Abstract
Objective:
We aimed to study the long-term cognitive abilities of patients surviving out-of-hospital cardiac arrest who were treated with therapeutic hypothermia (TH).
Methods:
We prospectively identified and examined consecutive survivors of out-of-hospital cardiac arrest who underwent TH at our institution from June 2006 to May 2011. The results of brain imaging, serum neuron-specific enolase (NSE) measurements, and EEGs were recorded. We assessed cognitive domains using the modified Telephone Interview for Cognitive Status. An education-adjusted score of ≥32 was considered normal.
Results:
Of 133 total patients, 77 (58%) were alive at a median follow-up of 20 months (interquartile range 14–24 months). We interviewed 56 patients (73% of those alive). Median age was 67 years (range 24–88 years). Fifty-one patients (91%) were living independently. Modified Telephone Interview for Cognitive Status scores ranged from 16 to 41. Thirty-three (60%) were considered cognitively normal and 22 (40%) were cognitively impaired. The time to assessment did not differ among the cognitive outcomes (p = 0.557). The median duration of coma was 2 days, possibly indicating that patients with severe anoxic injury were not included. Eighteen patients were not working at the time of their cardiac arrest (17 were retired and 1 was unemployed). Of the 38 patients who were working up to the time of the cardiac arrest, 30 (79%) returned to work. Cognitive outcome was not associated with age, time to return of spontaneous circulation, brain atrophy, or leukoaraiosis.
Conclusions:
The majority of surviving patients who underwent TH after cardiac arrest in this series had preserved cognitive function and were able to return to work.
Out-of-hospital cardiac arrest (OHCA) strikes suddenly and is frequently fatal. Over the past decade, people who undergo cardiac arrest and cardiopulmonary resuscitation are increasingly surviving.1–3 During a cardiac arrest, neuronal injury begins to occur because blood flow to the brain ceases, causing loss of consciousness. Even after restoration of effective circulation, patients may remain comatose, and ongoing injury to the brain occurs during reperfusion injury with release of free radicals and excessive excitatory neurotransmitters.4,5
Induction of moderate therapeutic hypothermia (TH) is the only neuroprotective strategy that has been proven to improve neurologic outcome in comatose survivors of cardiac arrest.6,7 Studies of patients undergoing TH for OHCA have mostly focused on endpoints such as hospital discharge disposition,6 functional scales according to level of dependence,7–10 or a dichotomized outcome in which merely recovery of consciousness defines a good outcome.11 The cognitive sequelae of conscious OHCA survivors are a vital concern that has not been well studied, particularly in patients treated with TH. The best data available are from 3 prospective studies and 1 population-based study, which altogether comprise 217 patients, only 3 of whom received TH.12–15 In these studies, most survivors of OHCA had cognitive impairment that most frequently affected long-term memory followed by executive domains. The conclusion of a systematic review indicated that “cognitive problems … seem common in survivors.”16 Nevertheless, it has been shown that as many as 85% of patients are still able to function independently after surviving OHCA.13 In the current study, we aimed to evaluate the long-term cognitive abilities of patients surviving OHCA who were treated with TH. We hypothesized that most patients would have cognitive impairment as a sequela of their cardiac arrest.
METHODS
We prospectively identified consecutive adult comatose cardiac arrest survivors who were admitted to Saint Marys Hospital (Rochester, MN) and underwent treatment with our TH protocol from June 2006 to May 2011. Detailed methods of our TH protocol and predictors of mortality in most of this population have been previously reported.9,17 During this study time period, candidates for the TH protocol included patients with ventricular fibrillation as the initial rhythm and those with witnessed pulseless electrical activity or asystolic arrest who responded to therapy with return of spontaneous circulation (ROSC), but who remained comatose. Patients who exhibited improving responsiveness (as demonstrated by spontaneous eye-opening, localizing a noxious stimulus, or following simple commands) shortly after the cardiac arrest were excluded from consideration. Our general approach is to wait approximately 30 minutes after ROSC to observe for evidence of awakening, so as not to start TH protocol unnecessarily.
