Abstract
Background
The impact of extranodal extension (ENE) of metastatic papillary thyroid carcinoma (PTC) on short- and long-term clinical outcomes, including biochemical testing, has not been reported.
Methods
This single-institution National Cancer Institute-designated Comprehensive Cancer Center cohort study included patients with macroscopic metastases and excluded patients with gross residual disease after surgery, distant disease, or poorly differentiated papillary carcinoma. A suppressed or stimulated thyroglobulin (Tg) <1 ng/mL, without suspicious imaging or anti-thyroglobulin antibodies, after radioactive iodine (RAI) treatment was termed an excellent or “complete biochemical response” (CR).
Results
Of 89 subjects included, 60 previously untreated patients underwent total thyroidectomy and therapeutic neck dissection; 29 additional patients underwent a neck dissection for persistence or recurrence after prior surgery and RAI administration. ENE, identified in 29 patients (33%), was associated with T4 classification (p=0.02) and involvement of a greater number of nodes (median 11 vs. 5, p=0.03). ENE was associated with a 20% increased risk of nodal persistence necessitating additional surgery (p=0.02). In a multivariable analysis, ENE, T4 classification, and recurrence/persistence proved to be independent predictors of systemic disease progression (ENE: hazard ratio [HR] 4.3 [95% confidence interval (CI) 1.2–15], p=0.02; T4 classification: HR 4.2 [CI 1.3–14], p=0.01; recurrent/persistent status: HR 3.6 [CI 1.1–12], p=0.035). Nodal or systemic disease progression was rare after a biochemical CR; in contrast, in previously untreated patients, stimulated Tg levels (sTg) >50 ng/mL prior to initial RAI administration, heralded the progression of nodal disease, and also predicted the eventual development of systemic disease (p=0.0001). Of those with a sTg >50 ng/mL, over 70% underwent surgery for nodal persistence within five years. The presence of ENE diminished the odds of a biochemical CR (odds ratio 3.5% [CI 1.3–10], p=0.02), and increased the probability that the sTg levels after surgery will exceed 50 ng/mL (odds ratio 5 [CI 1.2–21], p=0.03). Following surgery for tumor persistence, 25% of those with ENE were rendered biochemically free of disease.
Conclusions
ENE diminishes the probability of a biochemical CR after treatment for regional metastatic PTC, and increases the probability of tumor persistence after initial resection, likely from abundant metastasis. ENE and nodal persistence independently predict eventual systemic disease progression.
Introduction
Growth of thyroid cancer through a tissue barrier may be a feature of the primary cancer itself or of a metastatic deposit in a lymph node, where it is described as extranodal extension (ENE). Extrathyroidal extension of the primary tumor increases T-stage (1). ENE is not recognized in any staging system. Nevertheless the presence of ENE of papillary thyroid cancer has been associated with an increased risk of nodal recurrence, distant metastases, and cancer-related death (2–7). In contrast to BRAF (recognized as a marker of enhanced potential for tumor invasion and metastasis), the development of ENE is believed to be a late event in the progression pathway for papillary carcinoma (7).
Currently, biochemical assessments of thyroglobulin (Tg) levels are better predictors of short-term clinical outcomes such as nodal disease recurrence than any histopathologic staging criterion alone (8,9). A postoperative, stimulated, preablation Tg level (sTg) above 50 ng/mL predicts nodal persistence (defined as disease not eradicated with initial treatment) with a positive predictive value of 97% (10). A complete biochemical response, using a “dynamic” risk-stratification approach and assessed 6–24 months after the completion of treatment is associated with the subsequent development of structurally identifiable disease in 0–4% of cases (9). Thus, patients who achieve a complete biochemical response after treatment are at a very low risk of developing a recurrence (defined as the development of disease after prior biochemical and structural eradication of disease). We hypothesized that metastatic nodes with ENE were more subject to nodal persistence and less likely to exhibit a complete biochemical response after treatment, accounting for the adverse outcomes associated with the ENE phenotype, a clinicopathologic marker readily available at most institutions. Recognizing patients at risk of nodal persistence could inform treatment decisions in the perioperative period.
