Neurol 2009;22:246–255 [DOI] [PubMed] [Google Scholar]
- 28.Bermejo-Pareja F, Benito-León J, Vega S, Medrano MJ, Roman GC. Incidence and subtypes of dementia in three elderly populations of central Spain. J Neurol Sci 2008;264:63–72 [DOI] [PubMed] [Google Scholar]
- 29.Maggi S, Zucchetto M, Grigoletto F, et al. The Italian Longitudinal Study on Aging (ILSA): design and methods. Aging 1994;6:464–473 [DOI] [PubMed] [Google Scholar]
- 30.Salemi G, Savettieri G, Rocca WA, et al. Prevalence of essential tremor: a door-to-door survey in Terrasini, Sicily. Sicilian Neuro-Epidemiologic Study Group. Neurology 1994;44:61–64 [DOI] [PubMed] [Google Scholar]
- 31.Martínez-Martin P, Gil-Nagel A, Gracia LM, Gomez JB, Martínez-Sarries J, Bermejo F. Unified Parkinson’s disease rating scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord 1994;9:76–83 [DOI] [PubMed] [Google Scholar]
- 32.Louis ED, Barnes L, Wendt KJ, et al. A teaching videotape for the assessment of essential tremor. Mov Disord 2001;16:89–93 [DOI] [PubMed] [Google Scholar]
- 33.Benito-León J, Louis ED, Vega S, Bermejo-Pareja F. Statins and cognitive functioning in the elderly: a population-based study. J Alzheimers Dis 2010;21:95–102 [DOI] [PubMed] [Google Scholar]
- 34.Benito-León J, Mitchell AJ, Vega S, Bermejo-Pareja F. A population-based study of cognitive function in older people with subjective memory complaints. J Alzheimers Dis 2010;22:159–170 [DOI] [PubMed] [Google Scholar]
- 35.Benito-León J, Louis ED, Mitchell AJ, Bermejo-Pareja F. Elderly-onset essential tremor and mild cognitive impairment: a population-based study (NEDICES). J Alzheimers Dis 2011;23:727–735 [DOI] [PubMed] [Google Scholar]
- 36.Benito-León J, Louis ED, Posada IJ, et al. Population-based case-control study of cognitive function in early Parkinson’s disease (NEDICES). J Neurol Sci 2011;310:176–182 [DOI] [PubMed] [Google Scholar]
- 37.Prieto G, Contador I, Tapias-Merino E, Mitchell AJ, Bermejo-Pareja F. The Mini-Mental-37 test for dementia screening in the Spanish population: an analysis using the Rasch Model. Clin Neuropsychol 2012;26:1003–1018 [DOI] [PubMed] [Google Scholar]
- 38.Bain PG, Findley LJ, Atchison P, et al. Assessing tremor severity. J Neurol Neurosurg Psychiatry 1993;56:868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benito-León J. Essential tremor: from a monosymptomatic disorder to a more complex entity. Neuroepidemiology 2008;31:191–192 [DOI] [PubMed] [Google Scholar]
- 40.Louis ED, Benito-León J, Bermejo-Pareja F. Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. Eur J Neurol 2007;14:1138–1146 [DOI] [PubMed] [Google Scholar]