Abstract
Objective:
To explore whether traumatic brain injury (TBI) may be a risk factor for subsequent ischemic stroke.
Methods:
Patients with any emergency department visit or hospitalization for TBI (exposed group) or non-TBI trauma (control) based on statewide emergency department and inpatient databases in California from 2005 to 2009 were included in a retrospective cohort. TBI was defined using the Centers for Disease Control definition. Our primary outcome was subsequent hospitalization for acute ischemic stroke. The association between TBI and stroke was estimated using Cox proportional hazards modeling adjusting for demographics, vascular risk factors, comorbidities, trauma severity, and trauma mechanism.
Results:
The cohort included a total of 1,173,353 trauma subjects, 436,630 (37%) with TBI. The patients with TBI were slightly younger than the controls (mean age 49.2 vs 50.3 years), less likely to be female (46.8% vs 49.3%), and had a higher mean injury severity score (4.6 vs 4.1). Subsequent stroke was identified in 1.1% of the TBI group and 0.9% of the control group over a median follow-up period of 28 months (interquartile range 14–44). After adjustment, TBI was independently associated with subsequent ischemic stroke (hazard ratio 1.31, 95% confidence interval 1.25–1.36).
Conclusions:
In this large cohort, TBI is associated with ischemic stroke, independent of other major predictors.
Ischemic stroke and traumatic brain injury (TBI) are common,1,2 costly,3,4 and leading causes of severe disability in adults.1,5 In particular, both stroke and TBI are responsible for substantial disability in working-age adults—approximately 20% of strokes6 and more than 40% of TBI7 occur in adults younger than 65 years.
In the recent past, no specific stroke mechanism was identified for many strokes in the young.8 Although the proportion of unexplained stroke may be decreasing based on more recent data,9 a large proportion of stroke risk is unexplained by the frequently used stroke prediction models.10 Identifying novel risk factors has the potential to improve stroke prevention and outcomes. TBI is a potential unrecognized stroke risk factor as trauma to the head and neck may increase stroke risk through vascular dissection,11 microvascular injury, or abnormal coagulation.12
A recent observational study in Taiwan based on administrative data13 suggested an association between TBI and all stroke types. However, the association was strongest for known components of TBI (subarachnoid and intracerebral hemorrhage), and a large proportion of stroke risk occurred in the first month. Therefore, it is possible that some events classified as incident stroke were merely sequelae of the TBI and that the magnitude of the observed association may have been overstated.
In the current study, we explored whether the findings in the Taiwanese study extend to a representative region in the United States while limiting to cases of ischemic stroke and accounting for additional confounders. In secondary analyses, we explored whether the TBI-stroke relationship differed by severity of trauma, subtype of TBI, and when excluding early recurrent stroke.
METHODS
This retrospective cohort study was based on emergency department (ED) visits and inpatient discharges for the state of California from 2005 to 2009 from the State Inpatient Databases (SID),14 State Emergency Department Databases (SEDD),15 Healthcare Cost and Utilization Project (HCUP), and Agency for Healthcare Research and Quality. SID and SEDD capture all inpatient discharges and all ED visits that do not result in admission, respectively, within a given year. California was selected for this analysis because of its large population and because it allows for linkage of SEDD and SID records over multiple years using HCUP revisit files.16 We compared TBI patients with non-TBI trauma patients (controls) while accounting for a variety of other variables that may confound the association between TBI and ischemic stroke.
Standard protocol approvals, registrations, and patient consents.
The study protocol, which does not rely on human subjects, was deemed not regulated by the University of Michigan Institutional Review Board because it relied on private coded information that cannot be linked to specific individuals by the investigators.
Patient selection.
Adults 18 years or older were entered into our cohort if they survived either an inpatient admission (SID) or an ED visit (SEDD) for TBI or trauma at any time from 2005 to 2009. TBI was defined using the Centers for Disease Control and Prevention criteria: ICD-9-CM 800.0–801.9, 803.0–804.9, 850.0–854.1, or 959.01 in any discharge diagnosis field.17,18 Our TBI definition was designed to maximize differentiation of TBI and non-TBI trauma. Trauma is inherently a multisystem process, so limiting our diagnosis to only a subset of TBI claims (e.g., principal diagnoses only) would risk misclassifying patients with new TBI as non-TBI trauma. The non-TBI trauma group was composed of patients who had a fracture, excluding fractures of the head and neck: ICD-9-CM 807.0–807.9, 812–819.9, 822–822.9, or 823–827.9 in any position on the discharge record. If a patient had both TBI and non-TBI trauma codes or separate visits with both TBI and non-TBI trauma, they were classified as TBI. Individuals with a visit (ED or inpatient) with stroke (ICD-9-CM 433.x1, 434.x1, 436)19 before TBI or trauma were excluded from the cohort. Similarly, if a patient had multiple TBI or non-TBI trauma visits, they were entered into the cohort with their first visit. In addition, given the known role of arterial dissection as a mediator of ischemic stroke risk in trauma patients, we excluded all patients with carotid (ICD-9-CM 433.21) or vertebral (433.24) dissection at the index visit (n = 66).