Materials and Methods
Subjects
We retrospectively reviewed the charts of 294 patients with papillary thyroid carcinoma (PTC) treated at the Fox Chase Cancer Center between February 1, 2000, and March 9, 2010. This study was approved by the Fox Chase Cancer Center Institutional Review Board. Patients with poorly differentiated thyroid cancers (poorly differentiated papillary thyroid carcinoma, insular carcinoma, or anaplastic thyroid carcinoma), medullary carcinoma, or other nonfollicle-derived thyroid cancers were excluded. Patients with gross residual tumor or known distant disease at the time of resection were also excluded. A minimum of 24 months follow-up was required for entry into the study unless the patient had a clinical event (such as a recurrence). The indication for neck dissection was macroscopic nodal disease in all cases. A compartment-oriented dissection of levels II through V, including Level IIb (11,12) was performed. All patients received thyrotropin (TSH) suppressive therapy, underwent at least one follow-up neck ultrasound per year, and were submitted to at least one radio-iodine scan during the initial 24 months after surgery.
Risk-stratification
Patients were classified according to the American Joint Commission of Cancer Staging (AJCC) 7th edition (1) and the new American Thyroid Association (ATA) risk of recurrence system (9). T4 classification (reflecting tumor invasion of the subcutaneous soft tissues, larynx, esophagus, trachea, or recurrent laryngeal nerve) corresponded to high-risk stratification in the ATA staging system based on macroscopic tumor invasion. Extranodal extension was assigned pathologically by tumor cells extending beyond the lymph-node capsule into the perinodal fibroadipose tissue—thus, microscopic or gross disease beyond nodal capsule resulted in diagnosis. All specimens included in the series were reviewed at our institution, and the presence of ENE characterized by a pathologist (D.F.) for the purpose of this analysis.
Endpoints
Stimulated Tg levels were obtained postoperatively after withdrawal of thyroid hormone, and with a TSH of at least 30. A complete response to treatment was defined as a suppressed or stimulated Tg of <1 ng/mL, in the absence of findings suspicious for structural disease on neck ultrasound, cross-sectional or nuclear medicine imaging, 6–24 months after radioiodine treatment, consistent with an “excellent” biochemical response as defined by Tuttle et al. (9). By definition, patients with persistent antithyroglobulin antibodies were excluded from this group. Regional control was defined as survival without clinical or radiographic evidence of structural disease at any neck level. In the absence of distant metastases, patients with structural disease in the neck underwent surgery for persistence or recurrence.
Systemic disease progression was defined as the development of structural or measurable disease, either in the presence or absence of locoregional control. Confirmation of the development of systemic disease on cross-sectional imaging such as CT scan or 18-FDG-PET-CT was required. The decision to initiate systemic therapy (doxorubicin, or more recently, novel, multitargeted tyrosine kinase inhibitors) was undertaken at the discretion of the treatment team, most commonly in response to progression of distant disease on a CT scan in the setting of iodine-refractory cancer.
Statistical methods
Categorical comparisons were performed using the Fisher exact test. Nonparametric testing (Mann–Whitney U-test) was used to assess quantitative differences between Tg levels. The probability of a complete biochemical response or postoperative sTg level >50 ng/mL based on predictor variables was assessed using logistic regression. Survival times were calculated from the date of neck dissection. The Kaplan–Meier method was used to estimate survival. Univariable differences in survival were calculated using the log rank test. Deaths from causes other than thyroid cancer were censored at that time. Multivariable Cox proportional hazard regression was performed using SPSS software V20 (IBM, Armonk, NY).
Results
Sixty patients with newly diagnosed, previously untreated papillary carcinoma were managed with a total thyroidectomy, central compartment dissection, lateral neck dissection, and postoperative radioiodine. An additional 29 patients treated with neck dissection for nodal disease after prior treatment with surgery and radioiodine ablation were also included. All patients had structural evidence of nodal disease. Median primary tumor size was 2 cm (0.1–8 cm). Of 89 papillary carcinomas, 80 (90%) were multifocal. Clinical characteristics are listed in Table 1. Thirty-four patients (40%) achieved a complete biochemical response. Thirteen patients with antithyroglobulin antibodies were considered to have an incomplete biochemical response.
Table 1.
