Abstract
Previous studies have reported that mild induced hypothermia (MIH) treatment has positive effects on traumatic brain injury (TBI) outcomes, which have recently been linked to β-amyloid (Aβ)-induced secondary brain injury (SBI) extent in hippocampal tissues. We therefore investigate the relationship between MIH treatment and expression of Aβ and related proteins following TBI. Adult Sprague-Dawley rats were randomly divided into three equal groups (S: sham-operated, N: normothermia, and H: mild hypothermia). After TBI induced by fluid percussion, group N remained at normal temperature, and group H underwent MIH (32°C) for 6 hours. Behavioral scale scores were then assessed. All rats were sacrificed 24 hours and hippocampal tissues were harvested, stained with hematoxylin and eosin. mRNA and protein expressions of Aβ, β-amyloid protein precursor (APP), and β-secretase (BACE) were analyzed. Our results revealed significantly improved behavioral scale scores and the surviving neuron numbers were observed in group H compared to group N (p<0.05). Additionally, group N increased APP, Aβ, and BACE levels compared to group S (all p<0.05). Reduced expression of APP-, Aβ-, and BACE were apparent in group H compared to group N (all p<0.05). However, no statistically significant difference was observed between groups H and S in behavioral scale scores and the expression of APP-, Aβ-, and BACE (p>0.05). In conclusion, MIH treatment significantly improves the survival of neuron and reduced Aβ, BACE, and APP upregulation after TBI, which may provide a better understanding of the mechanisms by which hypothermia reduces SBI in TBI patients.
Introduction
Traumatic brain injury (TBI), an extremely complex neurological condition, is the leading cause of death in persons younger than 45 years of age (Okonkwo, 2008), accounting for up to 80% of all trauma-related deaths (Hansson, 2005). TBI occurs in large numbers in both the civilian and military populations, and it is often related to occupations hazards (Risdall and Menon, 2011). In recent years, TBI has been increasingly addressed by clinical research groups. Despite these efforts, mortality for TBI patients in emergency care facilities remains between 25% and 30% (Tu et al., 2012), and no successful Phase III trials of pharmacological agents for TBI have been reported to date (Okonkwo, 2008). Because of the increasing prevalence and high mortality rate of TBI (Margulies and Hicks, 2009), improved early diagnostic methods and treatment strategies for TBI are urgently required in clinical settings, posing a challenge for neurological researchers.
During TBI, primary brain injury results from physical displacement of structures during the initial insult; however, the most devastating effects of TBI are often associated with the progressive and complex processes of secondary brain injury (SBI) occurring well after initial damage (Scalea et al., 1994). Expression and abnormal release of various cellular factors during SBI can damage brain cells, cause dysfunction of the blood–brain barrier, aggravate cerebral edema, and interfere with intracellular signaling in cerebral tissues (Hansson, 2005). Recently, the β-amyloid (Aβ) protein has been identified as an important marker of SBI, indicating the extent of pathogenesis, severity, subsequent cognitive dysfunction, and potentially symptomatic pathophysiological alterations in the central nervous system of TBI patients (Mitani et al., 2012). Thus, Aβ may be useful as a marker in the clinical research of SBI mediating treatment strategies.
The Aβ protein, which is best known for its role in the cerebral plaque formations that contribute to senility in Alzheimer disease patients (Goedert and Spillantini, 2006), exhibits a strong tendency for aggregation and high neurotoxicity that can promote SBI following TBI (Loane et al., 2009). As a result, Aβ protein aggregates are extremely difficult to dissolve in nerve cells, particularly in the presence of β-secretase (BACE) that acts as the key rate-limiting enzyme in Aβ protein formation by hydrolysis of the β-amyloid protein precursor (APP) at the amino terminus (Uryu et al., 2007; Mannix et al., 2011). When Aβ aggregates outside of nerve cells, it can also promote abnormalities in neuronal cytoskeleton, resulting in cellular damage (Loane et al., 2009). In addition, Aβ may also be responsible for abnormal and potentially adverse metabolic activities, such as low glucose levels, elevated neuronal excitability, oxidative stress in various tissues, intracellular calcium imbalance, and promotion of inflammatory response processes (Lindholm et al., 2006).
Therapeutic hypothermia induced by either surface or endovascular cooling in clinical settings has been proven to exhibit both neuroprotective and edema blocking effects following cerebral ischemia (Song and Lyden, 2012). More recently, mild induced hypothermia (MIH) has also been shown to significantly reduce the incidence of SBI (van der Worp et al., 2007). In fact, therapeutic temperature modulation using MIH has been reported to attenuate SBI following TBI in a variety of clinical settings when applied on an individual patient basis with consideration for the rate of edema progression (Urbano and Oddo, 2012). As a result, the American Association of Neurological Surgeons recommended MIH treatment in its Guidelines for the Management of Severe Traumatic Brain Injury (2008) (Brain Trauma Foundation et al., 2007).
