Abstract
Purpose
Secondary glaucoma can be induced by a variety of local ocular problems. Intraocular tumors may initially present as secondary glaucoma.
Methods
8 consecutive patients with secondary glaucoma were found to have uveal melanoma. Thorough examination included detailed history, fundus examination with scleral depression, B scan ultrasonography, and CT/MRI scanning techniques.
Results
A single case presented with spontaneous hyphema, two patients presented with secondary glaucoma, extraocular melanoma and metastases, a single case was found to have angle block by an iridociliary ring melanoma and 4 cases presented with neovascular glaucoma. Enucleation was necessary in all 8 cases.
Conclusions
General ophthalmologists should be aware of these rare initial manifestations of intraocular tumors as secondary glaucoma. Enucleation would be recommended in most cases of intraocular malignancy manifesting as secondary glaucoma. One should be extremely cautious in doing a penetrating surgery in such cases.
Keywords: Glaucoma, Melanoma, Neovascular glaucoma, Orbital melanoma
Introduction
Malignant melanoma of the uveal tract involves tumors of the iris, ciliary body and most commonly the choroid. Unusual presentation of posterior uveal melanoma includes vitreous hemorrhage,1,2 rhegmatogenous retinal detachment,3 RPE detachment,4 cystoid macular edema,4 choroidal detachment,5 ocular inflammation,6,7 and secondary glaucoma. Secondary glaucoma due to intraocular malignancy may be due to acute angle closure glaucoma,8 direct angle invasion by ring melanoma of ciliary body,9–11 neovascular glaucoma,12 seeding of anterior chamber angle by malignant cells or phagocytosed melanin pigments in melanomalytic glaucoma13,14 and recurrent attacks of ocular inflammation.6,7 In this report, we review eight consecutive cases of uveal melanoma that presented with secondary glaucoma.
Materials and methods
Eight consecutive cases of uveal melanoma presenting at the EyeWorld Hospital, Giza, Egypt were retrospectively reviewed. Cases presenting with high intraocular pressure were assessed with detailed history, ocular and systemic evaluation. Ocular examination included slit lamp and fundus examination, ultrasonography, CT or MRI studies when necessary. Systemic evaluation was done by an oncologist to rule out metastases. Histopathologic evaluation both grossly and microscopically was performed for the enucleated globes to confirm diagnosis. Furthermore, thorough tumor evaluation of cell type, mitotic activity, lymphocytic infiltrates, tumor necrosis, vascular pattern, tumor emboli, optic nerve invasion and degree of tumor extension to the surroundings was done. Institutional review board approval was not required as the study adheres with the tenets of the Declaration of Helsinki.
Results
Case 1
A 43 year-old male presented with one month history of spontaneous hyphema (Fig. 1A) with an IOP 40 mmHg and ciliary conjunctival injection OD. Vision was NLP OD. The patient was on topical antiglaucoma measures for 2 weeks. There was no history of trauma, bleeding tendency or anticoagulant use. Left eye examination was normal with 6/6 vision on Snellen’s acuity chart. Ultrasonography was promptly performed revealing a large nasal ciliochoroidal melanoma measuring 18 × 17 × 12 mm associated with an exudative retinal detachment and ciliochoroidal effusion (Fig. 1B). There was inflammation in the posterior subtenon’s space with positive T sign and myositis suspecting extraocular extension. MRI confirmed the presence of the ciliochoroidal melanoma with the presence of inflammatory signs around the globe and no extraocular extension. Enucleation was performed with the insertion of a hydroxyapatite implant. Histopathology revealed a large necrotic melanoma with shadow cells and epithelioid cells (Fig. 1C and D). The patient did well with no evidence of liver or lung metastases 18 months following enucleation.
Figure 1.
