Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) has evolved over the past two decades to become the standard of care for hematologic and lymphoid malignancies. Major ocular complications after allogeneic HSCT have been increasing in number and severity. Graft-versus-host disease (GVHD) remains a major cause of ocular morbidity after allogeneic HSCT. The main objective of this review is to elucidate the ocular complications in patients developing GVHD following HSCT.
Ocular complications secondary to GVHD are common and include dry eye syndrome, acquisition of ocular allergy from donors with allergic disorders. Eyelid changes may occur in GVHD leading to scleroderma-like changes. Patients may develop poliosis, madarosis, vitiligo, lagophthalmos, and entropion. The cornea may show filamentary keratitis, superficial punctate keratitis, corneal ulcers, and peripheral corneal melting which may lead to perforation in severe cases. Scleritis may also occur which can be anterior or posterior. Keratoconjunctivis sicca appears to be the most common presentation of GVHD. The lacrimal glands may be involved with mononuclear cell infiltration of both the major and accessory lacrimal glands and decrease in tear production.
Severe dry eye syndrome in patients with GVHD may develop conjunctival scarring, keratinization, and cicatrization of the conjunctiva.
Therapy of GVHD includes systemic immunosuppression and local therapy. Surgical treatment in refractory cases includes surgical intervention to improve the manifestation of GVHD of the eye. This may include tarsorrhapy, prose lenses, punctal occlusions and corneal transplantation.
Keywords: Transplantation, Graft-versus-host disease, Ocular
Introduction
Hematopoietic stem cell transplantation (HSCT) has evolved as a promising curative therapy for hematopoietic disorders and some metabolic disorders. More recently, HSCT has been investigated for its potential in establishing tolerance for organ transplantation. For the treatment of malignant disorders, the goal is to eradicate the cancer through a combination of cytotoxic therapy and graft-versus-cancer effect. This approach is limited by conditioning induced organ toxicity, graft-versus-host disease (GVHD), and the ability of the malignancy to survive.1
GVHD occurs when donor T cells respond to genetically defined proteins on recipient host cells. The most important proteins are Human Leukocyte Antigens (HLA) which are highly polymorphic and are encoded by the major histocompatibility complex (MHC).2 Class I HLA (A, B, and C) proteins are expressed on almost all nucleated cells of the body at varying densities. Class II proteins (DR, DQ, and DP) on the other hand, are primarily expressed on hematopoietic cells (B cells, dendritic cells, and monocytes), but their expression can be induced on many other cell types following inflammation or injury.3 Despite HLA matching identity between a patient and donor, significant proportion of patients receiving HLA-identical grafts develop acute GVHD due to genetic differences that lie outside the HLA loci, or “minor” histocompatibility antigens (HA).4,5 The release of cytokines from tissues damaged by underlying disease, prior infections, and the transplant conditioning regimen is believed to promote the activation and proliferation of donor immune cells.6,7
Based largely on animal experimental models, the development of acute GVHD can be described in three sequential steps or phases: (1) activation of the antigen presenting cells (APCs) by the cytokines released from damaged tissues, (2) donor T cell activation, proliferation, differentiation and migration; and (3) target tissue damage by activated T cells.8
Experimental studies have generated several theories to explain the pathophysiology of chronic graft-versus-host-disease (cGVHD): (1) Thymic damage and the defective negative selection of T cells, (2) Regulatory T cell deficiencies, (3) auto-antibody production by aberrant B cells, and (4) the formation of profibrotic lesions.9
Chronic GVHD (cGVHD) remains the most common late complication of allogeneic stem cell transplantation (SCT). Although improvements have been made in the prevention of acute GVHD, these advances have not been reflected on the incidence of cGVHD.10 Several factors have contributed to the high incidence of cGVHD including increased upper age limit of transplant recipients, use of unrelated donors and incomplete HLA matched related donors, use of peripheral blood as a source of stem cells, and use of donor lymphocyte infusion to treat relapsed disease or to achieve full donor chimerism.11
Clinical presentation and staging of Graft-versus-host-disease (GVHD)
Graft-versus-host-disease (GVHD) occurring from the day of transplantation (day 0) to day 100 after the transplantation is referred as “acute” GVHD, whereas GVHD occurring after day 100 is termed “chronic” GVHD. These divisions, however, are thought to be relatively arbitrary, as acute and chronic patterns of GVHD can occur in chronic and acute settings, respectively.12 Acute patterns of GVHD include skin bullae desquamation, severe bile duct injury, and extensive gastrointestinal crypt drop-out.13 Chronic GVHD features are more complex, including clinical manifestations of mixed autoimmune disorders, with the hallmark of excessive fibrosis, stenosis, and atrophy of tissues in the skin, lung, and mucous membranes (mouth, vagina, and eyes).14 Acute GVHD has historically been graded by the culmination of the GVHD staging systems for three organs (skin, gastrointestinal tract, and liver).15 Chronic GVHD can be classified according to the type of onset, need for systemic immunosuppressive therapy, or mortality risk. The majority of patients with chronic GVHD have suffered from acute GVHD. Their disease may evolve directly from acute GVHD (progressive) that has a bad prognosis, or may follow a period of resolution (quiescent or interrupted), with an intermediate prognosis. Patients may develop chronic GVHD with no history of acute GVHD (de novo) which usually have a good prognosis.16 Based on data from the International Bone Marrow Transplant Registry (IBMTR), the distribution of chronic GVHD onset for HLA-matched siblings is progressive in 20–30%, interrupted in 30–40% and de novo in 35% of patients.17 The most commonly employed staging system is the ‘limited/extensive’ classification, proposed by the Seattle group in 1980, based on a retrospective clinical and pathological review of 20 patients with chronic GVHD.18 Localized skin involvement with or without hepatic dysfunction (limited disease) was associated with less severe disease and decreased rate of infections. Generalized skin involvement or limited disease with eye involvement, oral involvement, hepatic dysfunction with abnormal liver histology, or involvement of any other target organ was classified as extensive disease and was associated with more frequent infections.19 The Seattle group has developed the revised clinical criteria for limited and extensive chronic GVHD in order to clarify ambiguities of the original definition. In the revised classification, prolonged treatment with systemic immunosuppression is indicated for patients with clinically extensive chronic GVHD or anyone with high-risk features (i.e., platelets count <100 × 109/l, progressive onset, or receiving treatment with corticosteroids at the time of the diagnosis of chronic GVHD).14
Ocular manifestations of GVHD
Acquisition of ocular allergy occurred in patients receiving HSCT from donors with allergic disorders.20 Ocular complications secondary to GVHD are frequent. Major ocular complications occurred in 80 (13%) of 620 patients after HSCT (Table 1). The most common ocular complication was cGVHD.21 While ocular GVHD usually occurs in the setting of other systemic GVHD, it may also be the initial presentation of the patient’s systemic GVHD. The eye may be involved in both acute and chronic GVHD, although it is more common in the chronic form of the disease with more severe presentation.22
Table 1.