Outcome.
Our primary outcome was any hospitalization with a discharge diagnosis of ischemic stroke: ICD-9-CM 433.x1, 434.x1, and 436 from 2005 to 2009.19 This combination of diagnosis codes has been previously validated relative to medical record review and found to have a positive predictive value of 90% and sensitivity of 86%.20 ED visits that did not result in admission for ischemic stroke were not included in the primary outcome because of concerns about the accuracy of coding.
Covariates.
Our primary analysis adjusted for known and possible stroke predictors including demographics, vascular risk factors, comorbidities, trauma severity, and trauma mechanism. Age was divided into quartiles because of the known nonlinear relationship between age and stroke.1 Vascular risk factors were defined using the HCUP single-level clinical classification system.21 Comorbidities were defined based on diagnosis codes listed on the discharge record using the modified Charlson definition, and all Charlson comorbidities were included in our model.22 Trauma severity was estimated with the Abbreviated Injury Scale (ICD/AIS)23 using the software package ICDMAP-9024—a validated algorithm for assigning trauma severity using ICD-9 codes.25 Mechanism of trauma was accounted for by including major external cause of injury group codes (E codes), which describe the intent, mechanism, and circumstances of injuries independently of the anatomical location of an injury.26,27
Primary analysis.
Demographics and baseline characteristics of the TBI and the non-TBI trauma groups were summarized using descriptive statistics. Subsequent stroke was compared by TBI status with Kaplan-Meier estimates and the log-rank test. We were unable to determine whether a patient died outside the context of a hospitalization and thus cases were not censored at death. Cumulative hazard of stroke was estimated at different time intervals using the Nelson-Aalen method and the differences in cumulative hazard were calculated. Confidence intervals (CIs) of the differences were estimated using bootstrapping.
Our primary adjusted analysis relied on Cox proportional hazards modeling.28 We examined the association of TBI and stroke after adjusting for demographics, payer, vascular risk factors (hypertension, hyperlipidemia, diabetes, atrial fibrillation), all Charlson comorbidities, trauma severity, whether a patient had multiple visits for trauma/TBI, and trauma mechanism, while accounting for clustering at the hospital level. To explore how covariate groups (demographics, comorbidities, vascular risk factors, trauma severity, trauma mechanism) affected the TBI–ischemic stroke association, we also developed models in which covariate groups were added serially. Given the increased incidence of epilepsy in patients with TBI29 and the potential for misdiagnosing seizure as stroke,30 we included a covariate that represented whether a patient had any ED visit or admission for epilepsy (ICD-9-CM 345.x) or convulsions (780.3x).31 The proportional hazards assumption central to Cox modeling was tested by visually inspecting plots of the cumulative hazards function and plots of Schoenfeld residuals and no violations of the proportional hazards assumption were found.32
Secondary analyses.
We performed a series of post hoc secondary analyses to assess the robustness of the association between TBI and ischemic stroke that either added covariates to our primary analysis or stratified our primary analysis on covariates of interest. First, to determine whether there was a relationship between specific TBI types and ischemic stroke, we analyzed the risk of ischemic stroke for TBI subtypes identifiable by ICD-9-CM codes: skull fracture (800–801.9, 803–804.9), concussion (850–850.9), cerebral laceration/intracranial hemorrhage (851–853.9), other intracranial injury (854–854.9), and unspecified TBI (959.01).33 Second, to assess whether the TBI–ischemic stroke association may differ depending on overall trauma severity, we repeated our primary analysis stratified over injury severity tertiles. In addition, to assess for possible missed early stroke diagnosis (i.e., stroke present at initial trauma/TBI presentation and not diagnosed at that time), we repeated our primary analysis excluding all ischemic stroke hospitalizations that occurred within 7, 30, or 60 days of trauma. Next, to characterize the relative temporal association between TBI and stroke, we repeated our primary analysis by including only strokes that occurred in the first year after the index event and then again by excluding all strokes that occurred within the first year. We also explored the role of age on the TBI-stroke association by stratifying our analysis at age 50 years. In addition, to assess for alcohol and drug abuse/dependency as possible mediators of the relationship between TBI and ischemic stroke, we repeated our primary analysis adjusting for any alcohol/drug abuse diagnoses (ICD-9-CM 291–292.9, 303–304.9). Finally, to assess for the role of other potential stroke risk factors (e.g., vasculitis) and risk factors that are suboptimally measured (e.g., smoking34), we repeated our primary analysis and estimated the stroke-TBI association after adjusting for the following: hypercoagulable disorders, prior venous thromboembolism, obesity, vasculitis, arrhythmias other than atrial fibrillation, valvular disease, patent foramen ovale, and smoking.