Whereas the benefits of MIH treatment for TBI patients are widely accepted, the link between Aβ expression and hypothermia treatment has not been documented. In the current study, a rat model of TBI was treated with MIH to determine the influence of MIH treatment on the expression of Aβ and related proteins that have been linked with SBI. A better understanding of the mechanisms by which hypothermia reduces SBI in TBI patients may provide a basis for the future development of SBI prevention strategies that limit Aβ neurotoxicity and improve overall nerve cell recovery.
Materials and Methods
Animal subjects
Adult male Sprague-Dawley (SD) rats (n=60; mean body weight 370±30 g) aged 3–6 months were obtained from the Laboratory Animal Center of The Academy of Military Medical Sciences (Beijing, China) and housed at 20°C–25°C and 50%±5% humidity with ad libitum access to food and water and a 12-hour light/12-hour dark cycle. All procedures and animal experiments were approved by the Animal Ethics Committee of the Medical College of Chinese Armed Police Forces and conducted in accordance with all state regulations.
Study grouping
All 60 rats were randomly divided into three equal groups (S: sham-operated, N: normothermia, and H: mild hypothermia). A rat model of TBI was induced by fluid percussion. Rats of the S group underwent skull drilling and tube insertion under anesthesia, but TBI was not induced. Rats of the H group were subjected to immediate MIH by external surface cooling with ice until a body temperature of 32°C±0.5°C was achieved, in accordance with recommended MIH of 32°C–35°C provided by the American Association of Neurological Surgeons Guidelines for the Management of Severe Traumatic Brain Injury (2008) (Brain Trauma Foundation et al., 2007). After 6 hours of MIH, these animals were gradually warmed to 37°C±0.3°C within 1 hour using a heat lamp, as previously described (Feng et al., 2010). Rats of the N group were maintained at normal body temperature (37°C±0.3°C) following TBI induction.
Induction of TBI using fluid percussion
The N and H group rats underwent induced TBI by fluid percussion, as previously described (Tu et al., 2012). Briefly, rats were anesthetized with a nitrous oxide/oxygen mixture (70%/30%) containing 2% halothane and endotracheally intubated for mechanical ventilation and placed in a stereotactic apparatus exposing the skull under sterile conditions. A round window (4 mm diameter) was opened in the skull at a position 2.0 mm posterior to the fontanelle and 2.0 mm right of the center line. An injury tube was fixed on the edge of the bone window, while maintaining cerebral dura mater integrity. A fluid percussion device was connected to the injury tube and kept air-tight to induce TBI at a peak percussion of 0.15–0.25 MPa at 20 ms mean intervals. Rats undergoing cardiac and/or respiratory arrests were treated immediately with a small animal breathing apparatus (RWD407; RWD Biologic Apparatus Facilities Ltd. Co., Shenzhen, China).
Neurological assessment
Neurological function was assessed 24 hours after TBI according to the procedure described by Faden et al. (1989). Briefly, the performance in seven individual tests graded on a scale of 0 (severe impairment) to 5 (acceptable normal function) were used to produce a total neurological score ranging from 0 to 35. Tests included three inclined plane tests measuring the ability of subjects to maintain right- and left-horizontal and vertical distance when placed on an inclined plane, two flexion tests measuring right and left forearm flexion when suspended by the tail, and two resistance tests measuring the degree of resistance in response to right- and left-lateral pulsion.
Specimen preparation and pathological assessment
After neurological assessment, all rats were anesthetized with 4% sodium pentobarbital, cardiac regions were exposed by thoracotomy, and left ventricular cannulation extending to the ascending aorta was performed. Then, rats were perfused with 200 mL of buffered saline solution to flush the blood followed by 700 mL of 4% paraformaldehyde in a phosphate-buffered solution (PBS; pH 7.2–7.4) for perfusion fixation. Following complete perfusion, all rats were decapitated and whole brains were extracted. Whole brain tissues were fixed overnight in fresh 4% paraformaldehyde at 4°C and then embedded in paraffin. The dentate gyrus was selected for coronal serial sections, hematoxylin and eosin stained, and observed by bright-field microscopy using an Olympus BX51 microscope (Olympus Corp., Tokyo, Japan).
Immunohistochemical staining
A streptavidin-peroxidase immunohistochemical kit (Zhong Shan Biological Technology Co., Ltd., Beijing, China) was used according to the manufacturer's instructions. Briefly, tissues sections were incubated with 3% H2O2 and deionized water for 15 minutes to block endogenous peroxidase, washed with PBS, and incubated with goat serum for 20 minutes. Sections were incubated with the rabbit monoclonal antibody (mAb) against Aβ (dilution, 1:100), BACE (1:100), and APP (1:50) primary antibody (Epitomics, Inc., Burlingame, CA), respectively, overnight at 4°C, washed with PBS, labeled with biotinylated secondary antibodies, and incubated for 20 minutes at room temperature. Specimens were then washed with PBS and incubated with horseradish peroxidase-labeled streptavidin for 15 minutes at room temperature. Next, samples were incubated in the 3,3′-diaminobenzidine color development reagent, washed, restained with hematine, and dehydrated with ethanol. A transparent xylene solution was then added to the section sealed with neutral balata. Brown particles indicating positive immunohistochemical staining in morphologically intact cells were counted in five random, nonrepeated visual fields (magnification, ×100) (Itoh et al., 2009; Li et al., 2012).
