Abstract
Purpose
To review the features and prognosis of uveal melanoma in children.
Methods
Retrospective case series.
Results
Of 122 children with uveal melanoma, there were 53 (43%) male and 69 (57%) female patients. In this group, the mean age at presentation was 15 years (median 16 years, range 3–20 years). Age at presentation was 0 to 5 years in 4 (3%), 5.1 to 10 years in 14 (11%), 10.1 to 15 years in 43 (35%), and 15.1 to ⩽20 in 61 (50%). Associated ocular melanocytosis was present in 4 (3%). The melanoma was primarily located in the iris (n = 30, 25%), ciliary body (n = 10, 8%), or choroid (n = 82, 67%). The mean tumor basal dimension was 9.8 mm and mean thickness was 5.0 mm. The tumor color was pigmented (brown) (n = 102, 84%), nonpigmented (yellow) (n = 19, 16%), or mixed (n = 25, 21%). Subretinal fluid (n = 66, 54%) and hemorrhage (n = 9, 7%) were noted. Primary treatment involved laser photocoagulation (n = 3, 2%), transpupillary thermotherapy (n = 17, 14%), local tumor resection (n = 26, 21%), plaque radiotherapy (n = 42, 34%), or enucleation (n = 54, 44%). Kaplan Meier 5, 10, and 20-year estimates for uveal melanoma-related metastasis were 9%, 9%, and 20%, respectively, for children compared to 15%, 25%, and 36% for all ages.
Conclusion
Uveal melanoma in children tends to occur most often in the teenage years as a pigmented tumor involving the choroid or iris and with mean thickness of 5 mm. Prompt treatment is advised.
Keywords: Eye, Melanoma, Children, Pediatric, Uvea, Iris, Ciliary body, Choroid
Introduction
Uveal melanoma presents at an average age of 58 years and is rare in children.1,2 Based on a large series of 8033 patients with uveal melanoma, only 1% occurred at the age of 20 years or younger.1,3 According to the database of 2493 cases of uveal melanoma from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER), uveal melanoma at all ages was more common in males (4.9 per million) compared to females (3.7 per million). The mean age at presentation was 60 years, ranging from 6 years to 100 years.4 The age-specific incidence of uveal melanoma per million population for 10–14 year old persons was 0.2 for males and 0 for females, compared to 20–24 years at 0.4 for males and 0.6 for females, and this peaked at 70–74 years at 24.5 for males and 17.8 for females.4
In this report, we specifically focus on a large cohort of uveal melanoma in children and teenagers of age 20 years or younger. We will review tumor clinical features, treatments and outcomes. A review of the literature will be provided.
Methods
This retrospective, nonrandomized, interventional case series included all patients classified as children of age 20 years or younger and with a clinical diagnosis of uveal melanoma managed at the Ocular Oncology Service, Wills Eye Hospital, Thomas Jefferson University between August 25, 1970, and August 27, 2008. Institutional review board approval was obtained for this study.
The following data were extracted from the medical records including patient age at diagnosis (years), gender, and race. Each child was evaluated for unequivocal ocular or oculodermal melanocytosis involving eyelid, temporal fossa, palate, sclera, iris, ciliary body, choroid, and orbit (when possible). Additional data included intraocular pressure (mmHg), location of tumor epicenter (iris, ciliary body, choroid), largest tumor basal dimension and thickness (mm), tumor configuration (dome, mushroom, tapioca, plateau), color (pigmented, non-pigmented), subretinal fluid, intraocular hemorrhage, and extraocular extension. Tumor thickness was measured by B-scan ocular ultrasonography. All findings were documented with a large color-coded drawing, anterior segment and posterior segment photography, fluorescein angiography when appropriate, anterior and posterior segment optical coherence tomography when available, ultrasound biomicroscopy (for anterior segment tumors) and ultrasonography (for posterior segment tumors).
The proposed treatment was discussed with the patient and family and risks and benefits were explained. A signed informed consent was obtained. Systemic monitoring and screening for metastasis were performed by a medical physician or oncologist with twice-yearly physical examination and liver function tests (lactate dehydrogenase, alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase), once-yearly liver imaging (magnetic resonance imaging, computed tomography, or ultrasonography), and chest imaging (chest radiograph or computed tomography). The date and interval to systemic metastasis were recorded.