RESULTS
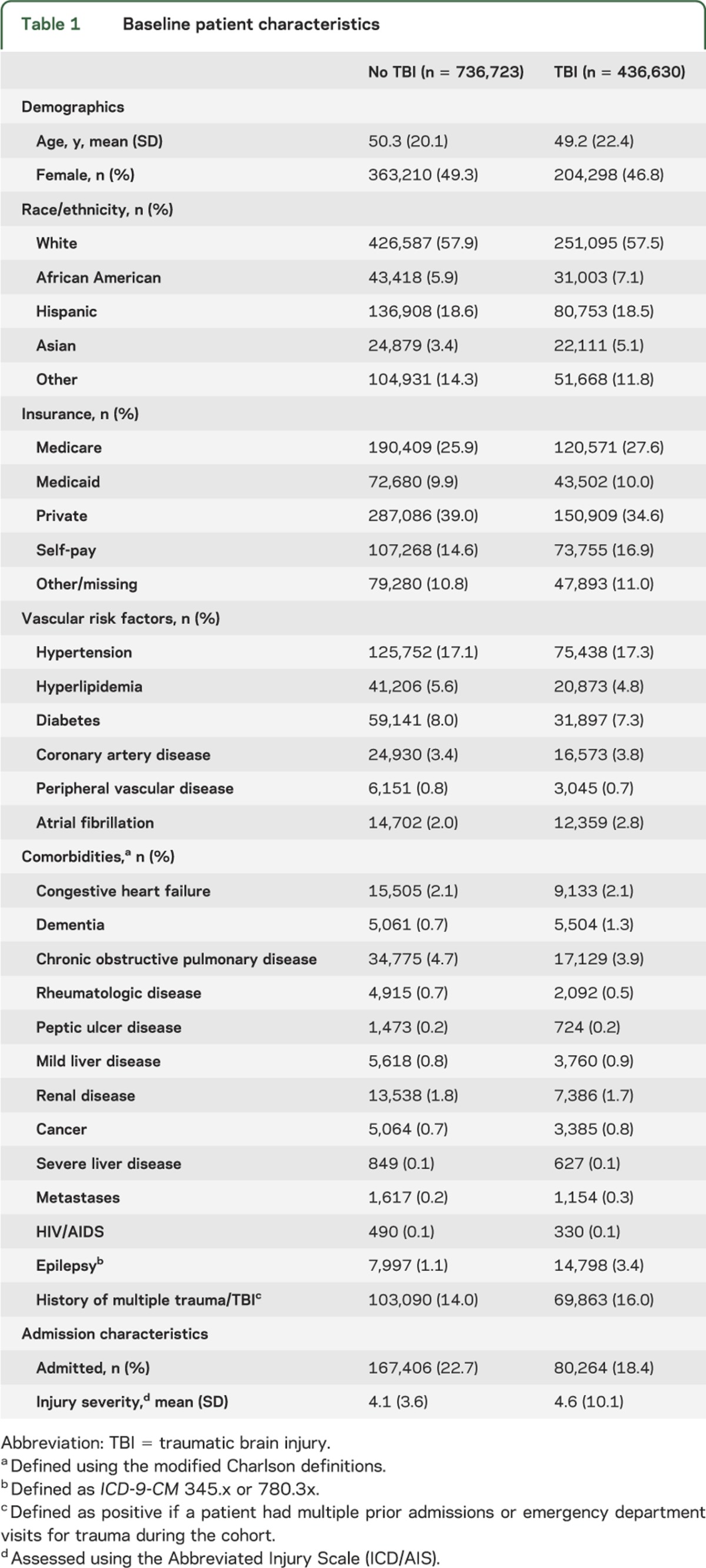
The study cohort included 1,173,353 total trauma subjects, 436,630 (37%) with TBI. The median duration of follow-up was 28 months (interquartile range 14–44), with a total of 11,229 (1%) ischemic strokes identified during this timeframe—1.1% in the TBI group and 0.9% in the non-TBI trauma group. The patients with TBI were slightly younger than controls (mean age 49.2 vs 50.3 years), less likely to be female (46.8% vs 49.3%), and had a higher mean injury severity score (4.6 vs 4.1). Further details of the study population are summarized in table 1.
Table 1.
Baseline patient characteristics
Association between TBI and ischemic stroke hospitalization.
Kaplan-Meier survival curves for survival free from ischemic stroke after TBI and non-TBI trauma are illustrated in the figure. The TBI group was more likely to be hospitalized for ischemic stroke than the non-TBI trauma group (log-rank test, p < 0.01). The difference between the unadjusted Nelson-Aaler cumulative hazard function in the TBI group compared with the non-TBI trauma group was 0.07% (0.06%–0.09%) at 90 days and 0.21% (0.18%–0.24%) at 2 years.
Figure. Kaplan-Meier curves demonstrating the proportion of the cohort with stroke-free survival in traumatic brain injury (TBI) and non-TBI trauma patients.
