Abstract
Objective:
We investigated whether interictal epileptiform discharges (IED) in the human hippocampus are related to impairment of specific memory processes, and which characteristics of hippocampal IED are most associated with memory dysfunction.
Methods:
Ten patients had depth electrodes implanted into their hippocampi for preoperative seizure localization. EEG was recorded during 2,070 total trials of a short-term memory task, with memory processing categorized into encoding, maintenance, and retrieval. The influence of hippocampal IED on these processes was analyzed and adjusted to account for individual differences between patients.
Results:
Hippocampal IED occurring in the memory retrieval period decreased the likelihood of a correct response when they were contralateral to the seizure focus (p < 0.05) or bilateral (p < 0.001). Bilateral IED during the memory maintenance period had a similar effect (p < 0.01), particularly with spike-wave complexes of longer duration (p < 0.01). IED during encoding had no effect, and reaction time was also unaffected by IED.
Conclusions:
Hippocampal IED in humans may disrupt memory maintenance and retrieval, but not encoding. The particular effects of bilateral IED and those contralateral to the seizure focus may relate to neural compensation in the more functional hemisphere. This study provides biological validity to animal models in the study of IED-related transient cognitive impairment. Moreover, it strengthens the argument that IED may contribute to cognitive impairment in epilepsy depending upon when and where they occur.
Temporal lobe epilepsy (TLE) is the most common focal epilepsy in adults, and is associated with memory impairment,1,2 which affects psychosocial functioning and quality of life.3 These deficits are attributed to etiologic changes in the hippocampus such as cell death and synaptic reorganization.4 However, dynamic factors such as interictal abnormalities evident on EEG may impart an independent contribution to neuropsychological outcome.5
Interictal epileptiform discharges (IED) in the cortex, detected with routine scalp EEG recordings, are associated with transient cognitive impairment.6–12 By extension, it is likely that IED in the mesial temporal lobes may affect cognition given the role of these structures in learning and memory. One study13 showed a 6% decline in working memory performance related to mesial temporal IED. However, as demonstrated in previous studies of cortical IED,9,10 the degree of cognitive impact could have been underestimated since the authors did not consider 1) the precise timing of the IED within the memory task trials and 2) the specific components involved in memory processing.
