Abstract
Diffuse anterior retinoblastoma is a rare variant of diffuse infiltrating retinoblastoma which occurs in up to 1–2% of cases of retinoblastoma. In diffuse anterior retinoblastoma there is a small focus of tumor in the peripheral retina from which free tumor cells enter the aqueous humor and implant on the ciliary body, iris, lens and trabecular meshwork. Patients most commonly present with pseudouveitis, pseudohypopyon and increased intraocular pressure. The differential diagnosis is broad and all of the reported cases relied upon aspirates from the aqueous humor in order to make the diagnosis prior to proceeding with treatment. Treatment involves enucleation and, depending upon the extent of the tumor, may require systemic chemotherapy or external beam radiation. This review summarizes the 7 previously reported cases of diffuse anterior retinoblastoma, discusses pathologic features, and addresses the challenges of early diagnosis and future directions.
Keywords: Retinoblastoma, Diffuse, Anterior, Uveitis
Introduction
The most common primary intraocular malignancy in children is retinoblastoma. Retinoblastoma comprises 2–4% of all childhood tumors and has an annual incidence in the United States of 11.8 per million in children under the age of four.1,2 Retinoblastoma can be classified as sporadic or familial and as unilateral or bilateral.3 In familial cases, parents carrying a mutation of the RB1 gene (13q4) have a 50% chance of passing the mutant allele onto their offspring and 40% of those who inherit the mutant allele will develop retinoblastoma.4 The tumors are unifocal or multifocal, discrete yellow–white retinal masses with prominent feeding vasculature.5 Growth patterns of retinoblastoma include endophytic, exophytic, mixed endophytic-exophytic, and diffuse.4,6 Endophytic retinoblastomas grow from the inner retina toward the vitreous and exophytic tumors grow from the outer retina toward the choroid.6
Diffuse infiltrating retinoblastoma is a rare growth pattern that occurs in 1–2% of retinoblastoma cases in which the tumor grows horizontally with minimal vertical growth. This form is the most difficult to diagnose as there is no well-defined retinal mass and it can mimic an inflammatory process.7 These tumors tend to be unilateral, sporadic, and more commonly occur in children around the ages of 6–8 years.8 Diffuse anterior retinoblastoma, an uncommon variant of diffuse infiltrating retinoblastoma, results from a small focus of intraretinal tumor at the far periphery of the retina which seeds tumor cells into the aqueous humor in the region of the vitreous base and ciliary body.4 As a result, these tumors tend to resemble uveitis and diagnosis is often delayed.
Historical review
Diffuse infiltrating retinoblastoma was first described almost two centuries after Hayes described a neuroepiblastic tumor of the retina, later referred to as retinoblastoma, in 1767.9 While Schofield published the first case titled “Diffuse infiltrating retinoblastoma” in 1960, it was Ashton who is credited with suggesting the term via personal communication with Schofield in 1958.9 Two similar cases representing likely diffuse infiltrating retinoblastoma had previously been described by Manschot in 1956 and Weizenblatt in 1957.9 Reeser presented the first case of what would later be called diffuse anterior retinoblastoma at the Verhoeff Society meeting in April of 1975.6 Garner et al. published the first case report in 1987 of a 7 year old girl with unilateral retinoblastoma that was initially misdiagnosed as granulomatous uveitis since no retinal mass was seen on examination to suggest retinoblastoma. However, two small foci of tumor at the far periphery of the retina were later identified under microscopy.10 Grossniklaus et al. were the first to suggest the name “Diffuse Anterior Retinoblastoma” in their case report of a 6 year old child with unilateral retinoblastoma which was also initially misdiagnosed as Toxocara endophthalmitis.7 Overall a total of seven cases have been reported.4,6–8,10–12 Out of these seven cases, only one germline mutation of the RB1 gene mutation has been reported. This is significant because up until 2009, it was believed that diffuse anterior retinoblastoma was a sporadic form of retinoblastoma, but Crosby et al. showed that this form may in fact be heritable.4