Immunofluorescent histochemical staining
Specimens were dewaxed and hydrated, treated with 2% rabbit serum (Epitomics, Inc.), and incubated with the BACE primary antibody (Epitomics, Inc.) (1:100) overnight at 4°C. Then, slides were washed three times with a washing buffer for 5 minutes each and stained with a rhodamine-labeled secondary antibody (1:50) in the dark for 20 minutes at 37°C. After washing with 10 μM PBS three times for a total of 15 minutes, 4′,6-diamidino-2-phenylindole (DAPI) was used to stain the nucleus. Finally, sections were sealed and observed by fluorescence microscopy with an 80i (Nikon, Tokyo, Japan).
Reverse transcription–polymerase chain reaction
DNA markers and the reverse transcription–polymerase chain reaction (RT-PCR) (TransGen Biotech, Beijing, China) were applied according to the instructions provided by the manufacturer. Total RNA from tissue samples was extracted with TRIzol (Invitrogen Corp., Carlsbad, CA). The concentration and purity of RNA were estimated by spectrophotometric analysis (OD260/280). cDNA synthesis was performed using the M-MLV Reverse Transcriptase enzyme (Invitrogen) and Oligo-dT primers. All primers were synthesized by Dinguo Biotech Co., Ltd. (Beijing, China). For APP, sense 5′-GTG CGC ATG GTG GAC CCC AA-3′ and antisense 5′-CCC AGA CCC TGG TCG AGT GGT-3′ yielded an expected product length of 429 bp. For BACE, sense 5′-AGG GCA TCC TAG GGC TGG CC-3′ and antisense 5′-CTC CGT CGA GGA GGC TGC CT-3′ yielded an expected product length of 419 bp. For β-actin, the internal reference, sense 5′-GGA CTT CGA GCA AGA GAT GG-3′ and antisense 5′-GAA GCA TTT GCG GTG GA-3′ yielded an anticipated product length was 503 bp. The reaction mixture had a total volume of 25 μL (1 μL of each primer, 1 μL of cDNA, 12.5 μL of 2× Easy Taq PCR Super Mix, and 9.5 μL of ddH2O). The reaction conditions included predenaturation for 5 minutes at 95°C followed by 30 cycles of denaturation for 30 seconds at 95°C, annealing for 30 seconds at 58°C, extension for 30 seconds at 72°C. RT-PCR products were electrophoresed in 1% agarose gel stained with ethidium bromide. The experiment was repeated three times. All bands were scanned and analyzed using an ultraviolet gel imager (Bio-Gel; Bio-Rad, Hercules, CA) and mean optical band densities were recorded. The relative content of target gene mRNA was calculated in relation to β-actin.
Western blotting
The tissue samples were lysed with 100 μL of 2× sodium dodecyl sulfate (SDS) buffer (1 M Tris–HCl, 1% SDS, 50% glycerol, pH 6.8) for every 100 mg of tissue on ice, while grinding for 10 minutes, and then centrifuged at 14,000 rpm for 20 minutes. The supernatant was removed and stored at −80°C for further use. The protein concentration was measured using a BCA Quantification Kit (Beyotime Institute of Biotechnology, Jiangsu, China). Briefly, protein samples (50 μg) were electrophoretically transferred to a nitrocellulose membrane (Bio-Rad) after 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) or tricine-SDS-PAGE (for Aβ) (Schagger, 2006). The membrane was blocked for 2 hours in the presence of 5% skimmed milk at room temperature, followed by addition of rabbit mAb against APP (dilution, 1:50), Aβ (1:500), BACE (1:200), and β-actin (1:2000) primary antibodies. After incubation overnight at 4°C, the membrane was washed three times using the PBS/Tween 20 buffer and further incubated with the goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (dilution, 1:2000) (KPL, Inc., Gaithersburg, MD) for 2 hours at room temperature. An enhanced chemiluminescence (ECL) kit (Beyotime Institute of Biotechnology) was used for color development. Optical density was measured and analyzed using a gel imaging system with Quantity One software (Bio-Rad).
Statistical analysis
All data were processed using SPSS software version 13.0 (SPSS, Inc., Chicago, IL) and analyzed by the t-test and single factor analysis of variance (ANOVA) followed by Tukey's post hoc analysis. All data are expressed as mean±standard deviations. p values less than 0.05 were considered statistically significant (p<0.05).