Using a rodent model of TLE,14 we recently found that hippocampal IED were related to considerable decrements in short-term memory retrieval but not memory encoding or maintenance. Therefore, the cognitive impact of IED in the hippocampus may be substantially more important than previously documented. We performed a translational study to assess the hypothesis that spontaneous hippocampal IED can dynamically disrupt short-term memory processes in patients with epilepsy.
METHODS
Patients.
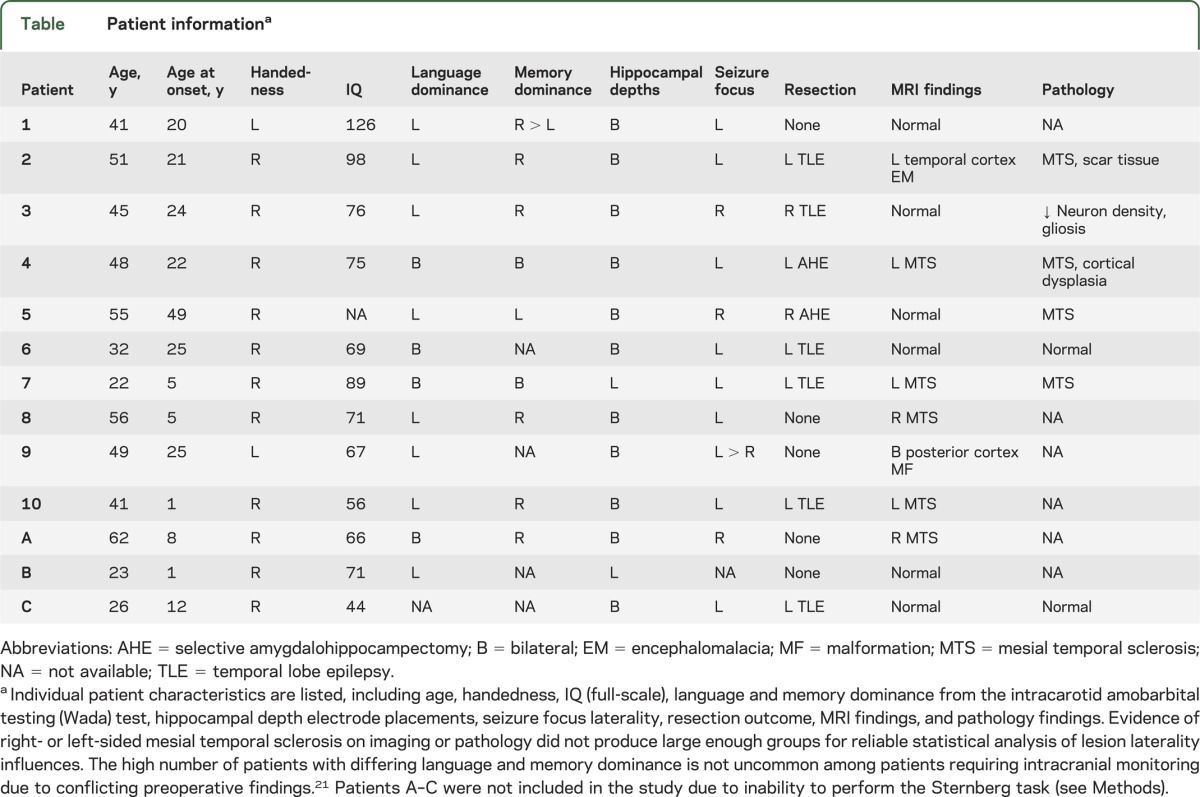
Ten patients with refractory seizures participated in the study. All had depth electrodes implanted into the hippocampus for intracranial epilepsy surgery evaluation of the seizure focus. Patients were monitored in the Peter D. Williamson Epilepsy Unit at the Dartmouth-Hitchcock Epilepsy Center between 2009 and 2011. Demographics and clinical characteristics are listed in the table.
Table.
Patient informationa
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Committee for the Protection of Human Subjects at Dartmouth College. Written consent was obtained for data collection from all participants or their durable power of attorney.
Equipment.
Depth electrodes were inserted via a posterior stereotactic neurosurgical approach, extending from the occipital cortex anteriorly through the longitudinal axis of the hippocampus. Electrode placement was confirmed with coregistration of postoperative CT to preoperative MRI (figure 1A). Electrodes were verified within the hippocampus. The anterior-most contact was within or close to the amygdala. Of note, no IED occurred in isolation in the amygdala. Cognitive testing began 3–5 days following the implantation of electrodes when pain was significantly controlled and antiepileptic medications had been withdrawn to induce seizures.
Figure 1. Examples of depth electrode placement and hippocampal interictal epileptiform discharges.
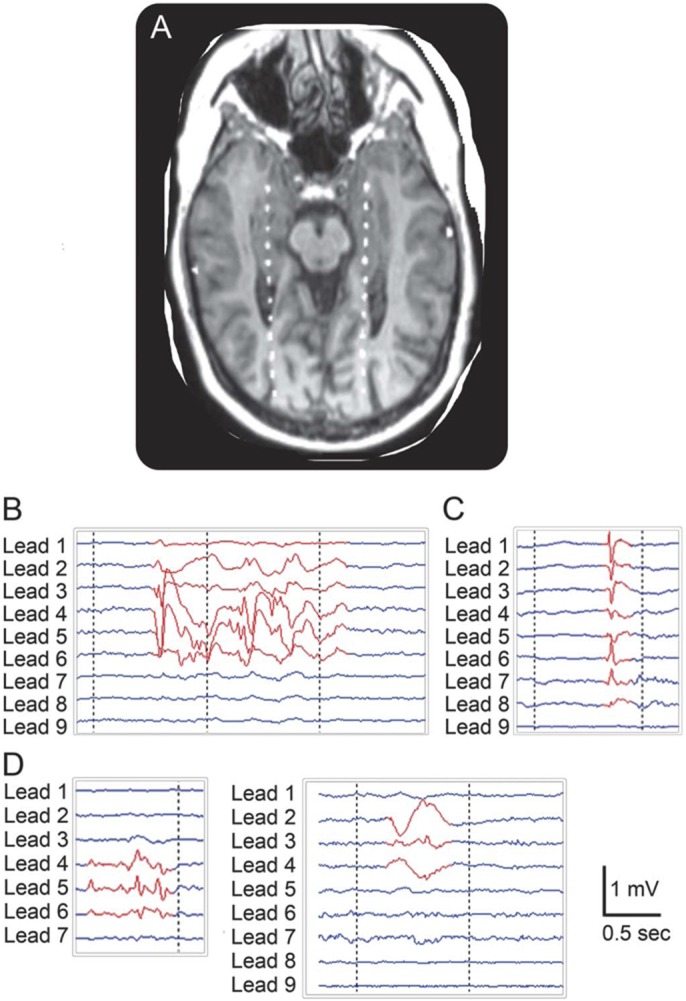
(A) Bilateral depth electrodes visualized by coregistered MRI and CT imaging. From anterior to posterior, contacts are located in the amygdala, hippocampal formation, lateral ventricles, white matter, and occipital cortex. (B) EEG signals illustrate a type 1 IED: repetitive spike-wave complex. From top to bottom, the depth-EEG signals shown are from contacts oriented from anterior to posterior, as illustrated in (A) (i.e., lead 1 is most anterior). (C) Type 2 IED: single spike or spike-wave. (D) Type 3 IED: shown are 2 examples of IED that did not show classical spike or spike-wave morphology. The left panel illustrates aberrant rhythmic activity, while the right panel shows a broad-based high-amplitude sharp wave.
