Abstract
Background
Low serum selenium concentrations have been associated with a diagnosis of differentiated thyroid cancer in small studies in selenium deficient areas. We conducted a pilot study to explore associations between selenium concentrations and the diagnosis of thyroid cancer in an area of selenium sufficiency in the United States. As low 25-hydroxyvitamin D concentrations have been associated with several malignancies, we also examined 25-hydroxyvitamin D levels.
Methods
This study was designed as a pilot study of prediagnostic selenium and 25-hydroxyvitamin D concentrations. We identified 65 euthyroid patients at an academic medical center who were scheduled for thyroidectomy for thyroid cancer, suspicion of thyroid cancer, or nodular disease. Blood samples were obtained two to four weeks prior to thyroidectomy. Samples were analyzed for thyrotropin (TSH), free thyroxine, total triiodothyronine, selenium, and 25 hydroxyvitamin D levels. Concentrations of these analytes were correlated with whether the patient was diagnosed with benign or malignant disease following their thyroidectomy. In patients with thyroid cancer, the concentrations of selenium and 25-hydroxyvitamin D were correlated with various prognostic features.
Results
Although selenium concentrations were not significantly lower in patients with thyroid cancer, serum selenium concentrations were inversely correlated with disease stage (p=0.011). There were no associations between vitamin D concentration and a diagnosis of thyroid cancer. Within the thyroid cancer patients, vitamin D concentrations were not associated with disease stage or any other prognostic features. In contrast, TSH concentrations were significantly higher in patients with thyroid cancer, and were positively correlated with the number of involved lymph nodes (p=0.011) and disease stage (p=0.022).
Conclusion
These data confirm the association between serum TSH and advanced thyroid cancer. In addition, they also suggest a potential association between selenium concentrations and higher thyroid cancer stage. No such association was seen for 25-hydroxyvitamin D concentrations. Larger prospective studies will be required to confirm this association. If confirmed, future studies would need to determine if the association is causative in nature. If causation exists, it seems likely that selenium concentrations would influence thyroid cancer development via an independent mechanism from that of TSH.
Introduction
Selenium (Se) serves as an essential micronutrient. Incorporated into selenoproteins within cells, it is vital for the removal of damaging peroxides, reduction of oxidized proteins and membranes, regulation of intracellular reduction–oxidation signaling, and thyroid hormone metabolism. Dietary Se is available from seafood, organ meats, and plant sources, with plant Se content being based upon soil Se concentrations. The recommended daily allowance for Se is 55 μg, as this is the intake that appears to maximize serum concentrations of glutathione peroxidase and selenoprotein P (1). Reference intervals for serum Se values are available from several databases, including the National Health and Nutrition Examination Survey (NHANES) III (1–6). Reference intervals are affected by geographic region, Se soil and water content, nutritional status, age, sex, reproductive status, smoking status, and ethnicity (1–4,7,8). Se supplements have been popular since the 1980s. Supplements generally provide 50–100 μg Se on a daily basis. Some studies show that supplement use raises the average Se concentration by approximately 7.8 ng/mL (9), although other studies show little impact of supplementation (5). NHANES III data show relatively stable serum levels in the U.S. population (1,5).
Se concentration is higher within the thyroid than in other tissues (10), and Se is important for thyroid hormone metabolism (11,12). All three deiodinases that catalyze the conversion of thyroxine into triiodothyronine contain selenocysteine. Glutathione peroxidase and thioredoxin reductase, which serve as powerful cellular antioxidants, also act to protect the thyrocytes from oxidative damage. Conditions of iodine deficiency and serum thyrotropin (TSH) elevation appear to be associated with increased levels of peroxide and oxidative stress. Se-containing enzymes appear to be protective against cellular damage in such circumstances. In conditions of Se sufficiency, glutathione peroxidase effectively controls the iodination process by degrading hydrogen peroxide. However, in Se deficiency states, the decreased activity of glutathione reductase results in increased generation of hydrogen peroxide and activity of thyroid peroxidase (13,14).
Thyroid tissue would be expected to be particularly prone to subsequent damage, since it relies on hydrogen peroxide production for thyroid hormone synthesis. There seems to be an association between autoimmune thyroid disease and Se deficiency (13–15); moreover, Se supplementation may mitigate the progression of Hashimoto's thyroiditis.
There is some limited evidence suggesting that Se is implicated in thyroid tumorigenesis. Low levels of Se were found in the serum and thyroid tissue of individuals in Poland with differentiated thyroid cancer (DTC) (16). Eighty-seven patients with various thyroid disorders were studied, of whom 21 had DTC. Another study, also conducted in Poland, showed low Se levels in the thyroid tissue of individuals with DTC (17), although this was not replicated in another study from Italy (18). Data from the Janus Serum Bank, a serum bank maintained in Norway, also suggested a role of low Se levels in the development of thyroid cancer (19,20). Within this database, serum Se levels were lower in 43 individuals who were subsequently diagnosed with DTC compared with 129 matched controls who did not develop DTC (20). In addition, Se concentrations were lower in females than in males, regardless of their status as either cases or controls. Furthermore, there was a trend for Se levels to be lowest in thyroid cancer patients who were within seven years of their DTC diagnosis. These European countries had widely varying Se levels compared with the United States at the time these studies were conducted, ranging from lower levels in some parts of Norway to higher levels in some areas of Poland (21).
Higher serum TSH levels are a risk factor for thyroid cancer in individuals with thyroid nodules (22). Not only are those individuals with higher TSH values more likely to have thyroid cancer than benign thyroid histology (22–25), but also within those individuals with malignancy, higher TSH concentrations are associated with extrathyroidal extension (24,26) and lymph-node metastases (26,27). In contrast to the well-established link between TSH levels and thyroid malignancy, the little data concerning vitamin D levels and thyroid cancer are scant and inconsistent (28,29).