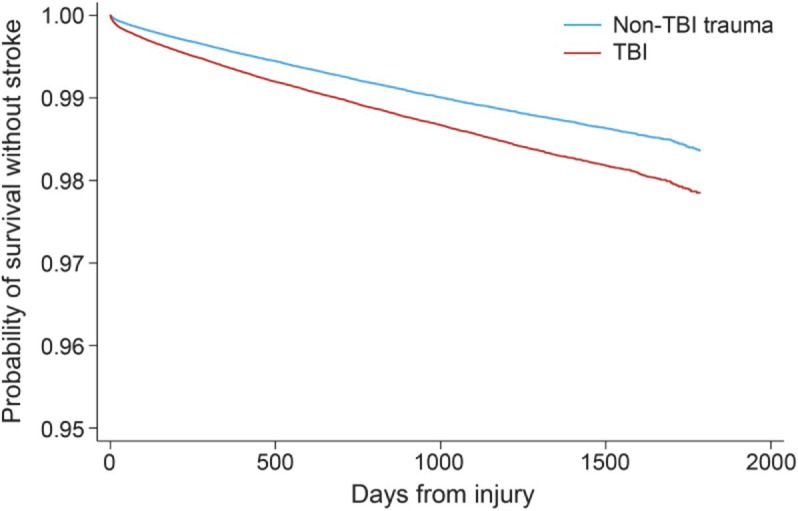
Note that these curves are presented to demonstrate the likely minimum difference in stroke risk between TBI and non-TBI trauma patients. The absolute risk estimates from this curve may be inaccurate because of lack of death censoring (see Discussion section). Log-rank test: p < 0.00001.
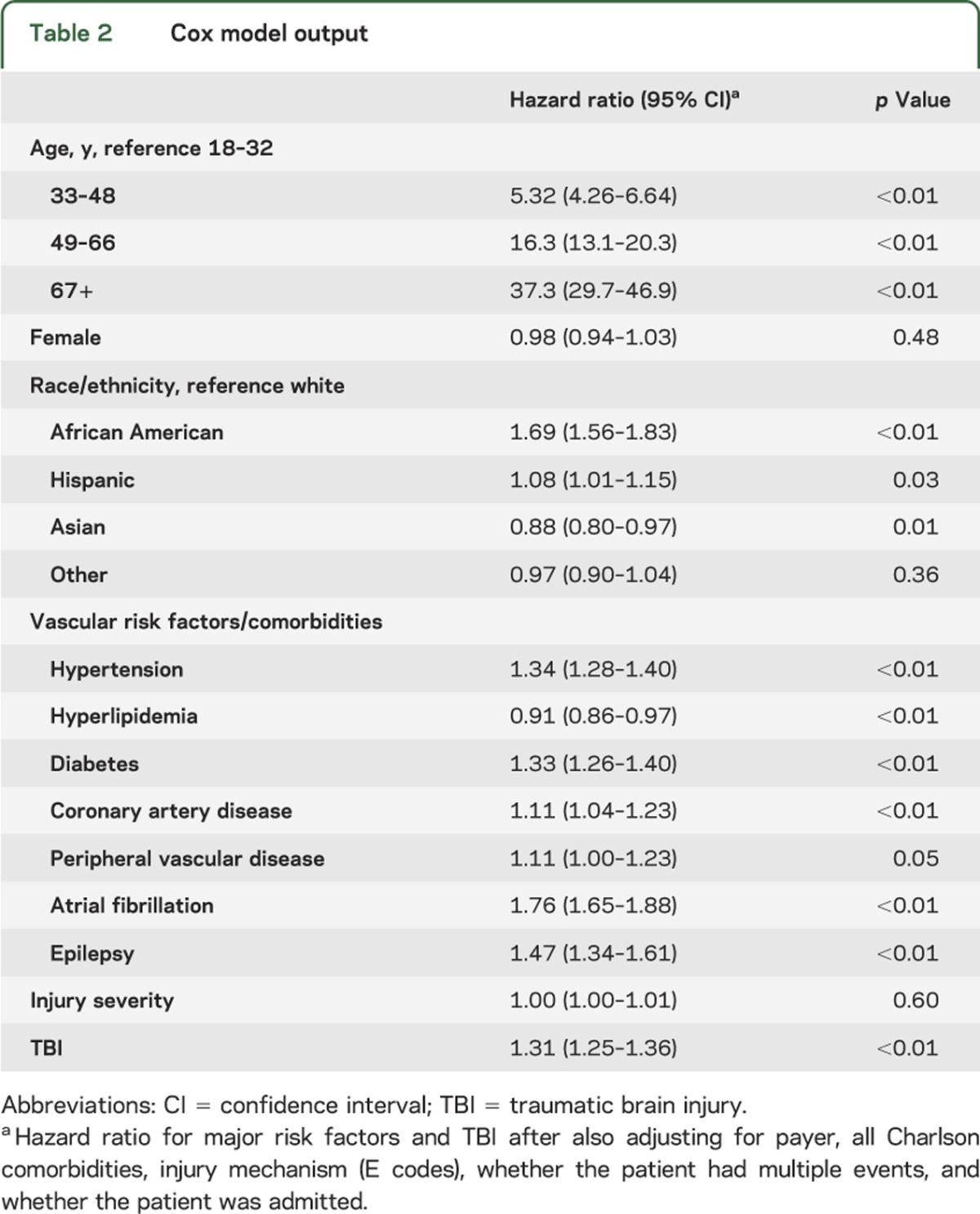
After adjustment for all covariates (table 2), TBI was associated with ischemic stroke hospitalization (hazard ratio [HR] = 1.31, 95% CI 1.25–1.36). This association only changed slightly when covariate groups were serially added: demographics only (HR = 1.34, 95% CI 1.28–1.39), addition of vascular risk factors (HR = 1.30, 95% CI 1.25–1.35), addition of comorbidities (HR 1.30, 95% CI 1.25–1.35), and addition of injury severity and trauma mechanism (HR = 1.31, 95% CI 1.25–1.36).