Our prospective databank includes a measure of functional outcome (the Cerebral Performance Category [CPC] score) at the time of hospital discharge. For the purpose of this study, we contacted surviving patients to assess their cognitive abilities using the Telephone Interview for Cognitive Status, modified (TICS-m), a validated tool used to screen for dementia (table 1).18,19 To minimize bias, we analyzed functional outcome at hospital discharge of patients that we could not reach by telephone. We administered the TICS-m according to published methods20 and using a standardized script. The maximum unadjusted score is 50 points. We adjusted the score for years of education according to published methods as follows: +5 points for education <8 years, +2 points for 8 to 10 years, 0 points for 11 to 15 years, and −2 points for ≥16 years.21 An education-adjusted TICS-m score ≥32 was considered normal and scores <32 were taken to indicate cognitive impairment.19 In a validation study of the TICS-m scale, this has been identified as the optimal score to separate patients with mild cognitive impairment or dementia from cognitively normal patients. A score ≤27 has been considered to separate those with dementia from those without dementia, but using the more stringent cutoff should minimize falsely classifying a participant as cognitively normal.19
Table 1.
Items and scoring of the TICS-m questionnairea
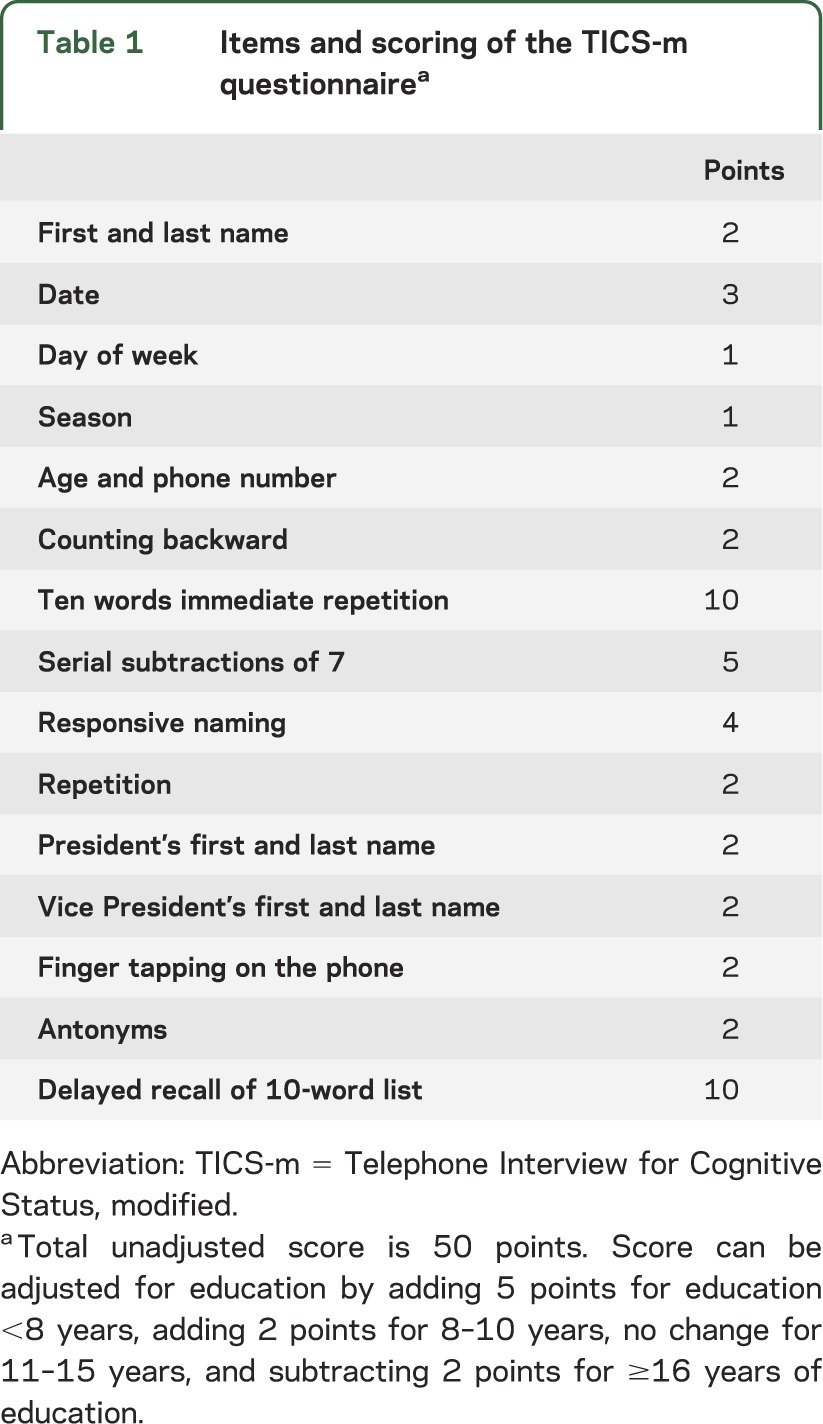
We reviewed the medical records and recorded the results of brain imaging, serum neuron-specific enolase (NSE) measurements, and EEGs. All brain imaging scans were interpreted by certified neuroradiologists, and the findings of atrophy and leukoaraiosis were confirmed by one of the authors (J.E.F.). All EEGs were read by certified electrophysiologists. A CPC score of ≤2 at hospital discharge was considered a favorable functional outcome. This scale consists of 5 categories with 1 indicating good recovery, 2 moderate disability, 3 severe disability, 4 vegetative state, and 5 death.
Statistical analyses.
Categorical variables are presented as counts and frequencies. Continuous variables are described with means or medians as appropriate, given the distribution of data.