Cognitive task.
We sought to assess short-term memory because it has been established as a hippocampal-dependent function.15 For this purpose we used the Sternberg task,16 which incorporates distinct short-term memory processes to perform delayed information recall. Patients performed up to 120 trials in a daily testing session with breaks between every 30 trials to avoid fatigue. For each trial (figure 2), a group of consonant letters (the “List”) was displayed for 2 seconds on a computer screen. A delay of 6 seconds followed, during which the patient fixated on a dot in the middle of the screen. A single consonant letter (the “Test”) was then shown on the screen for 2 seconds. The patients were instructed to respond with a left mouse click (“Yes”) if they believed that the Test letter was in the previous group (figure 2A), and with a right mouse click (“No”) if not (figure 2B). There was an intertrial interval of 3 seconds followed by a “+” sign displayed before the next trial commenced. To minimize anticipatory responding, the delay intervals and intertrial intervals were made variable by adding a jitter of ±200 milliseconds. Approximately 20% of trials were “Lure” trials, which did not require working memory. These consisted of repeated vowels (figure 2C) and were used for stimulus control to ensure patients were attending to the task. To ensure that patients were able to perform the task, patients performing below 65% cutoff for accuracy were excluded a priori. All 10 patients included in the analyses herein averaged above this cutoff. An additional 3 patients were excluded due to poor attention or lack of understanding the task. Patients did not participate in the study on days when they had a seizure.
Figure 2. Layout of the Sternberg task.
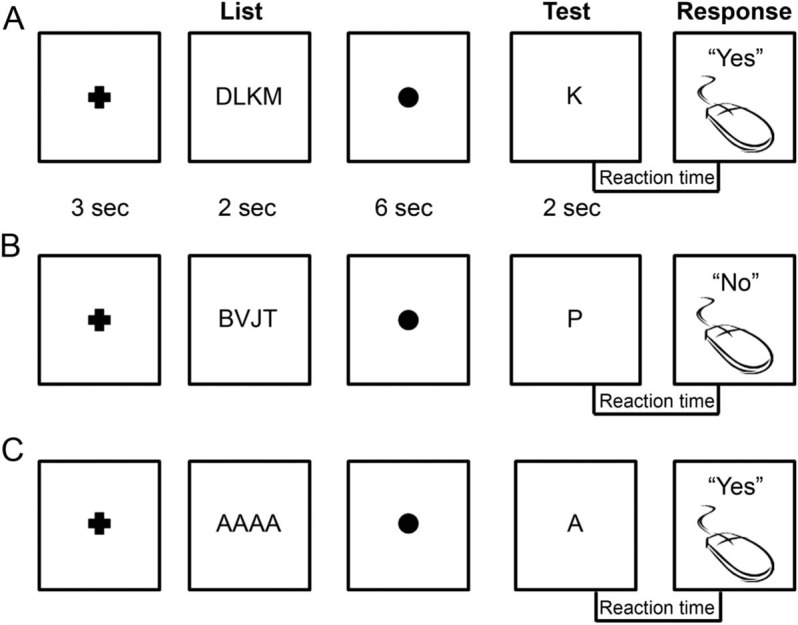
(A) Sternberg trial in which the Test letter is in the previous List sequence. During the intertrial interval (3 seconds), a cross is displayed, followed by the List sequence (2 seconds). A fixation dot is then shown (6 seconds), after which the Test letter is displayed (2 seconds). A correct response in this case would be a left mouse click (“Yes”). (B) A Sternberg trial in which the Test letter is not in the previous List sequence. A correct response in this case would be a right mouse click (“No”). (C) A Lure trial in which the List is always “AAAA” and the Test letter is always “A.” The patient must simply respond with a left mouse click (“Yes”) as soon as the Test letter is shown.
Task software and hardware interface.
Letter sequences were randomly generated using custom MATLAB software (The Mathworks, Inc., Natick, MA), and integrated into the Presentation program (Neurobehavioral Systems, Inc., Albany, CA). Task events and patient responses were synchronized with EEG data collected with equipment from Grass Technologies (West Warwick, RI) and exported afterward for further analysis.