Major ocular complications following Hematopoietic Stem Cell Transplantation (HSCT) among 620 patients.21
| No. | (%) | |
|---|---|---|
| Graft-versus-host-disease | 34 | (5.4) |
| Dry eye syndrome | 30 | (4.8) |
| Corneal ulcers | 15 | (2.4) |
| Steroid-induced cataract/glaucoma | 10 | (1.6) |
| Infectious retinitis | 4 | (0.6) |
| Acquisition of allergic conjunctivitis | 4 | (0.6) |
| Other (uveitis, endophthalmitis) | 4 | (0.6) |
The National Institutes of Health (NIH) working group published consensus document to establish standardized criteria for the diagnosis of cGVHD and to propose tools for scoring cGVHD organ involvement and assessing overall severity (Table 2).
Table 2.
NIH consensus criteria: Ocular GVHD staging.
| Score | Definition |
|---|---|
| 0 | No symptoms |
| 1 | Mild dry eye symptoms not affecting daily activities (requiring eye drops ⩽3x per day) or asymptomatic signs of KCS |
| 2 | Moderate dry eye symptoms partially affecting daily activities (requiring drops >3x per day or punctal plugs) without vision impairment |
| 3 | Severe dry eye symptoms significantly daily activities (special eyewear to relieve pain) or unable to work because of ocular symptoms or loss of vision caused by KCS |
GVHD: Graft-versus-host disease; KCS: Keratoconjunctivitis sicca; NIH: National Institutes of Health.
According to this consensus document, the diagnosis of cGVHD requires: (1) distinction from acute GVHD as well as other possible diagnoses, and (2) the presence of at least one diagnostic clinical sign of cGVHD or the presence of at least one distinctive manifestation confirmed by pertinent biopsy or other relevant tests.23 For cGVHD of the eye, there is no recommendation for obtaining samples for histopathologic diagnosis. However, distinctive manifestations of cGVHD include new onset of dry, gritty, or painful eyes, cicatricial conjunctivitis, keratoconjunctivitis sicca, and confluent areas of punctate keratopathy. Other features include photophobia, periorbital hyperpigmentation, difficulty in opening the eyes in the morning because of mucoid secretions, and blepharitis. New ocular sicca documented by low Schirmer test values with a mean value of both eyes ⩽5 mm at 5 min or a new onset of keratoconjunctivitis sicca by slit-lamp examination with mean values of 6 to 10 mm on the Schirmer test is sufficient for the diagnosis of chronic GVHD, if accompanied by distinctive manifestations in at least one other organ.23 (Figs. 1–3).
Figure 1.
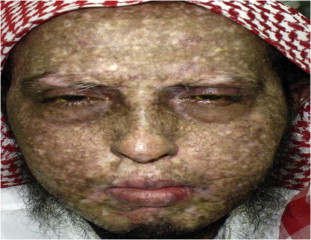
Graft-versus-host-disease (GVHD) with keratoconjunctivitis sicca showing cutaneous changes with scleroderma-like skin lesions and areas of pigmentations and depigmentations.
Figure 2.
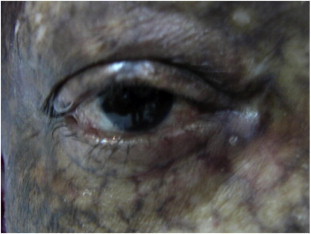
Dry eye syndrome with entropion and loss of eyelashes in GVHD.
Figure 3.
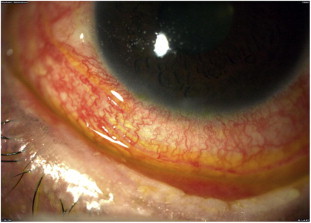
A 32 year-old male developed GVHD. He had bilateral conjunctivitis and dry eye syndrome.
Inamoto Y et al. tried to validate the measurement scales in ocular graft-versus-host- disease.24 They recommended using the NIH eye score to assess response in clinical trials for chronic GVHD because it is easy, quick and non-sophisticated test (Table 2). Besides, it correlates well with both clinician- and patient-reported symptom changes.24
Several other studies are in agreement with such recommendations because of the low reproducibility of Schirmer test and the inter- and intra-rater variability in reporting the severity of symptoms.25–27
Ocular GVHD is a spectrum of clinical manifestations, affecting all layers of the eye, including the lid, lacrimal gland, conjunctiva, cornea, and even the vitreous and the choroids, although the posterior involvement of ocular GVHD is exceedingly rare.28 Posterior scleritis, choroidal thickening, and serous detachments that respond to high-dose steroid are manifestations of ocular acute GVHD.29 Keratoconjunctivitis sicca appeared to be more severe in patients with GVHD than in patients without GVHD21 (Table 1).
Eyelid skin changes are similar to skin findings in acute GVHD and include maculopapular, erythematous exanthema. The cutaneous manifestations of GVHD may affect the eyelids. Dermatitis, lagophthalmos, ectropion, poliosis, madarosis, and vitiligo have been reported in cGVHD28 (Figs 1 and 2]. Anterior segment manifestations of acute GVHD include conjunctivitis, anterior uveitis, superior limbic keratoconjunctivitis, and episcleritis30–32 (Fig. 3).