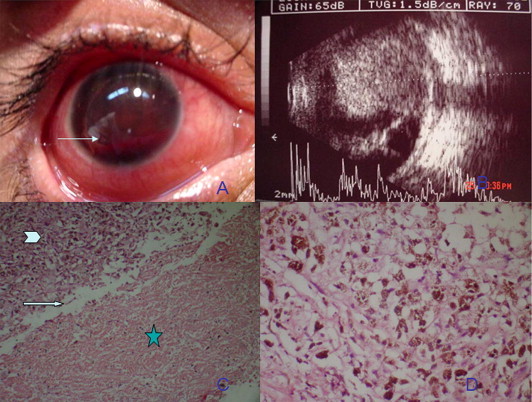
(A) Anterior segment photography of case 1 showing hyphema filling the anterior chamber with level (arrow). (B) A and B scan ultrasonography showing a large choroidal melanoma occupying the posterior pole with associated retinal detachment. (C) Photomicrograph of the melanoma showing ghost cells in the hemorrhagic component (asterisk), sharp demarcation between melanoma cells (arrowhead) and ghost cells (arrow) (hematoxylin and eosin × 100). (D) Photomicrograph of epithelioid melanoma cells containing melanin pigment (Hematoxylin and eosin × 500).
Case 2
A 55 year old female presented with a superior epibulbar mass OS for 3 months. Past surgical history was significant for subscleral trabeculectomy OS eight months prior. The right eye was normal with 6/6 vision and normal IOP. On examination, a brownish mulberry like epibulbar mass was projecting from the superotemporal limbus measuring 10 × 10 × 7 mm and partially encroaching on the cornea (Fig. 2A). Tumor base was partially infiltrating the surrounding sclera. Gonioscopy showed superior tumor cells infiltrating the angle. Ocular ultrasound revealed a diffuse ciliochoroidal mass with criteria of melanoma OS. The tumor was extensive and filling 1/3 of the globe, with no evidence of retrobulbar extension. There was no lymphadenopathy or other swellings in the body. Oncology evaluation including abdominal ultrasound, and chest X-ray was free. The patient was offered modified enucleation and this was carried out 6 weeks later due to patient hesitation. During this period, the epibulbar mass grew significantly reaching 15 × 15 × 10 mm in size and encroaching on most of the cornea (Fig. 2B and C). The globe and the conjunctiva surrounding the mass were en bloc excised. Histopathology revealed an epithelioid type melanoma with tumor extension along the site of trabeculectomy (Fig. 2D). The patient was lost to follow up and 8 months following surgery, she presented with liver metastases. She passed away 6 months later.
Figure 2.
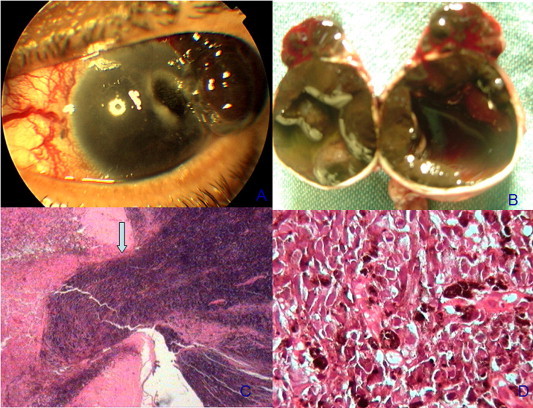
(A) Anterior segment photography of a case of melanoma with extraocular extension inducing an epibulbar and corneal gutter. (B) Gross picture of the open globe following enucleation showing a diffuse intraocular melanoma occupying the choroid and ciliary body and extending on the surface of the globe. (C) Photomicrograph showing exit of melanoma cells from the trabeculectomy site. (Hematoxylin and eosin × 200). (D) Photomicrograph showing epithelioid melanoma cells. (Hematoxylin and eosin × 400).