We conducted a pilot study to examine serum Se levels in patients undergoing thyroidectomy in the United States. We wished to determine whether preliminary data suggested that low Se concentrations were associated with a diagnosis of DTC in a country that has well-established Se levels (5) and is generally Se sufficient. In contrast with the previous studies, we also wished, when examining Se concentrations as a thyroid cancer risk factor, to perform an analysis that also took serum TSH levels, a known risk factor for thyroid cancer (22–25), into account.
Methods
We recruited patients who were already scheduled for thyroidectomy. Prospective participants were screened by telephone or in person to exclude the following diagnoses: hypothyroidism, hyperthyroidism, nonthyroid malignancies, diabetes, and chronic cardiac, pulmonary, hepatic, or renal disease. Smokers and those taking multivitamins, Se supplements, or vitamin D supplements within the last year were also excluded. Participants who had resided outside of the Washington, DC, metropolitan area or who had been pregnant or lactating within the last year were also excluded. Chart review was also conducted to exclude those known to have positive thyroid peroxidase antibodies. Other exclusion criteria were a family history of papillary thyroid cancer (PTC) or autoimmune thyroid disease.
Participants were recruited and underwent thyroidectomy during the period 2008–2010. Anthropometric data were collected for all patients. Participants completed a questionnaire detailing use of vitamins and over-the-counter supplements, family history, medications, medical conditions, and recent places of residence. A 6 mL blood sample was obtained two to four weeks prior to surgery. A sample of 2 mL of blood was drawn into a royal-blue-top trace element blood-collection tube for Se determination. After allowing the specimen to clot for 30 minutes, it was then centrifuged to separate the serum, which was poured into a trace metal-free, screw-capped, polypropylene vial, avoiding transfer of the cellular components. The specimen was stored in a freezer at −70°C. Four mL of blood was also obtained in a serum separator tube for the remainder of the analytes. After allowing clotting, the serum was transferred into polypropylene storage vials prior to storage in a freezer at −70°C. The other analytes determined were TSH, free thyroxine (FT4), total triiodothyronine (TT3), and 25-hydroxyvitamin D (vit D). Samples were analyzed in 2012 by Quest Diagnostics Laboratories or NMS Laboratories. All TSH, FT4, and TT3 assays were performed by Quest Diagnostics. The reference range for the thyroid assays were TSH 0.4–4.5 mIU/L (immunometric assay), FT4 0.8–1.8 ng/dL (immunoassay), and TT3 76–181 ng/dL (immunoassay). The remainder of the assays were performed by one or other of the two laboratories. For Quest Diagnostics, the reference ranges for the other analytes were as follows: Se 63–160 μg/L (atomic spectroscopy) and vit D 30–100 ng/mL (tandem mass spectrometry). For NMS laboratories, the reference ranges were Se 50–140 μg/L (inductively coupled mass spectrometry) and total vit D 30–100 ng/mL (tandem mass spectrometry).
Statistical analysis
First, we included all patients in the analysis to assess the association between laboratory values and diagnosis of DTC. Characteristics of participants and their laboratory values were reported as mean (standard deviation) for continuous variables and frequency (proportion) for the categorical variables according to the diagnosis of DTC. Unadjusted and adjusted odds ratios and corresponding 95% confidence intervals for thyroid cancer according to Se values, thyroid analytes, and vit D concentrations were calculated using a logistic regression model. Second, we included those patients with a diagnosis of thyroid cancer in an analysis to assess the association of Se, TSH, and vit D concentrations with various prognostic features and stage of thyroid cancer. For stage of thyroid cancer, the National Thyroid Cancer Treatment Cooperative Study Group (NTCTCSG) stage was used in addition to the TNM stage (developed by the American Joint Committee on Cancer [AJCC]) because of its excellent performance (30,31). Mean values (standard deviation) for continuous variables and frequency (proportion) for categorical variables were calculated according to the tertiles of Se, TSH, and vit D separately. The linear association of tertiles of Se, TSH, and vit D with various prognostic features and stage of thyroid cancer was evaluated using analysis of variance (ANOVA) or the Mantel–Haenszel chi-square test. Spearman correlation coefficients of Se, TSH, and vit D with various prognostic features and stage of thyroid cancer also were calculated. Since this was a pilot study, all of the analyses were treated as preliminary. This pilot study was designed to explore the potential association of selenium, vit D, and TSH with thyroid cancer (yes/no) and cancer characteristics/stage. The limited clinical data from this study could be used to determine the need for larger studies in the future. Our multiple comparisons increase the experiment-wise level of α (the type I error rate). As this was a pilot study, traditional levels of α and β (the type II error rate) may be inappropriate, since the objective of the research was not to provide definitive support for the relationship of selenium, vit D, and TSH with thyroid cancer and cancer characteristics/stage (32). The type I error has therefore been relaxed to 0.2. As there are three potential predictors (selenium, vitamin D, and TSH) and two primary outcomes (thyroid cancer: yes/no; and cancer characteristics/stage), a two-sided p value of <0.03 was considered statistically significant with adjustment for multiplicity with use of a Bonferroni correction (i.e., 0.2/6≈0.03).