Clinical/Pathologic Characteristics of 89 Patients with Metastatic Papillary Thyroid Carcinoma
| Primary (n=60): number (%) | Recurrent (n=29): number (%) | p-Value | |
|---|---|---|---|
| Age | |||
| Median (range) | 44 (20–85) | 42 (22–82) | 0.35 |
| Sex | |||
| Female | 37 (61.7%) | 22 (75.9%) | 0.17 |
| Histologic variant | |||
| Classic papillary carcinoma | 45 (75%) | 21 (72.4%) | 0.74 |
| Follicular variant | 6 (10%) | 2 (6.9%) | |
| Tall cell variant | 9 (15%) | 6 (20.7%) | |
| Overall stagea | |||
| Stage I | 33 (55%) | 17 (68%) | .007 |
| Stage II | 0 (0%) | 2 (8%) | |
| Stage III | 1 (1.7%) | 2 (8%) | |
| Stage IV | 26 (43.3%) | 4 (16%) | |
| T-classificationa | |||
| T1 | 17 (28.3%) | 2 (6.9%) | 0.072 |
| T2 | 8 (13.3%) | 8 (27.6%) | |
| T3 | 27 (45%) | 14 (48.3%) | |
| T4 | 8 (13.3%) | 5 (17.2%) | |
| Nodal metastases | |||
| Lateral compartment | 44 (73%) | 25 (86%) | 0.28 |
| Central compartment | 56 (93%) | 22 (76%) | 0.04 |
| M-stagea | |||
| Distant disease present | 0 (%) | 0 (0%) | 1 |
| ATA staging systemb | |||
| High risk | 8 (13.3%) | 5 (17.2%) | 0.75 |
| Intermediate risk | 52 (86.7%) | 24 (82.8%) | |
| Extranodal extensionc | |||
| ENE present | 16 (26.7%) | 13 (45%) | 0.09 |
| Metastatic lymph nodes | |||
| Lateral compartment | |||
| Median positive (range) | 4 (1–18) | 5 (1–14) | 0.98 |
| Total harvested (range) | 44 (17–80) | 41 (8–78) | |
| Central compartment | |||
| Median positive (range) | 3 (0–20) | 3 (0–12) | 0.24 |
| Total harvested (range) | 9 (2–32) | 3 (0–15) | |
| Radioiodine treatment, median (range) | |||
| Before surgery | NA | 156 mCi (0–452) | |
| After surgery | 150 mCi (93–250) | 115 mCi (0–250) | |
| External beam radiation after surgery | 7 (11.7%) | 3 (10.3%) | 1 |
With a median follow-up of 59 months, 17 patients developed nodal metastases (a median of 39 months after the index procedure), which were treated with additional surgery. In addition, 12 patients developed distant metastases (five in the primary group and seven in the recurrence/persistence group) detected a median of 43 months after the index nodal dissection. Eight patients began systemic therapy a median of 54 months after surgery, and six subsequently died of the disease.
The clinical and pathologic characteristics of patients with nodal recurrence/persistence, initially treated elsewhere, and newly diagnosed patients are compared in Table 1. Patients with recurrence or persistence of thyroid cancer were more likely to have had an initial AJCC Stage I PTC (p=0.01). They were also slightly less likely to have primary cancers ≤2 cm in diameter, and more likely to exhibit ENE in nodal metastases, but these differences were not statistically significant. All but one patient with recurrence/persistence were previously treated with RAI, and some had been treated several times.
Patients with and without ENE were compared in order to ascertain whether ENE was associated with factors known to affect prognosis (Table 2). ENE was associated with T4-classification/high-risk ATA features (p=.02), and a greater total number of nodes involved (median 11 vs. 5, p=0.03; Table 2).
Table 2.
Clinicopathologic Differences Between Patients With or Without Extranodal Extension in Metastatic Papillary Carcinoma
| No ENE (n=60) (%) | ENE (n=29) (%) | p-value | |
|---|---|---|---|
| Age | |||
| Median (range) | 40 (19–83) | 49 (22–71) | 0.23 |
| Sex | |||
| Male | 19 (32%) | 11 (38%) | 0.63 |
| Histologic variant | |||
| Tall cell variant versus other | 10 (17%) | 5 (17%) | 1.0 |
| T-classificationa | |||
| T4 versus T1–3 | 5 (8%) | 8 (29%) | 0.02 |
| ATA staging systemb | |||
| High risk | 5 (8%) | 8 (29%) | 0.02 |
| Intermediate risk | 55 (92%) | 20 (71%) | |
| Metastatic lymph nodes | |||
| Lateral compartment | |||
| Median positive (range) | 3.5 (1–18) | 6 (0–14) | 0.29 |
| Total harvested (range) | 42 (21–80) | 42 (13–70) | |
| Central compartment | |||
| Median positive (range) | 3 (0–20) | 6 (0–19) | 0.018 |
| Total harvested (range) | 7 (0–32) | 9.5 (1–26) | |
| Recurrent status | |||
| Recurrence/persistence | 16 (27%) | 13 (45%) | 0.09 |
| Radioiodine treatment, median (range) | |||
| Before surgery | 136 mCi (0–250) | 150 mCi (0–246) | 0.61 |
| After surgery | 155 mCi (0–350) | 195 mCi (100–452) | 0.55 |
| External beam radiation after radiation | 4 (7%) | 6 (21%) | 0.07 |
Clinicopathologic predictors of regional recurrence, distant progression, and systemic therapy use for the entire cohort are listed in Table 3. Of the clinicopathologic features tested, only ENE predicted diminished regional control—although not as well as biochemical approaches. Of the 17 patients with nodal disease after initial operation, only one achieved a complete biochemical response after treatment. Thus, 16 of the 17 patients likely had nodal persistence rather than recurrence.