Conjunctiva
Conjunctival involvement in acute GVHD is rare, but when present, it is a poor prognostic factor and is a marker for severe systemic involvement. Conjunctival disease ranges from mild erythema to pseudomembranous and cicatrizing conjunctivitis similar to ocular cicatricial pemphigoid33 (Fig. 4). The conjunctivitis of acute GVHD is more often ulcerative and hemorrhagic. Several hemorrhagic stage episodes alternating with exudative stages, which are characterized by sterile, purulent discharge of polymorphonuclear leukocytes with pseudomembrane formation may show ulceration and laser scarring, and fibrosis of the conjunctiva.33
Figure 4.
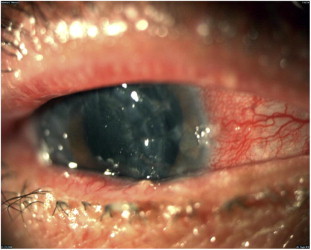
Conjunctival cicatrization and shrinkage in a patient with GVHD.
In the chronic form of GVHD, dry eye is the most frequent complication occurring in 40% to 76% of patients and its severity is correlated to the severity of cGVHD.21,34 The main cause of keratoconjunctivitis sicca in GVHD is lymphocytic infiltration of the accessory and major lacrimal glands. This may lead to fibrosis of the acini and ductules.34 Additionally, radiation effects, chemotherapy, lid dysfunction, meibomian gland dysfunction, and the underlying disease process may all contribute to dry eyes in GVHD patients.21 In most patients, keratoconjunctivitis sicca persists after the remission of GVHD. Patients with aqueous tear deficiency may rarely have normal tear function after 4 years of follow-up.31,32 Several complications may follow, such as punctate keratitis, corneal filaments, persistent epithelial defects, corneal keratinization, ulceration, and perforation in spite of adequate tear substitutes35 (Fig. 5). A characteristic non-infectious pseudomembranous conjunctivitis has been described in association with GVHD.31,32 Four stages of conjunctival GVHD were described. Stage 1 is conjunctival hyperemia without other changes. Stage 2 includes a chemotic response or serosanguinous exudates or both. Stage 3 is characterized by the presence of conjunctival pseudomembranes and Stage 4 disease occurs when patients with pseudomembranous conjunctivitis undergo cicatrization and conjunctival shrinkage with loss of the corneal epithelium and indolent corneal ulcer.
Figure 5.
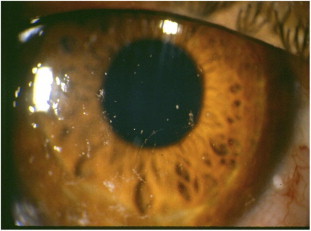
An 18 year-old female who developed GVHD following bone marrow transplantation. Figure shows keratoconjunctivitis sicca and corneal filaments.
Although patients may progress consecutively from lower to higher stages, they frequently present with Stage 3 disease. Development of Stage 4 ocular GVHD was found to be associated with a more severe form of both acute and chronic systemic GVHD.33
Corneal involvement
Corneal involvement is found typically more commonly in cGVHD than in acute GVHD. In acute GVHD, there may be corneal epithelial keratitis or filamentary keratitis secondary to the conjunctival cicatrization, but not a direct cause and effect of graft-versus-host tissue reaction. Rarely, peripheral corneal melting occurs which may lead to corneal perforation21,32 (Fig. 6).
Figure 6.
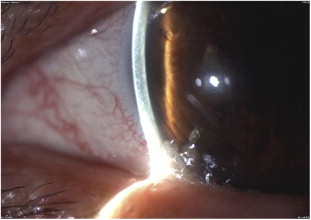
Peripheral corneal melting in a patient with GVHD and dry eye syndrome.
Chronic GVHD is commonly associated with keratoconjunctivitis sicca. The dry eye of GVHD is often severe and can lead to filamentary keratitis, corneal neovascularization, corneal ulceration, melting of the cornea, and ultimately corneal perforation if not treated36 (Fig. 6).
Lacrimal Gland involvement
In cGVHD disease, there is a histologic evidence of mononuclear cell infiltration of the major and accessory lacrimal of Kraus and Wolfring leading to tear dysfunction. T cell mediated cytotoxic effects may damage the periductal epithelial cells which impair the lacrimal gland exocrine functions.34 The mononuclear infiltration is similar to the findings seen in Sjögren syndrome. Subsequent fibrosis may occur resulting in irreversible dry eye syndrome (Fig. 7).
Figure 7.
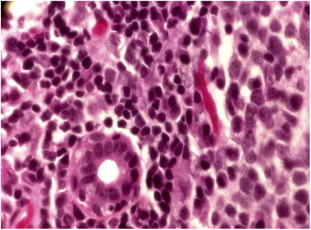
Labial Accessory salivary gland in a patient with GVHD showing mononuclear cell infiltration surrounding ductules with destruction of salivary acini.
Lacrimal gland dysfunction probably is the most common ocular manifestation of ocular GVHD. Histopathologic analysis demonstrates periodic acid-Schiff-positive material clogging and distending lacrimal gland ducts, further impairing the ability to secrete tears. In addition to lacrimal gland dysfunction, the presence of cicatricial lagophthalmos or ectropion may lead to poor tear film distribution and abnormal ocular surface disease.35–37 The labial accessory salivary glands show mononuclear infiltration similar to the changes in the lacrimal glands (Fig. 7).
