Abstract
Apical surgery has become a standard of care for tooth maintenance if conventional endodontic retreatment is not possible or associated with risks. However, in certain situations, the outcome of apical surgery may be compromised due to the extent or location of the periapical or periradicular lesions. The present review article including clinical and experimental studies reports and discusses the outcome of regenerative techniques (RT) in conjunction with apical surgery, with regard to the type of periradicular lesions:
Apical lesions
The majority of studies have shown no benefit for healing in test sites treated with RT compared to control sites treated without RT. The use of a radio-opaque bone filler/substitute may even compound the radiographic interpretation of periapical healing. Currently, the use of RT for lesions <10 mm limited to the apical area is not warranted.
Through-and-through lesions
All reviewed studies demonstrated a better outcome for test sites with RT compared to the control sites without RT; hence the use of RT for treatment of tunnel lesions in apical surgery is recommended.
Apico-marginal lesions
All clinical studies assessed cohorts without controls, and, therefore, no firm conclusion about the benefit of RT for treatment of apico-marginal lesions in conjunction with apical surgery can be drawn. However, the experimental animal studies have shown that healing of teeth with apico-marginal lesions appears to benefit from RT.
Keywords: Apical surgery, Regenerative technique, Bone regeneration, Bone defect
1. Introduction
Apical surgery refers to the surgical management of a tooth with a periapical or periradicular lesion that cannot be resolved with an orthograde endodontic approach. Apical surgery is often considered as a last resort to preserve a tooth when conventional endodontic retreatment is not feasible or is associated with therapeutic risks (Kim and Kratchman, 2006). The treatment alternative would be tooth extraction, or in multi-rooted teeth, root or tooth resection.
Indications for apical surgery include (European Society of Endodontology): (I) radiological findings of apical periodontitis and/or symptoms associated with an obstructed canal; (II) extruded material with clinical or radiological findings of apical periodontitis and/or symptoms continuing over a prolonged period; (III) persisting or emerging disease following root-canal treatment; and (IV) perforation of the root or the floor of the pulp chamber and where it is impossible to treat from within the pulp cavity. According to Wu et al. (2006) surgical intervention is warranted in cases with infection remaining in inaccessible apical areas, extraradicular infection including apically extruded dentin debris with bacteria present in dentinal tubules, true radicular cysts, and foreign body reactions.
Contraindications for apical surgery include the following: the tooth has no function (no antagonist, no strategic importance serving as a pillar for a fixed prothesis), the tooth cannot be restored, the tooth has compromised periodontal support, or the tooth has a vertical root fracture. Additional general contraindications may be an uncooperative patient or a patient with a compromised medical condition that precludes from an oral surgical intervention.
The main objective of apical surgery is to create an optimal environment for periradicular tissue healing. This is usually accomplished by removing pathology or removing inaccessible parts of the root canal system, and by preventing reinfection from the root canal system. For this purpose, a retrograde cavity is prepared following root-end resection, and a filling material is placed into this cavity to completely seal the root canal system at the resection level (European Society of Endodontology, 2006). The healing outcome of apical surgery is normally assessed by a clinical and radiographic re-examination 1 year post-surgery (Zuolo et al., 2000).
The technique of apical surgery was considerably enhanced via the introduction of microsurgical principles in the mid-1990s (von Arx, 2005). Among those principles, the most important developments include the use of microsurgical instruments for root-end cavity preparation (von Arx and Walker, 2000) and the utilization of magnification tools, such as the surgical microscope or endoscope, as visual aids (Kim and Kratchman, 2006). Both innovations have simplified the surgical technique and have improved the treatment outcome of apical surgery (Kim and Kratchman, 2006). Successful healing has been reported to be more frequent for the microsurgical technique than for the conventional technique (von Arx et al., 2010).
Following the establishment of (guided) tissue regeneration techniques in periodontology and implant dentistry, there has been growing interest in using this treatment option as an adjunct in apical surgery. An increasing number of clinicians and researchers have advocated the use of regenerative techniques (RT) in apical surgery (for review, see von Arx and Cochran, 2001).
The objective of the present paper is to provide the reader with information and data from clinical and experimental studies on the outcome and benefit of RT in apical surgery.
2. Clinical relevance of RT in apical surgery
Tissue regeneration is defined as reproduction or reconstruction of a lost, injured or surgically removed part such that the architecture and function of the lost, injured or removed tissues are completely restored (Karring et al., 2003). While it is obvious that the resected root end cannot be restored, tissue regeneration in apical surgery means the following: (I) regrowth of alveolar and peri-radicular bone, (II) re-establishment of a periodontal ligament at the resection plane and at the surgically exposed root surface, and (III) formation of new cementum at the cut root-face. However, the nature of regenerated periapical tissues after the use of RT in apical surgery remains unknown, despite radiographs showing some evidence of PDL space. Therefore, histology would be the ultimate standard in evaluating true tissue regeneration, but this is not possible in clinical studies that usually assess the healing outcome with clinical and radiographic parameters (Zuolo et al., 2000). In contrast, experimental studies would allow for a histological and histomorphometrical evaluation of healing, but observation periods of healing (in experimental studies) are limited. From a clinical perspective, it would be interesting to correlate radiographic with histological (and histomorphometrical) healing assessment. However, specific long-term effects (coronal leakage, crack formation) cannot be implemented in animal studies that normally last to a maximum of 6 months. Therefore, caution must be exercised when transferring data from experimental animal studies (with research-driven study designs) to the clinical situation in humans with numerous confounding factors that may influence the treatment outcome.
Tissue regeneration techniques are based on cell differentiation, cell proliferation, and induction and/or conduction of tissue formation. These effects are obtained with various protocols: the use of bone substitutes, barrier membranes, growth factors, or a combination of such agents and materials. Since it is beyond the scope of this paper to review the biological principles of tissue regeneration, readers are encouraged to read the pertinent literature in order to understand the basic principles (Bashutski and Wang, 2009; Lin et al., 2010).
The reasons for using RT in apical surgery are: (I) to accelerate periapical or periradicular healing, and (II), to allow healing in compromised clinical situations. Accelerated healing is the aim for teeth with provisional restorations that should be replaced by definitive restorations as soon as successful healing has been confirmed clinically and radiographically. Clinical data show that the healing outcome of apical surgery in teeth with permanent restorations appears to be better than in teeth with temporary restorations (Wang et al., 2004). However, from a cost perspective, placement of a new single crown or fixed partial prosthesis on a tooth that has undergone apical surgery should be postponed until a final assessment of healing can be made.
Several compromised clinical situations may be encountered during apical surgery. These include (I) large apical (cystic) lesions, (II) through-and-through (“tunnel”) lesions, and (III) apico-marginal lesions. In large apical (cystic) lesions, and in particular, in periapical lesions—also called “tunnel” lesions—extending from the facial to the lingual bone plates, the fast proliferation of soft tissue from the facial and lingual aspects may interfere with bone ingrowth from approximal bone surfaces, resulting in a fibrous connective tissue core. This type of healing (also called “scar tissue healing” or “incomplete healing”) is frequently observed after treatment of through-and-through defects in maxillary lateral incisors, and also following enucleation of large cystic lesions (Molven et al., 1996). Consequently a radiographic diagnosis is difficult to establish in such cases with “scar tissue healing”. In certain situations with unclear clinical signs and symptoms, the clinician might tend to perform a resurgery only to encounter scar tissue but no inflammatory tissue was present at the apical area of a tooth previously treated with apical surgery.
An apico-marginal lesion, or endo-perio lesion, carries the risk of epithelial downgrowth along the denuded root surface following apical surgery (Skoglund and Persson, 1985). The apical extension of the junctional epithelium may result in the establishment or recurrence of the communication between the marginal periodontium and the apical area, thus compromising the healing outcome and carrying a risk of gingival recession with esthetic concern.
3. Classification of periapical or periradicular lesions
Classification of periradicular lesions as they are encountered during apical surgery is helpful (I) to categorize the type of lesion, (II) to select the appropriate treatment, and (III) to use a defined classification of lesions for comparing different treatment approaches in clinical or experimental studies. Various classifications of periradicular lesions have been described by von Arx and Cochran (2001), Dietrich et al. (2002), and Kim and Kratchman (2006).
For the present review, clinical and experimental studies have been divided into three groups according to the type of periradicular lesion:
-
–
The lesion is limited to the periapical area (Fig. 1).