Clinical presentation
Diffuse anterior retinoblastoma is a unilateral form of retinoblastoma that affects males and females at equal rates and is usually diagnosed in children between 5 and 12 years of age.7 The average age of diagnosis based upon the seven previously reported cases is 6.4 years (Table 1). Most commonly, children present with pseudouveitis, pseudohypopyon and increased intraocular pressure.11 Slit-lamp examination is likely to show a prominent cellular reaction in the anterior chamber, keratic precipitates, and white fluffy exudates mimicking a hypopyon (Fig. 1). The dilated fundus exam is likely to be normal as only 1 case report identified a small peripheral retinal mass that was visible on examination.4 Concordantly, B-scan echography did not reveal a retinal mass or calcification in any case reports. Two of the seven reported cases were initially misdiagnosed due to a lack of well-defined retinal mass and the tumor cells mimicking an inflammatory process.7,10 The differential diagnosis for diffuse anterior retinoblastoma is broad and includes medulloepithelioma, sarcoidosis, idiopathic uveitis, metastatic neuroblastic tumor, fungal endophthalmitis, pars planitis, Toxocara endophthalmitis, leukemia, lymphoma, juvenile rheumatoid arthritis-associated uveitis, and retinoblastoma.8 While it is widely known that fine-needle aspiration biopsy (FNAB) is contraindicated in known cases of retinoblastoma, each of the published cases performed a FNAB of the anterior chamber infiltrate through the clear cornea in order to narrow the differential and make the diagnosis.4,7,8,10–12 It should be emphasized that a FNAB should only be performed as a last resort due to the risk of seeding viable tumor cells along the needle tract, as was reported by Langmuir et al.8
Table 1.
Published cases of diffuse anterior retinoblastoma through 2012.
| Article author (Year) | Eye | Age/Sex | Presenting symptom | Eye exam |
US imaging | Diagnosis | Treatment | Retinal involvement under microscopy | Immuno-histochemical stains | Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior segment | Posterior segment | ||||||||||
| Garner et al. (1987) | OD | 7yo/ F | Redness, Blurring of vision | “Severe anterior uveitis with large iris nodules and cells and opacities in the anterior viteous” | – | – | Biopsy of iris, lens excision | Topical corticosteroids, oral prednisone 5 mg TID, sub-Tenon’s injection of methylprednisolone, lens excision, enucleation, orbital radiotherapy, adjuvant chemotherapy | 2 foci at extreme periphery of retina, minimal thickening, <1.5 mm in diameter | +NSE, −S100, −GFAP | Recurrent orbital retinoblastoma 8 mos after enucleation; No sign of further recurrence at 12 mos |
| Grossniklaus et al. (1998) | OD | 6yo/F | Unknown | Keratic precipitates, 4 + cells, IOP28 mmHg | Normal appearing retina, cells in inferior vitreous | Posterior vitreous detachment, no retinal abnormality | Anterior chamber fine needle aspiration biopsy | Topical prednisolone, dexamethasone 0.1%, betaxolol HCl 0.5%, Thiabendazole for 3 days, oral prednisone | Intraretinal tumor in peripheral retina | +NSE, + vimentin, -MAK-6, -AE 1,3, -GFAP, -S-100 protein | Unknown |
| 4 + cells, small “hypopyon”, IOP 34 mmHg | 1–2 + Vitreous cells | Topical prednisolone q1–2H, Dorzolamide HCl TID, Timolol maleate 0.25% qHS, Diclofenac sodium QID, dexamethasone qHS | |||||||||
| 4 + cells, dense “hypopyon” | 4 + vitreous cells | Rimexolone q1–2H, alpha clonidine 0.5% TID | |||||||||
| – | – | Enucleation | |||||||||