Results
MIH treatment improves neurological function and behavioral scoring in rat model of TBI
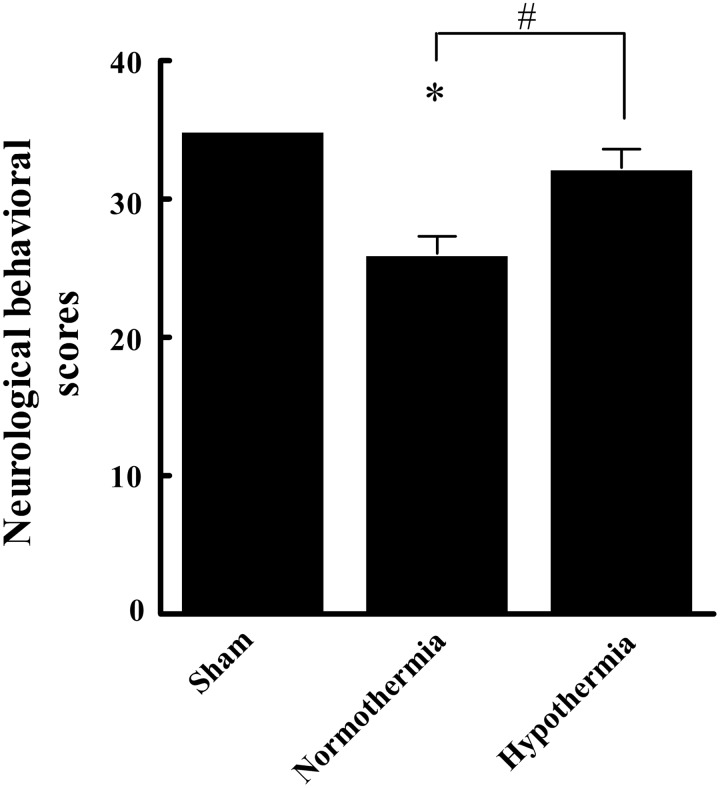
As shown in Figure 1, neurological function and behavioral scores in group N were significantly lower than those of group S (26.08±1.23 vs. 35.00±0.00, p<0.05). Conversely, scores in group H was significantly higher than those of group N (32.29±1.31 vs. 26.08±1.23, p<0.05), but nonsignificantly lower than those of the S group (p>0.05). Thus, the most apparent adverse neurological effects (from most to least severe) were observed in the N group>H group>S group.
FIG. 1.
Comparison of neurological behavioral scores in three groups. All data are expressed as mean±SD (n=20), *p<0.05 versus the sham group; #p<0.05 versus the normothermia group.
Effects of MIH treatment on rat hippocampal histopathological changes following TBI
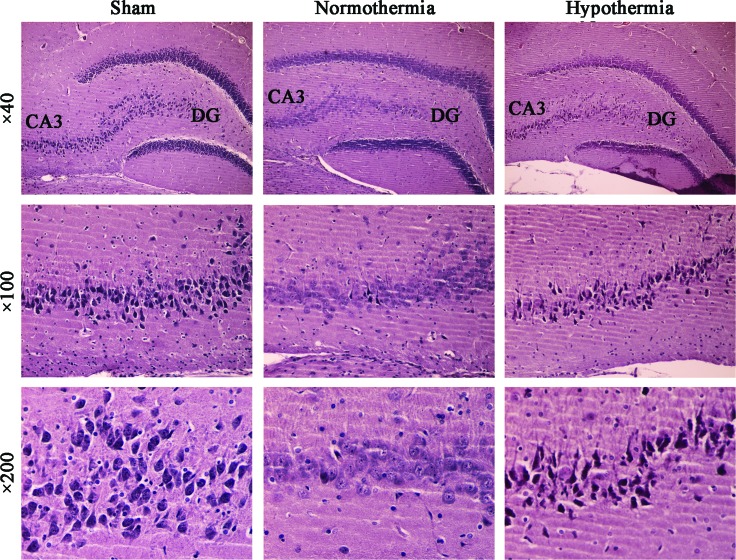
The pyramidal cell layer of the hippocampus CA3 area in group S specimens clearly revealed a neat and compact alignment, with highly intact neuronal structures. In group N specimens, however, a significant number of necrotic neurons were observed. The N group specimens exhibited an increased number of neurons with abnormal morphology, increased intercellular space, nonstructurally intact pyramidal cells, reduced cell body volume, disappearance of the nucleoli, and neuronal residues. Furthermore, a significantly lower number of dead neurons were observed in the H group specimens compared with those observed in the N group, and the vast majority of the H group specimens were revealed to exhibit a predominantly normal neuronal structure (Fig. 2).
FIG. 2.