Analysis methods and statistics.
Data processing utilized custom software written in MATLAB. IED were detected manually with a graphical user interface by a board-certified clinical neurophysiologist (B.C.J.). The reviewer was blinded to all Sternberg trial information and was shown only continuous stretches of EEG traces from the depth electrodes. The voltage amplitude (i.e., gain) and time scale displayed on the software interface during IED detection remained constant across all patients to further prevent observer bias. The temporal duration and the number of EEG leads involved were documented for each IED. IED were classified into 3 subtypes (figure 1, B–D). Subtype 1 consisted of repetitive, high-amplitude interictal spike-wave complexes including polyspikes. Subtype 2 consisted of high-amplitude isolated interictal spikes. Subtype 3 consisted of atypical epileptiform activity that did not have a classical spike or spike-wave morphology but was considered abnormal and epileptiform. Most often this represented rhythmic, low-amplitude sharp theta/alpha activity or high-amplitude broad-based sharp waves. IED were also classified according to laterality, meaning whether they occurred ipsilateral or contralateral to the seizure focus, or bilaterally. Subtype classification and randomly selected spike markings were reviewed by a second blinded reviewer (G.L.H.) to assure consistency. Interrater reliability was greater than 95%.
Based on our previous rodent study,14 3 stages of short-term memory processing were inferred in the task a priori: encoding (time window from 2 seconds before to 2 seconds after the List presentation onset), maintenance (the subsequent 4-second window), and retrieval (4-second window immediately prior to the patient's response). IED were classified according to whether or not they fell within these epochs during the Sternberg trials (e.g., retrieval IED were those that occurred within the 4-second window preceding the patient's response). IED that overlapped with multiple epochs (e.g., encoding and maintenance) were analyzed with separate tests for each epoch.
Statistical analyses were performed in STATA 11 (StataCorp LP, College Station, TX) and in SPSS (version 20; Chicago, IL). Correlations were obtained with Pearson or Spearman tests depending upon data distributions. Logistic regression was performed using generalized estimating equations (GEE) to account for within-individual correlation, test the effect of potential confounders, and adjust for uneven data (patients each performed between 30 and 480 trials). This multivariable regression method was used to investigate whether the presence and timing of IED during trials affected the likelihood of correct responses. GEE enabled a single analysis for each time epoch by using multiple factors (e.g., IED laterality and IED subtype) as covariates, minimizing the number of statistical tests used. Reaction time data were compared between trials with and without IED using a paired t test on log-transformed values following within-patient averaging to adjust for differing numbers of trials between patients.
RESULTS
Accuracy.
Sternberg task performance averaged 86% correct across patients overall, ranging from 67% to 98%. We investigated whether an IED in a given trial produced transient cognitive impairment by decreasing the likelihood of giving a correct response answer in that trial. We used reaction time as a covariate given its relation to accuracy (see Reaction time section).
Laterality of IED.
During the retrieval epoch (figure 3), IED that were contralateral to the seizure focus (odds ratio [OR] 0.544; 95% confidence interval [CI] 0.336–0.883; p < 0.05) and bilateral IED (OR 0.645; 95% CI 0.501–0.830; p < 0.001) decreased the likelihood of a correct response, while ipsilateral IED did not (OR 1.090; 95% CI 0.855–1.391). In the encoding epoch, ipsilateral (OR 0.913; 95% CI 0.559–1.489), contralateral (OR 0.723; 95% CI 0.346–1.513), or bilateral (OR 0.810; 95% CI 0.357–1.838) IED did not influence accuracy. In the maintenance epoch, bilateral IED decreased the likelihood of a correct response (OR 0.557; 95% CI 0.386–0.805; p < 0.01), but not IED that were ipsilateral (OR 1.165; 95% CI 0.807–1.683) or contralateral (OR 1.071; 95% CI 0.659–1.743). It should be noted that according to intracarotid amobarbital testing (IAT), only one patient had memory dominance ipsilateral to the seizure focus (table). All other patients had memory dominance on the contralateral side or comparable test scores bilaterally.
Figure 3. Influence of interictal epileptiform discharges during memory retrieval on accuracy.