Cataract
Increased cataract formation has been noted in patient post-HSCT, but this is thought to be related to treatment with corticosteroids or total body irradiation, not as a result of the GVHD reaction in itself.21,38–42 Patients with ocular GVHD who have developed cataracts may undergo phacoemulsification with intraocular lens implantation as long as their ocular surface disease is adequately managed.40
Posterior segment manifestations
A variety of posterior segment complications may be seen in GVHD patients. Micro-vascular retinopathy with cotton-wool spots, intra-retinal and vitreous hemorrhage, and infection may occur, which may be related to the initial disease, its treatment or conditioning regimen.43
Central serous chorioretinopathy (CSCR) is an uncommon complication in HSCT patients.44,45 Because of its response to systemic corticosteroids, it was suggested that GVHD itself may affect the choroidal vasculature, leading to choroidal hyperpermeability and the development of CSCR.46
Posterior scleritis has been reported to be associated with GVHD.45,46 Vitritis is an uncommon manifestation of GVHD.47
Systemic Therapy
Although systemic immune suppression is the corner stone in the management of GVHD, the role of topical treatment cannot be overlooked. Local treatment modalities might be needed even if systemic therapy is not indicated.48
Acute ocular GVHD can be treated as part of systemic disease without the need for local modalities. However, in Stage 2 pseudo-membranous conjunctivitis, topical steroids, cyclosporine or tacrolimus may reduce cicatricial and fibrovascular scarring and prevent progression to Stage 4 disease. Calcineurin inhibitors are found to ameliorate the sequential signs and manifestations of chronic ocular GVHD.49–51
In chronic ocular GVHD, several systemic therapeutic modalities are available including steroids, as cyclosporine A and mycophenolate mofetil, and agents targeting B-cells such as rituximab.49 Systemic tacrolimus has shown efficacy in lacrimal gland dysfunction secondary to ocular chronic GVHD.52
Several committees have published guidelines for the management of cGVHD including National Institutes of Health (NIH) consensus development project, Consensus Recommendations of Experts from Germany, Austria, and Switzerland, the British Committee for Standards in Haematology, and the British Society for Bone Marrow Transplantation.48,53,54
Corticosteroids are usually recommended as a first line therapy based on randomized trial.54 Most guidelines recommend a prednisolone dose of 1 mg/kg/day for 2 weeks with gradual tapering to 1 mg/kg every other day over 6–8 weeks and then tapered by 10–20% per month for a total duration of 9 months48,53–55
This regimen was also combined with additional immunosuppressive therapy like cyclosporine and mycophenolate mofetil. In such patients who are receiving other immunosuppressive agents, it is recommended to start tapering off steroids first. Other immunosuppressive agents can be tapered one at a time over a 3–9 month period with dose reductions every 2–4 weeks depending on clinical response. The median duration of immunosuppressive therapy is 2–3 years.56 With prolonged steroid therapy for cGVHD, patients and physician should be aware of the hazards of developing cataract as well as bone density loss, infectious diseases and other complications.
Second-line therapy for steroid refractory GVHD is based on only phase II trials and retrospective analyses.57 Several agents can be used in combination with each other in such case. In general, no more than three immunosuppressive agents should be combined, as combination of more drugs often does not lead to improved efficacy but results in a significantly increased risk of side effects and infections.57
Improved tear secretion, improved ocular surface findings and increase in Schirmer test values were reported with systemic tacrolimus therapy.58,59 In addition steroid sparing agents might allow steroid dose reduction with substantial reduction of toxicity.57
The systemic immunosuppression must be optimally titrated and maximized when ocular GVHD with systemic GVHD (skin, liver, and gut) is not controlled. The ideal approach to the local and systemic management of ocular GVHD is a multidisciplinary approach with the proper understanding of the patient’s overall GVHD clinical status.53
Local Therapy
The National Institutes of Health cGVHD consensus workshop had recently summarized the management of cGVHD, including chronic ocular GVHD. The group emphasized the importance of organ specific treatment in addition to the systemic immune suppression.48
Although systemic immunosuppressive therapies for chronic GVHD can improve dry eye symptoms, functional improvement might be limited, especially if lacrimal gland dysfunction has been long term.60
The aim of supportive care for the eye is to lubricate and decrease tear from the surface of the eye, hence improving ocular surface moisture and decreasing ocular surface inflammation and minimize the need for increasing systemic immunosuppressive therapy.
Decreasing Ocular Surface Inflammation
Judicious use of topical steroids to decrease ocular surface inflammation may be necessary. Usually local immunosuppressants are reserved for the control of ocular GVHD exacerbation that might happen during tapering of systemic immunosuppression, control of conjunctival inflammation associated with cicatricial conjunctivitis, and in the early treatment of Stage 4 acute ocular GVHD.60 Topical steroids help to avoid adverse events of prolonged intense systemic immune suppression and maximize the benefit of GVL effect.61,62 However, its use carries the risk of steroid-related complications which include increased intraocular pressure, cataract formation, and silent infectious keratitis.60
Topical cyclosporine can decrease surface ocular inflammation and inhibit surface apoptosis but with minimal effect on lacrimal gland function.63–65
Ocular surface inflammation may also be decreased with autologous and allogeneic serum eye drops, but this treatment is not available for a wide scale use.66,67
The use of preservative-free artificial tears has been shown to minimize superficial punctuate keratopathy and improve the quality of vision by coating and protecting the integrity of the ocular surface.68
Slowly dissolving preparations like 5-mg pellets of hydroxypropyl methylcellulose might decrease the rate of application from once hourly to twice per day and is more convenient.69 Hydroxypropyl cellulose ophthalmic inserts are reported to improve the symptoms of dry eye, ability to perform activities of daily living, and quality of life.70
Although some oral medications (as selective muscarinic agonists such as cevimeline or pilocarpine) increase lubrication by stimulating aqueous tear flow in autoimmune diseases, yet drug interactions, toxicities and patient comorbid conditions might limit therapeutic benefit in cGVHD.71
Meibomian glands produce the outer oil layer of the tear film. Measures to maximize the output of meibomian glands include warm compresses, lid care and use of moisture chamber goggles to minimize local evaporation.72 Doxycycline can be used to treat rosacea blepharitis thereby decreasing inflammation and evaporation.73
Several types of contact lenses have been used in ocular GVHD to reduce friction pain and control evaporation from the inflamed surface including silicon hydrogel, rigid, and gas permeable scleral contact lenses.74
Surgical treatment
In refractory cases, several surgical interventions improve the manifestations of cGVHD of the eye. Tarsorrhaphy decreases the exposed surface area, minimizing dryness and evaporation.75 Scleral lenses may also be beneficial in severe cases, but this treatment is available in only a limited number of centers.76
Temporary or permanent occlusion of the tear-duct puncta may provide additional benefit for patients with severe ocular GVHD to decrease drainage from the surface of the eye. As the temporary occlusion fall out repeatedly, permanent punctal occlusion by lacrimal punctal cauterization was effective with no recanalization and significant improvements in subjective symptoms and the ocular surface environment in cGVHD-related dry eye.77
Patients with cGVHD related severe dry eyes and calcareous corneal degeneration, even with little perforation may benefit from multilayer amniotic membrane transplantation. It may be considered as an alternative for reconstruction of the ocular surface in such patients.78,79 Lamellar or penetrating keratoplasty can be used to correct defects secondary to eye involvement like corneal ulceration or perforation.80 Amniotic membranes may be used for cases with indolent corneal ulcers.