| Crosby et al. (2009) | OS | 9yo/ F | Blurry vision, redness, discoloration of iris | Pseudohypopyon, IOP 34 mmHg | “Possible small, inferior, peripheral mass in her left retina” | – | Anterior chamber fine needle aspiration biopsy | Enucleation | 3 × 1 mm tumor in peripheral inferior retina | Tumor Seeds: + TGF-β, + VEGF, -iNOS, -HIF1α | Unknown |
| Retina: + VEGF,-iNOS, -HIF1α | |||||||||||
| Longmuir et al. (2010) | OD | 8.5 yo/M | Unknown | 3–4 + cells, IOP 46 mmHg, “less prominent flare”, small hypopyon, multiple nodules on iris | Normal appearing retina | Iris root thickening to 1 mm for 360°, “mild anterior vitreous opacities” | Anterior chamber fine needle aspiration biopsy | Topical prednisolone 1% q1H, topical dorzolamide HCl, Timolol maleate, Brimonidine, Scopolamine HBr 0.25%, oral prednisone 30 mg PO q day, Enucleation, 6 cycles of vincristine, carboplatin, etoposide, external beam radiation (4140 cGy total) | No retinal involvement identified on 487 slides | +Synaptophysin , - Leukocyte common antigen, -CD34 | No recurrence at 5 years |
| Khetan et al. (2011) | OS | 3yo/F | “Unresolving anterior uveitis with secondary glaucoma” | Conjunctiva l congestion, pupil sluggishly reactive to light, “white, fluffy exudates”, IOP 31 mrnHs | Normal appearing retina | Normal | Anterior chamber fine needle aspiration biopsy | Topical prednisolone 1% q4H, Homatropine BID, Timolol maleate 0.5% + Brimondine acetate 0.2% BID, Enucleation | No retinal involvement identified | Unknown | No recurrence at time of publication |
| Herwig et al. (2011) | OS | 3yo/M | Discoloration of iris | Pseudohypopyon, IOP 26 mmHg | Normal appearing retina | No mass or calcification | Anterior chamber fine needle aspiration biopsy | Enucleation, 6 cycles of carboplatin, etoposide, vincristine | No retinal involvement identified | +Synaptophysin ,+NSE,-S-100 | No recurrence at 5 mos |
Figure 1.
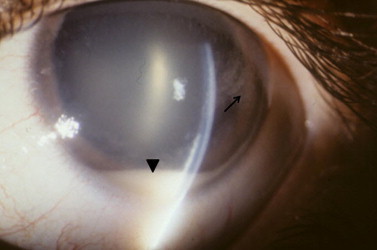
Slit lamp appearance of diffuse anterior retinoblastoma. A white pseudohypopyon is present (arrowhead). There are also aggregates of white, fluffy tumor on the posterior corneal surface (arrow). The eye is otherwise white and quiet.
Pathology
On gross examination, white fluffy material can be seen within the anterior chamber coating the iris, pars plicata, pars plana and lens zonules as described in the case reports (Fig. 2). It may also be possible to see white material in the vitreous base. Only one case report described a small focus of retinal tumor located close to the ora serrata that could be identified on gross examination of the enucleated specimen.4
Figure 2.
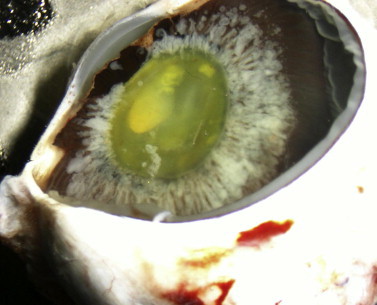
Gross appearance of enucleated eye with diffuse anterior retinoblastoma. There is white, fluffy material forming a corona around the lens. This represents tumor in the space between the anterior hyaloid face and ciliary body.
On microscopic examination, round basophilic tumor cells are likely to be seen infiltrating 360° around the lens zonules, ciliary smooth muscle, and iris stroma (Fig. 3). Tumor cells can also be seen diffusely throughout the anterior chamber, in the anterior vitreous, as well as lining the posterior cornea. In each case, the patients presented with increased intraocular pressure which can be explained by the combination of neovascularization of the iris and infiltration of tumor cells into Schlemm’s canal and the trabecular meshwork (Fig. 4). In Longmuir et al., tumor cells were even seen invading into the inner portion of the sclera along the region of the angle.8 Numerous pyknotic and necrotic nuclei can be seen in addition to stippled and hyperchromatic nuclei. Homer Wright rosettes, a ring of nuclei around a tangle of neural filaments with no central lumen, were present in two cases.4,8 Similarly, Flexner-Wintersteiner rosettes, a ring of columnar cells surrounding a central lumen, were present in two cases.8,11
Figure 3.