Univariate comparisons between dichotomous subgroups were performed with χ2 or 2-sided Fisher exact test. For comparisons of continuous variables, we used t tests or the Mann-Whitney U test. Probability values <0.05 were considered statistically significant. We used JMP 9.0, a SAS-based statistical package (SAS Institute, Inc., Cary, NC), to analyze the data.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Mayo Foundation Institutional Review Board. Informed consent was obtained from all patients participating in the study.
RESULTS
A total of 133 comatose survivors of OHCA and cardiopulmonary resuscitation underwent the TH protocol within the study period. Of these, 77 (58%) were alive at a mean follow-up of 21 months (range 2–59 months). Eighteen patients were not able to be reached by telephone despite multiple attempts and 3 were not interviewed because they did not speak English. We contacted the remaining 56 patients (73% of those alive) by telephone to assess their cognitive status. The mean age at the time of assessment was 64 years (range 24–88 years) and 37 (66%) were male. Twenty-one patients (37.5%) had received outpatient medical care at Mayo Clinic before their hospitalization for cardiac arrest. Only one patient had a premorbid history of mild cognitive impairment. None had a documented history of dementia.
Initial hospital course.
The first documented rhythm was ventricular fibrillation in 52 patients (93%). The median time from collapse to ROSC was 20 minutes (range 5–50 minutes). The cardiac arrest was witnessed in 52 cases (93%). Brain imaging was obtained during the hospitalization for 39 patients (70%). Thirty-four patients had CT and 11 had MRI. CT scans were obtained during the hypothermia protocol in 21 patients (62% of those with CT scans) and after rewarming in 13 (38%). Of the latter, the median time to imaging was 2 days (interquartile range [IQR] 2–2). Brain atrophy, as noted by the neuroradiologist, was present in 19 patients (49% of those imaged), and 11 patients (28% of those imaged) had leukoaraiosis. The background activity on all EEGs performed (n = 20, 36%) showed diffuse, nonspecific slowing. Two also had episodes of burst suppression during hypothermia. Thirteen of the EEGs were continuous recordings during the hypothermia period through rewarming and 7 were spot EEGs obtained after rewarming. Serum NSE was measured in 42 patients (75%) within the first 3 days after admission. Of these, the median maximum value was 24 ng/mL (range 11–95 ng/mL). Seven patients had a serum NSE level >33 ng/mL within the first 3 days (16.7% of those with measurements).