Frequent recurrences and severe dessication are the major drawback of these surgeries.81 Episcleral cyclosporine A implants had been tested in several immune mediated disorders in the eye, such as uveitis and in high-risk keratoplasty rejection. However, its broad utilization in cGVHD of the eye is still under investigations with promising results.82
Conclusion
Graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation. Ocular GVHD is a major cause of long-term morbidity in GVHD. The diagnosis and treatment of acute and chronic systemic GVHD require a multidisciplinary approach for optimal outcome, probably through a combined hematology and ophthalmology visits. Tailoring the immunosuppression, proper assessment of the response and utilization of maximal local and topical measures can improve the ability to perform activities of daily living and enhance the quality of life.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Antin J.H. Acute graft-versus-host disease: inflammation run amok? J Clin Invest. June 2001;107(12):1497–1498. doi: 10.1172/JCI13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara J.L., Levine J.E., Reddy P., Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinen J., Buckley R.H. Transplantation immunology: solid Organ and bone marrow. J Allergy Clin Immunol. February 2010;125(2 Suppl 2):S324–S335. doi: 10.1016/j.jaci.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleakley M.R. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer. 2004;4:371–380. doi: 10.1038/nrc1365. [DOI] [PubMed] [Google Scholar]
- 5.Goulmy E., Schipper R., Pool J., Blokland E., Falkenburg J.H., Vossen J. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med. 1996;334:281–285. doi: 10.1056/NEJM199602013340501. [DOI] [PubMed] [Google Scholar]; Lee S.J., Schubert M.M. Graft-vs-host disease. Crit Rev Oral Biol Med. 1997;8:201–216. doi: 10.1177/10454411970080020701. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara J.L., Deeg H.J. Graft-versus-host disease. N Engl J Med. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]; Parkman R. Chronic graft-versus-host disease. Curr Opin Hematol. 1998;5:22–25. doi: 10.1097/00062752-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 8.Goker H., Haznedaroglu I.C., Chao N.J. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29:259–277. doi: 10.1016/s0301-472x(00)00677-9. [DOI] [PubMed] [Google Scholar]
- 9.Min Chang.-Ki. The pathophysiology of chronic graft-versus-host disease: the unveiling of an enigma. Korean J Hematol. June 2011;46(2):80–87. doi: 10.5045/kjh.2011.46.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo S.B., Lee S.J., Shubert M.M. Graft versus host disease. Crit Rev Oral Biol Med. 1997;8:201–216. doi: 10.1177/10454411970080020701. [DOI] [PubMed] [Google Scholar]
- 11.Lee S.J., Vogelsang G., Flowers M.E. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 12.Cutler C., Giri S., Jeyapalan S., Paniagua D., Viswanathan A., Antin J.A. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001;19:3685–3691. doi: 10.1200/JCO.2001.19.16.3685. [DOI] [PubMed] [Google Scholar]
- 13.Couriel D., Caldera H., Champlin R., Komanduri K. Acute graft-versus-host disease: pathophysiology, clinical manifestations, and management. Cancer. 2004 Nov 1;101(9):1936–1946. doi: 10.1002/cncr.20613. [DOI] [PubMed] [Google Scholar]
- 14.Lee S.J., Klein J.P., Barrett A.J., Ringden O., Antin J.H., Cahn J.Y. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. Jul 15 2002;100(2):406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 15.Sung A.D., Chao N.J. Concise review: acute graft-versus-host disease: immunobiology, prevention, and treatment. Stem Cells Transl Med. Jan 2013;2(1):25–32. doi: 10.5966/sctm.2012-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogelsang B. Chronic graft versus host disease. Br J Haematol. 2004;125:435–454. doi: 10.1111/j.1365-2141.2004.04945.x. [DOI] [PubMed] [Google Scholar]
- 17.Champlin R.E., Schmitz N., Horowitz M.M., Chapuis B., Chopra R., Cornelissen J.J. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR histocompatibility and stem cell sources working committee and the european group for blood and marrow transplantation (EBMT) Blood. Jun 15 2000;95(12):3702–3709. [PubMed] [Google Scholar]
- 18.Shulman H.M., Sullivan K.M., Weiden P.L., McDonald G.B., Striker G.E., Sale G.E. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 19.Martin P.J., Carpenter P.A., Sanders J.E., Flowers M.E. Diagnosis and clinical management of chronic graft-versus-host disease. Int J Hematol. Apr 2004;79(3):221–228. doi: 10.1532/ijh97.03176. [DOI] [PubMed] [Google Scholar]
- 20.Tabbara K.F., Nassr A., Ahmed S.O., Al-Mohareb F., Aljurf M. Acquisition of vernal and atopic keratoconjunctivitis after bone marrow transplantation. Am J Ophthalmol. 2008;146(3):462–465. doi: 10.1016/j.ajo.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Tabbara K.F., AlGhamdi A., AlMohareb F., Ayas M., Chaudhri N., Alsharif F. Ocular findings following allogeneic hematopoietic stem cell transplantation (HSCT) Ophthalmology. 2009;116(9):1624–1629. doi: 10.1016/j.ophtha.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 22.Franklin R.M., Kenyon K.R., Tutschka P.J., Saral R., Green W.R., Santos G.W. Ocular manifestations of graft-vs-host disease. Ophthalmology. 1983;90:4–13. doi: 10.1016/s0161-6420(83)34604-2. [DOI] [PubMed] [Google Scholar]