Statistical analysis
The clinical features and outcomes (tumor-related metastasis) were then statistically analyzed. Kaplan–Meier analysis was performed to estimate the cumulative probability of tumor-related metastasis at 3, 5, 10 and 20 years.
Results
Of a comprehensive database on 8101 patients with uveal melanoma managed on the Oncology Service, Wills Eye Hospital during this 38-year period, 122 (1.5%) were young patients of age 20 years or less. In this group, the mean age at presentation was 15 years (median 16 years, range 3–20 years). The youngest patient, age 3 years, demonstrated a small choroidal melanoma and the next youngest, a 4 year old had a large, pigmented iris melanoma with angle seeding and secondary glaucoma. Melanoma by year is listed in Table 1. Of the 122 pediatric patients with melanoma, age was 0 to 5 years in 4 (3%), 5.1 to 10 years in 14 (11%), 10.1 to 15 years in 43 (35%), and 15.1 to ⩽20 in 61 (50%). Alternatively, the tumor occurred in children of preteen years (0 to13 years) in 41 (34%) and in those of teenage years (13.1 to ⩽20 years) in 81 (66%).
Table 1.
Uveal melanoma in 122 children: Age at presentation by year.
| Age (years) | Number (%) |
|---|---|
| 0–1 | 0 (0) |
| 1.1–2 | 0 (0) |
| 2.1–3 | 1 (1) |
| 3.1–4 | 1 (1) |
| 4.1–5 | 2 (2) |
| 5.1–6 | 3 (2) |
| 6.1–7 | 0 (0) |
| 7.1–8 | 4 (3) |
| 8.1–9 | 2 (2) |
| 9.1–10 | 5 (4) |
| 10.1–11 | 1 (1) |
| 11.1–12 | 5 (4) |
| 12.1–13 | 17 (14) |
| 13.1–14 | 9 (7) |
| 14.1–15 | 11 (9) |
| 15.1–16 | 10 (8) |
| 16.1–17 | 9 (7) |
| 17.1–18 | 6 (5) |
| 18.1–19 | 13 (11) |
| 19.1–20 | 23 (19) |
| Total | 122 (100) |
The patient race was Caucasian (n = 118, 97%), Hispanic (n = 3, 2%), or Middle Eastern (n = 1, 1%). There were 53 (43%) male patients and 69 (57%) female patients. The tumor involved the right eye (n = 54, 44%) or the left eye (n = 68, 56%).
The melanoma features are listed in Table 2. The melanoma was primarily located in the iris (n = 30, 25%), ciliary body (n = 10, 8%), or choroid (n = 82, 67%). (Figs. 1–3) The mean tumor basal dimension was 9.8 mm and mean thickness was 5.0 mm. The tumor color was pigmented (brown) (n = 102, 84%), nonpigmented (yellow) (n = 19, 16%), or mixed (n = 25, 21%). Associated subretinal fluid was present in 66 (54%) cases and hemorrhage in 9 (7%).
Table 2.
Uveal melanoma in 122 children: Clinical features.
| Feature | Number (%) |
|---|---|
| Location (n = 122) | |
| Iris | 30 (25) |
| Ciliary body | 10 (8) |
| Choroid | 82 (67) |
| Tumor quadrant (n = 122) | |
| Superior | 24 (20) |
| Nasal | 26 (21) |
| Inferior | 42 (34) |
| Temporal | 23 (19) |
| Diffuse | 4 (3) |
| Macula | 3 (2) |
| Distance to foveola (mm), mean (median) [range] | 8.6 (4.5), [0 to 25) |
| Distance to optic disk (mm), mean (median) [range] | 8.5 (4.5), [0 to 25] |
| Tumor base (mm) mean (median) [range] | 9.8 (9.0), [2.5 to 24] |
| Tumor thickness (mm) mean (median) [range] | 5.0 (3.7), [0.5 to 14] |
| Tumor configuration (n = 121) | |
| Plateau | 12 (10) |
| Dome | 88 (73) |
| Mushroom | 16 (13) |
| Tapioca | 5 (4) |
| Color (n = 121) | |
| Pigmented | 102 (84) |
| Non-pigmented | 19 (16) |
| Related features (n = 122) | |
| Subretinal fluid | 66 (54) |
| Intraocular hemorrhage | 9 (7) |
| Rupture of Bruch’s membrane | 16 (13) |
| Extraocular extension | 1 (1) |
Figure 1.