Table 3.
Univariable Predictors of Regional Control, Systemic Disease Progression, and Initiation of Systemic or Chemotherapy Among Patients With Metastatic Papillary Thyroid Carcinoma
| |
Regional control |
Survival without systemic disease |
Survival without systemic or chemotherapy |
|||
|---|---|---|---|---|---|---|
| Predictors | 5-year rate | p-value | 5-year rate | p-value | 5-year rate | p-value |
| Age (at least 45 years) | 66% vs. 81% | 0.47 | 81% vs. 92% | 0.16 | 82% vs. 97% | 0.09 |
| Tall cell variant | 58% vs. 75% | 0.15 | 67% vs. 96% | 0.02 | 63% vs. 98% | 0.02 |
| T4 classificationa | 81% vs. 73% | 0.95 | 67% vs. 92% | 0.001 | 73% vs. 95% | 0.008 |
| Extranodal extensionb | 61% vs. 82% | 0.02 | 70% vs. 93% | 0.003 | 73% vs. 100% | 0.0001 |
| Recurrent status | 76% vs. 75% | 0.73 | 83% vs. 89% | 0.02 | 90% vs. 92% | 0.03 |
| Complete biochemical response | 94% vs. 64% | 0.004 | 100% vs. 81% | 0.008 | 100% vs. 89% | 0.09 |
| Stimulated Tg >50 ng/mL | 23% vs. 85% | 0.01 | 55% vs. 100% | 0.001 | 79% vs. 100% | 0.02 |
AJCC, 7th ed. (1). Staging for patients with recurrences refer to initial stage.
ENE present at the time of the neck dissection.
In addition to diminishing regional control, ENE was also associated with systemic disease progression and with increased systemic therapy use (Table 3). In a multivariable analysis, ENE, T4 classification/high-risk ATA stage, and recurrence/persistence were identified as independent predictors of systemic disease progression (ENE: hazard ratio [HR] 4.3 [95% confidence interval (CI) 1.2–15], p=0.02; T4 classification: HR 4.2 [CI 1.3–14], p=0.01; recurrent status: HR 3.6 [CI 1.1–12], p=0.035). Tall cell variant papillary carcinoma was also an independent predictor of distant failure, but the magnitude of the effect was smaller (HR 2.4 [CI 1.3–4.7], p=0.007). Thus, the increased risk of systemic disease progression among patients with ENE is independent of the risk conferred by the presence of persistent or recurrent disease, T4 classification/high-risk ATA features, or tall cell variant papillary carcinoma.
However, biochemical studies were found to be more effective in predicting clinical outcomes, particularly in the short term. A complete biochemical response, achieved in 41% of previously untreated patients and 37% of patients with recurrence or persistence, largely predicted freedom from nodal disease, systemic disease progression, and systemic or chemotherapy use (Table 3). In fact, three patients with T4 classification/high-risk ATA features (25% of patients with those features), and six patients with ENE (21%) achieved a complete biochemical response after treatment.
For previously untreated patients, the best predictor of persistent nodal disease was the stimulated thyroglobulin level measured after resection but before RAI administration. This portion of the analysis was limited to 40 patients, none of whom had cross-reacting anti-Tg antibodies, and for whom initial stimulated Tg levels were available (representing 75% of evaluable patients). The measured stimulated thyroglobulin was significantly lower in patients who went on to achieve a complete biochemical response (Table 4). A pre-RAI sTg level >50 ng/mL was associated with a biochemical incomplete response to treatment in every case, and was highly predictive of adverse outcomes (Table 3 and Fig. 1).
Table 4.