Results
Seventy eligible participants were recruited, signed a consent form, and completed a questionnaire regarding medical conditions, concurrent medications, family history, and place of residence during the last 10 years. Eighty-eight patients were found to be ineligible during screening, primarily because of consumption of Se or vit D supplemental multivitamins, or because of diagnoses of hypothyroidism or hyperthyroidism. Five patients were excluded after thyroidectomy because of histology showing lymphocytic thyroiditis. The 65 remaining participants were 71% female and had an average age of 45 years. Of the entire group, 48 (74%) were found to have DTC, and 17 (26%) had benign thyroid disease. The characteristics of study participants and their laboratory data are shown in Table 1, with the group divided into those with malignant and benign diagnoses. Elevated body mass index (BMI), increased TSH concentration, and lower TT3 concentrations were each significantly associated with having DTC. The breakdown of the thyroid cancer histology was as follows: 69% (33 patients) had PTC, 8% (4 patients) had follicular thyroid cancer, 19% (9 patients) had follicular variant of PTC, and 4% (2 patients) had other variants of PTC. The breakdown of NTCTCSG stage was as follows: 46% (22 patients) stage 1, 23% (11 patients) stage 2, 27% (13 patients) stage 3, and 4% (2 patients) stage 4.
Table 1.
Characteristics of Participants and Their Laboratory Values Divided by Malignant or Benign Diagnosis
| Thyroid cancer(n=48) | Benign(n=17) | Difference | p (t test) | p (Wilcoxon) | |
|---|---|---|---|---|---|
| Age at diagnosis (years) | 45.9±13.5 | 52.5±12.8 | −6.7 | 0.08 | 0.05 |
| Height (m) | 1.67±0.08 | 1.72±0.09 | −0.05 | 0.05 | 0.08 |
| Weight (kg) | 74.9±12.4 | 71.4±10.9 | 3.5 | 0.31 | 0.32 |
| BMI (kg/m2) | 26.8±3.5 | 24.1±2.3 | 2.7 | 0.005 | 0.004 |
| Selenium, both labs (μg/L) | 115.8±13.6 | 117±9.8 | −1.2 | 0.75 | 0.82 |
| Selenium, NMS (μg/L) | 117.1±13.9 (n=40) | 119.4±10.1 (n=9) | −2.3 | 0.64 | 0.65 |
| Selenium, QUEST (μg/L) | 109.3±10.5 (n=8) | 114.1±9.1 (n=8) | −4.9 | 0.34 | 0.50 |
| TSH (mIU/L) | 1.46±0.58 | 0.97±0.54 | 0.49 | 0.0032 | 0.0029 |
| FT4 (ng/dL) | 1.05±0.18 | 1.15±0.21 | −0.10 | 0.076 | 0.25 |
| TT3 (ng/dL) | 116±24 | 134±30 | −18 | 0.016 | 0.05 |
| Vitamin D (ng/mL) | 26.5±9.9 | 31.2±8.6 | −4.7 | 0.089 | 0.089 |
BMI, body–mass index; TSH, thyrotropin; FT4, free thyroxine; TT3, total triiodothyronine.
Table 2 shows the unadjusted odds ratios (ORs) with 95% confidence intervals (CIs) for having thyroid cancer based on Se, TSH, FT4, TT3, and vit D values. The OR adjusted for age, sex, and BMI is also shown. Se analysis was carried out either (i) using the absolute value for each laboratory, without adjusting for the slight difference in reference intervals; (ii) using only the values from NMS labs (39 patients); or (iii) by stratifying the values by tertiles based on the respective laboratory references ranges. The only analyte that was associated with an increased risk of having a diagnosis of DTC was serum TSH (OR 7.7 [CI 1.8–32.3]). The increased risk of having DTC associated with an elevated TSH concentration remained after adjustment for age, sex, and BMI (OR 8.2 [CI 1.4–46.9]).
Table 2.
Odds Ratio of Thyroid Cancer According to Selenium Values, Thyroid Analytes, and Vitamin D Concentration Using a Logistic Regression Model
| Univariate OR [CI] | OR [CI] adjusted for age, sex, and BMI | |
|---|---|---|
| Seleniuma (μg/L) | 0.99 [0.95–1.04] | 1.01 [0.95–1.08] |
| Selenium, NMSb (μg/L) | 0.99 [0.93–1.04] | 0.99 [0.92–1.08] |
| Selenium, stratified by labc (μg/L) | 0.99 [0.93–1.03] | 0.98 [0.92–1.05] |
| TSH (mIU/L) | 7.70 [1.84–32.31] | 8.23 [1.45–46.93] |
| FT4 (ng/dL) | 0.07 [0.00–1.44] | 0.04 [0.00–2.87] |
| TT3 (ng/dL) | 0.97 [0.95–0.99] | 0.97 [0.95–0.99] |
| Vitamin D (ng/mL) | 0.95 [0.90–1.01] | 0.96 [0.89–1.03] |
Values from both labs without adjusting for reference range.
Values from NMS labs only.
Values from both labs stratified by tertile based on reference range.
OR, odds ratio; CI, 95% confidence interval.
Analyses were performed for both the NTCTCSG and TNM staging systems. These two staging systems were highly correlated (Spearman correlation coefficient=0.9, p<0.0001) and yielded similar results. Multiplicity was not a major issue in this case because of this degree of correlation (33). However, for simplicity, we removed the analyses for the TNM stage from the reported results. The NTCTCSG staging was retained, as this staging system generally has a good performance (30,31). Table 3 shows the association of Se concentrations (divided by tertiles) with tumor size, extrathyroidal extension, presence of multifocality, number of tumor foci, number of cervical lymph nodes, presence of distant metastases, and disease stage. DTC stage was significantly associated with the tertile of serum Se concentration using the values from NMS labs. This association became of borderline significance when both labs were combined. Table 4 shows the same associations of tumor characteristics and stage with serum TSH and vit D, again divided by tertiles. In this case, no significant associations were found, although there was a trend for disease stage in each case with p=0.07.
Table 3.