-
–
The lesion has eroded the lingual/palatal cortex (with or without erosion of buccal cortex), resulting in a through-and-through (tunnel) defect (Fig. 2).
-
–
An apico-marginal lesion is present with complete denudation of the buccal root surface (Fig. 3).
Figure 1.
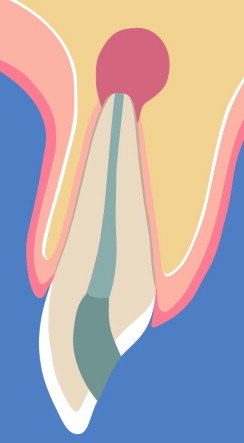
Schematic illustration of a lesion limited to the periapical area.
Figure 2.
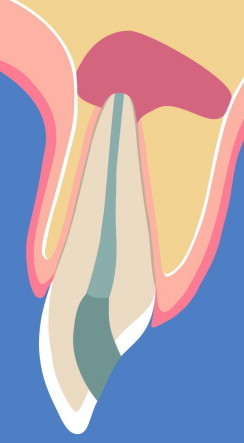
Schematic illustration of a through-and-through (tunnel) lesion.
Figure 3.
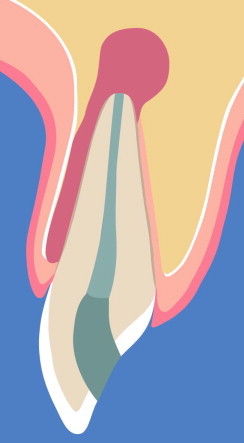
Schematic illustration of an apico-marginal lesion with complete denudation of the buccal root surface.
4. Methodology of literature search
The main inclusion criterion for the selection of a clinical or experimental study was that it had evaluated the outcome of apical surgery in relation to the use of regenerative techniques. The literature search with PubMed and Cochrane databases was conducted in April 2010, including papers published in English since 1980. The search strategy was based on the following MeSH terms: “(apical surgery) OR (apical microsurgery) OR (periapical surgery) OR (periradicular surgery) OR apicoectomy OR apicectomy OR (tooth apex surgery) NOT (case report OR case reports) NOT (in vitro) AND (regenerative technique OR guided bone regeneration OR guided tissue regeneration OR membrane OR growth factor OR bone filler OR bone substitute)”.
Additionally, a hand search was performed of the following journals: Journal of Endodontics, International Endodontic Journal, Oral Surgery Oral Medicine Oral Pathology (name changed to Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics in 1995), Endodontics and Dental Traumatology (name changed to Dental Traumatology in 2001), Journal of Oral and Maxillofacial Surgery, and International Journal of Oral and Maxillofacial Surgery.
Inclusion criteria: The assessed outcome had to be periapical healing based on radiographic and clinical parameters for clinical studies, or periapical healing based on radiographic, histologic or histomorphometric parameters for experimental studies; the studies had to have a minimum of 10 teeth with a minimum follow-up period of 6 months for clinical studies and of 8 weeks for experimental studies.
A total of 11 clinical and 10 experimental studies fulfilled the inclusion criteria (Tables 1–3) with one clinical study (Taschieri et al., 2007) and one experimental study (Murashima et al., 2002) each reporting the outcome of RT in apical surgery on various types of periradicular lesions. Table 4 summarizes the characteristics of the regenerative materials used in the reviewed studies.
Table 1.
Regenerative techniques in cases with apical surgery of lesions limited to the periapical area.
| Author(s) | Species (initial n/final n) | Teeth (initial n/final n) | Study design | Follow-up | Assessment | Regenerative technique (n sites) | Successful outcome | Comments | Strengths of study | Weaknesses of study |
|---|---|---|---|---|---|---|---|---|---|---|
| Stassen et al. (1994) | Humans (89/78) | 112/101 | Randomized clinical trial | 27 months | Clinical and radiographic healing | Test: IBBMa (Bio-Oss®) (45); Control (56) | Test: 89%, Control: 64% (p = 0.057) | Randomization of treatment; large sample size per group | No standardization of radiographic evaluation | |
| Pecora et al. (1995) | Humans (20/20) | 20/20 | Randomized clinical trial | 6 and 12 months | Clinical and radiographic healing | Test: ePTFEa membrane (Gore-tex®) (10); Control (10) | Test 6 ma: 80%, Test 12 m: 90%, Control 6 m: 20%, Control 12 m: 90% | Randomization of treatment; standardization of radiographic evaluation | Small sample size per group; three different root-end filling materials were used, but not specified | |
| Tobon et al. (2002) | Humans (28/25) | 30/26 | Randomized clinical trial | 12 months | Radiographic healing; histology | Test 1: ePTFE membrane (Gore-Tex®) (9); Test 2: resorbable hydroxyapatite (OsteoGen®) and ePTFE membrane (Gore-Text®) (8); Control (9) | Test 1: 77.8%, Test 2: 100%, Control: 88.9% | Histology: Test 1: 62.5% bone, 12.5% scar tissue, 25% granuloma; Test 2: 100% bone; Control: 25% bone, 25% scar tissue, 50% granuloma | Randomization of treatment; study included histology | Small sample size per group; two patients each had two surgical sites; no standardization of radiographic evaluation |
| Garrett et al. (2002) | Humans (25/13) | 25/13 | Randomized clinical trial | 3, 6, and 12 months | Radiographic change of densitometric ratio | Test: polylactide membrane (Guidor®) (9); Control (4) | Test 3 m: 0.89, Test 6 m: 0.94, Test 12 m: 0.97, Control 3 m: 0.82, Control 6 m: 0.97, Control 12 m: 0.91 (p > 0.05) | Ratio = density of apical area (region of interest) divided by density of normal bone (region of reference) | Randomization of treatment; standardization of radiographic evaluation | Small sample size per group; root-end filling was not always placed; large drop-out rate (48%) |
| Taschieri et al. (2007) | Humans (NAa/NA) | NA/38 | Randomized clinical trial | 12 months | Clinical and radiographic healing | Test: IBBM (Bio-Oss®) and collagen membrane (Bio-Gide®) (16); Control (22) | Test: 87.5%; Control: 81.8% (p N/A) | Data extracted from pooled results of apical and through-and-through lesions | Randomization of treatment | Initial sample size per group not specified; two centers involved; no standardization of radiographic evaluation |
| Pantchev et al. (2009) | Humans (131/NA) | 186/147 | Retrospective clinical study | >33 months | Clinical and radiographic healing | Test: Alloplastic glass particulate (PerioGlas®) (68); Control (79) | Test: 73.5%, Control: 83.5% (p > 0.05) | Definition of “uncertain” healing: incomplete but some bone regeneration and presence of some radiolucency | Large study sample | Retrospective study; in many patients, more than one tooth was treated; no standardization of radiographic evaluation; drop-out rate 21% |
| Maguire et al. (1998) | Cats (12/12) | 24/24 (maxillary cuspids) | Experimental | 12 weeks | Histology, histomorphometry | Test 1: polylactide membrane (Guidor®) (8); Test 2: osteogenic protein (hOP-1) (8); Control (8) | Test 1: 14.4%, Test 2: 18.4%, Control: 16.6% (percentages = osseous healing within region of interest 1.6 mm apical to resected root end) (p > 0.05) | Root-canal treatments and apical surgeries were performed in same session | Control group; experimental pulpal infection; standardization of defects | Short study period; small sample size per group |
| Murashima et al. (2002) | Dogs (11/11) | 22/22 (mandibular premolars #4) | Experimental split-mouth (randomized) | Two subgroups of healing: 8 weeks and 16 weeks | Histology, histomorphometry | Test: Calcium sulfate (Surgiplaster®) (11); Control (11) | Histology at 16 weeks: cortical bone in test sites with little concavity; control sites with obvious concavity. Histomorphometry: bone volume per tissue volume at 16 weeks: Test: 73.0%, Control: 55.6% (p < 0.001) | Root-canal treatment, creation of periapical defects, and apical surgery with root-end filling were all carried out in the same session (this study also assessed through-and-through defects, see Table 2, and apico-marginal defects, see Table 3) | Control group; standardization of defects | Short study period; surgically created defects; different types of lesions tested within same study |