Case 3
A 57 year old gentleman presented on February 2004 with a blind painful right eye with proptosis for 2 years. His past medical history revealed the presence of a temporal posterior pole melanoma diagnosed in 1997, measuring at that time 14 × 14 × 7 mm by ultrasonography. On presentation, axial proptosis measuring 28 mm OD was noted with limitation of ocular motility in horizontal gaze. Ocular examination revealed dilated episcleral vessels, an intraocular pressure of 40 mmHg, neovascularization of the iris with peripheral anterior synechiae and a total cataract (Fig. 3A). Vision OD was NLP, and left ocular examination was normal with 6/6 vision. Gonioscopy revealed the presence of neovascularization on the iris and in the angle inducing peripheral anterior synechiae. A and B scan ultrasound revealed a dome shaped peripapillary choroidal melanoma with 6 mm thickness and massive extrascleral extension (Fig. 3B). There was no associated lymphadenopathy or other swellings in the body. CT scan of the orbit and brain revealed a soft tissue mass extending temporal to the globe OS, and infiltrating the lateral rectus muscle. The soft tissue mass shadow was contiguous with an intraocular soft tissue mass shadow (Fig. 3C). There was no bony erosion. Systemic evaluation revealed multiple nodules in the liver and lungs suggestive of melanoma metastases (Fig. 3D). The patient was suffering from severe pain OD, and a palliative lid sparing modified exenteration was performed. Despite being treated with orbital radiotherapy and systemic chemotherapy, the patient had an aggressive orbital recurrence and he passed away from metastases 6 months following the surgery.
Figure 3.
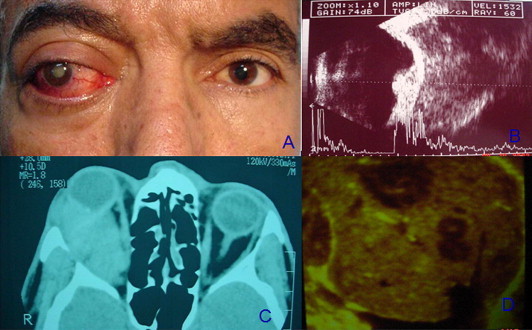
(A) Facial photograph showing right axial proptosis with congested episcleral vessels OD. (B) A and B ultrasound scans showing a dome shaped choroidal melanoma with massive extrascleral extension. (C) Axial CT scan showing an isodense vitreous OD with a minimally elevated intraocular mass with massive retrobulbar extension OD. (D) B scan of the liver showing multiple echolucent masses within liver parenchyma denoting metastases.
Case 4
A 55 year old lady presented with a localized epibulbar mass OD for 6 months. Ocular examination revealed visual acuity of 6/12. Slit lamp examination showed a superotemporal brownish subconjunctival epibulbar mass OD measuring 6 × 4 × 3 mm and encroaching slightly on the cornea. The mass was fixed to the globe (Fig. 4A). IOP was 40 mmHg and gonioscopy revealed heavy pigmentation of the angle by abnormal brown cells at 360 degrees of the angle. Ultrasound biomicroscopy revealed a ring shaped iridociliary soft tissue mass (Fig. 4B). The mass was infiltrating the angle and extending extraocularly. Ocular examination OS was normal with 6/6 vision and the patient was systemically healthy. A modified enucleation was performed with the insertion of a biointegrated medpor implant. Histopathology confirmed the presence of a mixed spindle and epithelioid cell melanoma of the ciliary body, iris and angle (Figs. 4C and D). The site of extraocular extension coincided with a perforating anterior ciliary channel. The patient was alive and well 10 months following surgery.
Figure 4.
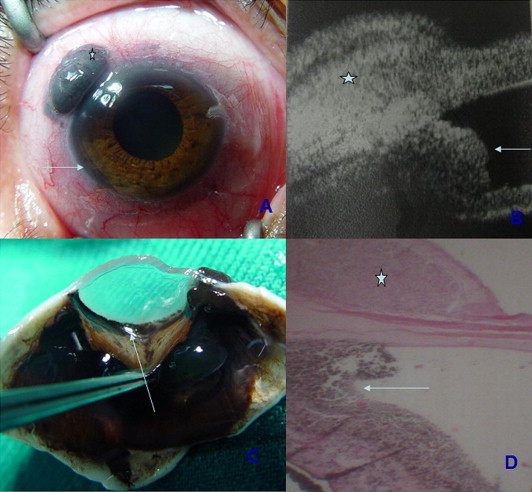
(A) Photograph of the right eye of case – showing an epibulbar mass OD (asterisk), with a ring angle infiltration (arrow). (B) Ultrasound biomicroscopy of the same eye clearly delineating the epibulbar melanoma (asterisk) and the angle infiltration (arrow). (C) Gross photograph of the dissected specimen showing diffuse angle infiltration by melanoma cells. (arrow). (D) Photomicrograph of the enucleated globe showing correlation between UBM and histopathology showing the epibulbar melanoma 9asterisk and angle infiltration (arrow). (Hematoxylin and eosin × 100).