The median duration of postarrest coma was 2 days (range 1–5 days). All patients were intubated and mechanically ventilated on admission. The median duration of mechanical ventilation was 2 days (IQR 2–4 days). Mean APACHE (Acute Physiology, Age, Chronic Health Evaluation) III score was 101 (±33). Systemic complications included acute kidney injury (n = 18, 32%), sepsis (n = 3, 5%), and acute respiratory distress syndrome (n = 1, 2%). Forty-seven patients (84%) achieved a favorable functional outcome (CPC score ≤2) at the time of discharge from the acute hospitalization. Of the 9 patients with a CPC score ≥3 at discharge from the hospital, 8 (89%) recovered to a CPC score of 2 after a stay in our inpatient rehabilitation unit.
Follow-up assessment.
The median time to telephone interview from cardiac arrest was 19.5 months (IQR 14.3–24 months). At the time of the telephone assessment, 46 patients (82%) were living in a house, 6 (11%) in an assisted living facility, 5 (9%) in an apartment or townhome, and 2 (4%) in a retirement community. The majority (n = 40, 71%) were married, 7 (13%) had never been married, 5 (9%) were widowed, and 4 (7%) were divorced. Eighteen patients were not working at the time of their cardiac arrest (17 were retired and 1 was unemployed). Of the remaining 38 patients who were working up to the time of the cardiac arrest, 30 (79%) had returned to work, 5 (13%) had not returned to work, and information was not provided for 3 patients (8%).
Of the 56 total patients, we were able to calculate a TICS-m score for 55 patients (98%). One patient was aphasic and his cognitive performance could not be accurately assessed. The education-adjusted TICS-m scores ranged from 16 to 41 points of 50 maximum possible points. Median score for immediate recall was 4 points (range 0–8) of a maximum of 10 points. Performance was the poorest on delayed recall, with a median score of 3 points (range 0–6) of a maximum of 10 points. Based on previously validated thresholds of the education-adjusted TICS-m, cognitive status was considered normal in the majority of patients (n = 33, 60%). Twenty-two patients (40%) were classified as having cognitive impairment. Ten had scores that were in the range consistent with dementia (≤27). The time to assessment was not significantly different between the cognitive outcome groups. We found no difference in education-adjusted TICS-m scores in patients evaluated at an earlier time point (within 1 year of arrest) and those evaluated at a later time point (≥1 year). Among patients who were evaluated within the first year, the mean TICS-m score was 31.7 ± 3.5, whereas among those evaluated ≥1 year after the cardiac arrest, the mean TICS-m score was 32.1 ± 5.4 (p = 0.694, Mann-Whitney U test).
Among patients with cognitive impairment (score <32 points on the TICS-m), 8 of 12 with information regarding work status had returned to work. Among those with scores of ≤27 on the TICS-m, 6 patients (60%) had achieved a CPC score at hospital discharge of ≤2. Although most patients who had a CPC score of 3 at hospital discharge (but a score of 2 after completion of rehabilitation) were ultimately classified as cognitively impaired, a fair proportion (44%) were cognitively normal at the follow-up assessment (figure).
Figure. Long-term cognitive status according to CPC score at hospital discharge.