Iris melanoma in a 6 year-old child that showed evidence of growth from 2007 (A) to 2011 (B), at which time secondary glaucoma and extensive angle seeding were detected. Following plaque radiotherapy the tumor regressed (C) and the glaucoma was controlled with topical therapy. There was no metastasis on 6 year follow up.
Figure 2.
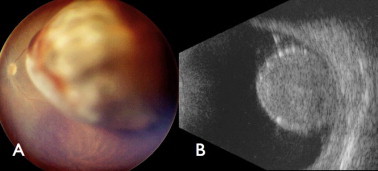
Choroidal melanoma in a 19 year-old girl appearing as a lightly pigmented mass (A) and measuring 11 mm thickness (B). The tumor responded following plaque radiotherapy. Liver metastases were detected 2 years later.
Figure 3.
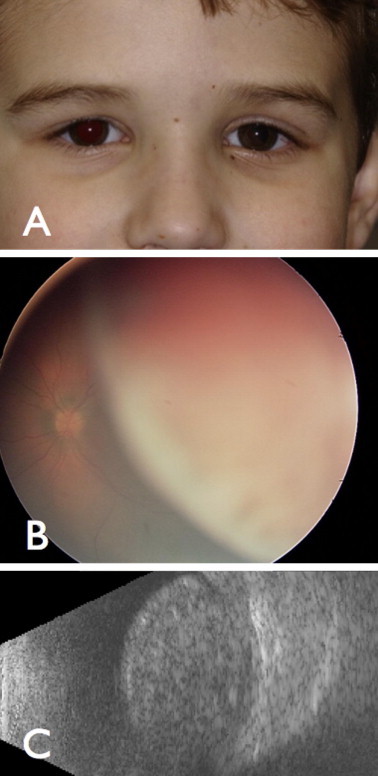
Ciliochoroidal melanoma in a 5 year-old boy (A) appearing as a nonpigmented (B) mass with thickness of 10 mm (C) and managed with enucleation. There was no metastasis on 7 year follow up.
Presence and location of ocular melanocytosis is listed in Table 3. Melanocytosis was present in 4 patients (3%) and involved the sclera (n = 2, 2%), iris (n = 1, 1%), and/or choroid (n = 2, 2%). Primary treatment involved laser photocoagulation (n = 3, 2%), transpupillary thermotherapy (n = 17, 14%), local tumor resection (n = 26, 21%), plaque radiotherapy (n = 42, 34%), or enucleation (n = 54, 44%).
Table 3.
Uveal melanoma in 122 children: Related ocular or oculodermal melanocytosis.
| Ocular/Oculodermal melanocytosis | Number (%) |
|---|---|
| Melanocytosis (n = 122 patients) | |
| Ocular/Oculodermal | 4 (3) |
| None | 118 (97) |
| Site of melanocytosis (n = 122 patients)⁎ | |
| Eyelid | 0 (0) |
| Temporal fossa | 0 (0) |
| Palate | 0 (0) |
| Sclera | 2 (2) |
| Iris | 1 (1) |
| Choroid | 2 (2) |
| Orbit | 0 (0) |
Some may have more than one site.
Kaplan Meier estimates for melanoma related metastasis based on age (children versus all ages) are listed in Table 4. Overall, metastasis at 10 years was 8.8% for children versus 25% for all ages and metastasis at 20 years was 20.2% for children and 36% for all ages. Of the 4 in this series with melanoma and melanocytosis, there were no deaths by mean 3 years follow up (range <1–7 years).
Table 4.