Biochemical Studies: The Postoperative Stimulated Thyroglobulin
| Predictors/outcomes | Median values (ng/mL) | (range in ng/mL) | p-value |
|---|---|---|---|
| Effects of pathologic variables on sTg | |||
| Tall cell variant | 10 vs. 1.8 | (0.1–601 vs. 0.1–500) | 0.20 |
| T4 classificationa | 15 vs. 1.9 | (0.5–601 vs. 0.1–500) | 0.24 |
| Extranodal extensionb | 20 vs. 1.3 | (0.1–601 vs. 0.1–500) | 0.03 |
| Pre-ablation sTg and outcomes | |||
| Incomplete biochemical response | 11 vs. 1 | (0.1–600 vs. 0.1–3.1) | 0.002 |
| Nodal recurrence | 82 vs. 1.3 | (0.1–601 vs. 0.1–483) | 0.003 |
| Distant metastases | 491 vs. 1.8 | (11.2–601 vs. 0.1–179) | 0.0001 |
n=40. Sample restricted to previously untreated patients after surgery but before initial RAI treatment.
AJCC, 7th ed. (1).
ENE present at the time of the neck dissection.
FIG. 1.
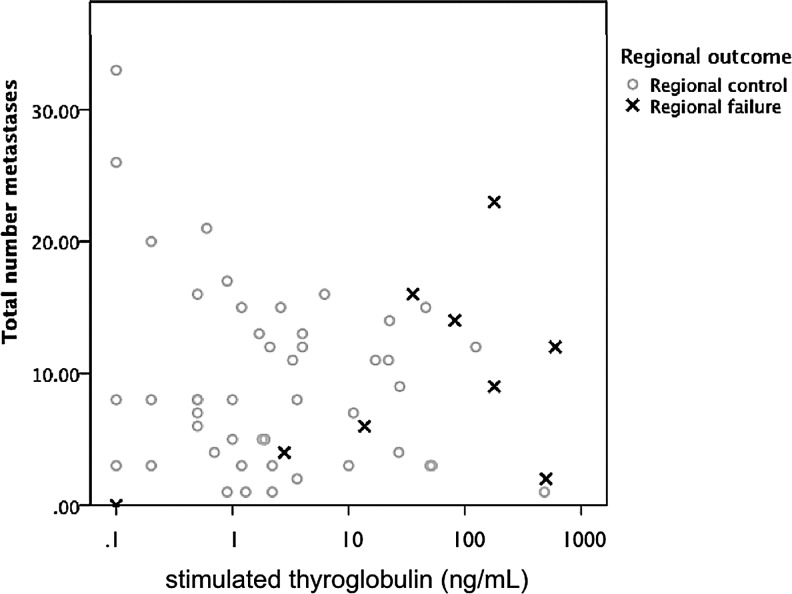
Scatterplot of preradioactive iodine (RAI) stimulated thyroglobulin (sTg) plotted against the number of nodal metastases harvested. Black ×'s indicate patients who subsequently underwent surgery for nodal disease. An increased preablation sTg rather than the number of metastatic nodes is associated with clinically significant nodal persistence requiring additional surgery.
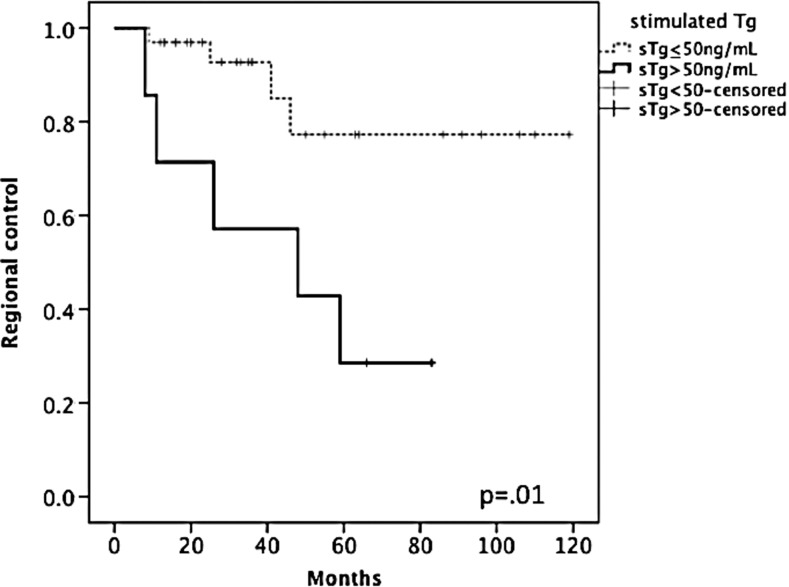
Indeed, almost 80% of patients with pre-RAI sTg >50 ng/mL required additional surgery for persistent disease within five years (p=0.01; Fig. 2). Of the seven patients with sTg >50 ng/mL, five underwent additional surgery for nodal metastases, and three eventually developed pulmonary metastases. In contrast, four of 33 patients with lower sTgs required subsequent lymphadenectomy. Of these, three had sTgs <10 ng/mL and two had sTgs <3 ng/mL. Thus, elevated pre-RAI sTgs are very specific for clinically significant nodal persistence. The converse, however, is not true: a low sTg did not guarantee a complete biochemical response or freedom from recurrence.