Hematoxylin and eosin (HE) staining of hippocampal tissue samples from three groups. HE staining of the pyramidal cell layer of the hippocampus CA3/DG (dentate gyrus) regions from the sham, normothermia, and hypothermia groups (magnification, ×40). Higher magnification (×100 and ×200) revealed a typical neuronal structure (n=10). Color images available online at www.liebertpub.com/ther
Effects of MIH treatment on rat hippocampal APP-, Aβ-, and BACE-positive cell number following TBI
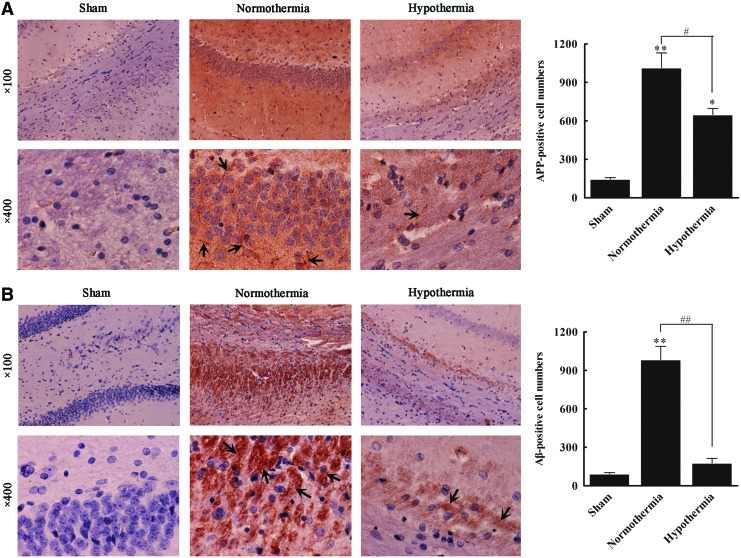
Both the APP- and Aβ-positive cell number in hippocampal samples from group N were significantly greater than those observed in group S (all p<0.01). Compared with group N, group H exhibited a significantly decreased number of Aβ-positive cells (984±10 vs. 178±35; p<0.01), although there was no significant difference between group H and group S (p>0.05) (Fig. 3A). The APP-positive cell number in group N was significantly greater (1014±115) than that observed in group S (145±11, p<0.01); however, the APP-positive cell number was clearly reduced in group H (549±48) compared with group N (p<0.01) (Fig. 3B).
FIG. 3.
Immunohistochemical staining of β-amyloid protein precursor (APP) and β-amyloid (Aβ) proteins in three groups. Representative immunohistochemical staining of APP (A) and Aβ (B) proteins in the sham, normothermia, and hypothermia groups. Positive expression of APP and Aβ are indicated by yellowish-brown particles and brown particles, respectively. The positive cell number was calculated. All data are expressed as mean±SD (n=10). *p<0.05, **p<0.01 versus the sham group; #p<0.05, ##p<0.01 versus the normothermia group. Color images available online at www.liebertpub.com/ther
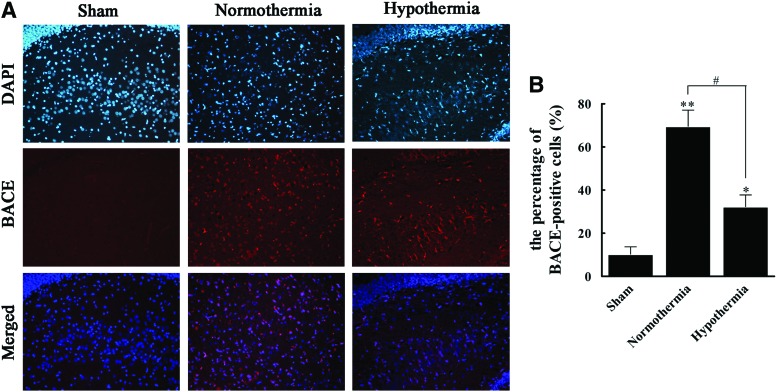
Immunofluorescence revealed that BACE staining was positive in 10.3% of cells in group S and 69.5% of cells in group N, indicating that TBI was likely associated with increased BACE. Compared to this elevation in the N group, the H group exhibited a much lower BACE-positive rate of 32.3% (Fig. 4), suggesting that MIH treatment may be correlated with BACE.
FIG. 4.