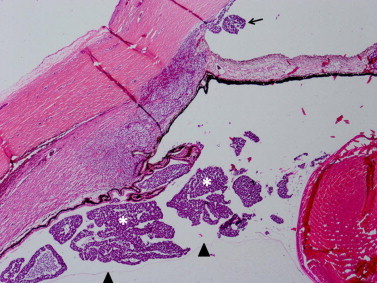
Diffuse anterior retinoblastoma in the posterior compartment. Tumor (asterisks) is present between the anterior hyaloid face (arrowheads) and the ciliary body. Tumor has percolated through the pupil and lodged in the peripheral anterior chamber (arrow). (Hematoxylin and eosin, 10X).
Figure 4.
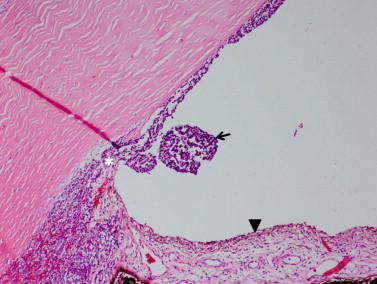
Diffuse anterior retinoblastoma in the anterior chamber. Tumor (arrow) is in the peripheral anterior chamber and plastered against the posterior corneal surface. There is neovascularization of the iris (arrowhead) present. Tumor has also infiltrated the trabecular meshwork (asterisk). (Hematoxylin and eosin, 100X).
From the published cases, 50% of cases identified a small focus of retinal tumor under microscopy while no retinal tumor could be identified in the other half case reports. When retinal involvement was detected, the tumors were located at the far periphery of the retina close to the ora serrata and minimal thickening of the retina was noted. Initially no retinal involvement was identified in Garner et al., but after examining multiple levels, 2 small foci of tumor, each less than 1.5 mm in total diameter, were identified at 11–12 o’clock and 6–7 o’clock meridians.10 Similarly, after examining serial step sections, Grossniklaus et al. identified a small intraretinal tumor at the periphery of the retina.7 Crosby et al. reported the largest known intraretinal tumor in diffuse anterior retinoblastoma, which still only measured 3 × 1 mm, and did not invade into either the pigment epithelium or the choroid.4 Longmuir et al. examined 487 slides of 4 μm sections, Herwig et al. examined 422 sections, and Khetan et al. examined an unreported number of serial sections, and no retinal focus was identified in any of these cases.8,11,12 Possible explanations for a lack of intraretinal tumor include recession or involution of a small focus of retinal tumor by the time of enucleation, a retinal tumor so small that it was missed on serial sections, or lastly that the tumors arose from a stem cell located within the anterior segment.8,11
Varying immunohistochemical stains were performed on the tissue specimens in each case report and the results were consistent between all case reports that used a given stain. For example, neuron specific enolase (NSE), which stains cells of neuronal origin, was positive in each of the three case reports that used the NSE stain.7,10,11 Likewise, synaptophysin was positive in two cases indicating that neuronal differentiation was present.8,11 Vimentin, which stains cells that have undergone epithelial-to-mesenchymal transition, was positive in Grossniklaus et al.7 The remainder of the stains that were tested was found to be negative. S-100 stains schwann cells and was negative in three case reports.7,10,11 Glial fibrillary acidic protein (GFAP), mouse monoclonal anti-cytokeratin (MAK-6), AE1, AE3, leukocyte common antigen, and cluster of differentiation molecule 34 (CD34) each stained negatively in one case report.7,8,10 On electron microscopy, tumor cells have a high nuclear-to-cytoplasmic ratio, rare nucleoli, glycogen granules, intercellular junctions, sparse intracytoplasmic filaments, chromatin clumping, and cilia in the 9 + 0 configuration that is characteristic of retinoblastoma and retinal photoreceptors.7,10
Cell signaling & survival mechanisms
Crosby et al. investigated the cell signaling and cell survival mechanisms involved in diffuse anterior retinoblastoma by performing a series of immunofluorescent stains on the intraretinal tumor and on tumor cells in the aqueous humor.4 The immunofluorescent stains that were chosen stained for tumor growth factor β (TGF-β), vascular endothelial growth factor (VEGF), inducible nitric oxide synthase (iNOS), and hypoxia inducible factor 1 (HIF1). TGF-β can have opposing effects on tumor growth dependent upon the type of cell and the genotypic phenotype of the proteins acting in the signal transduction pathway.4 For example, through effects on cyclin-dependent kinase inhibitors, the RB1 gene can regulate the expression of TGF-β. TGF-β can have tumor suppression effects by affecting cell apoptosis, replication potential and proliferation.4 Alternatively, TGF-β can promote tumor growth by aiding tumor cell invasion, migration, and angiogenesis.4 The authors also stained for VEGF, a signaling protein in the tyrosine kinase platelet-derived growth factor family that is involved in vasculogenesis, angiogenesis and survival of the cell.4 iNOS is an enzyme that converts l-arginine to nitric oxide in order to mediate angiogenesis, malignant transformation, and metastasis. When the retina becomes ischemic, iNOS is expressed thereby modulating intraretinal angiogenesis to intravitreal angiogenesis.4 Lastly, HIF1 is a transcription factor that serves as the main mediator in hypoxic environments that serves to promote angiogenesis.4