- 23.Filipovich A.H., Weisdorf D., Pavletic S., Socie G., Wingard J.R., Lee S.J. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. Dec 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Inamoto Y., Chai X., Kurland B.F., Cutler C., Flowers M.E., Palmer J.M. Chronic GVHD consortium. Ophthalmology. 2012;119(3):487–493. [Google Scholar]
- 25.Mitchell S.A., Jacobsohn D., Thormann Powers K.E., Carpenter P.A., Flowers M.E., Cowen E.W. A multicenter pilot evaluation of the national institutes of health chronic graft-versus-host disease (cGVHD) therapeutic response measures: feasibility, interrater reliability, and minimum detectable change. Biol Blood Marrow Transplant. Nov 2011;17(11):1619–1629. doi: 10.1016/j.bbmt.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sáles C.S., Johnston L.J., Ta C.N. Long-term clinical course of dry eye in patients with chronic graft-versus-host disease referred for eye examination. Cornea. Feb 2011;30(2):143–149. doi: 10.1097/ICO.0b013e3181e9b3bf. [DOI] [PubMed] [Google Scholar]
- 27.Nichols K.K., Mitchell G.L., Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23:272–285. doi: 10.1097/00003226-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Kim S.K. Ocular graft versus host disease. In: Krachmer J.H., Mannis M.J., Holland E.J., editors. Cornea. Mosby; St Louis: 2004. pp. 879–885. [Google Scholar]
- 29.Kaiserman I., Or R. Laser photocoagulation for central serous retinopathy associated with graft-versus-host disease. Ocul Immunol Inflamm. 2005;13:249–256. doi: 10.1080/09273940590928625. [DOI] [PubMed] [Google Scholar]
- 30.Lew J., Smith J.A. Ocular graft-versus-host disease. Contemporary Ophthalmology. 2007;6(21):1–8. [Google Scholar]
- 31.Jack M.K., Jack G.M., Sale G.E., Shulman H.M., Sullivan K.M. Ocular manifestations of graft-versus-host disease. Arch Ophthalmol. 1983;101:1080–1084. doi: 10.1001/archopht.1983.01040020082014. [DOI] [PubMed] [Google Scholar]
- 32.Hirst L.W., Jabs D.A., Tutschka P.J., Green W.R., Santos G.W. The eye in bone marrow transplantation. I. Clinical study. Arch Ophthalmol. 1983;101:580–584. doi: 10.1001/archopht.1983.01040010580010. [DOI] [PubMed] [Google Scholar]
- 33.Jabs D.A., Wingard J., Green W.R., Farmer E.R., Vogelsang G., Saral R. The eye in bone marrow transplantation. III. Conjunctival graft vs- host disease. Arch Ophthalmol. 1989;107:1343–1348. doi: 10.1001/archopht.1989.01070020413046. [DOI] [PubMed] [Google Scholar]
- 34.Tuchocka-Piotrowska A., Puszczewicz M., Kołczewska A., Majewski D. Graft-versus-host disease as the cause of symptoms mimicking Sjögren’s syndrome. Ann Acad Med Stetin. 2006;52(Suppl 2):89–93. [PubMed] [Google Scholar]
- 35.Karwacka E., Oldakowska-Jedynak U., Brydak-Godowska J., Paczek L., Kecik D. Pemphigoid-like ocular lesions in patients with graft-versus- host disease following allogeneic bone marrow transplantation. Transplant Proc. 2006;38:292–294. doi: 10.1016/j.transproceed.2005.12.035. [DOI] [PubMed] [Google Scholar]; Hassan A.S., Clouthier S.G., Ferrara J.L., Stepan A., Mian S.I., Ahmad A.Z. Lacrimal gland involvement in graft-versus-host disease a murine model. Invest Ophthalmol Vis Sci. 2005;46:2692–2697. doi: 10.1167/iovs.05-0040. [DOI] [PubMed] [Google Scholar]
- 36.Yeh P.T., Hou Y.C., Lin W.C., Wang I.J., Hu F.R. Recurrent corneal perforation and acute calcareous corneal degeneration in chronic graft-versus-host disease. J Formos Med Assoc. 2006;105:334–339. doi: 10.1016/S0929-6646(09)60125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammadpour M. Progressive corneal vascularization caused by graft-versus-host disease. Cornea. 2007;26:225–226. doi: 10.1097/01.ico.0000243956.22275.8c. [DOI] [PubMed] [Google Scholar]
- 38.Tichelli A., Gratwohl A., Egger T., Roth J., Prunte A., Nissen C. Cataract formation after bone marrow transplantation. Ann Intern Med. 1993;119:1175–1180. doi: 10.7326/0003-4819-119-12-199312150-00004. [DOI] [PubMed] [Google Scholar]
- 39.Suh D.W., Ruttum M.S., Stuckenschneider B.J., Mieler W.F., Kivlin J.D. Ocular findings after bone marrow transplantation in a pediatric population. Ophthalmology. 1999;106:1564–1570. doi: 10.1016/S0161-6420(99)90454-2. [DOI] [PubMed] [Google Scholar]
- 40.Penn E.A., Soong H.K. Cataract surgery in allogeneic bone marrow transplant recipients with graft-versus-host disease(1) J Cataract Refract Surg. 2002;28:417–420. doi: 10.1016/s0886-3350(01)01165-8. [DOI] [PubMed] [Google Scholar]
- 41.Dunn J.P., Jabs D.A., Wingard J., Enger C., Vogelsang G., Santos G. Bone marrow transplantation and cataract development. Arch Ophthalmol. 1993;111:1367–1373. doi: 10.1001/archopht.1993.01090100075031. [DOI] [PubMed] [Google Scholar]
- 42.Kawase E., Azuma N., Shioda Y., Kumagai M. Infantile case of occlusive microvascular retinopathy after bone marrow transplantation. Jpn J Ophthalmol. 2005;49:318–320. doi: 10.1007/s10384-004-0205-5. [DOI] [PubMed] [Google Scholar]