Association of Selenium Tertiles with Prognostic Factors and Thyroid Cancer Stage
| Tertile 1 | Tertile 2 | Tertile 3 | pa | |
|---|---|---|---|---|
| Selenium (both labs) | ||||
| n in tertile (min–max values) | 10 (92–107) | 25 (110–120) | 13 (124–140) | — |
| Tumor size (cm) | 2.1±1.9 | 2.7±1.8 | 1.8±0.8 | 0.62 |
| Extrathyroidal extension | 6 (60%) | 12 (48%) | 6 (46%) | 0.53 |
| Multifocality | 5 (50%) | 11 (44%) | 8 (62%) | 0.53 |
| Number of foci | 2.6±2.3 | 1.9±1.2 | 2.4±1.6 | 0.75 |
| Number of positive lymph nodes | 3.8±7.5 | 1.4±2.6 | 4.6±6.6 | 0.71 |
| Distant metastases | 1 (10%) | 1 (4%) | 0 (0%) | —b |
| NTCTCSG Stage 1 | 3 (30%) | 10 (40%) | 9 (69%) | 0.061c |
| NTCTCSG Stage 2 | 3 (30%) | 6 (24%) | 2 (15%) | |
| NTCTCSG Stage 3 | 3 (30%) | 8 (32%) | 2 (15%) | |
| NTCTCSG Stage 4 | 1 (10%) | 1 (4%) | 0 (0%) | |
| Selenium (NMS) | ||||
| n in tertile (min–max values) | 18 (92–110) | 10 (115–120) | 12 (130–140) | — |
| Tumor size | 2.5±2.1 | 2.5±1.7 | 1.7±0.8 | 0.23 |
| Extrathyroidal extension | 9 (50%) | 3 (30%) | 5 (42%) | 0.59 |
| Multifocality | 10 (56%) | 4 (40%) | 7 (58%) | 0.96 |
| Number of foci | 2.5±1.8 | 1.5±0.9 | 2±1.47 | 0.56 |
| Number of positive lymph nodes | 2.6±5.8 | 2.2±3.2 | 3.4±5.2 | 0.68 |
| Distant metastases | 2 (11%) | 0 (0%) | 0 (0%) | —b |
| NTCTCSG Stage 1 | 5 (28%) | 6 (60%) | 9 (75%) | 0.0046c |
| NTCTCSG Stage 2 | 3 (17%) | 4 (40%) | 1 (8%) | |
| NTCTCSG Stage 3 | 8 (44%) | 0 (0%) | 2 (17%) | |
| NTCTCSG Stage 4 | 2 (11%) | 0 (0%) | 0 (0%) | |
Data are mean±SD or n (%).
p Value for linear trend.
The test is not reliable due to the small event number (n=2 with distant metastases).
Exact test.
Table 4.
Association of TSH and Vitamin D Tertiles With Prognostic Features and Thyroid Cancer Stage
| Tertile 1 | Tertile 2 | Tertile 3 | pa | |
|---|---|---|---|---|
| TSH | ||||
| n in tertile (min–max values) | 16 (0.50–1.17) | 17 (1.18–1.50) | 15 (1.60–3.70) | — |
| Tumor size | 1.8±1.3 | 2.4±1.8 | 2.7±1.7 | 0.13 |
| Extrathyroidal extension | 6 (38%) | 9 (53%) | 9 (60%) | 0.21 |
| Multifocality | 9 (56%) | 8 (47%) | 7 (47%) | 0.59 |
| Number of foci | 2.1±1.2 | 2.1±1.5 | 2.4±2.0 | 0.56 |
| Number of positive lymph nodes | 2.0±4.8 | 1.7±3.0 | 4.9±7.1 | 0.13 |
| Distant metastases | 0 (0%) | 0 (0%) | 2 (13%) | —b |
| NTCTCSG Stage 1 | 11 (69%) | 5 (29%) | 6 (40%) | 0.072c |
| NTCTCSG Stage 2 | 2 (12%) | 6 (35%) | 3 (20%) | |
| NTCTCSG Stage 3 | 3 (19%) | 6 (35%) | 4 (27%) | |
| NTCTCSG Stage 4 | 0 (0%) | 0 (0%) | 2 (13%) | |
| Vitamin D | ||||
| n in tertile (min–max values) | 16 (8–19) | 17 (21–30) | 15 (32–50) | — |
| Tumor size | 2.8±2.2 | 1.9±1.1 | 2.3±1.3 | 0.34 |
| Extrathyroidal extension | 9 (56%) | 9 (53%) | 6 (40%) | 0.37 |
| Multifocality | 9 (56%) | 8 (47%) | 7 (47%) | 0.59 |
| Number of foci | 2.3±1.3 | 2.2±1.9 | 2.1±1.6 | 0.84 |
| Number of positive lymph nodes | 0.8±1.6 | 4.2±6.8 | 3.3±5.4 | 0.19 |
| Distant metastases | 2 (13%) | 0 (0%) | 0 (0%) | —b |
| NTCTCSG stage 1 | 7 (44%) | 7 (41%) | 8 (53%) | 0.13c |
| NTCTCSG stage 2 | 2 (13%) | 4 (24%) | 5 (33%) | |
| NTCTCSG stage 3 | 5 (31%) | 6 (35%) | 2 (13%) | |
| NTCTCSG stage 4 | 2 (13%) | 0 (0%) | 0 (0%) | |
Data are mean±SD or n (%).
p Value for linear trend.
The test is not reliable due to the small event number (n=2 with distant metastases).
Exact test.