| Yoshikawa et al. (2002) | Dogs (12/12) | 48/48 (mandibular premolars #3 and premolars #4) | Experimental, randomized | 4, 8 and 16 weeks (each with four dogs) | Histology, histomorphometry | Test 1: ePTFE membrane (Gore-Tex®) (n N/A); Test 2: PLGAa membrane (product and n N/A); Test 3: collagen membrane (product and n N/A); Test 4: calcium sulfate (Surgiplaster®) (n N/A); Control (n N/A) | Results after 16 weeks: Test 1: 54.8%, Test 2: 21.2%, Test 3: 34.0%, Test 4: 48.9%, Control: 37.4% (percentages = osseous healing of access window) Test 1 better than Test 2 (p < 0.01), Test 3 (p < 0.05) and control (p < 0.05); Test 4 better than Test 2 (p < 0.01) and control (p < 0.05) | Root-canal treatments and apical surgeries were performed in same session | Control group; standardization of defects | Short study period; surgically created defects; sample size per group not specified |
| Apaydin and Torabinejad (2004) | Dogs (4/4) | 24/24 (mandibular premolars #2–4) | Experimental, randomized | 16 weeks | Histology, histomorphometry (region of interest 1.6 × 2.2 mm apical of resected root end) | Test: calcium sulfate (Capset®) (12); Control (12) | Test: 54.6%, Control: 53.1% (percentages = bone density in region of interest) | Apical surgeries were performed 2 weeks after root-canal treatment | Control group; split-mouth design; standardization of defects | Short study period; surgically created defects |
| Bergenholtz et al. (2006) | Monkeys (6/6) | 48/46 (maxillary and mandibular central and lateral incisors) | Experimental, randomized | 1 month (mandibular incisors); 4.5 months (maxillary incisors) | Radiographs, histology, histometry | Test: rhBMPa-2 (23); Control (23) | No difference in the extent of bone fill or extent and quality of cementum regeneration was observed when comparing test with control | Root-canal treatments and apical surgeries were performed in same session | Control group; split-mouth design; sample size per group; experimental pulpal infection | Short study period; defect size not mentioned; no information about root-end filling material |
| Bernabé et al. (2010) | Dogs (6/6) | 48/43 (roots) (mandibular premolars #2–3) | Experimental, comparative (no randomization) | 6 months | Histology Histomorphometry | Test 1: bovine cortical membrane (Gen-Derm®) (n N/A), Test 2: IBBM (Gen-Ox®) (n N/A), Test 3: IBBM (Gen-Ox®) and bovine cortical membrane (Gen-Derm®) (n N/A) Control (n N/A) | No statistically significant differences were noted for inflammatory cell infiltrate and periapical healing when comparing test and control sites | Root-canal treatments and apical surgeries were performed in same session | Control group; experimental pulpal infection; standardization of defects | No randomization of treatment; sample size per group not specified |
IBBM, inorganic bovine bone mineral; ePTFE, expanded polytetrafluoroethylene; NA, not available; PLGA, polylactic-co-glycolic-acid; rhBMP, recombinant human bone morphogenetic protein; m, months; N/A, not available.
Table 2.
Regenerative techniques in cases with apical surgery of through-and-through (“tunnel”) lesions.
| Author(s) | Species (initial n/final n) | Teeth (initial n/final n) | Study design | Follow-up | Assessment | Regenerative technique (n sites) | Successful outcome | Comments | Strengths of study | Weaknesses of study |
|---|---|---|---|---|---|---|---|---|---|---|
| Pecora et al. (2001) | Humans (20/18) | 20/18 | Randomized clinical trial | 12 months | Radiographic healing | Test: Calcium sulfate (Surgiplaster®) (9); Control (9) | Test: complete healing (7/9), incomplete healing (2/9); Control: complete healing (3/9), incomplete healing (5/9), unsatisfactory healing (1/9) | Randomization of treatment | Small sample size per group; no standardization of radiographic evaluation | |
| Taschieri et al. (2007) | Humans (NAa/NA) | NA/21 | Randomized clinical trial | 12 months | Clinical and radiographic healing | Test: IBBMa (Bio-Oss®) and collagen membrane (Bio-Gide®) buccally only (8);Control (13) | Test: 75.0%; Control: 61.5% (p N/A) | Data extracted from pooled results of apical and through-and-through lesions | Randomization of treatment | Initial sample size per group not specified; two centers involved; small final sample size per group; no standardization of radiographic evaluation |
| Taschieri et al. (2008) | Humans (27/25) | 34/31 | Randomized clinical trial | 12 months | Clinical and radiographic healing | Test: IBBM (Bio-Oss®) and collagen membrane (Bio-Gide®) buccally only (17); Control (14) | Test: 88.0%, Control: 57.1% (p = 0.02) | Cases with incomplete and uncertain radiographic healing were pooled because of difficult differentiation due to opacity of filler material | Randomization of treatment | Some patients had more than one tooth treated; no standardization of radiographic evaluation |
| Dahlin et al. (1990) | Monkeys (7/6) | 14/12 (maxillary lateral incisors) | Experimental, split-mouth (non-randomized) | 3 months | Histology | Test: ePTFEa (Gore-Tex®) membrane facially and palatally (6); Control (6) | Test: all defects had healed with almost complete closure by newly formed bone; Control: defects were filled with fibrous connective tissue | Maxillary lateral incisors were treated endodontically 3 months before apicectomy and creation of transosseous defects (Ø 8 mm) | Control group; standardization of defects | Short study period; small sample size per group; surgically created defects; no root-end fillings placed |
| Baek and Kim (2001) | Ferrets (8/8) | 16/16 (mandibular premolars) | Experimental | Two subgroups of healing: 6 weeks and 12 weeks | Histology, radiography | Test 1: ePTFE membrane (Gore-Tex®) buccally and lingually (4); Test 2: Polyglactin 910 (PGLAa) membrane (Vicryl®) buccally and lingually (4);Test 3: polylactide membrane (Guidor®) buccally and lingually (4); Control (4) | Histology at 12 weeks: Test 1: defects were filled with regenerated immature bone, Test 2: defects showed extensive lamellar bone healing, Test 3: only limited fibered bone regeneration, Control: connective tissue infiltration Radiography (%) of tissue regeneration after 12 weeks: Test 1: 95%, Test 2: 95%, Test 3: 90%, Control: 80% | Root-canal treatment, creation of transosseous defects, and apicectomies were all carried out in the same session | Control group; standardization of defects | Short study period; small sample size per group; surgically created defects; no root-end fillings placed |
| Murashima et al. (2002) | Dogs (11/11) | 22/22 (mandibular premolars #4) | Experimental split-mouth (randomized) | Two subgroups of healing: 8 weeks and 16 weeks | Histology, histomorphometry | Test: Calcium sulfate (Surgiplaster®) (11); Control (11) | Histology at 16 weeks: Test and control defects almost closed with newly formed bone; cortical bone in control sites more concave than in test sites. Histomorphometry: Bone volume per tissue volume at 8 weeks: Test: 68.4%, Control: 51.6% (p < 0.001); Bone volume per tissue volume at 16 weeks: Test: 81.3%, Control: 64.5% (p < 0.001) | Root-canal treatment, creation of periapical defects, and apical surgery with root-end filling were all carried out in the same session. This study also assessed periapical defects (see Table 1) and apico-marginal defects (see Table 3) | Control group; standardization of defects | Short study period; surgically created defects; different types of lesions tested within same study |
IBBM, inorganic bovine bone mineral; ePTFE, expanded polytetrafluoroethylene; NA, not available; PLGA, polylactic-co-glycolic-acid.
Table 3.