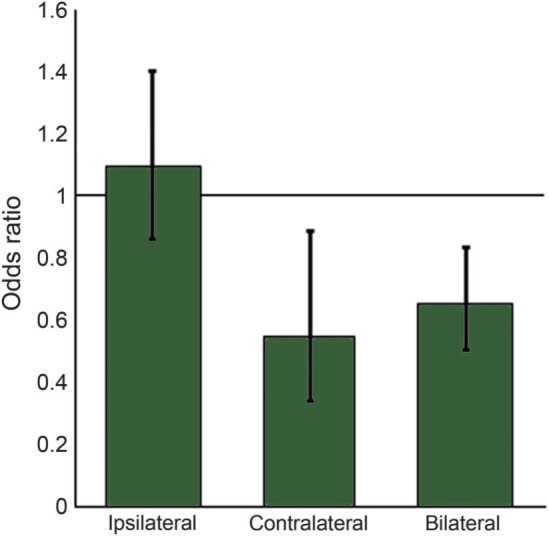
Predicted odds ratios are shown for the influence of interictal epileptiform discharges that were ipsilateral or contralateral to the seizure focus, or bilateral. Error bars indicate 95% confidence intervals. Contralateral and bilateral spikes during retrieval significantly decreased the likelihood of responding correctly during a trial.
The laterality analyses were repeated by designating IED as left or right hemisphere instead of ipsilateral or contralateral to the seizure focus. The results for left- and right-sided IED were similar to ipsilateral and contralateral IED, respectively, likely since most patients had left-sided seizure foci (see table). Specifically, right-sided IED during the retrieval period decreased the likelihood of a correct answer (OR 0.623; 95% CI 0.411–0.945; p < 0.05), while left-sided IED did not (OR 1.167; 95% CI 0.928–1.468).
Without laterality taken into account, IED that occurred during memory encoding (OR 1.054; 95% CI 0.723–1.535), maintenance (OR 0.973; 95% CI 0.730–1.298), or retrieval (OR 1.135; 95% CI 0.874–1.476) did not influence the likelihood of a correct response. Multiple IED in a trial, including IED that overlapped with multiple epochs, did not decrease the likelihood of a correct response.
IED subtypes.
Separating IED into subtypes revealed that both type 1 (OR 0.542; 95% CI 0.296–0.992; p < 0.05) and type 3 IED (OR 0.776; 95% CI 0.604–0.998; p < 0.05) decreased the likelihood of a correct response when they fell in the retrieval period. The mean duration (in seconds) of type 1 IED in the memory maintenance epoch further decreased the likelihood of a correct response (OR 0.300; 95% CI 0.138–0.656; p < 0.01), but not during encoding (OR 0.364; 95% CI 0.204–1.793) or retrieval (OR 1.016; 95% CI 0.488–2.114). There were no other effects of the individual IED subtypes on encoding, maintenance, or retrieval. To control for multiple comparisons, we then carried out multivariable regression for each epoch using subtype, laterality, and reaction time as independent covariables. The results confirmed the significant results reported above for IED subtype and laterality influences, and showed that these variables covaried with each other.
IED correlations.
The total number of IED in a given Sternberg session was not related to overall accuracy in that session (Spearman rho = −0.50, p = 0.14), nor was it related to IQ or other neuropsychological measures in the California Verbal Learning Test (p > 0.05).
Reaction time.
The time between Test letter presentation and the patient's response (i.e., reaction time) was shorter for correct trials compared to incorrect trials (p < 0.001). Lure trials had shorter reaction times than both correct and incorrect trials (p < 0.001 for both). Average reaction times were 1.42 seconds in correct trials, 1.76 seconds in incorrect trials, and 0.76 seconds in Lure trials. The placement of the Test letter in the List sequence (first through fourth place) was significantly related to reaction time (p < 0.001), but not significantly related to accuracy (OR 0.785; 95% CI 0.570–1.080). Reaction times were longer for trials where the Test letter was in the List (see figure 2A) compared to trials where it was not in the List (see figure 2B; p < 0.001), though there was no significant difference in accuracy (OR 1.385; 95% CI 0.661–2.902).
The transient impact of IED on reaction time was analyzed only among correct trials to control for the influence of accuracy. If IED occurred during the retrieval period, the reaction time was similar to trials without any IED during this epoch (p = 0.324; figure 4). This lack of difference in reaction time between trials with and without IED was consistent regardless of IED laterality or subtype.
Figure 4. Reaction time in trials with and without interictal epileptiform discharges.