Table 5 and 6 show the correlation between the concentration of Se, TSH, and vit D (as continuous variables), and the tumor characteristics and tumor stage using the Spearman correlation coefficient. For serum Se, calculations were performed for both labs combined, NMS labs only, or Quest labs only (where the small numbers permitted the analysis). Serum Se was correlated with cancer stage (Spearman correlation coefficient −0.39, p=0.0058), and this held true after adjustment for age, sex, and BMI (Spearman correlation coefficient −0.41, p=0.011). TSH concentration was correlated both with the number of positive lymph nodes and the thyroid cancer stage (Spearman correlation coefficient 0.31, p=0.03; Spearman correlation coefficient 0.30, p=0.036 respectively). Again this correlation remained following adjustment for age, sex, and BMI (Spearman correlation coefficient 0.42, p=0.011; Spearman correlation coefficient 0.37, p=0.022 respectively). No significant correlations were found for vit D concentrations.
Table 5.
Correlation Between Selenium, TSH, and Vitamin D Concentrations and Tumor Characteristics and Stage
| SeleniumaSpearman correlation coefficient (p value) | Selenium (NMS) Spearman correlation coefficient (p value) | Selenium (QUEST) Spearman correlation coefficient (p value) | TSH Spearman correlation coefficient (p value) | Vitamin D Spearman correlation coefficient (p value) | |
|---|---|---|---|---|---|
| Number | 48 | 40 | 8 | 48 | 48 |
| Tumor size | −0.02 (0.90) | −0.04 (0.79) | 0.65 (0.083) | 0.19 (0.21) | 0.02 (0.88) |
| Extrathyroidal extension | −0.17 (0.25) | −0.15 (0.37) | 0.25 (0.56) | 0.19 (0.19) | −0.06 (0.67) |
| Multifocality | 0.04 (0.80) | −0.06 (0.72) | 0.51 (0.20) | −0.07 (0.64) | −0.02 (0.90) |
| Number of foci | −0.05 (0.74) | −0.16 (0.34) | 0.49 (0.21) | 0.01 (0.92) | −0.05 (0.73) |
| Number of positive lymph nodes | 0.15 (0.29) | 0.06 (0.71) | 0.55 (0.16) | 0.31 (0.03) | 0.23 (0.12) |
| Distant metastases | −0.22 (0.14) | −0.25 (0.12) | N/A | 0.25 (0.08) | −0.17 (0.26) |
| NTCTCSG stage | −0.39 (0.0058) | −0.45 (0.0040) | 0.24 (0.57) | 0.30 (0.036) | −0.16 (0.29) |
Using selenium concentrations from both labs without adjustment for laboratory reference range.
Table 6.
Correlation Between Selenium, TSH, and Vitamin D Concentrations and Tumor Characteristics and Stage, After Adjusting for Age, Sex, and BMI
| Seleniuma Spearman correlation coefficient (p value) | Selenium (NMS) Spearman correlation coefficient (p value) | TSH Spearman correlation coefficient (p value) | Vitamin D Spearman correlation coefficient (p value) | |
|---|---|---|---|---|
| Tumor size | −0.09 (0.80) | −0.09 (0.60) | 0.25 (0.13) | 0.04 (0.82) |
| ETE | −0.10 (0.58) | −0.10 (0.57) | 0.20 (0.24) | 0.02 (0.91) |
| Multifocality | −0.08 (0.63) | −0.08 (0.63) | 0.04 (0.83) | −0.04 (0.84) |
| Number of foci | −0.17 (0.30) | −0.17 (0.30) | 0.15 (0.36) | −0.07 (0.69) |
| Number of positive LN | 0.08 (0.62) | 0.08 (0.62) | 0.42 (0.011) | 0.16 (0.35) |
| Distant metastases | −0.26 (0.12) | −0.26 (0.12) | 0.23 (0.18) | −0.22 (0.19) |
| NTCTCSG stage | −0.41 (0.011) | −0.41 (0.012) | 0.37 (0.022) | −0.08 (0.66) |
Using selenium concentrations from both labs without adjustment for laboratory reference range. Adjusted Spearman correlation coefficient is not applicable for Selenium (QUEST) due to the small sample size.
Finally, Figure 1 shows the distribution of the analytes for benign disease and NTCTCSG stages I–IV of DTC using box plots. The decreasing Se concentration and increasing TSH concentration with advancing disease stage can be seen. Although vit D concentrations appear to trend down with advancing stage, there is wide variation in values, as seen from the percentile values.
FIG. 1.
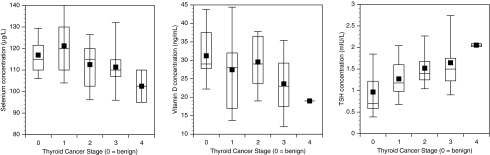
Serum selenium, vitamin D, and thyrotropin concentrations displayed according to benign diagnosis (labeled as 0) and thyroid cancer stages 1–4. The top, bottom, and line through the box correspond to the 75th percentile, 25th percentile, and 50th percentile (median), respectively. The whiskers extend from the 10th percentile to the 90th percentile. The filled square indicates the arithmetic mean.
Discussion
Based on this analysis, we cannot confirm the previously reported association between low serum Se levels and a diagnosis of DTC found in studies from Europe. However, within the group of patients diagnosed with thyroid cancer, we did find an association between more advanced stage and lower Se concentrations. However, the design of our study did not allow us to determine whether Se deficiency is associated with the pathogenesis of thyroid cancer, or alternatively whether advanced thyroid cancer promotes Se deficiency (34), or, indeed, whether unmeasured confounders exist. With respect to a mechanism whereby low Se levels could promote carcinogenesis, it has been hypothesized that an impaired antioxidative defense associated with low Se levels could lead to the generation of reactive oxygen species within thyroid tissue and lead to subsequent carcinogenesis, as may also occur with mutation of the RAS oncogene (14). However, whether such reactive oxygen species may be one of the triggers of thyroid carcinogenesis remains to be elucidated. Interestingly, a recent in vitro study found that the growth of several DTC cell lines was inhibited by treatment with Se (35).