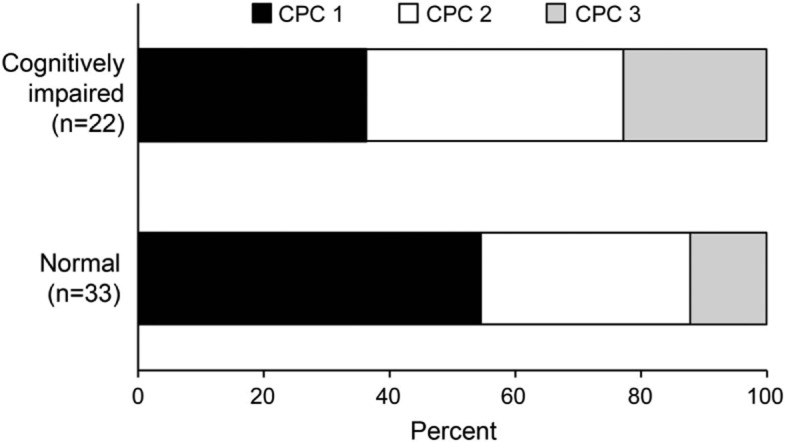
Distribution of cerebral performance category (CPC) score at hospital discharge according to cognitive outcome. Cognitive status was assessed using the modified Telephone Interview for Cognitive Status. An education-adjusted score of ≥32 points was considered normal.
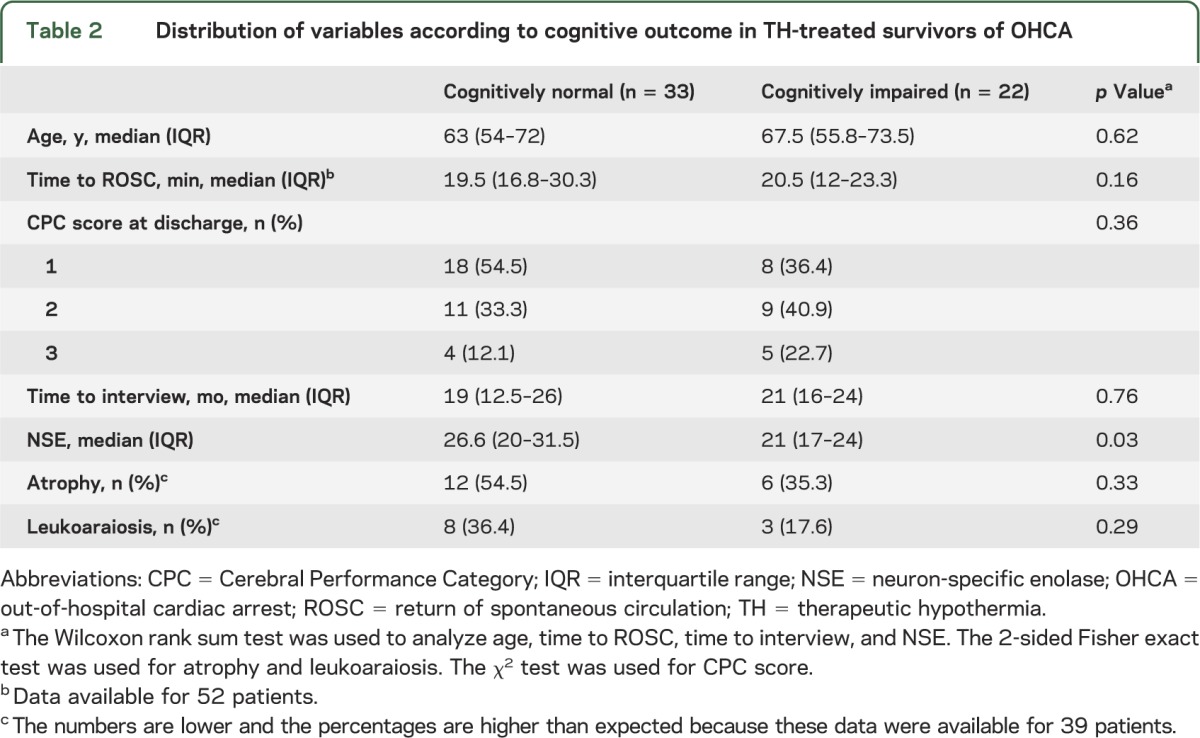
We did not find evidence to support an association between cognitive outcome and any of the following variables: patient age at time of cardiac arrest, time to ROSC, time to telephone interview, brain atrophy, or leukoaraiosis. NSE levels were higher on average in the patients who regained normal cognitive function compared with those who remained cognitively impaired, but lower than the commonly accepted prognostic threshold of 33 ng/dL in both groups (table 2).