Cases 5–8
A series of 4 patients presented with neovascular glaucoma secondary to uveal melanoma. Ages varied from 27 to 55 years. There were two males and two females. Case 7 has undergone cataract extraction OD with implant insertion one year prior to presentation with no improvement in vision. The patient developed worsening of visual acuity to LP with development of NVG. On examination a large ciliochoroidal melanoma measuring 18 × 16 × 12 mm was noticed temporally.
All patients were managed with enucleation with insertion of a biointegrated medpor implant. Patient’s demographic data, tumor criteria, management, histopathologic findings and follow up are described in Table.
Discussion
Uveal melanoma is a locally destructive disease in addition to its ability for distant metastases. Local tumor effects originate from compression of surrounding structures, direct invasion of ocular structures, associated exudative retinal detachment, tumor necrosis and hemorrhage. All local tumor effects can result in secondary glaucoma of varying pathogeneses. Direct angle invasion and spontaneous hyphema would result in trabecular outflow obstruction.11–13 Long standing exudative retinal detachment with the development of ischemia results in neovascular glaucoma.14,15 Tumor necrosis and hemorrhage result in outflow obstruction by melanomalytic and red blood cells,2,7,14 or might induce ciliary body engorgement with anterior displacement of iris/lens diaphragm inducing acute angle closure glaucoma.8
In our first case, the patient was a 44 year-old gentleman who presented with spontaneous hyphema, and glaucoma secondary to spontaneous choroidal melanoma necrosis. In previous reports,1,2 vitreous hemorrhage was a rare presentation of choroidal melanoma and was significantly associated with increased tumor thickness.2 Abi-Ayad et al. described a case of ring melanoma presenting with spontaneous hyphema.13 In our case, tumor thickness was 8 mm, and was invading Bruch’s membrane, with massive tumor necrosis. Histopathology showed epithelioid cells, with vitreous hemorrhage, hyphema, and red blood cells invading the angle of anterior chamber. A high index of suspicion with prompt ultrasound evaluation of the globe is indicated to avoid the risk of performing surgery on a globe harboring malignancy.
Cases 2 and 3 presented with extraocular melanoma and glaucoma. Case 2, was erroneously diagnosed with unilateral glaucoma and subscleral trabeculectomy was done elsewhere 8 months prior to presentation. A rapidly growing epibulbar mass was noticed 3 months following surgery. A modified enucleation with en bloc excision of the mass with the conjunctiva was successful in local tumor control. The tumor was predominantly epithelioid, with a basal diameter of 18 mm. However, the patient developed liver metastases, and passed away 14 months after exenteration. Singer et al.17 described a similar case scenario in a 61-year-old lady that was diagnosed with acute angle-closure glaucoma and despite prompt enucleation following Scheie filtration surgery, she developed a local orbital recurrence and fatal metastatic disease 30 months later. The authors concluded a possible causal relationship between the filtering surgery and the local and systemic recurrence of the disease.17 Besides iatrogenic openings in globe, ocular melanoma can spread outside the eye along natural openings in the sclera. Orbital extension was described along the short posterior ciliary vessels18,19 and along the long posterior ciliary nerve.21
Case 3 is a typical presentation of the natural history of choroidal melanoma. Six years after being diagnosed with choroidal melanoma, and declining treatment, a 57 year-old male presented with proptosis, cataract, and glaucoma. Imaging studies revealed a temporal orbital soft tissue mass in extension with the intraocular choroidal mass. Systemic evaluation revealed liver and lung metastases. A palliative exenteration revealed the presence of a mixed type, spindle and epithelioid cell melanoma. The patient passed away 6 months following exenteration. In a report about choroidal melanoma extending to the orbit, Rini et al.21 described four patients with choroidal melanoma extending to the orbit. All patients died of systemic dissemination. Interestingly enough, the patients who survived the longest had only a simple biopsy or no surgery done at all.21
Case 4 had an iridociliary melanoma who presented with localized extraocular extension, and 360° angle involvement with secondary glaucoma. Enucleation revealed direct angle invasion by malignant spindle cells. In a review of almost 9000 cases of uveal melanoma, Demirci et al.,11 recorded an incidence ring melanoma of anterior chamber angle to be 0.2%. All cases had secondary glaucoma and twelve out of thirteen cases were managed with enucleation. Histopathology revealed involvement of Schlemm’s canal in all cases. Metastases developed in 25% of cases at a mean follow up of 6 years.11 Ring melanoma was misdiagnosed in four cases of refractory glaucoma as angle recession, iridocorneal endothelial syndrome and melanocytoma of ciliary body.9 Because of the epibulbar mass and the gonioscopy performed, the diagnosis of ring melanoma was well established in our case. Ultrasound biomicroscopy beautifully demonstrated the ciliary body mass, the iris and angle involvement and the contiguity with the epibulbar mass through the sclera. Our patient did well with no evident metastases 18 months following enucleation.