As a result of epidemiologic studies suggesting an inverse relationship between Se and cancer incidence or a possible relationship between low Se levels and increased cancer mortality, there has long been interest in Se as a chemoprotective agent (36,37). However, results of subsequent trials of Se supplementation have generally been disappointing or mixed (38) for cancers as diverse as basal and squamous-cell carcinomas of the skin (39), prostate cancer (40), and lung cancer (41). Given the complexity of the factors affecting thyroid carcinogenesis, it is likely that similar mixed results would be obtained if similar trials of chemoprevention were indicated for thyroid cancer based on additional data confirming an association between DTC and serum Se levels.
We did not find either an association between low vit D levels and a diagnosis of DTC in our study population, or an association between lower vit D levels and higher stage disease within patients with DTC. However, caution is indicated, as our sample size was quite small. A recent report suggested an association between vit D deficiency and a diagnosis of thyroid cancer (29). A single small study showed lower 1,25-dihydroxyvitamin D concentrations in patients with DTC in Poland compared with control patients (42). Vit D status is known to be intimately related to body weight and BMI, with vit D levels decreasing with increasing BMI (43). In turn, increasing BMI has been shown to be a risk factor for DTC (44–46). Thus, it is possible that the effect of vit D levels reported by Roskies et al. (29) is confounded by patient BMI. However, vit D did not appear to be a risk factor for thyroid cancer in our study, either before or after the performance of an adjustment for BMI. If, in the future, vit D levels are confirmed to influence DTC development, independent of an effect mediated through BMI and obesity, we postulate that this would be due to an effect on the immune system and immune surveillance, and is unlikely to be specific to DTC. For example, low vit D concentrations have been associated with diagnoses of breast cancer and colon cancer in epidemiologic studies (47,48).
We did, however, confirm the now well-established relationship between elevated TSH concentrations and DTC. The findings of higher TSH levels and advanced stage have been reported previously (24). A finding of higher TSH levels and the presence of lymph-node metastases has also been reported before (26,27,49). Our data also suggest that TSH concentrations are associated with the number of positive lymph nodes. Thus, data appear to be accumulating that link prediagnostic TSH levels not only with the presence of disease, but also with more advanced or aggressive disease.
The weaknesses of our study include our small sample size, multiplicity, and the cross-sectional nature of our data. Regarding the multiplicity, however, we used a Bonferroni correction to control for the type I error rate. Regarding the cross-sectional nature, these data are not truly prediagnostic, as they were, for example, in the study by Glattre et al., where serum samples were obtained several years before the DTC diagnosis (20). Although our samples were obtained immediately prior to thyroidectomy, thus not truly being prediagnostic, if we were to assume stable Se levels based on lack of intermittent use of various supplements and based on stable residence in one geographic area, it is possible that these Se levels could be extrapolated to several years earlier. NHANES data, in fact, do show relatively stable Se levels in the overall U.S. population (5). The prior studies of prediagnostic Se concentrations did not document whether patients were taking supplements or whether such supplements were in use in those countries during the relevant time period. These studies may be difficult to interpret if the participants' status fluctuated as they initiated and discontinued vitamin and trace element supplementation.
The strength of our study includes the fact that our subjects were selected to have not used supplements at all in the year preceding their DTC diagnosis and to have been resident in the Washington, DC, metropolitan area during this time. Thus, we predict that they had relatively stable Se and vit D levels. We also concurrently accounted for serum TSH, a known risk factor for thyroid cancer, in our study.
Based on our small study, it seems that although serum Se levels do not differ between those with thyroid cancer and those with benign thyroid disease, lower Se levels may, nevertheless, be associated with a more advanced DTC diagnosis. In summary, within those individuals diagnosed with DTC, low Se concentrations may be a risk factor for more advanced disease, but this needs to be confirmed. Should this association be borne out in larger studies, it is possible that Se may protect from thyrocyte damage that ultimately culminates in more advanced DTC, in the same way that Se supplementation appears to mitigate the inflammatory activity associated with autoimmune thyroid disease (50).
Acknowledgments
This study was funded in part with federal funds (Grant # UL1TR000101 previously UL1RR031975) from the National Center for Research Resources, NIH, through the Clinical and Translational Science Awards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, Re-Engineering the Clinical Research Enterprise.
Author Disclosure Statement
The authors have no conflicts to declare.