Table 2.
Cox model output
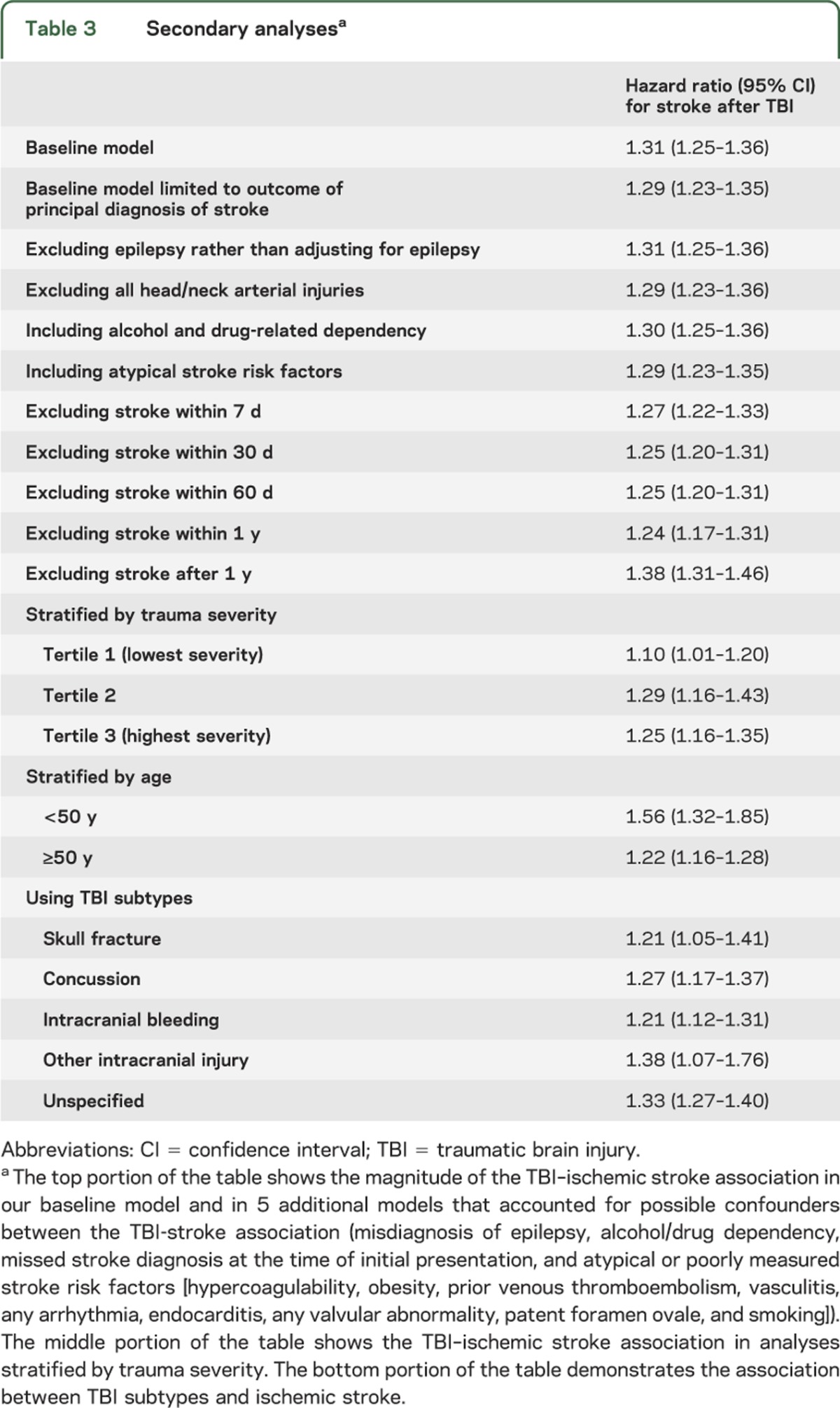
Secondary analyses.