Cases 5 to 8, all presented with neovascular glaucoma secondary to a large ciliochoroidal melanoma with a long standing exudative retinal detachment resulting in retinal ischemia. Ischemia triggers the release of factors that both inhibit and promote new vessel growth.23 Greater concentration in promoting factors results in neovascularization.24 Rubeosis iridis with secondary peripheral anterior synechiae and angle closure was present in all cases.
A recent report described the management of secondary glaucoma in eyes with initial presentation of uveal melanoma with cyclophtocoagulation.25 The target of the intervention was to preserve vision and relieve pain. The cohort included 27 patients, of them 14 cases (52%) died during follow up reflecting an advanced disease, 4 eyes were enucleated and 15 (65%) out of 23 preserved eyes had a visual acuity of NLP. Cyclophotocoagulation can be a useful palliative way in pain management in advanced intraocular melanoma.
In conclusion, uveal melanoma can mimic any form of secondary glaucoma, which is the presenting feature in only 3% of cases.12 Glaucoma was found to be an independent bad prognostic factor on multivariate analysis, perhaps related to delay in diagnosis and/or mismanagment.21 Despite being a rare presentation, it is important to exclude clinically and by thorough investigations intraocular malignancy before taking any decision for filtering surgery while dealing with a potentially fatal disease.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Cunliffe I.A., Rennie I.G. Choroidal melanoma presenting as vitreous hemorrhage. Eye. 1993;7:711–713. doi: 10.1038/eye.1993.162. [DOI] [PubMed] [Google Scholar]
- 2.Gailloud C., Zografos L., Uffer S. Uveal melanomas and vitreous hemorrhage. Diagnosis and treatment. Klin Monatsbl Augenheilkd. 1991;198:165–170. doi: 10.1055/s-2008-1045982. [DOI] [PubMed] [Google Scholar]
- 3.Haimovici R., Mukai S., Schachat A.P. Rhegmatogenous retinal detachment in eyes with uveal melanoma. Retina. 1996;16:488–496. doi: 10.1097/00006982-199616060-00004. [DOI] [PubMed] [Google Scholar]
- 4.Damato B.E., Foulds W.S. Tumor-associated retinal pigment epitheliopathy. Eye. 1990:382–387. doi: 10.1038/eye.1990.51. [DOI] [PubMed] [Google Scholar]
- 5.Sneed S.R., Byrne S.F., Mieler W.F. Choroidal detachment associated with malignant choroidal tumors. Ophthalmology. 1991;98:963–970. doi: 10.1016/s0161-6420(91)32195-x. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen Q.D., Foster C.S. Ciliary body melanoma masquerading as chronic uveitis. Ocular Immunol Inflamm. 1998;6:253–256. doi: 10.1076/ocii.6.4.253.4031. [DOI] [PubMed] [Google Scholar]
- 7.Fraser D.J., Jr., Font R.L. Ocular inflammation and hemorrhage as initial manifestations of uveal malignant melanoma. Incidence and prognosis. Arch Ophthalmol. 1979;97:1311–1314. doi: 10.1001/archopht.1979.01020020053012. [DOI] [PubMed] [Google Scholar]
- 8.Escalona-Benz E., Benz M.S., Briggs J.W., Budenz D.L., Parrish R.K., Murray T.G. Uveal melanoma presenting as acute angle-closure glaucoma: report of two cases. Am J Ophthalmol. 2003;136:756–758. doi: 10.1016/s0002-9394(03)00396-9. [DOI] [PubMed] [Google Scholar]