Regenerative techniques in cases with apical surgery of apico-marginal lesions.
| Author(s) | Species (initial n/final n) | Teeth (initial n/final n) | Study design | Follow-up | Assessment | Regenerative technique (n sites) | Successful outcome | Comments | Strengths of study | Weaknesses of study |
|---|---|---|---|---|---|---|---|---|---|---|
| Dietrich et al. (2003) | Humans (24/22) | 25/23 | Clinical cohort | 12 months | Clinical and radiographic healing | IBBMa (Bio-Oss®) and collagen membrane (Bio-Gide®) (23); No controls | 82.6% | Only 16% of cases received root-end fillings; 84% had orthograde root-canal filling during apical surgery | Low drop-out rate (8%) | No control group; no standardization of radiographic evaluation; majority of teeth did not receive root-end fillings |
| Marin et al. (2006) | Humans (30/30) | 30/30 | Comparative clinical study (randomized) | 12 months | Clinical and radiographic healing | Test 1: periosteal graft (15); Test 2: polyglactin 910 PGLAa membrane (Vicryl®) (15); No controls | Test 1: 87%, Test 2: 87% | No drop-outs; comparison of two different treatment options | No control group; no standardization of radiographic evaluation | |
| Kim et al. (2008) | Humans (NAa/NA) | 42/30 | Cohort study | 1–5 years | Clinical and radiographic healing | Lesion type E without filler (11); Lesion type F received calcium sulfate (product N/A) and collagen tape (CollaTape®) buccally (19) | Lesion type E: 63.6%, Lesion type F: 73.7% | Data for lesion types E and F extracted from study that assessed lesions A–F [lesion classification according to Kim and Kratchman (2006)] | No control group; initial sample size not specified; no standardization of radiographic evaluation; three different root-end filling materials used; different types of lesions received different treatments | |
| Douthitt et al. (2001) | Dogs (9/9) | 36/36 (mandibular premolars #3 and #4) | Experimental (randomized) | Two subgroups of healing: 9 weeks and 27 weeks | Histology, histomorphometry | Test: polylactide membrane (Guidor®) (18); Control (18) | Histology at 27 weeks: Test sites had greater width and height of new bone on the buccal root surface than control sites; an increased length of junctional epithelium was a frequent finding in the control group. Histomorphometry at 27 weeks over denuded root surface: Connective tissue attachment: test 4.15 mm, control 1.81 mm (p < 0.05); bone: test 2.49 mm, control 0.66 mm (p < 0.05). Complete bony fill of periradicular defect: test 89%, control 68.8% | Root-canal treatment, creation of apico-marginal defects, and apicoectomies were all carried out in the same session | Control group; fair sample size per group; standardization of defects | Surgically created defects; no root-end fillings were placed |
| Murashima et al. (2002) | Dogs (11/11) | 22/22 (mandibular premolars #3) | Experimental split-mouth (randomized) | Two subgroups of healing: 8 weeks and 16 weeks | Histology | Test: Calcium sulfate (Surgiplaster®) (11); Control (11) | Histology at 8 weeks: Histology at 16 weeks: No bone observed on buccal root surface in both test and control sites; some control defects exhibited epithelial downgrowth and inflammatory cell infiltration | Root-canal treatment, creation of periapical defects, and apical surgery with root-end filling were all carried out in the same session. This study also assessed periapical defects (see Table 1) and through-and-through defects (see Table 2) | Control group; standardization of defects | Short study period; surgically created defects; different types of lesions tested within same study |
| Britain et al. (2005) | Dogs (4/4) | (24/24) (mandibular premolars #2–4) | Experimental (non-randomized) | 6 months | Histology, histomorphometry | Test 1: collagen membrane (Bio-Gide®) (8); Test 2: IBBM (Bio-Oss®) and collagen membrane (Bio-Gide®) (8);Control (8) | Apical extension of junctional epithelium and buccal radicular bone height: No significant differences Percentage of buccal root surface with cementum: Test 1: 79.6%, Test 2: 83.8%, Control: 60.1% (both p < 0.05) | Pulpal necrosis was induced along with surgical removal of radicular buccal bone; after 6 weeks root-canal instrumentation (but without orthograde obturation) and apical surgery with root-end filling were performed | Control group; experimentally induced infection; standardization of defects | Sample size per group; surgically created defects |
IBBM, inorganic bovine bone mineral; ePTFE, expanded polytetrafluoroethylene; NA, not available; PLGA, polylactic-co-glycolic-acid.
Table 4.
Characteristics of regenerative materials used in the reviewed clinical and experimental studies.
| Regenerative material | Product name | Action | Clinical studies | Experimental studies |
|---|---|---|---|---|
| Membranes | ||||
| ePTFE-membrane (expanded Polytetra-fluoroethylene), synthetic and non-resorbable | Gore-Tex® | Barrier membrane to provide a protected space | Pecora et al. (1995) and Tobon et al. 2002 | Yoshikawa et al. (2002), Dahlin et al. (1990) and Baek and Kim (2001) |
| PLA-membrane (polylactide), synthetic and resorbable | Guidor® | Membrane to provide a temporary protected space | Garrett et al. (2002) | Maguire et al. (1998), Baek and Kim (2001) and Douthitt et al. (2001) |
| Collagen-membrane (collagen type I and III), porcine-derived and resorbable | Bio-Gide® | Bilayer membrane to provide a temporary protected space | Taschieri et al. (2007, 2008), Dietrich et al. (2003) and Britain et al. 2005 | |
| Lyophilized decalcified cortical bone membrane, bovine-derived and resorbable | Gen-derm® | Membrane to provide a temporary protected space | Bernabé et al. (2010) | |
| Polyglactin 910-membrane (co-polymer of 90% glycolide and 10% l-lactide), synthetic and resorbable | Vicryl® | Membrane to provide a temporary protected space | Marin et al. (2006) | Baek and Kim (2001) |
| Collagen tape (collagen type I), bovine-derived and resorbable | CollaTape® | Tape to provide a temporary protected space | Kim et al. (2008) | |
| Bone fillers | ||||
| IBBM particulate (inorganic bovine bone mineral), bovine-derived and non-resorbable | Bio-Oss® | Osteoconductive filler | Stassen et al. (1994), Taschieri et al. (2007, 2008), Dietrich et al. (2003) and Britain et al. (2005) | |
| Lyophilized bone matrix, bovine-derived | Gen-ox® | Osteoconductive and osteoinductive filler | Bernabé et al. (2010) | |
| Synthetic non-ceramic hydroxyapatite, resorbable | Osteogen® | Osteoconductive filler | Tobon et al. (2002) | |
| Synthetic glass particles (calcium phospho silicate) resorbable | PerioGlas® | Osteostimulative and osteoconductive filler | Pantchev et al. (2009) | |
| CS particulate (calcium sulfate), synthetic and resorbable | Surgiplaster®∗, Capset®∗∗ | Osteoconductive filler | ∗Pecora et al. (2001) | ∗Murashima et al. (2002), ⁎Yoshikawa et al. (2002) and ⁎⁎Apaydin and Torabinejad (2004) |
| Growth factors | ||||
| hOP-1 (human osteogenic protein) (carrier: collagen matrix) | – | hOP-1, also known as BMP-7, belongs to the TGF-beta superfamily (transforming growth factors) and is osteostimulative | Maguire et al. (1998) | |
| rhBMP-2 (recombinant human bone morphogenetic protein) (carrier: collagen matrix) | – | BMP-2 belongs to the TGF-beta superfamily (transforming growth factors) and is osteostimulative | Bergenholtz et al. (2006) | |
5. RT in cases undergoing apical surgery for lesions limited to the periapical area (Table 1)
5.1. Clinical studies
The very first comparative clinical study on the use of a regenerative technique in apical surgery was published by Stassen et al. (1994). Periapical defects of consecutive patients requiring apical surgery were randomly allocated to receive granular inorganic bovine bone mineral (IBBM) or nothing. Cases were followed for 2 years. Healing was assessed clinically and radiographically. Cases treated with IBBM demonstrated a healing rate of 64%, whereas 89% of the control cases healed (p = 0.057). Although the difference did not reach statistical significance, the difference in outcome is of clinical importance. The authors concluded that there was no benefit of IBBM placement in bony defects following apical surgery. (Strengths of study: randomized clinical trial; large study sample. Weakness of study: no standardization of radiographic evaluation.)
Pecora et al. (1995) assessed the advantages of guided tissue regeneration (GTR) in apical surgery of cases with radiographically measured periapical lesions (largest diameter ⩾10 mm). In test sites, ePTFE membranes (Goretex®) were placed to cover the bony defects, while control sites received no membrane. The treatment approach was chosen at random. At 6 months, only two of the 10 control cases had healed, whereas eight of 10 test cases with membrane applications had healed. However, at the 1-year follow-up, all except one case in each group showed healing with periapical radiography. Overall, the placement of an ePTFE membrane appeared to accelerate the postsurgical bony healing. (Strengths of study: randomized clinical trial; standardization of radiographic evaluation. Weaknesses of study: only 10 teeth per group; three different root-end filling materials were used, but not specified.)