In Crosby et al., the tumor seeds located in the aqueous humor stained positive for TGF-β and VEGF but were negative for iNOS and HIF1. Interestingly, the intraretinal tumor only stained positive for VEGF and was negative for TGF-β, iNOS and HIF1. The authors theorize that the expression of VEGF in the intraretinal tumor was likely not secondary to ischemia as iNOS and HIF1, components of the most common ischemic pathway, were negative.4 They also propose that the tumor seeds in the aqueous humor acquired expression of TGF-β as a survival mechanism to aid in tumor migration and invasion. This neoplastic transformation occurs when tumors acquire the capability to evade cell apoptosis, replicate limitlessly, evade antigrowth signals and produce self-sufficient cell signaling and angiogenesis.4
Treatment
Secondary to early misdiagnosis, the majority of patients in the case reports received a mixture of topical corticosteroids and medications to lower the intraocular pressure initially. An enucleation of the affected eye was performed in all cases ranging from 1 day (Longmuir et al.) up to 6 weeks (Khetan et al.) after the diagnosis of diffuse anterior retinoblastoma was made.8,12 Two case reports performed 6 cycles of systemic chemotherapy with carboplatin, etoposide and vincristine,8,11 and in one of those cases external beam radiation to the eye socket for a total of 4140 cGy was given.8 There is only one case reported in which there was a recurrence of the tumor approximately 8 months following enucleation.10 The patient presented with orbital swelling and an enlarging orbital mass that was treated with radiotherapy to the eye socket in addition to three courses of adjuvant chemotherapy.10 At 12 months, the patient was alive with no further signs of recurrence or metastatic disease.10 No recurrences of tumor were reported in any of the other case reports, and at the time of publication, all of the patients in the case reports were alive.4,7,8,11,12 It is difficult to extract an accurate survival rate from the reported cases as long-term follow up for the patients is not known but the lack of known recurrences at the times of publication is reassuring. However, the survival rate inclusive of all variants of diffuse infiltrating retinoblastoma has been reported and was found to be greater than 95% in children who receive an enucleation of the affected eye.13
Future directions & challenges
One of the most significant challenges in the management of patients with diffuse anterior retinoblastoma is making the correct diagnosis. Since this variant of retinoblastoma is extremely rare, misdiagnosis is common as the absence of an obvious retinal mass leads to clinical confusion. Diffuse anterior retinoblastoma can mimic a number of more common conditions including granulomatous uveitis as in Garner et al., Toxocara endophthalmitis as in Grossniklaus et al., or juvenile xanthogranuloma or tuberculosis as cited in Spencer.6,7,10 In addition, the reported cases relied upon a FNAB in order to make the diagnosis, which is contraindicated in known cases of retinoblastoma. Khetan et al. commented that in their experience ultrasonography was also not especially helpful as it failed to show any calcifications, retinal masses or other features of typical retinoblastoma.12 To further complicate the situation, physicians want confirmation of the diagnosis prior to advising the recommended treatment of enucleation to families. Similarly, parents are likely to opt for a confirmatory test, such as a FNAB, despite the risk of tumor spread prior to proceeding with an irreversible treatment that is associated with extremely high morbidity and decreased quality of life if the diagnosis of diffuse anterior retinoblastoma was incorrect. In the future, it would be ideal to develop a diagnostic tool that could be used to confirm the diagnosis that had minimal to no risk of tumor dissemination.