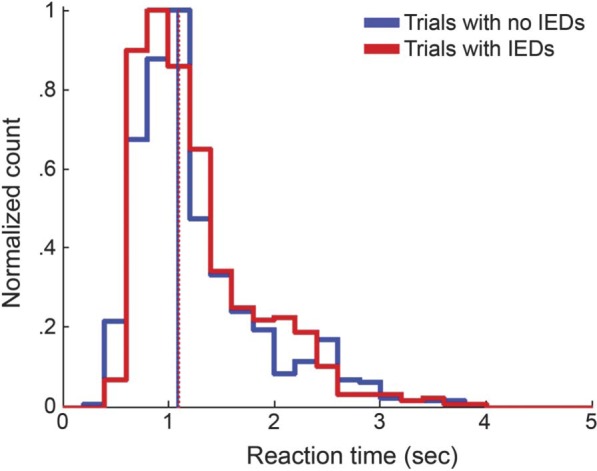
The figure shows histograms of the relative frequencies of reaction time in trials without interictal epileptiform discharges (IED) (thick solid blue line) and trials with IED (thick solid red line). The median reaction time was not significantly different between trials without IED (1.085 seconds; solid blue vertical line) and trials with IED (1.100 seconds; dotted red vertical line).
DISCUSSION
We show that hippocampal IED may be associated with greater decrements in memory performance than previously reported,13 depending on their timing and location. We also provide the first clinical translation of IED-associated transient cognitive impairment findings across species, from a rodent model14 to patients with epilepsy.
Neuropsychological test scores have been used to indirectly investigate whether IED relate to poorer cognitive outcomes by correlating performance with the cumulative number of IED in a given period. These types of studies have produced mixed findings.17–19 We showed no relation between the number of IED in a session and session accuracy, but there was a transient effect of an IED in a given trial. We attributed this to the concept put forth by some prior studies9,10 that an IED has to be in the right place at the right time to produce cognitive impairment. Hippocampal IED in any period outside of maintenance and retrieval (on a short-term memory task) may be inconsequential and would not support a correlation between IED frequency and accuracy. Future investigations of IED-induced cognitive impairment must account for the timing of IED to reveal their full consequence.
The laterality of IED influenced whether cognitive impairment was observed. A classic study on transient cognitive impairment due to cortical IED6 reported the lateralization of IED-induced impairments, with left-sided IED producing verbal task impairments and right-sided IED producing spatial task impairments. The Sternberg task involves combinations of letters, implying a possible verbal memory component. However, right-sided IED significantly affected cognitive performance while left-sided IED did not. Another potential explanation for the strong effect of right- but not left-sided IED may relate to the majority of our patients having a left-sided seizure focus. Faulty unilateral hippocampal circuitry may have resulted in a shift of memory workload to the right (or contralateral) side over time,20 consistent with variations in memory dominance laterality as reflected by our IAT results.21 Other researchers have corroborated this concept by showing increased activation in the hemisphere contralateral to the seizure focus.22 Thus, in the vast majority of patients, IED in the left (or ipsilateral) hemisphere would have little to no effect while right-sided (or contralateral) IED would compromise the compensatory networks. Bilateral IED would affect both hemispheres with additional memory circuits, and would therefore affect additional functions such as memory maintenance, as seen here. Indeed, they prominently disrupted both maintenance and retrieval processes in this study, similar to previous findings on cognitive impairment with bilateral hippocampal stimulation.23 Laterality of IED in our previous rat study14 did not influence accuracy. However, rats have questionable lateralization of function and show bilateral lesions after pilocarpine.
Although this study translates a number of our animal findings,14 type 2 events, which were similar in morphology to the IED in our rodent model, did not predict impairments here. On the other hand, type 1 and type 3 events did affect accuracy, consistent with a prior study that reported a greater impact of spikes with a “larger breadth of field” than small-amplitude focal spikes on scalp EEG in humans.8 The vulnerability of rats to focal interictal spikes may be a matter of species or due to a shorter amount of time (weeks to months) between pilocarpine epilepsy induction and the testing period. Patients in the current study had been diagnosed with epilepsy decades earlier (see table), perhaps allowing their brains to compensate for small-amplitude, focal IED.20
Hippocampal IED did not affect reaction time in this study, whereas hippocampal interictal spikes increased response latency (a similar measure) in the animal study. However, a previous study13 also showed that hippocampal IED do not affect reaction time in humans. It is likely that species and task differences account for the discrepancy.