References
- 1.Panel on Dietary Antioxidants and Related Compounds. Washington, DC: National Academy Press; 2000. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Food and Nutrition Board, Institute of Medicine. [Google Scholar]
- 2.Duffield-Lillico AJ. Reid ME. Turnbull BW. Combs GF., Jr Slate EH. Fischbach LA. Marshall JR. Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 3.Alegria A. Barbera R. Clemente G. Farre R. Garcia MJ. Lagarda MJ. Selenium and glutathione peroxidase reference values in whole blood and plasma of a reference population living in Valencia, Spain. J Trace Elem Med Biol. 1996;10:223–228. [PubMed] [Google Scholar]
- 4.Alfthan G. Neve J. Reference values for serum selenium in various areas—evaluated according to the TRACY protocol. J Trace Elem Med Biol. 1996;10:77–87. doi: 10.1016/S0946-672X(96)80015-0. [DOI] [PubMed] [Google Scholar]
- 5.Niskar AS. Paschal DC. Kieszak SM. Flegal KM. Bowman B. Gunter EW. Pirkle JL. Rubin C. Sampson EJ. McGeehin M. Serum selenium levels in the US population: Third National Health and Nutrition Examination Survey, 1988–1994. Biol Trace Elem Res. 2003;91:1–10. doi: 10.1385/BTER:91:1:1. [DOI] [PubMed] [Google Scholar]
- 6.Bleys J. Navas-Acien A. Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–410. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 7.Muntau AC. Streiter M. Kappler M. Roschinger W. Schmid I. Rehnert A. Schramel P. Roscher AA. Age-related reference values for serum selenium concentrations in infants and children. Clin Chem. 2002;48:555–560. [PubMed] [Google Scholar]
- 8.Ha EJ. Smith AM. Plasma selenium and plasma and erythrocyte glutathione peroxidase activity increase with estrogen during the menstrual cycle. J Am Coll Nutr. 2003;22:43–51. doi: 10.1080/07315724.2003.10719274. [DOI] [PubMed] [Google Scholar]
- 9.Grandjean P. Nielsen GD. Jorgensen PJ. Horder M. Reference intervals for trace elements in blood: significance of risk factors. Scand J Clin Lab Invest. 1992;52:321–337. doi: 10.1080/00365519209088366. [DOI] [PubMed] [Google Scholar]
- 10.Aaseth J. Frey H. Glattre E. Norheim G. Ringstad J. Thomassen Y. Selenium concentrations in the human thyroid gland. Biol Trace Elem Res. 1990;24:147–152. doi: 10.1007/BF02917202. [DOI] [PubMed] [Google Scholar]
- 11.Kohrle J. Selenium and the control of thyroid hormone metabolism. Thyroid. 2005;15:841–853. doi: 10.1089/thy.2005.15.841. [DOI] [PubMed] [Google Scholar]
- 12.Kohrle J. Jakob F. Contempre B. Dumont JE. Selenium, the thyroid, and the endocrine system. Endocr Rev. 2005;26:944–984. doi: 10.1210/er.2001-0034. [DOI] [PubMed] [Google Scholar]
- 13.Duntas LH. Environmental factors and autoimmune thyroiditis. Nat Clin Pract Endocrinol Metab. 2008;4:454–460. doi: 10.1038/ncpendmet0896. [DOI] [PubMed] [Google Scholar]
- 14.Duntas LH. The role of selenium in thyroid autoimmunity and cancer. Thyroid. 2006;16:455–460. doi: 10.1089/thy.2006.16.455. [DOI] [PubMed] [Google Scholar]
- 15.Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95:5180–5188. doi: 10.1210/jc.2010-0191. [DOI] [PubMed] [Google Scholar]
- 16.Kucharzewski M. Braziewicz J. Majewska U. Gozdz S. Concentration of selenium in the whole blood and the thyroid tissue of patients with various thyroid diseases. Biol Trace Elem Res. 2002;88:25–30. doi: 10.1385/BTER:88:1:25. [DOI] [PubMed] [Google Scholar]
- 17.Kucharzewski M. Braziewicz J. Majewska U. Gozdz S. Copper, zinc, and selenium in whole blood and thyroid tissue of people with various thyroid diseases. Biol Trace Elem Res. 2003;93:9–18. doi: 10.1385/BTER:93:1-3:9. [DOI] [PubMed] [Google Scholar]
- 18.Bellisola G. Bratter P. Cinque G. Francia G. Galassini S. Gawlik D. Negretti de Bratter VE. Azzolina L. The TSH-dependent variation of the essential elements iodine, selenium and zinc within human thyroid tissues. J Trace Elem Med Biol. 1998;12:177–182. doi: 10.1016/S0946-672X(98)80006-0. [DOI] [PubMed] [Google Scholar]
- 19.Jellum E. Andersen A. Lund-Larsen P. Theodorsen L. Orjasaeter H. Experiences of the Janus Serum Bank in Norway. Environ Health Perspect. 1995;103:85–88. doi: 10.1289/ehp.95103s385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glattre E. Thomassen Y. Thoresen SO. Haldorsen T. Lund-Larsen PG. Theodorsen L. Aaseth J. Prediagnostic serum selenium in a case-control study of thyroid cancer. Int J Epidemiol. 1989;18:45–49. doi: 10.1093/ije/18.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Haug A. Graham RD. Christophersen OA. Lyons GH. How to use the world's scarce selenium resources efficiently to increase the selenium concentration in food. Microb Ecol Health Dis. 2007;19:209–228. doi: 10.1080/08910600701698986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLeod DS. Watters KF. Carpenter AD. Ladenson PW. Cooper DS. Ding EL. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose–response meta-analysis. J Clin Endocrinol Metab. 2012;97:2682–2692. doi: 10.1210/jc.2012-1083. [DOI] [PubMed] [Google Scholar]
- 23.Boelaert K. Horacek J. Holder RL. Watkinson JC. Sheppard MC. Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2006;91:4295–4301. doi: 10.1210/jc.2006-0527. [DOI] [PubMed] [Google Scholar]
- 24.Haymart MR. Repplinger DJ. Leverson GE. Elson DF. Sippel RS. Jaume JC. Chen H. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93:809–814. doi: 10.1210/jc.2007-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonklaas J. Nsouli-Maktabi H. Soldin SJ. Endogenous thyrotropin and triiodothyronine concentrations in individuals with thyroid cancer. Thyroid. 2008;18:943–952. doi: 10.1089/thy.2008.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SS. Lee BJ. Lee JC. Song SH. Kim BH. Son SM. Kim IJ. Kim YK. Kang YH. Preoperative serum thyroid stimulating hormone levels in well-differentiated thyroid carcinoma is a predictive factor for lateral lymph node metastasis as well as extrathyroidal extension in Korean patients: a single-center experience. Endocrine. 2011;39:259–265. doi: 10.1007/s12020-010-9430-5. [DOI] [PubMed] [Google Scholar]