- 43.Coskuncan N.M., Jabs D.A., Dunn J.P., Haller J.A., Green W.R., Vogelsang G.B. The eye in bone marrow transplantation. VI. Retinal complications. Arch Ophthalmol. 1994;112:372–379. doi: 10.1001/archopht.1994.01090150102031. [DOI] [PubMed] [Google Scholar]
- 44.Cheng L.L., Kwok A.K., Wat N.M., Neoh E.L., Jon H.C., Lam D.S. Graft-versus-host disease associated conjunctivalchemosis and central serous chorioretinopathy after bone marrow transplant. Am J Ophthalmol. 2002;134:293–295. doi: 10.1016/s0002-9394(02)01464-2. [DOI] [PubMed] [Google Scholar]
- 45.Kim R.Y., Anderlini P., Naderi A.A., Rivera P., Ahmadi M.A., Esmaeli B. Scleritis as the initial clinical manifestation of graft-versus-host disease after allogenic bone marrow transplantation. Am J Ophthalmol. Jun 2002;133(6):843–845. doi: 10.1016/s0002-9394(02)01425-3. [DOI] [PubMed] [Google Scholar]
- 46.Strouthidis N.G., Francis P.J., Stanford M.R., Graham E.M., Holder G.E., Bird A.C. Posterior segment complications of graft versus host disease after bone marrow transplantation. Br J Ophthalmol. 2003;87:1421–1423. doi: 10.1136/bjo.87.11.1421-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adrean S.D., Puklin J.E. Perforated corneal ulcer with subsequent endophthalmitis in a patient with graft-versus-host disease. Cornea. Jan 2007;26(1):107–108. doi: 10.1097/01.ico.0000240101.66652.36. [DOI] [PubMed] [Google Scholar]
- 48.Couriel D., Carpenter P.A., Cutler C., Bolaños-Meade J., Treister N.S., Gea-Banacloche J. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. Apr 2006;12(4):375–396. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Shikari H., Antin J.H., Dana R. Ocular Graft-versus-host disease: a review. Surv Ophthalmol. May–Jun 2013;58(3):233–251. doi: 10.1016/j.survophthal.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Espana E.M., Shah S., Santhiago M.R., Singh A.D. Graft versus host disease: clinical evaluation, diagnosis and management. Graefes Arch Clin Exp Ophthalmol. May 2013;251(5):1257–1266. doi: 10.1007/s00417-013-2301-z. [DOI] [PubMed] [Google Scholar]
- 51.Westeneng A.C., Hettinga Y., Lokhorst H., Verdonck L., van Dorp S., Rothova A. Ocular graft-versus-host disease after allogeneic stem cell transplantation. Cornea. Jul 2010;29(7):758–763. doi: 10.1097/ICO.0b013e3181ca321c. [DOI] [PubMed] [Google Scholar]
- 52.Ahmad S.M., Stegman Z., Fructhman S., Asbell P.A. Successful treatment of acute ocular graft-versus-host disease with tacrolimus (FK506) Cornea. 2002;21:432–433. doi: 10.1097/00003226-200205000-00024. [DOI] [PubMed] [Google Scholar]
- 53.Dignan F.L., Amrolia P., Clark A., Cornish J., Jackson G., Mahendra P. Haemato-oncology task force of British committee for standards in haematology; British society for blood and marrow transplantation. Diagnosis and management of chronic graft-versus-host disease. Br J Haematol. Jul 2012;158(1):46–61. doi: 10.1111/j.1365-2141.2012.09128.x. [DOI] [PubMed] [Google Scholar]
- 54.Wolff D., Bertz H., Greinix H., Lawitschka A., Halter J., Holler E. The treatment of chronic graft-versus-host disease: consensus recommendations of experts from Germany, Austria, and Switzerland. Dtsch Arztebl Int. 2011 Oct;108(43):732–740. doi: 10.3238/arztebl.2011.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sullivan K.M., Witherspoon R.P., Storb R., Weiden P., Flournoy N., Dahlberg S. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood. Aug 1988;72(2):546–554. [PubMed] [Google Scholar]
- 56.Lee S.J., Flowers M.E. Recognizing and managing chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2008:134–141. doi: 10.1182/asheducation-2008.1.134. [DOI] [PubMed] [Google Scholar]
- 57.Wolff D., Schleuning M., von Harsdorf S., Bacher U., Gerbitz A., Stadler M. Consensus conference on clinical practice in chronic GVHD: second-line treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17:1–17. doi: 10.1016/j.bbmt.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 58.Masaoka T., Shibata H., Kakishita E., Kanamaru A., Takemoto Y., Moriyama Y. Phase II study of FK 506 for allogeneic bone marrow transplantation. Transplant Proc. 1991 Dec;23(6):3228–3231. [PubMed] [Google Scholar]
- 59.Aoki S., Mizote H., Minamoto A., Suzuki M., Mishima H.K., Tanaka H. Systemic FK506 improved tear secretion in dry eye associated with chronic graft versus host disease. Br J Ophthalmol. 2005 Feb;89(2):243–244. doi: 10.1136/bjo.2004.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson M.R., Lee S.S., Rubin B.I., Wayne A.S., Pavletic S.Z., Bishop M.R. Topical corticosteroid therapy for cicatricial conjunctivitis associated with chronic graft-versus-host disease. Bone Marrow Transplant. 2004 May;33(10):1031–1035. doi: 10.1038/sj.bmt.1704453. [DOI] [PubMed] [Google Scholar]
- 61.Marsh P., Pflugfelder S.C. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitissicca in Sjogren syndrome. Ophthalmology. 1999;106:811–816. doi: 10.1016/S0161-6420(99)90171-9. [DOI] [PubMed] [Google Scholar]
- 62.Robinson M.R., Lee S.S., Rubin B.I., Wayne A.S., Pavletic S.Z., Bishop M.R. Topical corticosteroid therapy for cicatricial conjunctivitis associated with chronic graft-versus-host disease. Bone Marrow Transplant. 2004;33:1031–1035. doi: 10.1038/sj.bmt.1704453. [DOI] [PubMed] [Google Scholar]