Table 2.
Distribution of variables according to cognitive outcome in TH-treated survivors of OHCA
DISCUSSION
We found that the majority of patients who survived OHCA did not have cognitive impairment according to the results of the validated, structured TICS-m. Furthermore, nearly 80% of those we interviewed who had been working up until the time of their cardiac arrest were able to return to their work. These findings support the ongoing emphasis on early, high-quality resuscitation interventions, including TH, for patients with OHCA because a high proportion of those who are resuscitated and survive to hospital discharge can return to a meaningful, functional life. As observed by others,13,14 a long time to ROSC did not preclude a good outcome in our study.
Our results are consistent with those obtained in a recent population-based study of long-term cognitive outcomes of OHCA survivors, in which 85% of survivors regained functional independence.13 Notably, however, only 3 patients in that series were treated with TH. Our results are also in line with a study that included 27 patients treated with TH and found that 67% of the TH-treated patients had normal cognition or very mild deficits.22 Two small series found that a larger proportion (approximately 50%) of TH-treated cardiac arrest survivors had cognitive impairment.23,24 One study of 43 patients found that 48% had cognitive impairment, half of whom had “mild” deficits.23 The Neurobehavioral Cognitive Status Examination (Cognistat) was used for the cognitive screening test. Still, the majority of patients in that study were functional with good quality of life, and 42% had returned to work.23
Another study included 26 patients who had been treated with TH, only evaluating those with a CPC score of 1 or 2, and found that 52% had cognitive dysfunction.24 Three prospective studies of patients who were not treated with TH found cognitive dysfunction in 42% to 50% of survivors.12,14,15 Our study cannot define the impact of TH on cognitive outcomes, but it shows that the majority of survivors regain good cognitive function. Studies using different durations of TH, different methods of cooling, and different supportive protocols may find different incidences of cognitive impairment, but families of patients treated with current 24 hours of moderate TH can be told that if the patient awakens, he or she has a substantial chance of recovering with good cognitive function.
The most common cognitive domain affected in our series was delayed recall. Most studies have similarly found that the most common cognitive domains affected after survival of cardiac arrest are memory and executive function.12,13,15,22,24 In a systematic review (largely of patients not treated with TH), memory problems, followed by attention and executive dysfunction, were frequently encountered.16 Clinicians who care for patients surviving OHCA should be aware of the possibility of cognitive deficits, even when neurologic outcome appears favorable in the acute phase. A good outcome according to CPC score at hospital discharge in our series did not correlate perfectly with cognitive outcome when assessed several months later. However, it is notable that although 16% of patients in our series had a CPC score of 3 upon discharge from the acute hospitalization, nearly all of them (8 of 9) recovered to a score of 2 after intensive rehabilitation efforts.
One limitation of this study is that the time to the telephone assessment was not uniform among the patients we interviewed. It is possible that cognitive abilities improve during the years after a cardiac arrest. Nevertheless, there was no difference in the time to assessment among our 2 cognitive outcome groups and we do not think this contributed in a major way to the results of our study. Our results could be biased if the patients who were lost to follow-up were more cognitively impaired than the ones who were interviewed. Most of the patients who were not interviewed achieved a good functional outcome at hospital discharge (median CPC score of 1), leading us to believe this is a less likely possibility, but one that cannot be entirely excluded. In addition, based on the short duration of postarrest coma and lack of malignant EEGs in these patients, our results may not be generalizable to a population of patients with more severe brain anoxia.