Uveal melanoma-related metastasis using Kaplan–Meier estimates.
| Metastasis | % at 5 years ± SE |
% at 10 years ± SE |
% at 20 years ± SE |
|||
|---|---|---|---|---|---|---|
| Children ⩽ 20 years | All patients⁎, all ages | Children ⩽ 20 years | All patients⁎, all ages | Children ⩽ 20 years | All patients⁎, all ages | |
| Iris melanoma | 9.1 ± 8.7 | 4.1 + 1.8 | – | 6.9 ± 2.7 | – | 9.5 ± 3.6 |
| Ciliary body melanoma | 0 | 19.0 ± 2.5 | 0 | 33.6 ± 4.1 | 0 | 55.3 ± 11.4 |
| Choroid melanoma | 9.4 ± 5.3 | 15.2 ± 0.6 | 9.4 ± 5.3 | 25.1 ± 0.9 | 20.7 ± 11.6 | 36.2 ± 1.7 |
| All sites uveal melanoma | 8.8 ± 4.3 | 15.0 ± 0.5 | 8.8 ± 4.3 | 25.0 ± 0.8 | 20.2 ± 11.3 | 36.0 ± 1.7 |
SE = standard error.
Compared to published data on 8033 patients with uveal melanoma.1
Discussion
In 1962, Apt reported on uveal melanoma in children from the Registry of Ophthalmic Pathology of the Armed Forces Institute of Pathology (AFIP) and identified 46 cases.5 He commented that nearly all patients were Caucasian and most cases occurred during the mid to late teenage years. He speculated that this acceleration was related to [unproven] hormonal influence in the development of melanoma. One important observation was the relative number of tumors in the iris, representing 19 (41%) of the 46 cases. Unfortunately, the cohort was not purely melanoma in that 8 (42%) of the 19 iris melanomas and 4 (15%) of the 27 posterior uveal melanomas proved to be benign spindle A nevus and not melanoma.
In 1981, Barr et al. revisited the files of the AFIP and found 78 cases of pediatric uveal melanoma.6 They identified 5, 10, and 15-year mortality for pediatric iris melanoma at 8%, 8%, and 13% and for pediatric choroid/ciliary body melanoma at 21%, 21%, and 25%. For iris melanoma, risks for death included glaucoma, posterior invasion into ciliary body/choroid, diffuse growth pattern, angle involvement, scleral invasion, necrosis, epithelioid cells, and high mitotic activity. For choroidal melanoma, factors related to death included painful red eye, extraocular extension, basal tumor diameter ⩾10 mm, tumor thickness >2 mm, tumor necrosis, high mitotic activity, Bruch’s membrane invasion, and iris/ciliary body invasion. There were 7 cases of erroneous clinical diagnosis with unsuspected melanoma before enucleation in that series.6 In fact, 4 patients had scleral buckle for retinal detachment and 2 had filtration surgery for glaucoma before the diagnosis of melanoma was established, with 3 of these 6 patients succumbing to metastatic disease. They concluded that uveal melanoma in children should be promptly recognized, avoiding misdirected therapy, and managed as aggressively as those in adults.
The previous reports emanated retrospectively from a tertiary pathology laboratory with little clinical detail.5,6 In 1965, Verdaguer et al. reported a clinical series of 17 children with uveal melanoma and noted no metastatic event over median 5-year follow up.7 In 1991, Shields et al. described a comprehensive clinical series of 40 children with uveal melanoma.8 They stated that this represented 1% of the 3706 consecutive patients managed with uveal melanoma in their tertiary clinical ocular oncology center. There was no previous misdiagnosis in this series and misdirected therapy was avoided. Overall cumulative survival was excellent with only 4% metastasis at 5 years. They stated that this early survival was encouraging but longer follow up was necessary to understand the true impact of uveal melanoma in children. Part of the favorable survival was likely related to the detection of the melanoma when small, avoidance of misdirected therapy, and management at an experienced center for uveal melanoma. Later, in 2000, an updated clinical database from this department by Singh et al. added 23 more patients for a total of 63 pediatric patients with uveal melanoma.9 They observed that oculo(dermal) melanocytosis was 9 times more common in this cohort than expected in the general population. An additional observation was that the early favorable prognosis appeared to normalize at the 15-year point, more similar to adults.