- 63.Kiang E., Tesavibul N., Yee R., Kellaway J., Przepiorka D. The use of topical cyclosporin A in ocular graft-versus-hostdisease. Bone Marrow Transplant. 1998;22:147–151. doi: 10.1038/sj.bmt.1701304. [DOI] [PubMed] [Google Scholar]
- 64.Sall K., Stevenson O.D., Mundorf T.K., Reis B.L. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–639. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 65.Prabhasawat P., Tesavibul N., Mahawong W. A randomized double-masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunction. Cornea. 2012 Dec;31(12):1386–1393. doi: 10.1097/ICO.0b013e31823cc098. [DOI] [PubMed] [Google Scholar]
- 66.Cho Y.K., Huang W., Kim G.Y., Lim B.S. Comparison of autologous serum eye drops with different diluents. Curr Eye Res. 2013 Jan;38(1):9–17. doi: 10.3109/02713683.2012.720340. [DOI] [PubMed] [Google Scholar]
- 67.Na K.S., Kim M.S. Allogeneic serum eye drops for the treatment of dry eye patients with chronic graft-versus-host disease. J Ocul Pharmacol Ther. Oct 2012;28(5):479–483. doi: 10.1089/jop.2012.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sacchetti M., Lambiase A., Mantelli F., Deligianni V., Leonardi A., Bonini S. Tailored approach to the treatment of vernal keratoconjunctivitis. Ophthalmology. Jul 2010;117(7):1294–1299. doi: 10.1016/j.ophtha.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 69.Hill J.C. Slow-release artificial tear inserts in the treatment of dry eyes in patients with rheumatoid arthritis. Br J Ophthalmol. 1989;73:151–154. doi: 10.1136/bjo.73.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luchs J.I., Nelinson D.S., Macy J.I. LAC-07-01 study group. Efficacy of hydroxypropyl cellulose ophthalmic inserts (LACRISERT) in subsets of patients with dry eye syndrome: findings from a patient registry. Cornea. 2010 Dec;29(12):1417–1427. doi: 10.1097/ICO.0b013e3181e3f05b. [DOI] [PubMed] [Google Scholar]
- 71.Akpek E.K., Lindsley K.B., Adyanthaya R.S., Swamy R., Baer A.N., McDonnell P.J. Treatment of Sjögren’s syndrome-associated dry eye an evidence-based review. Ophthalmology. Jul 2011;118(7):1242–1252. doi: 10.1016/j.ophtha.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 72.Madden L.C., Tomlinson A., Simmons P.A. Effect of humidity variations in a controlled environment chamber on tear evaporation after dry eye therapy. Eye Contact Lens. Mar 2013;39(2):169–174. doi: 10.1097/ICL.0b013e318283dfc6. [DOI] [PubMed] [Google Scholar]
- 73.Foulks G.N., Borchman D., Yappert M., Kakar S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea. Jan 2013;32(1):44–53. doi: 10.1097/ICO.0b013e318254205f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takahide Kikuchi, Parker Pablo.M., Michael Wu, Hwang William Y.K., Carpenter Paul A., Moravec Carina. Flowers. Use of fluid-ventilated gas-permeable scleral lens for management of severe keratoconjunctivitis sicca secondary to chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(9):1016–1021. doi: 10.1016/j.bbmt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alipour F., Kheirkhah A., JabarvandBehrouz M. Use of mini scleral contact lenses in moderate to severe dry eye. Cont Lens Anterior Eye. 2012 Dec;35(6):272–276. doi: 10.1016/j.clae.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 76.Cosar C.B., Cohen E.J., Rapuano C.J., Maus M., Penne R.P., Flanagan J.C. Tarsorrhaphy: clinical experience from a cornea practice. Cornea. Nov 2001;20(8):787–791. doi: 10.1097/00003226-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 77.Yaguchi S., Ogawa Y., Kamoi M., Uchino M., Tatematsu Y., Ban Y. Surgical management of lacrimal punctal cauterization in chronic GVHD-related dry eye with recurrent punctal plug extrusion. Bone Marrow Transplant. Nov 2012;47(11):1465–1469. doi: 10.1038/bmt.2012.50. [DOI] [PubMed] [Google Scholar]
- 78.Jain S., Rastogi A. Evaluation of the outcome of amniotic membrane transplantation for ocular surface reconstruction in symblepharon. Eye (Lond) Dec 2004;18(12):1251–1257. doi: 10.1038/sj.eye.6701379. [DOI] [PubMed] [Google Scholar]
- 79.Peris-Martínez C., Menezo J.L., Díaz-Llopis M., Aviñó-Martínez J.A., Navea-Tejerina A., Risueño-Reguillo P. Multilayer amniotic membrane transplantation in severe ocular graft versus host disease. Eur J Ophthalmol. Apr–Jun 2001;11(2):183–186. doi: 10.1177/112067210101100215. [DOI] [PubMed] [Google Scholar]
- 80.Vanathi M., Sharma N., Sinha R., Tandon R., Titiyal J.S., Vajpayee R.B. Indications and outcome of repeat penetrating keratoplasty in India. BMC Ophthalmol. Nov 2005;2(5):26. doi: 10.1186/1471-2415-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Claesson M., Armitage W.J. Clinical outcome of repeat penetrating keratoplasty. Cornea. July 2013;32(7):1026–1030. doi: 10.1097/ICO.0b013e31828a2810. [DOI] [PubMed] [Google Scholar]
- 82.Kim H., Csaky K.G., Gilger B.C., Dunn J.P., Lee S.S., Tremblay M. Preclinical evaluation of a novel episcleral cyclosporine implant for ocular graft-versus-host disease. Invest Ophthalmol Vis Sci. 2005;46:655–662. doi: 10.1167/iovs.04-1076. [DOI] [PubMed] [Google Scholar]