Indeed, the neural networks involved for the Sternberg task are likely different from those governing the delayed-match-to-sample task (DMTS) in the animal study. DMTS has a prominent hippocampal-dependent component according to lesion studies in rats.24 The hippocampus is also implicated in processing the Sternberg task25; however, it involves the prefrontal cortex and multiple other substrates in the limbic system.26 Both tasks require a component of short-term memory function that is hippocampal-dependent, and both were affected by IED in the retrieval period. Likewise, neither task was affected by IED during the encoding period, in contradiction to the effects of cortical IED.10,11 This process-specific vulnerability across species may relate to the intricate intrahippocampal circuits involved in memory recall,27,28 while memory encoding may be buffered by other cortical structures such as the prefrontal cortex29 or perhaps lingering representation in primary sensory areas. Thus, assessing the full impact of IED on cognition requires consideration of the characteristics of both the cognitive task and the IED themselves.8,11
More patients may have allowed better assessment of whether neural plasticity was truly involved in redirecting memory function to supplemental (e.g., contralateral) brain networks, and whether IED in these networks truly compromised this compensation. We were also limited in that we used only one cognitive task to assess hippocampal function, which may have been considerably verbal in nature. Furthermore, it did not allow adjustment of memory load, which can relate to the degree of hippocampal recruitment.30 Moreover, it is unclear whether IED-associated disruption during such a specific memory task relates to clinically relevant dysfunction.
Recurrent seizures are the cornerstone of epilepsy and the focus of its treatment, while IED are not currently incorporated into treatment strategies. Instead they are usually considered a harmless biomarker that assists in the diagnosis of epilepsy. Our study demonstrates that bilateral IED and IED contralateral to the seizure focus are probably the most clinically relevant to memory impairment and warrant attention. However, treatment options are limited. Pharmacologic treatment to reduce IED is a hotly debated topic,31 particularly since antiepileptic drugs impart their own cognitive side effects.32,33
Our previous findings in an animal model have been generally translated to patients in the current study. Thus we surmise that future studies of IED-associated cognitive impact can be probed with animal research. This study thereby encourages and facilitates future investigations into IED-induced effects and whether attenuating them might improve the lives of patients.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Terrance Darcey (equipment setup), Kandan Kulandaivel (data acquisition), Myrta Otero (data acquisition), and Robert Roth (equipment setup) for their assistance.
GLOSSARY
- CI
confidence interval
- DMTS
delayed-match-to-sample task
- GEE
generalized estimating equations
- IAT
intracarotid amobarbital testing
- IED
interictal epileptiform discharges
- OR
odds ratio
- TLE
temporal lobe epilepsy
Footnotes
Editorial, page 12
AUTHOR CONTRIBUTIONS
Dr. Kleen: study design, data acquisition, statistical analysis, study coordination, data interpretation, writing the manuscript. Dr. Scott: study design, statistical analysis, data interpretation, writing the manuscript. Dr. Holmes: study design, study coordination, data interpretation, writing the manuscript. Dr. Roberts: study design, data interpretation, writing the manuscript. M.M. Rundle: data acquisition, data interpretation, writing the manuscript. Dr. Testorf: data acquisition, data interpretation, writing the manuscript. Dr. Lenck-Santini: study design, data acquisition, study coordination, data interpretation, writing the manuscript. Dr. Jobst: study design, data acquisition, study coordination, data interpretation, writing the manuscript.
STUDY FUNDING
Supported by NIH grants F30NS064624, R21MH086833, R01NS044295, R01NS074450, and R01NS073083.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Helmstaedter C. Effects of chronic epilepsy on declarative memory systems. In: Thomas S, Asla P, eds. Progress in Brain Research. Philadelphia: Elsevier; 2002:439–453 [DOI] [PubMed] [Google Scholar]
- 2.Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol 1997;54:369–376 [DOI] [PubMed] [Google Scholar]
- 3.Hoppe C, Elger CE, Helmstaedter C. Long-term memory impairment in patients with focal epilepsy. Epilepsia 2007;48(suppl 9):26–29 [DOI] [PubMed] [Google Scholar]
- 4.Holmes GL. Effects of seizures on brain development: lessons from the laboratory. Pediatr Neurol 2005;33:1–11 [DOI] [PubMed] [Google Scholar]
- 5.Binnie CD. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol 2003;2:725–730 [DOI] [PubMed] [Google Scholar]
- 6.Aarts JH, Binnie CD, Smit AM, Wilkins AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain 1984;107(pt 1):293–308 [DOI] [PubMed] [Google Scholar]