The relatively small sample size may preclude our ability to detect statistically significant associations (type II error) between clinical characteristics and cognitive outcomes. The difference in average NSE levels observed in our analysis is unlikely to be clinically meaningful. Finally, the TICS-m, better used as a screening tool and not a stand-alone diagnostic evaluation, is not a substitute for an extensive, formalized, in-person neuropsychometric assessment. The majority of our patients did not have formal neuropsychological testing performed and we cannot completely exclude the possibility that some of the patients who were classified as cognitively normal in our study may have had subtle impairments found on more extensive neuropsychometric testing. However, it has been shown that 95% of patients older than 80 years who score >28 on TICS-m are cognitively normal,25 and the TICS-m performs reasonably well when dichotomized to separate those with normal cognition from those with impaired cognition. In our current study, only 4 patients who were classified as cognitively normal had formal neuropsychological testing performed after the cardiac arrest. Of these, only one patient—who was tested immediately after the hospitalization—was found to have mild deficits in learning and memory retention. This patient was functioning normally in later follow-up. The other 3 had no evidence of cognitive impairment in the measured domains.
Based on the results of this study, the majority of patients treated with TH after undergoing an OHCA and who survive to hospital discharge can recover cognitive function reasonably well, and, if working at the time of the cardiac arrest, most can return to their previous jobs. These findings should be confirmed in larger prospective studies utilizing formal neuropsychometric assessments of TH-treated OHCA survivors.
GLOSSARY
- CPC
Cerebral Performance Category
- IQR
interquartile range
- NSE
neuron-specific enolase
- OHCA
out-of-hospital cardiac arrest
- ROSC
return of spontaneous circulation
- TH
therapeutic hypothermia
- TICS-m
Telephone Interview for Cognitive Status, modified
AUTHOR CONTRIBUTIONS
Dr. Fugate: drafting/revising the manuscript, acquisition of data, statistical analysis, analysis or interpretation of data. Dr. Moore: drafting/revising the manuscript, acquisition of data. Dr. Knopman: drafting/revising the manuscript, analysis or interpretation of data, study supervision. Dr. Claassen: study concept or design, drafting/revising the manuscript, acquisition of data. Dr. Wijdicks: drafting/revising the manuscript, analysis or interpretation of data, study supervision. Dr. White: drafting/revising the manuscript, study supervision. Dr. Rabinstein: study concept or design, drafting/revising the manuscript, analysis or interpretation of data, study supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
J.E. Fugate serves as a member of the Resident & Fellow Section Editorial Team for Neurology®, S.A. Moore reports no disclosures. D.S. Knopman serves as Deputy Editor for Neurology®; served on a Data Safety Monitoring Board for Lilly Pharmaceuticals; served as a consultant to TauRx, and was an investigator in clinical trials sponsored by Baxter, Elan Pharmaceuticals, and Forest Pharmaceuticals; and receives research support from the NIH. D.O. Claassen, E.F.M. Wijdicks, and R.D. White report no disclosures. A.A. Rabinstein serves as a member of the Editorial Board for Neurology®. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Reinikainen M, Oksanen T, Leppanen P, Torppa T, Niskanen M, Kurola J. Mortality in out-of-hospital cardiac arrest patients has decreased in the era of therapeutic hypothermia. Acta Anaesthesiol Scand 2012;56:110–115 [DOI] [PubMed] [Google Scholar]
- 2.van der Wal G, Brinkman S, Bisschops LL, et al. Influence of mild therapeutic hypothermia after cardiac arrest on hospital mortality. Crit Care Med 2011;39:84–88 [DOI] [PubMed] [Google Scholar]