- 9.Lee V., Cree I.A., Hungerford J.L. Ring melanoma-a rare cause of refractory glaucoma. Br J Ophthalmol. 1999;83:194–198. doi: 10.1136/bjo.83.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ardjomand N., Eckhardt M., Langmann G., Faulborn J. Malignant melanoma of the ciliary body. A diagnostic problem. Ophthalmologe. 2001;98:406–408. doi: 10.1007/s003470170150. [DOI] [PubMed] [Google Scholar]
- 11.Demirci H., Shields C.L., Shields J.A., Honavar S.G., Eagle R.C., Jr. Ring melanoma of of anterior chamber angle: a report of 14 cases. Am J Ophthalmol. 2001;132:336–342. doi: 10.1016/s0002-9394(01)01051-0. [DOI] [PubMed] [Google Scholar]
- 12.Shields C.L., Materin M.A., Shields J.A., Gershenbaum E., Singh A.D., Smith A. Factors associated with elevated intraocular pressure in eyes with iris melanoma. Br J Ophthalmol. 2001;85:666–669. doi: 10.1136/bjo.85.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abi-Ayad N., Grange J.D., Watkin E. Ring melanoma revealed by spontaneous hyphema. J Fr Ophtalmol. 2007;30:729–732. doi: 10.1016/s0181-5512(07)91361-3. [DOI] [PubMed] [Google Scholar]
- 14.Shields C.L., Shields J.A., Shields M.B., Augsburger J.J. Prevalence and mechanisms of secondary intraocular pressure elevation in eyes with intraocular tumors. Ophthalmology. 1987;94:839–846. doi: 10.1016/s0161-6420(87)33537-7. [DOI] [PubMed] [Google Scholar]
- 15.Bianciotto C., Saornil M.A., Muiños Y. Ocular hypertension as the principal indicator of onset of uveal melanoma. Arch Soc Esp Oftalmol. 2005;80:27–34. doi: 10.4321/s0365-66912005000100006. [DOI] [PubMed] [Google Scholar]
- 17.Singer P.R., Krupin T., Smith M.E. Recurrent orbital and metastatic melanoma in a patient undergoing glaucoma surgery. Am J Ophthalmol. 1979;87:766–768. doi: 10.1016/0002-9394(79)90350-7. [DOI] [PubMed] [Google Scholar]
- 18.Sambuelli R., Luna J.D., Reviglio V.E. Small choroidal melanoma with massive extraocular extension: invsion through posterior scleral emissiary channels. Int Ophthalmol. 2001;24:213–218. doi: 10.1023/a:1022539129449. [DOI] [PubMed] [Google Scholar]
- 19.Shields C.L., Santos M.C., Shields J.A. Extraocular extension of unrecognized choroidal melanoma simulating a primary optic nerve tumor: report of two cases. Ophthalmology. 1999;106:1349–1352. doi: 10.1016/S0161-6420(99)00723-X. [DOI] [PubMed] [Google Scholar]
- 21.Rini F.J., Jakobiec F.A.A., Hornblass A. The treatment of advanced choroidal melanoma with massive orbital extension. Am J Ophthalmol. 1987;104:634–640. doi: 10.1016/0002-9394(87)90177-2. [DOI] [PubMed] [Google Scholar]
- 23.Casey R., Li W.W. Factors controlling ocular angiogenesis. Am J Ophthalmol. 1997;124:521–529. doi: 10.1016/s0002-9394(14)70868-2. [DOI] [PubMed] [Google Scholar]
- 24.Cairns J.E. Rationale for therapy in neovascular glaucoma. Trans Ophthalmol Soc UK. 1981;101:184–185. [PubMed] [Google Scholar]
- 25.Piirtola A., Puska P., Kivelä T. Red laser cyclophotocoagulation in the treatment of secondary glaucoma in eyes with uveal melanoma. J Glaucoma. 2012 doi: 10.1097/IJG.0b013e31825c0fb7. [DOI] [PubMed] [Google Scholar]