Tobon et al. (2002) compared three different RT in apical surgery: placement of an ePTFE membrane over the bony defect (test 1), placement of a resorbable hydroxyapatite filler into the bony defect with ePTFE membrane coverage (test 2), and a conventional approach without a filler/membrane (control). The radiographic healing after 1 year was determined as successful in 77.8% for test group 1, 100% for test group 2, and 88.9% for the control group. The histological evaluation of biopsies taken during the 1-year re-entry procedures (membrane removal) demonstrated marked differences between the groups: test group 1 had trabecular bone in 62.5% of the biopsy sample, scar tissue in 12.5%, and granuloma in 25%; test group 2 had trabecular bone in 100%; and controls had trabecular bone in 25%, scar tissue in 25%, and granuloma in 50%. (Strengths of study: randomized clinical trial; histological samples obtained. Weaknesses of study: only 10 teeth per group, and two patients had two surgical sites; no standardization of radiographic evaluation.)
Garrett et al. (2002) evaluated the effect of a bioresorbable polylactide membrane on changes in radiodensity following apical surgery. Three-month, 6-month, and 12-month recall radiographs were compared with radiographs taken immediately after surgery. Digital imaging was used to calculate a densitometric ratio that gave a numerical estimation of osseous healing. The densitometric ratio was similar (p > 0.05) for membrane cases (0.97) and control cases (0.91). The authors concluded that the use of a (polylactide) membrane had no beneficial effect on the bony healing of the periapical defects, and the additional expense to the patient was not warranted. (Strengths of study: randomized clinical trial; standardization of radiographic evaluation. Weaknesses of study: small sample size per group; root-end filling was only placed where necessary; large drop-out rate of 48%.)
Taschieri et al. (2007) assessed the treatment outcome 1 year after apical surgery in cases with periapical lesions (diameter ⩾10 mm), with or without RT. After completion of root-end filling, in test cases the bony defect was filled with anorganic bovine bone particulate (Bio-Oss®) and was covered with a collagen membrane (Bio-Gide®). In the control cases, neither a graft nor membrane materials were used. Healing rates did not significantly differ between the test group (87.5%) and the control group (81.8%). The conclusion was that guided tissue regeneration had no beneficial effect on healing outcome in large periradicular lesions of strictly endodontic origin. (Strength of study: randomized clinical trial. Weaknesses of study: initial sample size per group not specified; no standardization of radiographic evaluation, two centers involved.)
A possible benefit of placing alloplastic glass particles into the bony crypt following apical surgery was evaluated by Pantchev et al. (2009). Control sites remained empty. The retrospective study assessed healing after 9 months to 2 years (short-term) and after 33 months to more than 4 years (long-term). A statistically significant difference (p < 0.05) was observed over the short term, with success in 72% of the test sites and 56.4% of the control sites. At the long-term re-examination, 83.5% of the control sites and 73.5% of the test sites demonstrated healing (p > 0.05). The authors concluded that the glass particulate as bone substitute did not significantly improve the healing after apical surgery. The superior short-term success rate of the test compared to the control sites may be due to the radiopacity of the bony filler, giving a false impression of bony healing on periapical radiographs. (Strength of study: large study sample. Weaknesses of study: retrospective study; in a large number of patients, more than one tooth was treated; no standardization of radiographic evaluation; drop-out rate 21%.)
5.2. Experimental studies
Maguire et al. (1998) assessed two different RT in periapical surgery on maxillary cuspid teeth in cats. Before reapproximation of the surgical flaps, osteotomies were either covered with a resorbable polylactide membrane (test 1) or filled with human osteogenic protein-1 (hOP-1) on a collagen carrier (test 2). Control sites received no further treatment. After a healing period of 12 weeks, the animals were euthanized and the obtained specimens were examined histomorphometrically. Mean percentages of osseous healing were low but similar (p > 0.05) in all the three groups (test 1: 14.4%; test 2: 18.4%; control: 16.6%). Significantly more inflammation adjacent to the resected root ends was observed in sites treated with membranes than in sites without membranes. (Strengths of study: control group; experimental pulpal infection; standardized defects. Weaknesses of study: short study period; only 8 teeth treated.)
Murashima et al. (2002) evaluated calcium sulfate (CS) as a bone substitute in apical surgery for the treatment of three different types of osseous defects in mandibular third and fourth premolars of dogs. After root-canal treatment and apicectomy, defects created on both sides of the mandible included (I) large periapical defects, (II) through-and-through defects, and (III) apico-marginal defects. Experimental sites (random allocation) were filled with CS and the defects on the opposite side were left unfilled as controls. Results for periapical defects after 16 weeks were as follows (results for through-and-through defects and for apico-marginal defects are presented below in the corresponding sections): the test sites histologically showed complete bone regeneration with little concavity, whereas in the control sites it was obviously concave. The percentage of new bone formation in CS-treated sites (73.0%) was significantly (p < 0.01) higher than in control defects (55.6%). (Strengths of study: fair sample size; control group; standardized defects. Weaknesses of study: short study period; surgically created defects; multiple types of lesions tested in same study.)
Guided bone regeneration (GBR) using membranes or calcium sulfate following apicectomy was studied in dogs by Yoshikawa et al. (2002). The mandibular third and fourth premolars were root-canal treated, and apicectomies were performed. Osseous defects were randomly divided into five groups for treatment: bony defects were covered with ePTFE membranes (test 1), polylactide–polyglycolide membranes (test 2), or collagen membranes (test 3). In test 4 sites, defects were filled with calcium sulfate. Nothing was used in the controls. After 16 weeks of healing, greater percentages of new bone formation within the defect area were found in test 1 (54.8%) and test 4 (48.9%) groups compared to the other groups (test 2: 21.2%; test 3: 34.0%; control: 37.4%). However, newly formed cortical bone had closed the access defect of the cortical plate in all groups. (Strengths of study: fair sample size; control group; standardized defects. Weaknesses of study: short study period; surgically created defects.)
Apaydin and Torabinejad (2004) performed an experimental study to determine the effect of calcium sulfate (CS) on cementum deposition and osseous healing after apical surgery. The root canals of mandibular premolars in dogs were endodontically treated, followed 2 weeks later by apical surgery. The right or left side was assigned at random to receive CS or no material in the osteotomy sites before wound closure. The histological and histomorphometrical analyses after 4 months of healing demonstrated evidence of cementum deposition adjacent to the root-end filling and bone regeneration in the osteotomies in all samples. The authors concluded that the placement of CS into bony defects after apical surgery did not significantly improve periapical osseous healing. (Strengths of study: fair sample size; control group; split-mouth design; standardized defects. Weaknesses of study: short study period; surgically created defects.)
Bergenholtz et al. (2006) evaluated the ability of recombinant human bone morphogenetic protein-2 (rhBMP-2) to enhance bone healing after apical surgery. The experimental study was performed in monkeys. Pulpal infections were generated in maxillary and mandibular incisors. Four to seven months later, the teeth received conventional endodontic treatment immediately followed by apical surgery. In a randomized split-mouth design, contralateral apical bone defects received rhBMB-2 in an absorbable collagen sponge carrier or served as controls without placement of BMP. Histological and radiographic evaluations after one month (mandibular incisors) or after 4.5 months (maxillary incisors) showed no obvious differences in the healing of test and control sites. (Strengths of study: fair sample size; control group; split-mouth design; experimental pulpal infection. Weaknesses of study: short study period; defect size not mentioned; no information about root-end filling material.)
Bernabé et al. (2010) evaluated periapical healing after the use of guided tissue regeneration (membrane, bone graft or combinations) in apical surgery. Apical lesions were induced in mandibular premolars of dogs, and 3 months later, root-canal treatment and apical surgery were carried out. Periapical bone defects were standardized with a 5 mm trephine bur. Surgical sites were divided into four groups for treatment: (i) membrane coverage, (ii) bone graft, (iii) bone graft with membrane coverage, and (iv) blood fill as control. The animals were killed after 6 months. Histology and histomorphometry showed that the inflammatory infiltrate and the periapical healing process were similar in all groups. (Strengths of study: fair sample size; control group; experimentally induced infection; standardized defects; adequate study period. Weakness of study: no randomization of treatment.)