- 3.Fugate JE, Brinjikji W, Mandrekar JN, et al. Post-cardiac arrest mortality is declining: a study of the US National Inpatient Sample 2001 to 2009. Circulation 2012;126:546–550 [DOI] [PubMed] [Google Scholar]
- 4.Endoh M, Maiese K, Wagner J. Expression of the inducible form of nitric oxide synthase by reactive astrocytes after transient global ischemia. Brain Res 1994;651:92–100 [DOI] [PubMed] [Google Scholar]
- 5.Busl KM, Greer DM. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation 2010;26:5–13 [DOI] [PubMed] [Google Scholar]
- 6.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–563 [DOI] [PubMed] [Google Scholar]
- 7.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–556 [DOI] [PubMed] [Google Scholar]
- 8.Bouwes A, Binnekade JM, Kuiper MA, et al. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Ann Neurol 2012;71:206–212 [DOI] [PubMed] [Google Scholar]
- 9.Fugate JE, Wijdicks EF, Mandrekar J, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol 2010;68:907–914 [DOI] [PubMed] [Google Scholar]
- 10.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol 2010;67:301–307 [DOI] [PubMed] [Google Scholar]
- 11.Samaniego EA, Mlynash M, Caulfield AF, Eyngorn I, Wijman CA. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care 2011;15:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roine RO, Kajaste S, Kaste M. Neuropsychological sequelae of cardiac arrest. JAMA 1993;269:237–242 [PubMed] [Google Scholar]
- 13.Mateen FJ, Josephs KA, Trenerry MR, et al. Long-term cognitive outcomes following out-of-hospital cardiac arrest: a population-based study. Neurology 2011;77:1438–1445 [DOI] [PubMed] [Google Scholar]
- 14.van Alem AP, de Vos R, Schmand B, Koster RW. Cognitive impairment in survivors of out-of-hospital cardiac arrest. Am Heart J 2004;148:416–421 [DOI] [PubMed] [Google Scholar]
- 15.Sauve MJ, Doolittle N, Walker JA, Paul SM, Scheinman MM. Factors associated with cognitive recovery after cardiopulmonary resuscitation. Am J Crit Care 1996;5:127–139 [PubMed] [Google Scholar]
- 16.Moulaert VR, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation 2009;80:297–305 [DOI] [PubMed] [Google Scholar]
- 17.Fugate JE, Wijdicks EF, White RD, Rabinstein AA. Does therapeutic hypothermia affect time to awakening in cardiac arrest survivors? Neurology 2011;77:1346–1350 [DOI] [PubMed] [Google Scholar]
- 18.Crooks VC, Clark L, Petitti DB, Chui H, Chiu V. Validation of multi-stage telephone-based identification of cognitive impairment and dementia. BMC Neurol 2005;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knopman DS, Roberts RO, Geda YE, et al. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology 2010;34:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry Neuropsychol Behav Neurol 1993;6:103–110 [Google Scholar]
- 21.Gallo JJ, Breitner JC. Alzheimer's disease in the NAS-NRC Registry of aging twin veterans. IV. Performance characteristics of a two-stage telephone screening procedure for Alzheimer's dementia. Psychol Med 1995;25:1211–1219 [DOI] [PubMed] [Google Scholar]
- 22.Tiainen M, Poutiainen E, Kovala T, Takkunen O, Happola O, Roine RO. Cognitive and neurophysiological outcome of cardiac arrest survivors treated with therapeutic hypothermia. Stroke 2007;38:2303–2308 [DOI] [PubMed] [Google Scholar]
- 23.Cronberg T, Lilja G, Rundgren M, Friberg H, Widner H. Long-term neurological outcome after cardiac arrest and therapeutic hypothermia. Resuscitation 2009;80:1119–1123 [DOI] [PubMed] [Google Scholar]
- 24.Torgersen J, Strand K, Bjelland TW, et al. Cognitive dysfunction and health-related quality of life after a cardiac arrest and therapeutic hypothermia. Acta Anaesthesiol Scand 2010;54:721–728 [DOI] [PubMed] [Google Scholar]
- 25.Graff-Radford NR, Ferman TJ, Lucas JA, et al. A cost effective method of identifying and recruiting persons over 80 free of dementia or mild cognitive impairment. Alzheimer Dis Assoc Disord 2006;20:101–104 [DOI] [PubMed] [Google Scholar]