The association between TBI and ischemic stroke hospitalization was robust in that similar associations were observed under a variety of different modeling assumptions (table 3). All TBI subtypes had a similar magnitude of association with ischemic stroke, and when stroke hospitalization within 7, 30, 60, or 365 days of trauma was excluded from the outcome measure, the ischemic stroke–TBI association only modestly decreased (table 3). The age-stratified analysis was a secondary analysis in which the stroke-TBI association was most substantially altered, with a greater association observed in the population younger than 50 years (odds ratio [OR] 1.56, 95% CI 1.32–1.85) vs the population 50 years and older (OR 1.22, 95% CI 1.16–1.28).
Table 3.
Secondary analysesa
DISCUSSION
We found a robust association between TBI visits and subsequent hospitalization for ischemic stroke in California from 2005 to 2009, even after adjusting for a number of potential confounding variables. The magnitude of this association was substantial (HR 1.31) and was similar to the association between the leading stroke risk factor, hypertension (HR 1.34), and ischemic stroke. Given the higher prevalence of TBI in this trauma population, TBI was responsible for more ischemic stroke than hypertension. The TBI–ischemic stroke association persisted in secondary analyses after accounting for a variety of variables and assumptions that may alter the stroke-TBI relationship. Despite the robust association of TBI and ischemic stroke, the absolute ischemic stroke risk difference between TBI and non-TBI trauma patients in this low-risk cohort is small. Nonetheless, if further research definitively established TBI as a novel stroke risk factor, this would stimulate research to understand stroke pathophysiology after TBI and inform stroke prevention efforts in this young population with few vascular risk factors.
We found a similar association between ischemic stroke and TBI as in the prior Taiwanese study.13 In our study, the association persisted after selecting non-TBI trauma controls that are likely more similar to the TBI population than the age- and sex-matched controls in the Taiwanese study. We also found that the ischemic stroke–TBI association was similarly unaffected by accounting for potential confounders such as trauma severity and trauma mechanism. Interestingly, we found that the difference in ischemic stroke risk between the TBI and non-TBI trauma groups was not just attributable to a high early risk in patients with TBI. The risk of stroke after TBI persisted even when excluding cases of stroke within 60 days of trauma. This finding differs somewhat from the prior Taiwanese study that found a large early recurrence rate and a more modest effect after 30 days. We also found that the TBI-stroke association was of considerably greater magnitude in the population younger than 50 years (OR 1.56) vs those 50 years and older (OR 1.22), suggesting that TBI may be uniquely important in younger patients.
If the association between TBI and ischemic stroke is causal, a number of potential pathways may explain this relationship. For example, TBI causes alterations in the coagulation cascade, which in turn may increase stroke risk.12 However, given that these alterations last briefly, they likely explain at most a portion of the association found in this study. TBI is also known to cause vascular dissection—a well-described ischemic stroke mechanism.35,36 Our analysis excluded dissection-mediated stroke; however, given the small number of dissections identified by claims, it is likely that some dissections were undetected. Dissection is unlikely, however, to explain the entire association given the relatively low long-term risk of ischemic stroke after dissection,37 the high short-term recanalization rates after dissection,38 and the fact that the risk difference between TBI and non-TBI trauma patients appears to continue to increase even years after the initial injury. Although other novel pathophysiologic pathways may have a role, it is also possible that patients with TBI may accrue conventional vascular risk factors at a faster rate than patients with non-TBI trauma because of a more sedentary lifestyle after TBI.
This study has a number of important limitations. First, inaccuracy in administrative diagnosis coding may affect both stroke and TBI diagnoses. For example, sequelae of TBI could lead to a misdiagnosis of ischemic stroke based on neuroimaging studies. If this was the case, we would have expected a stronger association between TBI and ischemic stroke in any position on the record compared with the association between TBI and ischemic stroke as the principal diagnosis because principal position diagnoses generally have a higher specificity.39 However, we found a similar association between TBI and stroke regardless of the stroke's position on the claim, thus suggesting that the results are not attributable to diagnostic inaccuracy. Similarly, it is possible that patients presenting with focal neurologic symptoms after a seizure related to their TBI are misdiagnosed with stroke.30 However, our primary analysis adjusted for patients with any epilepsy diagnosis and in secondary analyses we excluded these patients, but the TBI–ischemic stroke association was not substantially affected. Similar potential limitations exist for claims-based diagnosis of mild TBI, which is relatively specific but insensitive.33 As a consequence, some of the patients in our non-TBI trauma control group likely had TBI. To the extent that this was the case, we would have expected the relationship between TBI and ischemic stroke to be biased toward the null. Similarly, because the control group may also be susceptible to an increased stroke risk relative to the general population (e.g., mediated through lack of mobility), it is possible that the reported association between TBI and stroke represents an underestimate. In addition, our estimates of the risk of ischemic stroke in both the TBI cases and controls are underestimates because we were unable to capture out-of-state stroke hospitalizations or to account for competing mortality. We do not expect, however, that either of these limitations would explain our primary findings. First, we were able to account for risk of mortality after trauma using the injury severity score. Second, TBI patients in general would be expected to have a higher mortality than non-TBI trauma patients40; therefore, failing to account for competing mortality would likely lead to underestimation of the true TBI–ischemic stroke association. As with all observational studies, unmeasured confounders (e.g., differences in baseline medications) may lead to biased estimates. Finally, this dataset enables only limited inferences about the possible mechanistic links between TBI and trauma given that many of clinical details (e.g., ischemic stroke subtype, localization, severity) are not measured.