Immunofluorescence staining of β-secretase (BACE) protein in three groups. (A) Samples were fixed, stained with an antibody against BACE followed by a rhodamine-conjugated secondary antibody (red) and analyzed by fluorescence microscopy. Nuclei were visualized by 4′,6-diamidino-2-phenylindole (DAPI) staining (blue) and are presented in merged images (magnification, ×200). (B) The percentage of BACE-positive cells in the sham, normothermia, and hypothermia groups were calculated. All data are expressed as mean±SD (n=10). *p<0.05, **p<0.01 versus the sham group; #p<0.05 versus the normothermia group. Color images available online at www.liebertpub.com/ther
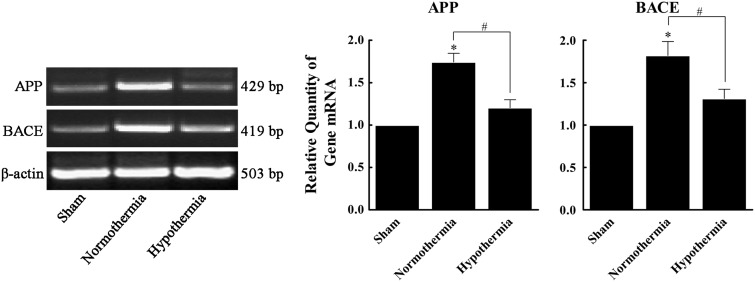
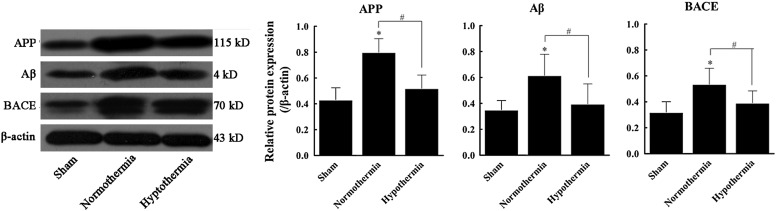
Effects of MIH treatment on rat hippocampal APP, Aβ, and BACE mRNA and protein expression following TBI
Increased APP and BACE mRNA expression was observed in group N compared with group S by 1.74- and 1.82-fold, respectively. APP and BACE mRNA expression was downregulated in group H compared to group N, and mRNA levels were only 69.1% and 72.1%, respectively, of those observed in group N (Fig. 5). Furthermore, Aβ, APP, and BACE protein expression were significantly increased in group N compared to group S (1.76-, 1.86-, and 1.67-fold, respectively; p<0.05). However, Aβ, APP, and BACE protein expression were significantly decreased in group H (64.2%, 65.1%, and 73.2% of group N; p<0.05). No statistically significant difference was observed between groups H and S (p>0.05) (Fig. 6).
FIG. 5.
Effect of mild induced hypothermia (MIH) treatment on rat hippocampal APP and BACE mRNA expressions following traumatic brain injury (TBI). Total RNA in the sham, normothermia, and hypothermia groups was extracted and assayed for APP and BACE mRNA by reverse transcription–polymerase chain reaction analysis. β-actin was used as an internal control. Results were relative to β-actin mRNA expression. All data are expressed as mean±SD (n=10). *p<0.05 versus the sham group; #p<0.05 versus the normothermia group.
FIG. 6.
Effects of MIH treatment on rat hippocampal APP, Aβ, and BACE protein expressions following TBI. Total protein in the sham, normothermia, and hypothermia groups was extracted and assayed for APP, Aβ, and BACE protein by western blotting analysis. β-actin was used as an internal control. Results are relative to β-actin protein expression. *p<0.05 versus the sham group; #p<0.05 versus the normothermia group.
Discussion
BACE expression was upregulated in rat hippocampal tissues following TBI in the present study. Furthermore, increasing APP expression promoted Aβ accumulation, inducing neurological dysfunction typical of SBI. Thus, high expression of APP and BACE may be related to poor prognosis in the 24-hour period following TBI. Immediate treatment with MIH did, however, mediate the effects of SBI, indicating that immediate hypothermia treatment may play a role in the expression of key proteins associated with SBI, such as BACE and APP. While further study will be required to confirm these results in human patients and design strategies for implementing MIH protocols, these preliminary findings confirm the effect of hypothermia on Aβ expression levels following TBI in rats and provide valuable clues about the underlying mechanism.
MIH involves the rapid application of mild hypothermia regimens, as suggested and confirmed by several recent studies (Brain Trauma Foundation et al., 2007; van der Worp et al., 2007; Song and Lyden, 2012; Urbano and Oddo, 2012). In a study of 1626 patients with severe TBI, Li and Jiang (2012) suggested that hyperthermia following TBI was a key detrimental factor to patient prognosis, accentuating delayed mechanisms that follow initial physical disruption during the primary injury and causing injury to hippocampal tissues. Conversely, hypothermic treatment has the opposite effect, prolonging TBI patient survival, reducing complication occurrence, improving prognosis, and reducing mortality (Li and Jiang, 2012). Whereas these previous studies have examined prevention of hyperthermia, only in the last decade has clinical administration of MIH been broadly clinically recommended, although employing these protocols remains challenging (Feng et al., 2010).
Studies of the pathogenesis of progressive SBI in TBI patients have focused primarily on particular enzymes and structural proteins, including neuron-specific enolase, glial fibrillary acidic protein, and S-100β molecules that directly indicate damage (Korfias et al., 2006). However, the application of modern molecular techniques for the separation and identification of protein degradation products has allowed researchers to identify other TBI-related proteins that have a greater involvement in SBI processes, such as Aβ proteins (Korfias et al., 2006). Aβ expression in the cerebrospinal fluid of TBI patients increases in patients with poor prognoses, potentially indicating axon damage (Lichtlen and Mohajeri, 2008). These findings are consistent with the current results, which indicate that Aβ elevation in tissues is linked with reduced neuronal function indicate of damage to neuronal structures.