5.3. Discussion and clinical recommendation
The reviewed clinical and experimental studies show no or only minimal benefits of using RT in apical surgery for the treatment of osseous defects limited to the periapical area (Fig. 1). By definition, these lesions have intact facial and lingual bony plates. Apical surgery with removal of facial bone to create the surgical access window results in a bone defect with a 4-wall configuration, i.e., with intact mesial, distal, lingual and basal bone structures. Bone would only be missing on the facial (access) window and at the cut root face, meaning that the risk of soft tissue proliferation into the defect is low, and unimpeded new bone formation can take place. None of the tested regenerative techniques or materials resulted in a better outcome when comparing test and control sites, except for ePTFE membranes with hydroxyapatite in Tobon’s study, and ePTFE membranes or calcium sulfate in Yoshikawa’s study. ePTFE membranes have been associated with an increased risk of wound dehiscence and site infection (Gher et al., 1994; Augthun et al., 1995; Machtei, 2001). In addition, the ePTFE membrane needs to be removed in a second surgery, increasing cost and patient morbidity. With regard to calcium sulfate (CS), this material has not been tested in a clinical study for lesions limited to the periapical area, hence it is not known whether CS enhances bone healing in patients following apical surgery. Inorganic bovine bone mineral (IBBM) or alloplastic glass particulate did not show improvement of osseous healing after apical surgery in two clinical studies (see Table 4).
In summary, taking into consideration the current data from clinical and experimental studies, the use of RT in apical surgery for treatment of lesions limited to the apical area is not warranted. Clinicians should also bear in mind that a radio-opaque bone substitute will make radiographic interpretation of periapical healing more difficult.
6. RT in cases with apical surgery of “through-and-through” lesions (Table 2)
6.1. Clinical studies
Pecora et al. (2001) assessed the use of calcium sulfate in the treatment of through-and-through lesions (>10 mm) during apical surgery. At the 1-year follow-up, of nine cases that had received calcium sulfate (CS) for filling of the bony lesion, seven showed complete radiographic healing and two showed incomplete healing. Of nine control cases without filling material, three showed complete healing, five showed incomplete healing, and one had unsatisfactory radiographic healing. The authors concluded that the addition of CS improved the clinical outcome in apical surgery with through-and-through lesions. (Strength of study: randomized clinical trial. Weaknesses of study: only 10 teeth per group; no standardization of radiographic evaluation.)
Taschieri et al. (2007) evaluated the outcome of apical surgery in large lesions (>10 mm) with or without guided tissue regeneration. The patients were divided into cases with 4-wall defects (data reported in previous section “RT in apical surgery with lesions limited to the periapical area”) and cases with through-and-through lesions. Test sites received a bone substitute (anorganic bovine bone mineral) and the bony window was covered with a collagen membrane. The control sites received neither filler nor membrane. At the 1-year follow-up, the success rates of the test sites (75.0%) and the control sites (61.5%) did not differ significantly. Overall, the outcome of the 4-wall defects (control and test sites combined), as reported in the previous section, was significantly better (p = 0.03) than the outcome of the through-and-through lesions (control and test sites combined). (Strength of study: randomized clinical trial. Weaknesses of study: initial sample size per group not specified; no standardization of radiographic evaluation; small sample size per group, two centers involved.)
The same authors published a different study a year later (Taschieri et al., 2008). Following root-end resection and retrograde filling, the bony defect in test cases was filled with an organic bovine bone mineral and covered with a collagen membrane. In the control cases, no filler or membrane was used. After 1 year, the rate of healed cases was significantly (p = 0.02) higher for the test group (88.2%) compared to the control group (57.1%). The authors also mentioned the difficulty of radiographic healing interpretation, since the radiopacity of the bone substitute compounded the radiographic differentiation between incomplete (“scar tissue”) and uncertain healing categories. (Strength of study: randomized clinical trial. Weaknesses of study: small sample size per group with some patients having more than one tooth treated; no standardization of radiographic evaluation, two centers involved.)
6.2. Experimental studies
The very first experimental study on RT in apical surgery was conducted by Dahlin et al. (1990). Transosseous defects were surgically created in conjunction with apicoectomy of the lateral maxillary incisors in seven monkeys. The teeth had their root canals previously filled. Following root-end resection, defects on the animals’ right side (test) were covered facially and palatally with ePTFE membranes, whereas contralateral defects (left side) served as controls. After a healing period of 3 months, all membrane-treated defects had healed with bony closure. In contrast, control defects had healed with fibrous connective tissue. (Strengths of study: control group; standardized defects. Weaknesses of study: small sample size; short study period; surgically created defects; no root-end fillings placed.)
Baek and Kim (2001) evaluated different barrier membranes for improvement of bone regeneration in experimentally created through-and-through mandibular defects in ferrets. Following root-canal treatment, tunnel defects (diameter: 3 × 5 mm) were made bilaterally in mandibular premolars at the level of the root apices that were subsequently resected. The transosseous defects were covered both buccally and lingually with either ePTFE (test 1), polyglactin 910 (test 2), or polylactide (test 3) membranes. Control defects received no membranes. Healing was assessed after 12 weeks. Radiographically, the control sites showed some bone growth, but large defects remained. Membrane-treated sites showed tissue regeneration in between 90% and 95% of baseline radiolucencies. Histologically, test 2 sites showed excellent new bone formation and test 1 sites demonstrated good bone formation. In contrast, test 3 sites (due to inflammatory responses to large membrane residues) and the control sites demonstrated only limited bone regeneration. (Strengths of study: control group; standardized defects. Weaknesses of study: small sample size; short study period; surgically created defects; no root-end fillings.)
Murashima et al. (2002) assessed calcium sulfate (CS) as a bone substitute in apical surgery for treatment of osseous through-and-through defects in mandibular third and fourth premolars of dogs. After root-canal treatment and apicectomy, tunnel defects were created on both sides of the mandible. Test sites (random allocation) were filled with CS and the defects on the opposite side served as the controls. After 16 weeks, the osseous defects on both sides were almost closed with newly formed bone. However, the cortical bone was more concave in the control sites compared to CS-treated sites. Also, the percentage of new bone formation in CS-treated sites (81.3%) was significantly (p < 0.01) higher than in the control sites (64.5%). (Strengths of study: fair sample size; control group; standardized defects. Weaknesses of study: short study period; surgically created defects; multiple types of lesions tested in same study.)
6.3. Discussion and clinical recommendation
A tunnel (or through-and-through) lesion is characterized by an eroded buccal and lingual bone plate, or the lesion results after creation of the buccal bony access window in cases with lesions that have eroded the lingual bony plate. The bony crypt typically has a three-wall configuration with mesial, distal, and caudal bone structures, but buccal and lingual bone walls are missing. Since new bone formation is slower compared to soft tissue proliferation, the latter will grow into the “unprotected” bony crypt, with a scar bridging the defect from buccal to lingual, thereby preventing or retarding bone formation. The reviewed clinical and experimental studies demonstrate that cases with tunnel lesions may benefit from the use of RT, in particular to reduce the amount of scar tissue formation (radiographically categorized as incomplete healing). Since incomplete and uncertain healing categories are sometimes difficult to differentiate radiographically from each other, patients may benefit from avoiding unnecessary explorative surgeries. However, as one study has shown, the use of a non-resorbable radio-opaque bone substitute may confound the radiographic assessment of periapical healing, and, therefore, such material is not preferred as a defect filler. Recommended alternatives would be to use resorbable calcium sulfate for defect fill, or to place resorbable membranes both on buccal and lingual aspects of the tunnel lesion to prevent the ingrowth of soft tissues. In large defects, buccally and lingually placed membranes may collapse toward the defect; therefore, placement of a non-opaque and resorbable filling material (calcium sulfate or collagen fleece) in the bony crypt is recommended to support non-rigid membranes.
7. RT in cases with apical surgery of apico-marginal lesions (Table 3)
7.1. Clinical studies
Dietrich et al. (2003) evaluated the periapical and periodontal healing after apical surgery of cases with apico-marginal defects (84% of teeth had apical surgery with orthograde root-canal treatment, 16% with retrograde filling). The defects were grafted with anorganic bovine bone mineral and were subsequently covered with a collagen membrane. No control treatment was studied. After 1 year, the clinical and radiographic assessment demonstrated a success rate of 82.6%. Overall, the baseline median probing pocket depth (PPD) decreased from 9 to 3 mm. The authors also reported that defects extending to a proximal root surface had a significantly greater residual PPD than defects not involving a proximal root surface. (Strength of study: low drop-out rate of 8%. Weaknesses of study: no control group; no standardization of radiographic evaluation; majority of teeth received no root-end filling.)