FIG. 2.
An sTg level >50 ng/mL after initial surgery was associated with clinically significant nodal disease requiring additional surgery.
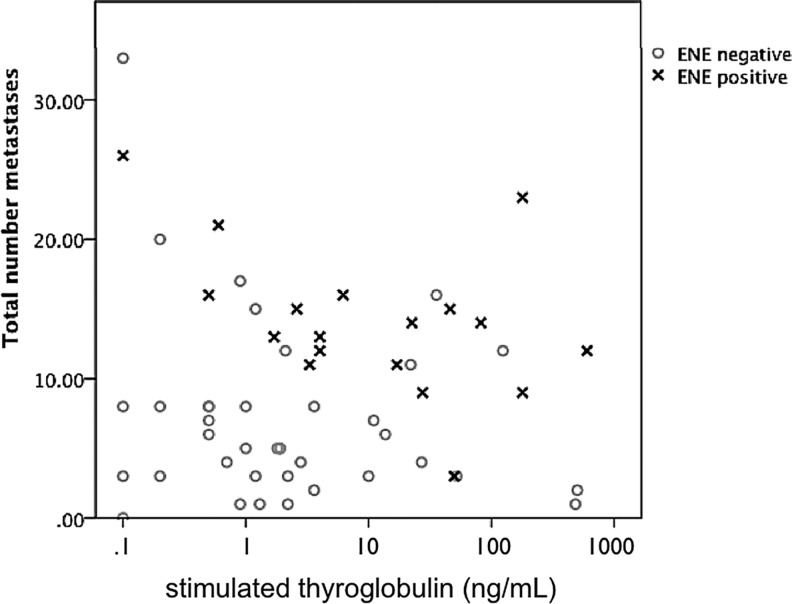
The presence of ENE increased the probability of nodal persistence. The presence of ENE in previously untreated patients increased the probability that pre-RAI stimulated Tg levels after resection would exceed 50 ng/mL some fivefold (odds ratio 5 [CI 1.2–21, p=0.03). No other clinicopathologic features were significantly associated with elevated postoperative sTg levels (Table 4). ENE was generally associated with higher median sTg levels after operation (20 ng/mL vs. 1.3 ng/mL, p=0.03), as well as higher metastatic nodal yields (median 11 vs. 5, p=0.03; Fig. 3).
FIG. 3.
Scatterplot of pre-RAI sTg plotted against number of nodal metastases harvested. Metastatic papillary thyroid carcinoma with extranodal extension (ENE; ×) generally is characterized by a larger number of metastases and higher sTg levels.
As expected, the presence of ENE also diminished the probability of a biochemical complete response for the entire cohort (odds ratio 3.5 [CI 1.3–10], p=0.01), from 49% (28/57) to 21% (6/28). ENE was the only variable significantly associated with this endpoint. As noted above, nodal recurrence occurring after a complete biochemical response was rare, and was seen in only one patient.
Seven of 13 patients found to have ENE in the persistence/recurrence category had no prior evidence of ENE. No nodes had been examined in four, and sampled nodes had no evidence of ENE in three. Of 29 patients treated for recurrent or persistent disease in the neck, 10 were rendered biochemically free of cancer. Of these, three-quarters exhibited no ENE. Only three of 12 patients with ENE (25%) treated for recurrence or persistence were biochemically free of disease after lymphadenectomy.