Aβ can be used to assess the efficacy of TBI treatments, such as MIH, but it is also potentially useful as a treatment target. Because inhibition of BACE gene expression presents a rapid and convenient method to reduce Aβ formation (Blasko et al., 2004), Aβ may also be able to attenuate the effects of SBI following TBI, although further research will be required to explore this promising hypothesis. In studies of rat TBI injury models, blocking β- or γ-secretase successfully prevented generation of Aβ from APP, improving movement and cognitive ability, while reducing neuronal cell death (Loane et al., 2009). Furthermore, dynamic monitoring of intercellular fluids in injured brain regions of TBI patients using microdialysis has demonstrated that Aβ accumulates occurred within the first several hours following the primary injury and persisted throughout recovery, affecting neurological functioning thereafter (Brody et al., 2008). Notably, the current study demonstrated positive preliminary findings that surface cooling techniques for MIH applied immediately following primary injury in TBI could effectively attenuate Aβ accumulation.
The full mechanism of the benefits of early hypothermic treatment to TBI patients remains unknown, however, it has been speculated that reduced cerebral oxygen consumption and inflammatory response inhibition are primarily responsible for reducing secondary damage to neuron structures (Truettner et al., 2005). The current study supports this finding, indicating reduced damage to these structures in rats treated with hypothermic treatments compared to those allowed to recover at normal body temperatures. Recently, Diller and Zhu (2009) reviewed a large body of articles linking formation of harmful factors to poor prognosis in TBI, highlighting the value of MIH in mediating many of these compounds. Consistent with these reports, the current study indicated that BACE expression after TBI was significantly reduced by hypothermia intervention.
Furthermore, current expression of the apoptosis gene caspase-3 after TBI was clearly enhanced by hypothermic treatment, indicating activation of the apoptotic signal transduction pathway. Caspase-3 significantly reduces BACE degradation by binding site GGA3 of the hydrolytic enzyme (Tesco et al., 2007), and caspase-3 has been demonstrated to be altered by MIH treatment (Huang et al., 2009). Hence, upregulation of BACE expression induced by TBI can be reduced to effectively decrease Aβ generation, thus attenuating SBI effects. Furthermore, protection of neurons and axons through hypothermia therapy after TBI can prevent extracellular APP accumulation and subsequent cascade reactions.
While these results are promising, further clinical study will be required to assess these effects in humans, where a larger body size and other extenuating factors make cooling processes more complex, and to address the issue of clinical implementation of MIH protocols in the field. While MIH protocols have been widely available since the 1950s, programs to widely implement these protocols for emergency response personnel have only appeared in the past decade, primarily focusing on cardiac arrest patients with minimal emphasis on TBI patients (Frampton, 2011). Thus, there is also a need to raise awareness and provide training for existent protocols. Furthermore, improvements in understanding the mechanism of pathological and physiological neuronal changes following TBI, including future research on Aβ expression levels, is required to assess optimal temperature for MIH, cooling methods, and rewarming protocols before optimal clinical recommendations can be generated.
Aβ, BACE, and APP expression in hippocampal tissues of TBI rat models were significantly increased. Furthermore, MIH treatment effectively reduced Aβ, BACE, and APP upregulation after TBI, limiting adverse effects associated with SBI extent. Thus, hypothermia treatment may enhance neuroprotective functions by inhibiting neurotoxicity through the suppression of Aβ and BACE expression. The biomarkers TBI, Aβ, and BACE may be useful in assessing and improving diagnostic criteria, treatment strategies, and prognoses of TBI patients. The exact relationship between MIH and Aβ and BACE expression, however, will require further investigation before elucidation of complete mechanisms and generation of optimal, viable clinical recommendations.
Acknowledgments
This study was supported by grants from the National Natural Scientific Fund of China (NSFC No. 31200809, 81271392) and the Logistics College of the Chinese People's Armed Police Forces grant (FYZ201201, WYM201119).
Disclosure Statement
The authors declare no conflicts of interest.