Marin et al. (2006) assessed the healing after apical surgery of teeth with total denudation of the buccal root surface in a clinical study without a control group. In one group of patients, a sliding periosteal graft was used to cover the defect, whereas in the other group a membrane of polyglactin 910 was placed over the defect. Healing rates after 1 year were identical (87%) for the two groups. Also, reduction of periodontal probing depths (6.3 and 5.8 mm, respectively) and gain of clinical attachment levels (6 and 5.6 mm, respectively) were similar. (Strength of study: no drop-outs; comparison of two cohorts. Weaknesses of study: no control group; no standardization of radiographic evaluation.)
Kim et al. (2008) evaluated the outcomes of apical surgery of teeth with a solely endodontic lesion and teeth with combined endodontic–periodontal lesions. For the present review, the data of endo-perio cases were extracted. In teeth with complete loss of the buccal bone plate, calcium sulfate (CS) was placed into the periradicular defect, and the exposed buccal root surface was covered with a collagen tape (lesion type F) [classification according to Kim and Kratchman (2006)]. Teeth with an endodontic–periodontal communication, but an intact buccal bone plate (lesion type E), or teeth with periapical lesions and periodontal pockets >4 mm without communication (lesion type D) did not receive any RT. 95.2% of lesion types A–C with isolated endodontic lesions had successful outcomes. In groups D–F, the success rate was 77.5% for endodontic–periodontal combined lesions (lesion type D: 100%; lesion type E: 63.6%; and lesion type F: 73.7%). (Strength of study: prospective study. Weaknesses of study: initial sample size not specified; no control group; no standardization of radiographic evaluation; three different root-end filling materials used; different types of defects received different regenerative treatments.)
7.2. Experimental studies
Douthitt et al. (2001) histologically assessed healing after the use of a bioresorbable polylactide membrane for the management of buccal bone loss concomitant with periapical surgery. The experimental study was performed in mandibular third and fourth premolars of nine dogs. Following root-canal treatment, the alveolar bone was completely removed from the buccal root surfaces and apicectomies were carried out. Sites were randomly assigned to the test or control groups. After a healing period of 27 weeks, membrane-treated teeth showed significantly (p < 0.05) longer new attachment on formerly denuded buccal root surfaces (4.15 mm) compared to control teeth (1.81 mm). The amount of regenerated alveolar bone was significantly greater (p = 0.001) in membrane-treated sites than in the control sites. After 27 weeks, periradicular bony healing was complete in 89% of the test sites and in 68.8% of the control sites. (Strengths of study: fair sample size; control group; standardized defects; study period. Weaknesses of study: surgically created defects; no root-end fillings.)
Calcium sulfate (CS) was evaluated as a bone substitute following apical surgery for treatment of osseous defects that communicated with the gingival sulcus in mandibular third and fourth premolars of dogs (Murashima et al., 2002). After root-canal treatment and apicectomy, apico-marginal defects were created on both sides of the mandible. Test sites (random allocation) were filled with CS, whereas the defects on the opposite side were left unfilled as controls. After 16 weeks, bone was not observed histologically on the buccal side of the root in both test and control teeth, although the apical part of the defect was filled with bone. In some test specimens, inflammatory cell infiltration was observed in the soft tissue along the root surface from the gingival sulcus to the apical area, and epithelial downgrowth was also present. (Strengths of study: fair sample size; control group; standardized defects. Weaknesses of study: short study period; surgically created defects; multiple types of lesions tested in same study.)
Britain et al. (2005) studied the use of guided tissue regeneration in apical surgery for generated chronic periodontic–endodontic lesions. Pulpal necrosis was induced in dogs along with surgical removal of radicular buccal bone. After 6 weeks, chronic lesions were surgically treated with root-canal instrumentation, apical surgery, and RT that included placement of a collagen membrane over the denuded buccal root and apical defect (test 1) or infill of the bony defect with anorganic bovine bone mineral and placement of a collagen membrane to cover the surgical site (test 2). The control defects received neither a bone substitute nor a membrane. Histologic and histomorphometric analysis after a healing period of 6 months showed statistically significantly (p < 0.05) more new cementum on the denuded buccal root surface in the teeth of both test groups compared to the control teeth. Mean radicular bone height on the buccal root surface was 2.2 mm in control teeth, 3.2 mm in membrane-treated teeth, and 3.5 mm in membrane-filler-treated teeth. (Strengths of study: control group; experimentally induced infection; standardized defects; adequate study period. Weaknesses of study: sample size; surgically created defects.)
7.3. Discussion and clinical recommendation
An apico-marginal lesion is the most challenging situation in apical surgery, particularly when the buccal bone plate is completely missing. In some cases, a thin facial bone plate is still present, but the buccal root surface is exposed. The main problem of an apico-marginal lesion is that healing is often characterized by epithelial downgrowth along the denuded root surface after apical surgery. As a consequence, a long junctional epithelium forms along the root surface, with an increased risk of a recurrent communication between marginal and apical tissues (Skoglund and Persson, 1985).
All three reviewed clinical studies did not have a control group, making a clinical conclusion impossible. Kim’s study used two different treatment modalities for two different types of apico-marginal lesions; therefore, no firm clinical conclusion can be drawn either (Kim et al., 2008). Two of three reviewed experimental studies (with control groups) demonstrated a beneficial effect of barrier membranes (with or without a bone filler) on healing in teeth with apico-marginal lesions. In contrast, calcium sulfate was found to cause an inflammatory cell response with epithelial downgrowth. No clinical or experimental study has so far evaluated the use of enamel matrix derivatives for treatment of apico-marginal lesions in conjunction with apical surgery. The clinician is advised to cautiously perform apical surgery in teeth with complete denudation of buccal (and/or proximal) root surfaces. In multi-rooted teeth, extraction or root/tooth resection should be considered as treatment alternatives.
Acknowledgements
The authors thank Ueli Iff, medical illustrator, School of Dental Medicine, University of Bern, Switzerland, for the schematic illustrations.
There was no financial support for this article other than departmental funds.
References
- Apaydin E.S., Torabinejad M. The effect of calcium sulfate on hard-tissue healing after periradicular surgery. J. Endod. 2004;30:17–20. doi: 10.1097/00004770-200401000-00003. [DOI] [PubMed] [Google Scholar]
- Augthun M., Yildirim M., Spiekermann H., Biesterfeld S. Healing of bone defects in combination with immediate implants using the membrane technique. Int. J. Oral Maxillofac. Implants. 1995;10:421–428. [PubMed] [Google Scholar]
- Baek S.H., Kim S. Bone repair of experimentally induced through-and-through defects by Gore-Tex, Guidor, and Vicryl in ferrets: a pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001;91:710–714. doi: 10.1067/moe.2001.115393. [DOI] [PubMed] [Google Scholar]
- Bashutski J.D., Wang H.L. Periodontal and endodontic regeneration. J. Endod. 2009;35:321–328. doi: 10.1016/j.joen.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Bergenholtz G., Wikesjö U.M., Sorensen R.G., Xiropaidis A., Wozney J.M. Observations on healing following endodontic surgery in nonhuman primates (Macaca fascicularis): effects of rhBMP-2. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101:116–125. doi: 10.1016/j.tripleo.2005.02.085. [DOI] [PubMed] [Google Scholar]