Discussion
The American Thyroid Association Risk of Recurrence Classification, in conjunction with biochemical and imaging studies obtained during the first two years of follow-up, has been shown to predict clinical outcomes significantly better than histopathologic assessments alone. Patients considered at intermediate or high risk of recurrence based on the initial ATA estimate, such as those in our cohort, are at very low risk of developing structurally identifiable disease if they achieve an excellent response to initial therapy (9). Conversely, a stimulated thyroglobulin level >50 ng/mL, obtained after initial surgery but prior to RAI administration, is associated with a high rate of residual nodal tumor, consistent with observations reported by Piccardo et al. (13). In our intermediate to high-risk cohort, persistent nodal disease after treatment, rather than recurrence after a complete biochemical response, accounted for a large proportion of treatment failures.
Since completeness of the initial surgical resection is the major determinant of the pre-RAI sTg, it is likely that disease-related outcomes are more affected by the initial surgical resection than by other variables. However, the sTg level is reliable only after a complete thyroidectomy with minimal residual thyroid tissue remaining. In that setting, the pre-RAI sTg level may serve as a metric for the adequacy of lymphadenectomy, and allow earlier identification of nodal persistence after neck dissection. The presence of an elevated preablation sTg, particularly in the setting of ENE, should trigger a meticulous search for retained nodal disease. Concerns that a high postoperative sTg reflects distant disease may be misplaced. Although systemic metastases were eventually detected in almost half of patients with highly elevated sTgs, this did not ensue for several years after lymphadenectomy. It is unclear whether early node dissection for patients with highly elevated stimulated Tg levels would have changed the outcome of patients with distant cancer. A multidisciplinary team with effective communication between endocrinology, surgery, nuclear medicine, and radiology is needed to determine the best course of action in the face of an elevated pre-RAI sTg value.
In this study, ENE diminished the probability of a complete biochemical response, and in previously untreated patients increased the probability that the sTg level after surgery would be highly elevated. ENE was also strongly associated with abundant regional metastases (more than twofold higher than with nodes lacking ENE). Interestingly, the number of nodes involved was not directly related to the postoperative sTg level, and did not appear to affect clinical outcomes. This may be because it is not the number of nodes removed but rather the nodal burden retained in the patient that defines “nodal persistence.”
Others have reported an association between ENE and both distant failure and cause-specific survival (4,5). ENE has previously been linked to extracapsular extension of the primary cancer (2) and T4 stage (2–4). In our study, ENE was frequently identified in those patients requiring neck dissection for macroscopic recurrence or persistence. The impact of ENE on the risk of developing distant metastases was independent of the nodal persistence itself. In at least half of these subjects, ENE was not appreciated during the initial resection. ENE is exceedingly unlikely with microscopic nodal disease, and can only be discerned when macroscopic tumor deposits are examined. Molecular markers of ENE might identify patients with more aggressive disease.
ENE has been associated with other prognostic markers, including poor differentiation, distant metastases on initial presentation, and unresectable disease (14), all of which served as exclusion criteria for this study. This eliminated many older patients from inclusion in the series. Younger patients were overrepresented among those treated for nodal recurrence. As a result, the sample population was relatively young. However, the outcomes of these patients were less favorable than those typically associated with younger age.
In the absence of certain high-risk features (poorly differentiated papillary carcinomas, unresectable cancer, and distant disease on initial presentation), ENE nevertheless differentiated patients with metastatic PTC into prognostically distinct groups, suggesting that ENE should be considered in the initial assessment of recurrence risk. Notably, ENE predisposed to nodal persistence rather than genuine nodal recurrence after a biochemical complete response.
Limitations of this study include its retrospective design and single-institution experience. Biochemical data were less complete early in the series. Although the absolute number of patients was relatively small, the cohort comprises an intermediate to high-risk group, with sufficient events and power to perform a multivariable analysis to assess determinants of systemic disease progression (but not the more delayed cancer-related death). Understanding factors associated with nodal persistence or recurrence after surgery should facilitate the development of strategies to improve clinical outcomes.
Acknowledgment
The authors thank Jonah D. Klein for help with database management.
Author Disclosure Statement
None of the authors reports a conflict of interest.