TBI is associated with ischemic stroke, and further work is needed to assess whether it may be a novel stroke risk factor. Prospective cohort and/or population-based, cross-sectional studies are needed to confirm the association, explore potential mechanisms for the association between TBI and ischemic stroke, and carefully characterize the clinical features of both TBI and subsequent stroke, including both TBI and stroke mechanism, size, and location.
GLOSSARY
- CI
confidence interval
- ED
emergency department
- HCUP
Healthcare Cost and Utilization Project
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, ninth revision, Clinical Modification
- OR
odds ratio
- SEDD
State Emergency Department Databases
- SID
State Inpatient Databases
- TBI
traumatic brain injury
AUTHOR CONTRIBUTIONS
Dr. Burke drafted the initial manuscript, participated in development of study design, performed the primary data analysis, and acquired the data. Dr. Stulc and Dr. Skolarus revised the manuscript for content, participated in development of the study design, and participated in data interpretation. Dr. Sears revised the manuscript for content, participated in development of the study design, and participated in data analysis. Dr. Zahuranec revised the manuscript for content, participated in development of the study design, and participated in data interpretation. Dr. Morgenstern revised the manuscript for content, initially developed the study concept, participated in formulation of the study design, and participated in data interpretation.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
J. Burke is supported by a VA Advanced Fellowship. J. Stulc reports no disclosures relevant to the manuscript. L. Skolarus is supported by NIH K23NS073685 from the National Institute of Neurological Diseases and Stroke. E. Sears reports no disclosures relevant to the manuscript. D. Zahuranec is supported by grant K23AG038731 from the National Institute on Aging. L. Morgenstern receives NIH funding (significant) from grants R01NS38916, R01NS062675, U01NS056975, U01NS062835, R18HS017690, R01NS073595, and R01HL098065. He receives significant research support from St. Jude Medical. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012;125:e2–e220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruns J, Hauser W. The epidemiology of traumatic brain injury: a review. Epilepsia 2003;44:2–10 [DOI] [PubMed] [Google Scholar]
- 3.Brown DL, Boden-Albala B, Langa KM, et al. Projected costs of ischemic stroke in the United States. Neurology 2006;67:1390–1395 [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein EA, Corso PS, MIller TR. Incidence and Economic Burden of Injuries in the United States. Oxford, UK: Oxford University Press; 2006 [Google Scholar]
- 5.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil 1999;14:602–615 [DOI] [PubMed] [Google Scholar]
- 6.Glozier N, Hackett ML, Parag V, Anderson CS; Auckland Regional Community Stroke (ARCOS) Study Group The influence of psychiatric morbidity on return to paid work after stroke in younger adults: the Auckland Regional Community Stroke (ARCOS) Study, 2002 to 2003. Stroke 2008;39:1526–1532 [DOI] [PubMed] [Google Scholar]
- 7.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths 2002–2006. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010 [Google Scholar]
- 8.Williams LS, Garg BP, Cohen M, Fleck JD, Biller J. Subtypes of ischemic stroke in children and young adults. Neurology 1997;49:1541–1545 [DOI] [PubMed] [Google Scholar]
- 9.Ji R, Schwamm LH, Pervez MA, Singhal AB. Ischemic stroke and transient ischemic attack in young adults: risk factors, diagnostic yield, neuroimaging, and thrombolysis—ischemic stroke/TIA in young adults. JAMA Neurol 2013;70:51–57 [DOI] [PubMed] [Google Scholar]
- 10.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke 1994;25:40–43 [DOI] [PubMed] [Google Scholar]
- 11.Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology 2001;57:1155–1160 [DOI] [PubMed] [Google Scholar]
- 12.Lu D, Mahmood A, Goussev A, et al. Atorvastatin reduction of intravascular thrombosis, increase in cerebral microvascular patency and integrity, and enhancement of spatial learning in rats subjected to traumatic brain injury. J Neurosurg 2004;101:813–821 [DOI] [PubMed] [Google Scholar]
- 13.Chen YH, Kang JH, Lin HC. Patients with traumatic brain injury: population-based study suggests increased risk of stroke. Stroke 2011;42:2733–2739 [DOI] [PubMed] [Google Scholar]