Conclusions
Diffuse anterior retinoblastoma is an uncommon variant of diffuse infiltrating retinoblastoma that occurs unilaterally in children between the ages of 3 and 9 years. The majority of cases are nonhereditary, however there is one reported case in a child with a germline mutation of the RB1 gene. Since the tumor can masquerade as a variety of conditions, diffuse anterior retinoblastoma should be considered in all cases of refractory uveitis in children. Fine needle aspiration biopsy should only be performed at highly specialized centers with experienced ophthalmologists and ophthalmic pathologists as a last resort to narrow the differential diagnosis due to the risk of tumor dissemination. Treatment involves enucleation of the involved orbit and in some cases may require external beam radiation or systemic chemotherapy.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
Supported in part by an unrestricted department grant from Research to Prevent Blindness, Inc.
References
- 1.Aziz H.A., LaSenna C.E., Vigoda M., Fernandes C., Feuer W., Aziz-Sultan M.A. Retinoblastoma treatment burden and economic cost: impact of age at diagnosis and selection of primary therapy. Clin Ophthalmol (Auckland, NZ) 2012;6:1601–1606. doi: 10.2147/OPTH.S33094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reis A.H., Vargas F.R., Lemos B. More epigenetic hits than meets the eye: microRNAs and genes associated with the tumorigenesis of retinoblastoma. Front Genet. 2012;3:284. doi: 10.3389/fgene.2012.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shields C.L., Shields J.A. Diagnosis and management of retinoblastoma. Cancer Control. 2004;11(5):317–327. doi: 10.1177/107327480401100506. [DOI] [PubMed] [Google Scholar]
- 4.Crosby M.B., Hubbard G.B., Gallie B.L., Grossniklaus H.E. Anterior diffuse retinoblastoma: mutational analysis and immunofluorescence staining. Arch Pathol Lab Med. 2009;133(8):215–218. doi: 10.5858/133.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields C.L., Ghassemi F., Tuncer S., Thangappan A., Shields J.A. Clinical spectrum of diffuse infiltrating retinoblastoma in 34 consecutive eyes. Ophthalmology. 2008;115(12):2253–2258. doi: 10.1016/j.ophtha.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 6.McLean I.W. Retinoblastomas, retinocytomas, and pseudoretinoblastomas. In: Spencer W.H., editor. 4th ed. vol. 2. Saunders; Philadelphia: 1996. pp. 1345–1351. (Ophthalmic pathology: an atlas and textbook). [Google Scholar]
- 7.Grossniklaus H.E., Dhaliwal R.S., Martin D.F. Diffuse anterior retinoblastoma. Retina. 1998;18(3):238–241. doi: 10.1097/00006982-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Longmuir SQ., Syed NA., Boldt HC. Diffuse anterior retinoblastoma without retinal involvement. Ophthalmology. 2010;117(10):2034–2038. doi: 10.1016/j.ophtha.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Schofield P.B. Diffuse infiltrating retinoblastoma. Br J Ophthalmol. 1960;44:35–41. doi: 10.1136/bjo.44.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garner A., Kanski J.J., Kinnear F. Retinoblastoma: report of a case with minimal retinal involvement but massive anterior segment spread. Br J Ophthalmol. 1987;71(11):858–863. doi: 10.1136/bjo.71.11.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herwig M.C., Hubbard G.B., Wells J.R., Grossniklaus H.E. Diffuse anterior retinoblastoma. Ophthalmologe. 2011;108(10):969–972. doi: 10.1007/s00347-011-2369-y. [DOI] [PubMed] [Google Scholar]
- 12.Khetan V., Sudrik S., Singh S., Gopal L., Krishnakumar S. Diffuse anterior retinoblastoma with undetectable retinal involvement. J Pediatr Ophthalmol Strabismus. 2011;48:e7–e9. doi: 10.3928/01913913-20110208-05. [DOI] [PubMed] [Google Scholar]
- 13.Bhatnagar R., Vine A.K. Diffuse infiltrating retinoblastoma. Ophthalmology. 1991;98(11):1657–1661. doi: 10.1016/s0161-6420(91)32072-4. [DOI] [PubMed] [Google Scholar]