- 27.Fiore E. Rago T. Provenzale MA. Scutari M. Ugolini C. Basolo F. Di Coscio G. Berti P. Grasso L. Elisei R, et al. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009;16:1251–1260. doi: 10.1677/ERC-09-0036. [DOI] [PubMed] [Google Scholar]
- 28.Laney N. Meza J. Lyden E. Erickson J. Treude K. Goldner W. The prevalence of vitamin D deficiency is similar between thyroid nodule and thyroid cancer patients. Int J Endocrinol. 2010;2010:805716. doi: 10.1155/2010/805716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roskies M. Dolev Y. Caglar D. Hier MP. Mlynarek A. Majdan A. Payne RJ. Vitamin D deficiency as a potentially modifiable risk factor for thyroid cancer. J Otolaryngol Head Neck Surg. 2012;41:160–163. [PubMed] [Google Scholar]
- 30.Sherman SI. Brierley JD. Sperling M. Ain KB. Bigos ST. Cooper DS. Haugen BR. Ho M. Klein I. Ladenson PW, et al. Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–1021. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Wong R. Bresee C. Braunstein G. Comparison with published systems of a new staging system for papillary, follicular thyroid carcinomas. Thyroid. 2012;2013;23:566–574. doi: 10.1089/thy.2012.0181. [DOI] [PubMed] [Google Scholar]
- 32.Schoenfeld D. Statistical considerations for pilot studies. Int J Radiat Oncol Biol Phys. 1980;6:371–374. doi: 10.1016/0360-3016(80)90153-4. [DOI] [PubMed] [Google Scholar]
- 33.Koch GG. Gansky SA. Statistical considerations for multiplicity in confirmatory protocols. Drug Inf J. 1996;30:523–533. [Google Scholar]
- 34.Glattre E. Nygard JF. Aaseth J. Selenium and cancer prevention: observations and complexity. J Trace Elem Med Biol. 2012;26:168–169. doi: 10.1016/j.jtemb.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 35.Kato MA. Finley DJ. Lubitz CC. Zhu B. Moo TA. Loeven MR. Ricci JA. Zarnegar R. Katdare M. Fahey TJ., 3rd Selenium decreases thyroid cancer cell growth by increasing expression of GADD153 and GADD34. Nutr Cancer. 2010;62:66–73. doi: 10.1080/01635580903191569. [DOI] [PubMed] [Google Scholar]
- 36.Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis. 1999;20:1657–1666. doi: 10.1093/carcin/20.9.1657. [DOI] [PubMed] [Google Scholar]
- 37.Rayman MP. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 38.Dennert G. Zwahlen M. Brinkman M. Vinceti M. Zeegers MP. Horneber M. Selenium for preventing cancer. Cochrane Database Syst Rev. 2011:CD005195. doi: 10.1002/14651858.CD005195.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark LC. Combs GF., Jr Turnbull BW. Slate EH. Chalker DK. Chow J. Davis LS. Glover RA. Graham GF. Gross EG, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 40.Hurst R. Hooper L. Norat T. Lau R. Aune D. Greenwood DC. Vieira R. Collings R. Harvey LJ. Sterne JA, et al. Selenium and prostate cancer: systematic review and meta-analysis. Am J Clin Nutr. 2012;96:111–122. doi: 10.3945/ajcn.111.033373. [DOI] [PubMed] [Google Scholar]
- 41.Fritz H. Kennedy D. Fergusson D. Fernandes R. Cooley K. Seely A. Sagar S. Wong R. Seely D. Selenium and lung cancer: a systematic review and meta analysis. PLoS One. 2011;6:e26259. doi: 10.1371/journal.pone.0026259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stepien T. Krupinski R. Sopinski J. Kuzdak K. Komorowski J. Lawnicka H. Stepien H. Decreased 1-25 dihydroxyvitamin D3 concentration in peripheral blood serum of patients with thyroid cancer. Arch Med Res. 2010;41:190–194. doi: 10.1016/j.arcmed.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Wortsman J. Matsuoka LY. Chen TC. Lu Z. Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 44.Kitahara CM. Platz EA. Freeman LE. Hsing AW. Linet MS. Park Y. Schairer C. Schatzkin A. Shikany JM. Berrington de Gonzalez A. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20:464–472. doi: 10.1158/1055-9965.EPI-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almquist M. Johansen D. Bjorge T. Ulmer H. Lindkvist BStocks T. Hallmans G. Engeland A. Rapp K. Jonsson H, et al. Metabolic factors and risk of thyroid cancer in the Metabolic syndrome and Cancer project (Me-Can) Cancer Causes Control. 2011;22:743–751. doi: 10.1007/s10552-011-9747-2. [DOI] [PubMed] [Google Scholar]
- 46.Leitzmann MF. Brenner A. Moore SC. Koebnick C. Park Y. Hollenbeck A. Schatzkin A. Ron E. Prospective study of body mass index, physical activity and thyroid cancer. Int J Cancer. 2010;126:2947–2956. doi: 10.1002/ijc.24913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin MH. Holmes MD. Hankinson SE. Wu K. Colditz GA. Willett WC. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–1311. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 48.Chung M. Lee J. Terasawa T. Lau J. Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:827–838. doi: 10.7326/0003-4819-155-12-201112200-00005. [DOI] [PubMed] [Google Scholar]
- 49.McLeod DS. Cooper DS. Ladenson PW. Ain KB. Bigos ST. Brierley JD. Fein HG. Haugen BR. Jonklaas J. Magner J, et al. Prognosis of differentiated thyroid cancer in relation to at-diagnosis TSH and thyroglobulin antibody status. 82nd Annual Meeting of the American Thyroid Association; Quebec. 2012. [Google Scholar]
- 50.Negro R. Greco G. Mangieri T. Pezzarossa A. Dazzi D. Hassan H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab. 2007;92:1263–1268. doi: 10.1210/jc.2006-1821. [DOI] [PubMed] [Google Scholar]