- 7.Binnie CD, Kasteleijn-Nolst Trenite DG, Smit AM, Wilkins AJ. Interactions of epileptiform EEG discharges and cognition. Epilepsy Res 1987;1:239–245 [DOI] [PubMed] [Google Scholar]
- 8.Shewmon DA, Erwin RJ. The effect of focal interictal spikes on perception and reaction time: I: general considerations. Electroencephalogr Clin Neurophysiol 1988;69:319–337 [DOI] [PubMed] [Google Scholar]
- 9.Shewmon DA, Erwin RJ. The effect of focal interictal spikes on perception and reaction time: II: neuroanatomic specificity. Electroencephalogr Clin Neurophysiol 1988;69:338–352 [DOI] [PubMed] [Google Scholar]
- 10.Shewmon DA, Erwin RJ. Focal spike-induced cerebral dysfunction is related to the after-coming slow wave. Ann Neurol 1988;23:131–137 [DOI] [PubMed] [Google Scholar]
- 11.Shewmon DA, Erwin RJ. Transient impairment of visual perception induced by single interictal occipital spikes. J Clin Exp Neuropsychol 1989;11:675–691 [DOI] [PubMed] [Google Scholar]
- 12.Kasteleijn-Nolst Trenite DG, Riemersma JB, Binnie CD, Smit AM, Meinardi H. The influence of subclinical epileptiform EEG discharges on driving behaviour. Electroencephalogr Clin Neurophysiol 1987;67:167–170 [DOI] [PubMed] [Google Scholar]
- 13.Krauss GL, Summerfield M, Brandt J, Breiter S, Ruchkin D. Mesial temporal spikes interfere with working memory. Neurology 1997;49:975–980 [DOI] [PubMed] [Google Scholar]
- 14.Kleen JK, Scott RC, Holmes GL, Lenck-Santini PP. Hippocampal interictal spikes disrupt cognition in rats. Ann Neurol 2010;67:250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen P. The Hippocampus Book. Oxford: Oxford University Press; 2007 [Google Scholar]
- 16.Sternberg S. High-speed scanning in human memory. Science 1966;153:652–654 [DOI] [PubMed] [Google Scholar]
- 17.Chauviere L, Rafrafi N, Thinus-Blanc C, Bartolomei F, Esclapez M, Bernard C. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J Neurosci 2009;29:5402–5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonseca LC, Tedrus GM, Tonelotto JM, Antunes Td Tde A, Chiodi MG. School performance in children with benign childhood epilepsy with centrotemporal spikes [in Portuguese]. Arq Neuropsiquiatr 2004;62:459–462 [DOI] [PubMed] [Google Scholar]
- 19.Weglage J, Demsky A, Pietsch M, Kurlemann G. Neuropsychological, intellectual, and behavioral findings in patients with centrotemporal spikes with and without seizures. Dev Med Child Neurol 1997;39:646–651 [DOI] [PubMed] [Google Scholar]
- 20.Voytek B, Davis M, Yago E, Barcelo F, Vogel EK, Knight RT. Dynamic neuroplasticity after human prefrontal cortex damage. Neuron 2010;68:401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovac S, Moddel G, Reinholz J, et al. Memory performance is related to language dominance as determined by the intracarotid amobarbital procedure. Epilepsy Behav 2009;16:145–149 [DOI] [PubMed] [Google Scholar]
- 22.Powell HW, Richardson MP, Symms MR, et al. Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia 2007;48:1512–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halgren E, Wilson CL. Recall deficits produced by afterdischarges in the human hippocampal formation and amygdala. Electroencephalogr clin Neurophysiol 1985;61:375–380 [DOI] [PubMed] [Google Scholar]
- 24.Hampson RE, Jarrard LE, Deadwyler SA. Effects of ibotenate hippocampal and extrahippocampal destruction on delayed-match and -nonmatch-to-sample behavior in rats. J Neurosci 1999;19:1492–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzuto DS, Madsen JR, Bromfield EB, Schulze-Bonhage A, Kahana MJ. Human neocortical oscillations exhibit theta phase differences between encoding and retrieval. Neuroimage 2006;31:1352–1358 [DOI] [PubMed] [Google Scholar]
- 26.Schon K, Quiroz YT, Hasselmo ME, Stern CE. Greater working memory load results in greater medial temporal activity at retrieval. Cereb Cortex 2009;19:2561–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci USA 2007;104:14495–14500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colgin LL, Denninger T, Fyhn M, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 2009;462:353–357 [DOI] [PubMed] [Google Scholar]
- 29.Lee I, Kesner RP. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J Neurosci 2003;23:1517–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Axmacher N, Haupt S, Cohen MX, Elger CE, Fell J. Interference of working memory load with long-term memory formation. Eur J Neurosci 2009;29:1501–1513 [DOI] [PubMed] [Google Scholar]
- 31.Stafstrom CE. Interictal spikes: memories forsaken. Epilepsy Curr 2010;10:135–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richens A, Yuen AW. Overview of the clinical efficacy of lamotrigine. Epilepsia 1991;32(suppl 2):S13–S16 [DOI] [PubMed] [Google Scholar]
- 33.Ben-Menachem E, Treiman DM. Effect of gamma-vinyl GABA on interictal spikes and sharp waves in patients with intractable complex partial seizures. Epilepsia 1989;30:79–83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