- 14.Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project Overview of the state inpatient databases [online]. Available at: http://www.hcup-us.ahrq.gov/sidoverview.jsp. Accessed June 12, 2012 [Google Scholar]
- 15.Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project Overview of the state emergency department databases [online]. Available at: http://www.hcup-us.ahrq.gov/seddoverview.jsp. Accessed June 12, 2012 [Google Scholar]
- 16.Healthcare Cost and Utilization Project Supplemental variables for revisit analyses [online]. Available at: http://www.hcup-us.ahrq.gov/toolssoftware/revisit/revisit.jsp. Accessed June 12, 2012 [Google Scholar]
- 17.Thurman DJ, Sniezek JE, Johnson D, Greenspan A. Guidelines for Surveillance of Central Nervous System Injury. Atlanta: U.S. Department of Health and Human Services, Public Health Service, Center for Disease Control and Prevention; 1995 [Google Scholar]
- 18.National Center for Injury Prevention Report to Congress. Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta: Centers for Disease Control and Prevention; 2003 [Google Scholar]
- 19.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke 1998;29:1602–1604 [DOI] [PubMed] [Google Scholar]
- 20.Tirschwell DL, Longstreth WT. Validating administrative data in stroke research. Stroke 2002;33:2465–2470 [DOI] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality (AHRQ) HCUP clinical classification software (CCS) for ICD-9-CM [online]. Available at: http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed June 12, 2012 [Google Scholar]
- 22.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–1139 [DOI] [PubMed] [Google Scholar]
- 23.Association for the Advancement of Automotive Medicine The Abbreviated Injury Scale [online]. Available at: http://www.aaam1.org/ais/index.php. Accessed June 5, 2012 [Google Scholar]
- 24. The Johns Hopkins University, Tri-Analytics. ICD-MAP-90 software.
- 25.Haas B, Xiong W, Brennan-Barnes M, Gomez D, Nathens AB. Overcoming barriers to population-based injury research: development and validation of an ICD10-to-AIS algorithm. Can J Surg 2012;55:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLoughlin E, Annest JL, Fingerhut LA, Berenholz A. Recommended framework for presenting injury mortality data. MMWR Morb Mortal Wkly Rep 1997;46:1–30 [PubMed] [Google Scholar]
- 27.LeMier M, Cummings P, West TA. Accuracy of external cause of injury codes reported in Washington State hospital discharge records. Inj Prev 2001;7:334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox D. Partial likelihood. Biometrika 1975;62:269–276 [Google Scholar]
- 29.Annegers JF, Coan SP. The risks of epilepsy after traumatic brain injury. Seizure 2000;9:453–457 [DOI] [PubMed] [Google Scholar]
- 30.Hand P, Kwan J, Lindley R, Dennis M. Distinguishing between stroke and mimic at the bedside the brain attack study. Stroke 2006;37:769–775 [DOI] [PubMed] [Google Scholar]
- 31.Jetté N, Reid AY, Quan H, Hill MD, Wiebe S. How accurate is ICD coding for epilepsy? Epilepsia 2010;51:62–69 [DOI] [PubMed] [Google Scholar]
- 32.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 1995;14:1707–1723 [DOI] [PubMed] [Google Scholar]
- 33.Bazarian JJ, Veazie P, Mookerjee S, Lerner EB. Accuracy of mild traumatic brain injury case ascertainment using ICD-9 codes. Acad Emerg Med 2006;13:31–38 [DOI] [PubMed] [Google Scholar]
- 34.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke 2005;36:1776–1781 [DOI] [PubMed] [Google Scholar]
- 35.Fisher CM, Ojemann RG, Roberson GH. Spontaneous dissection of cervico-cerebral arteries. Can J Neurol Sci 1978;5:9–19 [PubMed] [Google Scholar]
- 36.Caplan LR, Zarins CK, Hemmati M. Spontaneous dissection of the extracranial vertebral arteries. Stroke 1985;16:1030–1038 [DOI] [PubMed] [Google Scholar]
- 37.Touzé E, Gauvrit JY, Moulin T, et al. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology 2003;61:1347–1351 [DOI] [PubMed] [Google Scholar]
- 38.Baracchini C, Tonello S, Meneghetti G, Ballotta E. Neurosonographic monitoring of 105 spontaneous cervical artery dissections: a prospective study. Neurology 2010;75:1864–1870 [DOI] [PubMed] [Google Scholar]
- 39.Rector TS, Wickstrom SL, Shah M, et al. Specificity and sensitivity of claims-based algorithms for identifying members of Medicare+Choice health plans that have chronic medical conditions. Health Serv Res 2004;39(6 pt 1):1839–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cameron CM, Purdie DM, Kliewer EV, McClure RJ. Long-term mortality following trauma: 10 year follow-up in a population-based sample of injured adults. J Trauma 2005;59:639–646 [PubMed] [Google Scholar]