References
- Blasko I. Beer R. Bigl M. Apelt J. Franz G. Rudzki D. Ransmayr G. Kampfl A. Schliebs R. Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer's disease beta-secretase (BACE-1) J Neural Transm. 2004;111:523–536. doi: 10.1007/s00702-003-0095-6. [DOI] [PubMed] [Google Scholar]
- Brain Trauma Foundation, American Association of Neurological Surgeons, and Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. Erratum in: J Neurotrauma 2008;2025:2276–2278. [DOI] [PubMed] [Google Scholar]
- Brody DL. Magnoni S. Schwetye KE. Spinner ML. Esparza TJ. Stocchetti N. Zipfel GJ. Holtzman DM. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller KR. Zhu L. Hypothermia therapy for brain injury. Annu Rev Biomed Eng. 2009;11:135–162. doi: 10.1146/annurev-bioeng-061008-124908. [DOI] [PubMed] [Google Scholar]
- Faden AI. Demediuk P. Panter SS. Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Feng JF. Zhang KM. Jiang JY. Gao GY. Fu X. Liang YM. Effect of therapeutic mild hypothermia on the genomics of the hippocampus after moderate traumatic brain injury in rats. Neurosurgery. 2010;67:730–742. doi: 10.1227/01.NEU.0000378023.81727.6E. [DOI] [PubMed] [Google Scholar]
- Frampton WR. Implementing an induced hypothermia protocol. Neurosurgery. 2011;164:28–33. [Google Scholar]
- Goedert M. Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Huang T. Solano J. He D. Loutfi M. Dietrich WD. Kuluz JW. Traumatic injury activates MAP kinases in astrocytes: mechanisms of hypothermia and hyperthermia. J Neurotrauma. 2009;26:1535–1545. doi: 10.1089/neu.2008.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T. Satou T. Nishida S. Tsubaki M. Hashimoto S. Ito H. Improvement of cerebral function by anti-amyloid precursor protein antibody infusion after traumatic brain injury in rats. Mol Cell Biochem. 2009;324:191–199. doi: 10.1007/s11010-008-0013-1. [DOI] [PubMed] [Google Scholar]
- Korfias S. Stranjalis G. Papadimitriou A. Psachoulia C. Daskalakis G. Antsaklis A. Sakas DE. Serum S-100B protein as a biochemical marker of brain injury: a review of current concepts. Curr Med Chem. 2006;13:3719–3731. doi: 10.2174/092986706779026129. [DOI] [PubMed] [Google Scholar]
- Li J. Jiang JY. Chinese Head Trauma Data Bank: effect of hyperthermia on the outcome of acute head trauma patients. J Neurotrauma. 2012;29:96–100. doi: 10.1089/neu.2011.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Wang B. Kan Z. Zhang B. Yang Z. Chen J. Wang D. Wei H. Zhang JN. Jiang R. Progesterone increases circulating endothelial progenitor cells and induces neural regeneration after traumatic brain injury in aged rats. J Neurotrauma. 2012;29:343–353. doi: 10.1089/neu.2011.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtlen P. Mohajeri MH. Antibody-based approaches in Alzheimer's research: safety, pharmacokinetics, metabolism, and analytical tools. J Neurochem. 2008;104:859–874. doi: 10.1111/j.1471-4159.2007.05064.x. [DOI] [PubMed] [Google Scholar]
- Lindholm D. Wootz H. Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- Loane DJ. Pocivavsek A. Moussa CE. Thompson R. Matsuoka Y. Faden AI. Rebeck GW. Burns MP. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med. 2009;15:377–379. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix RC. Zhang J. Park J. Lee C. Whalen MJ. Detrimental effect of genetic inhibition of B-site APP-cleaving enzyme 1 on functional outcome after controlled cortical impact in young adult mice. J Neurotrauma. 2011;28:1855–1861. doi: 10.1089/neu.2011.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies S. Hicks R. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani Y. Yarimizu J. Saita K. Uchino H. Akashiba H. Shitaka Y. Ni K. Matsuoka N. Differential effects between gamma-secretase inhibitors and modulators on cognitive function in amyloid precursor protein-transgenic and nontransgenic mice. J Neurosci. 2012;32:2037–2050. doi: 10.1523/JNEUROSCI.4264-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo DO. Introduction: traumatic brain injury. Neurosurg Focus. 2008;25:E1. doi: 10.3171/FOC.2008.25.10.E1. [DOI] [PubMed] [Google Scholar]
- Risdall JE. Menon DK. Traumatic brain injury. Philos Trans R Soc Lond B Biol Sci. 2011;366:241–250. doi: 10.1098/rstb.2010.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalea TM. Maltz S. Yelon J. Trooskin SZ. Duncan AO. Sclafani SJ. Resuscitation of multiple trauma and head injury: role of crystalloid fluids and inotropes. Crit Care Med. 1994;22:1610–1615. [PubMed] [Google Scholar]
- Schagger H. Tricine-SDS-PAGE. Nat Protoc. 2006;1:16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- Song SS. Lyden PD. Overview of therapeutic hypothermia. Curr Treat Options Neurol. 2012;14:541–548. doi: 10.1007/s11940-012-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesco G. Koh YH. Kang EL. Cameron AN. Das S. Sena-Esteves M. Hiltunen M. Yang SH. Zhong Z. Shen Y. Simpkins JW. Tanzi RE. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truettner JS. Suzuki T. Dietrich WD. The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Brain Res Mol Brain Res. 2005;138:124–134. doi: 10.1016/j.molbrainres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Tu Y. Chen C. Sun HT. Cheng SX. Liu XZ. Qu Y. Li XH. Zhang S. Combination of temperature-sensitive stem cells and mild hypothermia: a new potential therapy for severe traumatic brain injury. J Neurotrauma. 2012;29:2393–2403. doi: 10.1089/neu.2012.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano LA. Oddo M. Therapeutic hypothermia for traumatic brain injury. Curr Neurol Neurosci Rep. 2012;12:580–591. doi: 10.1007/s11910-012-0304-5. [DOI] [PubMed] [Google Scholar]
- Uryu K. Chen XH. Martinez D. Browne KD. Johnson VE. Graham DI. Lee VM. Trojanowski JQ. Smith DH. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208:185–192. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Worp HB. Sena ES. Donnan GA. Howells DW. Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130:3063–3074. doi: 10.1093/brain/awm083. [DOI] [PubMed] [Google Scholar]