In 2012, Shields et al. reviewed 8033 cases of uveal melanoma to explore clinical differences in tumor based on age at presentation.1 Several significant findings (p < 0.05) identified children (compared to adults older than 60 years) as more likely to show iris location, smaller tumor size, further from foveola and optic disk, lack of pigmentation, and less subretinal fluid and extrascleral extension. Regarding metastasis and death, children and young adults combined showed more favorable prognosis than older adults (>60 years). In that analysis, specific metastasis per decade revealed 10-year rates for children 0–10 years old at 0% and 11–20 years old at 10% compared to adults 61–70 years old at 30%.1
To more clearly understand the prognosis of children versus adults with uveal melanoma, Kaliki et al. provided a matched retrospective cohort study on children (⩽20 years) versus mid-adults (21–60 years) versus older adults (>60 years).10 The 3 groups were matched for gender, tumor location, location of anterior margin of tumor, tumor basal diameter, tumor thickness, and extraocular extension. Kaplan–Meier estimates of melanoma metastasis at 3, 5, and 10 years were 1%, 8%, and 8% in children; 8%, 11%, and 26% in mid-adults; and 13%, 16%, and 24% in older adults. After exclusion of iris melanoma, melanoma metastasis at 3, 5, and 10 years was 2%, 11%, and 18% in children; 9%, 14%, and 21% in mid-adults; and 9%, 34%, and 33% in older adults. After adjusting for tumor diameter, the metastatic rate was found to be lower in children compared to mid-adults (0 = 0.042, HR 3.00) and older adults (p = 0.007, HR 4.20).
In our present cohort of 122 children with uveal melanoma evaluated herein, we found specific age at presentation was 0 to 5 years in 4 (3%), 5.1 to 10 years in 14 (11%), 10.1 to 15 years in 43 (35%), and 15.1 to ⩽20 in 61 (50%). Ocular melanocytosis was present in 4 (3%). The mean tumor basal dimension was 9.8 mm, 5.0 mm in thickness, and pigmented in 102 cases (84%). The tumor was located in the iris (n = 30, 25%), ciliary body (n = 10, 8%), or choroid (n = 82, 67%). The patients in this report have been included in previous smaller studies8,9 or a matched cohort study,10 with the purpose of this investigation to detail precise clinical features.
Ocular melanocytosis is a known precursor to uveal melanoma.11–13 This condition was noted in 3% of 7872 patients with uveal melanoma.13 In this analysis of children with uveal melanoma, melanocytosis was present in 3%. Based on published data, patients with uveal melanoma and ocular melanocytosis have double the risk for metastasis compared to those without melanocytosis.13 Of the 4 in this pediatric series with melanoma and melanocytosis, there were no deaths on relatively short mean 3-year follow up (range <1–7 years).
Genetic markers have become important in the prognostication of uveal melanoma.14–19 Evidence confirms that chromosomal mutations in 3, 6, and 8 or gene expression abnormalities can lead to an increased risk for melanoma metastasis.14,15,18,19 Further information on mutations in BAP1, GNAQ, and GNA11 could be major events leading to melanoma development or metastatic events.16,17 Levasseur et al. identified GNAQ mutation in an 18 month-old child with congenital uveal melanoma.20 Van Raamsdonk et al. found that 83% of uveal melanomas show somatic mutation in either GNAQ or GNA11, involved in the mitogen-activated protein kinase pathway.16 This could represent one of the earliest mutations in the ultimate development of melanoma.
In conclusion, uveal melanoma in children tends to be smaller and more often in the iris compared to older adults. Similar to adults, ocular melanocytosis can be a precursor condition. Our understanding of the prognosis of melanoma in this subset of patients has evolved over 50 years. It remains important for all patients, whether children or adults, to achieve early detection of melanoma when the tumor is small to minimize the risk for metastasis. Children with melanoma show slightly better systemic prognosis when matched to older adults. The reason for better prognosis in young age is unknown but could be partly due to smaller tumor, more robust immune system, and speculated fewer genetic alterations.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgements
Statistical analysis provided by Rishita Nutheti, Ph.D., Hyderabad, India. Support provided by the Eye Tumor Research Foundation, Philadelphia, PA (CLS). The funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review or approval of the manuscript. Carol L. Shields, M.D. has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. There is no proprietary or financial disclosure interest from any author.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Shields C.L., Kaliki S., Furuta M. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina. 2012;32:1363–1372. doi: 10.1097/IAE.0b013e31824d09a8. [DOI] [PubMed] [Google Scholar]
- 2.Shields J.A., Shields C.L. 2nd ed. Lippincott Williams and Wilkins; Philadelphia: 2008. Intraocular tumors. An atlas and textbook. [pp. 85–139] [Google Scholar]
- 3.Shields C.L., Furuta M., Thangappan A. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989–998. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 4.Singh A.D., Topham A. Incidence of uveal melanoma in the United States:1973–1997. Ophthalmology. 2003;110:956–961. doi: 10.1016/S0161-6420(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 5.Apt L. Uveal melanomas in children and adolescents. Int Ophthalmol Clin. 1962;2:403–410. [Google Scholar]
- 6.Barr C.C., McLean I.W., Zimmerman L.E. Uveal melanoma in children and adolescents. Arch Ophthalmol. 1981;99:2133–2136. doi: 10.1001/archopht.1981.03930021009003. [DOI] [PubMed] [Google Scholar]
- 7.Verdaguer J. Prepubertal and pubertal melanomas in ophthalmology. Am J Ophthalmol. 1965;60:1002–1011. doi: 10.1016/0002-9394(65)92807-2. [DOI] [PubMed] [Google Scholar]
- 8.Shields C.L., Shields J.A., Milite J. Uveal melanoma in teenagers and children. A report of 40 cases. Ophthalmology. 1991;98:1662–1666. doi: 10.1016/s0161-6420(91)32071-2. [DOI] [PubMed] [Google Scholar]
- 9.Singh A.D., Shields C.L., Shields J.A., Sato T. Uveal melanoma in young patients. Arch Ophthalmol. 2000;118:918–923. [PubMed] [Google Scholar]
- 10.Kaliki S., Shields C.L., Ganesh A., Mashayekhi A., Shields J.A. Influence of age on young patients with uveal melanoma: a matched retrospective cohort study. Eur J Ophthalmol. 2013;43:208–216. doi: 10.5301/ejo.5000200. [DOI] [PubMed] [Google Scholar]
- 11.Singh A.D., De Potter P., Fijal B.A., Shields C.L., Shields J.A., Elston R.C. Lifetime prevalence of uveal melanoma in Caucasian patients with ocular (dermal) melanocytosis. Ophthalmology. 1998;105:195–198. doi: 10.1016/s0161-6420(98)92205-9. [DOI] [PubMed] [Google Scholar]
- 12.Shields C.L., Qureshi A., Mashayekhi A., Park C., Sinha N., Zolotarev F. Sector (partial) oculo(dermal) melanocytosis in 89 eyes. Ophthalmology. 2011;118:2474–2479. doi: 10.1016/j.ophtha.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Shields C.L., Kaliki S., Livesey M., Walker B., Garoon R., Bucci M. Association of ocular and oculodermal melanocytosis with rate of uveal melanoma metastasis. Analysis of 7872 consecutive eyes. JAMA Ophthalmology. May 16 2013 doi: 10.1001/jamaophthalmol.2013.129. doi:10.1001/jamaophthalmol.2013.129. [DOI] [PubMed] [Google Scholar]
- 14.Harbour J.W. Molecular prognostic testing and individualized patient care in uveal melanoma. Am J Ophthalmol. 2009;148:823–829. doi: 10.1016/j.ajo.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damato B., Dopierala J.A., Coupland S.E. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe. Clin Cancer Res. 2010;16:6083–6092. doi: 10.1158/1078-0432.CCR-10-2076. [DOI] [PubMed] [Google Scholar]
- 16.Van Raamsdonk C.D., Griewank K.G., Crosby M.B. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbour J.W., Onken M.D., Roberson E.D. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shields C.L., Ganguly A., Bianciotto C.G., Turaka K., Shields J.A. Prognosis of uveal melanoma in 500 cases using genetic testing of needle aspiration biopsy specimens. Ophthalmology. 2011;118:396–401. doi: 10.1016/j.ophtha.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Shields C.L., Ganguly A., O’Brien J. Uveal melanoma trapped in the Temple of Doom. Editorial Am J Ophthalmol. 2012;154:219–221. doi: 10.1016/j.ajo.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Levasseur S.D., Paton K.E., Van Raamsdonk C.D., Heran M.K.S., White V.A. Mutation of GNAQ in a cytologically unusual choroidal melanoma in an 18 month old child. JAMA Ophthalmol. April 9 2013 doi: 10.1001/jamaophthalmol.2013.2483. doi:10.1001/jamaophthalmol.2013.2483. [DOI] [PubMed] [Google Scholar]