References
- 1.AJCC Cancer Staging Manual. In: Edge SB, editor; Byrd DR, editor; Compton CC, editor; Fritz AG, editor; Greene FL, editor; Trotti A, editor. 7th. Springer; New York: 2010. [Google Scholar]
- 2.Spires JR. Robbins KT. Luna MA. Byers RM. Metastatic papillary carcinoma of the thyroid: the significance of extranodal extension. Head Neck. 1989;11:242–246. doi: 10.1002/hed.2880110309. [DOI] [PubMed] [Google Scholar]
- 3.Ito Y. Hirokawa M. Jikuzono T. Higashiyama T. Takamura Y. Miya A. Kobayashi K. Matsuzuka F. Kuma K. Miyauchi A. Extranodal tumor extension to adjacent organs predicts a worse cause-specific survival in patients with papillary thyroid carcinoma. World J Surg. 2007;31:1194–1201. doi: 10.1007/s00268-007-9042-2. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita H. Noguchi S. Murakami N. Kawamoto H. Watanabe S. Extracapsular invasion of lymph node metastasis is an indicator of distant metastasis and poor prognosis in patients with thyroid papillary carcinoma. Cancer. 1997;80:2268–2272. [PubMed] [Google Scholar]
- 5.Yamashita H. Noguchi S. Murakami N. Toda M. Uchino S. Watanabe S. Kawamoto H. Extracapsular invasion of lymph node metastasis. A good indicator of disease recurrence and poor prognosis in patients with thyroid microcarcinoma. Cancer. 1999;86:842–849. doi: 10.1002/(sici)1097-0142(19990901)86:5<842::aid-cncr21>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Randolph G. Duh QY. Heller KS. Livolsi VA. Mandel SJ. Steward D. Tufano RP. Tuttle RM ATA Surgical Affairs Committee's Taskforce on Thyroid Cancer Nodal Surgery. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012;22:1144–1152. doi: 10.1089/thy.2012.0043. [DOI] [PubMed] [Google Scholar]
- 7.Ricarte-Filho J. Ganly I. Rivera M. Katabi N. Fu W. Shaha A. Tuttle RM. Fagin JA. Ghossein R. Papillary thyroid carcinomas with cervical lymph node metastases can be stratified into clinically relevant prognostic categories using oncogenic BRAF, the number of nodal metastases, and extra-nodal extension. Thyroid. 2012;22:575–584. doi: 10.1089/thy.2011.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb RC. Howard RS. Stojadinovic A. Gaitonde DY. Wallace MK. Ahmed J. Burch HB. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J Clin Endocrinol Metab. 2012;97:2754–2763. doi: 10.1210/jc.2012-1533. [DOI] [PubMed] [Google Scholar]
- 9.Tuttle RM. Tala H. Shah J. Leboeuf R. Ghossein R. Gonen M. Brokhin M. Omry G. Fagin JA. Shaha A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20:1341–1349. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccardo A. Arecco F. Morbelli S. Bianchi P. Barbera F. Finessi M. Corvisieri S. Pestarino E. Foppiani L. Villavecchia G. Cabria M. Orlandi F. Low thyroglobulin concentrations after thyroidectomy increase the prognostic value of undetectable thyroglobulin levels on levo-thyroxine suppressive treatment in low-risk differentiated thyroid cancer. J Endocrinol Invest. 2010;33:83–87. doi: 10.1007/BF03346558. [DOI] [PubMed] [Google Scholar]
- 11.Pingpank JF., Jr. Sasson AR. Hanlon AL. Friedman CD. Ridge JA. Tumor above the spinal accessory nerve in papillary thyroid cancer that involves lateral neck nodes: a common occurrence. Arch Otolaryngol Head Neck Surg. 2002;128:1275–1278. doi: 10.1001/archotol.128.11.1275. [DOI] [PubMed] [Google Scholar]
- 12.Kupferman ME. Patterson DM. Mandel SJ. LiVolsi V. Weber RS. Safety of modified radical neck dissection for differentiated thyroid carcinoma. Laryngoscope. 2004;114:403–406. doi: 10.1097/00005537-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Piccardo A. Arecco F. Puntoni M. Foppiani L. Cabria M. Corvisieri S. Arlandini A. Altrinetti V. Bandelloni R. Orlandi F. Focus on high-risk DTC patients: high postoperative serum thyroglobulin level is a strong predictor of disease persistence and is associated to progression-free survival and overall survival. Clinical Nucl Med. 2013;38:18–24. doi: 10.1097/RLU.0b013e318266d4d8. [DOI] [PubMed] [Google Scholar]
- 14.Arrangoiz R. Lango M. Li T. Veloski C. Galloway T. Mehra R. Ridge J. Extracapsular nodal disease is an independent predictor of death after lateral cervical lymphadenectomy for papillary thyroid cancer. Meeting of the American Association of Endocrine Surgeons; Iowa City, Iowa. 2012. [Google Scholar]