- Bernabé P.F., Gomes-Filho J.E., Cintra L.T., Moretto M.J., Lodi C.S., Nery M.J., Otoboni Filho J.A., Dezan E. Histologic evaluation of the use of membrane, bone graft, and MTA in apical surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010;109:309–314. doi: 10.1016/j.tripleo.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Britain S., von Arx T., Schenk R.K., Buser D., Nummikoski P.V., Cochran D.L. The use of guided tissue regeneration principles in endodontic surgery for induced chronic periodontic–endodontic lesions: a clinical, radiographic, and histologic evaluation. J. Periodontol. 2005;76:450–460. doi: 10.1902/jop.2005.76.3.450. [DOI] [PubMed] [Google Scholar]
- Dahlin C., Gottlow J., Linde A., Nyman S. Healing of maxillary and mandibular bone defects using a membrane technique. An experimental study in monkeys. Scand. J. Plastic Reconstr. Surg. Hand Surg. 1990;24:13–19. doi: 10.3109/02844319009004514. [DOI] [PubMed] [Google Scholar]
- Dietrich T., Zunker P., Dietrich D., Bernimoulin J.P. Apicomarginal defects in periradicular surgery: classification and diagnostic aspects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002;94:233–239. doi: 10.1067/moe.2002.123864. [DOI] [PubMed] [Google Scholar]
- Dietrich T., Zunker P., Dietrich D., Bernimoulin J.P. Periapical and periodontal healing after osseous grafting and guided tissue regeneration treatment of apicomarginal defects in periradicular surgery: results after 12 months. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003;95:474–482. doi: 10.1067/moe.2003.39. [DOI] [PubMed] [Google Scholar]
- Douthitt J.C., Gutmann J.L., Witherspoon D.E. Histologic assessment of healing after the use of a bioresorbable membrane in the management of buccal bone loss concomitant with periradicular surgery. J. Endod. 2001;27:404–410. doi: 10.1097/00004770-200106000-00009. [DOI] [PubMed] [Google Scholar]
- European Society of Endodontology, 2006. Quality guidelines for endodontic treatment: consensus report of the European Society of Endodontology. Int. Endod. J. 39, 921–930. [DOI] [PubMed]
- Garrett K., Kerr M., Hartwell G., O’Sullivan S., Mayer P. The effect of a bioresorbable matrix barrier in endodontic surgery on the rate of periapical healing: an in vivo study. J. Endod. 2002;28:503–506. doi: 10.1097/00004770-200207000-00003. [DOI] [PubMed] [Google Scholar]
- Gher M.E., Quintero G., Assad D., Monaco E., Richardson A.C. Bone grafting and guided bone regeneration for immediate dental implants in humans. J. Periodontol. 1994;65:881–891. doi: 10.1902/jop.1994.65.9.881. [DOI] [PubMed] [Google Scholar]
- Karring T., Lindhe J., Cortellini P. Regenerative periodontal therapy. In: Lindhe J., Karring T., Lang N.P., editors. Clinical Periodontology and Implant Dentistry. fourth ed. Blackwell Munksgaard; Oxford, UK: 2003. pp. 650–704. [Google Scholar]
- Kim S., Kratchman S. Modern endodontic surgery concepts and practice: a review. J. Endod. 2006;32:601–623. doi: 10.1016/j.joen.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Kim E., Song J.S., Jung I.Y., Lee S.J., Kim S. Prospective clinical study evaluating endodontic microsurgery outcomes for cases with lesions of endodontic origin compared with cases with lesions of combined periodontal–endodontic origin. J. Endod. 2008;34:546–551. doi: 10.1016/j.joen.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Lin L., Chen M.Y., Ricucci D., Rosenberg P.A. Guided tissue regeneration in periapical surgery. J. Endod. 2010;36:618–625. doi: 10.1016/j.joen.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Machtei E.E. The effect of membrane exposure on the outcome of regenerative procedures in humans. A meta-analysis. J. Periodontol. 2001;72:512–516. doi: 10.1902/jop.2001.72.4.512. [DOI] [PubMed] [Google Scholar]
- Maguire H., Torabinejad M., McKendry D., McMillan P., Simon J.H. Effects of resorbable membrane placement and human osteogenic protein-1 on hard tissue healing after periradicular surgery in cats. J. Endod. 1998;24:720–725. doi: 10.1016/S0099-2399(98)80161-1. [DOI] [PubMed] [Google Scholar]
- Marin M.L., Dominguez J.S., Arismendi J.A., Mesa A.L., Florez G.A., Tobon S.I. Healing response of apicomarginal defects to two guided tissue regeneration techniques in periradicular surgery: a double-blind, randomized clinical trial. Int. Endod. J. 2006;39:368–377. doi: 10.1111/j.1365-2591.2006.01081.x. [DOI] [PubMed] [Google Scholar]
- Molven O., Halse A., Grung B. Incomplete healing (scar tissue) after periapical surgery – radiographic findings 8 to 12 years after treatment. J. Endod. 1996;22:264–268. doi: 10.1016/s0099-2399(06)80146-9. [DOI] [PubMed] [Google Scholar]
- Murashima Y., Yoshikawa G., Wadachi R., Sawada N., Suda H. Calcium sulfate as a bone substitute for various osseous defects in conjunction with apicectomy. Int. Endod. J. 2002;35:768–774. doi: 10.1046/j.1365-2591.2002.00565.x. [DOI] [PubMed] [Google Scholar]
- Pantchev A., Nohlert E., Tegelberg A. Endodontic surgery with and without inserts of bioactive glass PerioGlas® – a clinical and radiographic follow-up. Oral Maxillofac. Surg. 2009;13:21–26. doi: 10.1007/s10006-008-0141-5. [DOI] [PubMed] [Google Scholar]
- Pecora G., Kim S., Celletti R., Davarpanah M. The guided tissue regeneration principle in endodontic surgery: one-year postoperative results of large periapical lesions. Int. Endod. J. 1995;28:41–46. doi: 10.1111/j.1365-2591.1995.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Pecora G., de Leonardis D., Ibrahim N., Bovi M., Cornelini R. The use of calcium sulphate in the surgical treatment of a “through and through” periradicular lesion. Int. Endod. J. 2001;34:189–197. doi: 10.1046/j.1365-2591.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- Skoglund A., Persson G. A follow-up study of apicoectomized teeth with total loss of the buccal bone plate. Oral Surg. Oral Med. Oral Pathol. 1985;59:78–81. doi: 10.1016/0030-4220(85)90120-3. [DOI] [PubMed] [Google Scholar]
- Stassen L.F., Hislop W.S., Still D.M., Moos K.F. Use of anorganic bone in periapical defects following apical surgery – a prospective trial. Br. J. Oral Maxillofac. Surg. 1994;32:83–85. doi: 10.1016/0266-4356(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Taschieri S., del Fabbro M., Testori T., Weinstein R. Efficacy of xenogeneic bone grafting with guided tissue regeneration in the management of bone defects after surgical endodontics. J. Oral Maxillofac. Surg. 2007;65:1121–1127. doi: 10.1016/j.joms.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Taschieri S., del Fabbro M., Testori T., Saita M., Weinstein R. Efficacy of guided tissue regeneration in the management of through-and-through lesions following surgical endodontics: a preliminary study. Int. J. Periodont. Restorative Dent. 2008;28:265–271. [PubMed] [Google Scholar]
- Tobon S.I., Arismendi J.A., Marin M.L., Mesa A.L., Valencia J.A. Comparison between a conventional technique and two bone regeneration techniques in periradicular surgery. Int. Endod. J. 2002;35:635–641. doi: 10.1046/j.1365-2591.2002.00523.x. [DOI] [PubMed] [Google Scholar]
- von Arx T. Failed root canals: the case for apicoectomy (periradicular surgery) J. Oral Maxillofac. Surg. 2005;63:832–837. doi: 10.1016/j.joms.2005.02.019. [DOI] [PubMed] [Google Scholar]
- von Arx T., Cochran D.L. Rationale for the application of the GTR principle using a barrier membrane in endodontic surgery: a proposal of classification and literature review. Int. J. Periodont. Restorative Dent. 2001;21:127–139. [PubMed] [Google Scholar]
- von Arx T., Walker W. Microsurgical instruments for root-end cavity preparation following apicoectomy: a literature review. Endod. Dent. Traumatol. 2000;16:47–62. doi: 10.1034/j.1600-9657.2000.016002047.x. [DOI] [PubMed] [Google Scholar]
- von Arx T., Penarrocha M., Jensen S.S. Prognostic factors in apical surgery with root-end filling: a meta-analysis. J. Endod. 2010;36:957–973. doi: 10.1016/j.joen.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Wang N., Knight K., Dao T., Friedman S. Treatment outcome in endodontics – the Toronto study. Phases I and II: apical surgery. J. Endod. 2004;30:751–761. doi: 10.1097/01.don.0000137633.30679.74. [DOI] [PubMed] [Google Scholar]
- Wu M.K., Dummer P.M.H., Wesselink P.R. Consequences of and strategies to deal with residual post-treatment root canal infection. Int. Endod. J. 2006;39:343–356. doi: 10.1111/j.1365-2591.2006.01092.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa G., Murashima Y., Wadachi R., Sawada N., Suda H. Guided bone regeneration (GBR) using membranes and calcium sulfate after apicectomy: a comparative histomorphometrical study. Int. Endod. J. 2002;35:255–263. doi: 10.1046/j.1365-2591.2002.00473.x. [DOI] [PubMed] [Google Scholar]
- Zuolo M.L., Ferreira M.O.F., Gutmann J.L. Prognosis in periradicular surgery: a clinical prospective study. Int. Endod. J. 2000;33:91–98. doi: 10.1046/j.1365-2591.2000.00263.x. [DOI] [PubMed] [Google Scholar]


