Abstract
Background: Spinal cord has a limited capacity to repair; therefore, medical interventions are necessary for treatment of injuries. Transplantation of Schwann cells has shown a great promising result for spinal cord injury (SCI). However, harvesting Schwann cell has been limited due to donor morbidity and limited expansion capacity. Furthermore, accessible sources such as bone marrow stem cells have drawn attentions to themselves. Therefore, this study was designed to evaluate the effect of bone marrow-derived Schwann cell on functional recovery in adult rats after injury. Methods: Mesenchymal stem cells were cultured from adult rats’ bone marrow and induced into Schwann cells in vitro. Differentiation was confirmed by immunocytochemistry and RT-PCR. Next, Schwann cells were seeded into collagen scaffolds and engrafted in 3 mm lateral hemisection defects. For 8 weeks, motor and sensory improvements were assessed by open field locomotor scale, narrow beam, and tail flick tests. Afterwards, lesioned spinal cord was evaluated by conventional histology and immunohistochemistry. Results: In vitro observations showed that differentiated cells had Schwann cell morphology and markers. In this study, we had four groups (n = 10 each): laminectomy, control, scaffold and scaffold + Schwann cells. Locomotor and sensory scores of cell grafted group were significantly better than control and scaffold groups. In histology, axonal regeneration and remyelination were better than control and scaffold groups. Conclusion: This study demonstrates that bone marrow-derived Schwann cells can be considered as a cell source for Schwann cells in SCI treatment.
Key Words: Rats, Spinal cord injuries (SCI), Bone marrow, Schwann cells, Cell transdifferentiation
INTRODUCTION
Spinal cord injuries (SCI) often cause to permanent neurological problems that in severe cases may lead to complete palsy of the lower and upper extremities. Unfortunately, effective therapies to repair spinal cord function have not been available yet. Recently, restoration of function of an injured mammalian spinal cord has been achieved in rat and mouse using embryonic spinal cord segment, intercostals nerve, mouse embryonic stem cells, olfactory ensheathing glia, fetal spinal cord with neurotrophins and collagen filaments [1]. One approach to SCI restoration is transplantation of Schwann cells [2]. Schwann cells which make peripheral myelin can produce a variety of neurotrophic factors and adhesion molecules, known to contribute to peripheral nervous system regeneration [3]. In fact, it has been reported that transplantation of Schwann cells can promote axonal regeneration of lesioned adult rat spinal cord [4]. However, the clinical use of Schwann cells is limited because it is hard to obtain them in sufficient large numbers. To overcome this difficulty, the capacity of various types of stem cells for differentiation into Schwann cells is under evaluation.
Bone marrow stem cells (BMSC) are interesting cell sources, because they are able to self-renew with a high growth rate, possess multi-potent differentiation properties and also they are easy accessible. BMSC have the potential to transdifferentiate into neuron like cells with various neuronal markers and functional neuronal activity [5]. Due to their high plasticity and low immunogenicity, application of mesenchymal stem cells (MSC) is considered as an attractive form of cell therapy for the injured nervous system [6]. Previous studies have demonstrated that differentiated Schwann cells help neurite outgrowth [7] and provide trophic support for surviving axons as well as remyelinating injured axons in vivo [8, 9].
The obvious benefits of MSC have led us to investigate whether BMSC can be a reliable source for harvesting Schwann cells for treatment of SCI.
MATERIALS AND METHODS
Cell isolation and culture. BMSC were obtained from adult male Wistar rats (n = 5, 250-300 g). Bone marrow donor animals were euthanized with diethyl ether. The bone marrow was flushed into sterile cryovials with DMEM and antibiotic (200 U/ml penicillin and 200 µg/ml streptomycin; PAA, Linz, Austria). After centrifugation, marrow suspensions were resuspended in media and placed in plastic tissue culture flasks. Cells were cultured at 37°C under 95% humidity and 5% CO2. Culture media consisted of DMEM (Sigma, USA), 10% FBS (Gibco, Life technologies, USA) and antibiotic agents (100 U/ml penicillin and 100 µg/ml streptomycin; PAA, Linz, Austria). Every 4 days, the media were replaced and cultures were split as needed to maintain a cell density of 80-90% confluency. The passage 3 was used for the experiments.
Characterization of mesenchymal stem cells
Multilineage differentiation. To confirm the MSC multipotency, the cell cultures were differentiated into osteoblasts and adipocytes according to the previously published protocols [10, 11]. The cell cultures were treated for 3 weeks with the differentiation media. When the differentiation processes were completed, the cultures were stained for osteogenic and adipogenic differentiation with von Kossa and Oil Red O, respectively.
Flow cytometry. Rat MSC were treated with trypsin and washed with PBS for 3 times. After blocking with 10% BSA (Sigma-Aldrich, USA), phycoerythrin (PE) antibodies against rat CD73 (Biocompare, USA), CD45, CD90 and CD44 (eBioscience, USA) were added and incubated away from light at room temperature for 45 min. Rat MSC were fixed with 10 g/L paraformaldehyde for 15 min after the cells were washed with PBS. Flow cytometer (Becton Dickinson, USA) was used to analyze the samples.
Differentiation of MSC into Schwann-like cells. First, growth medium of BMSC was replaced with the medium supplemented with 1 mM β-mercaptoethanol for 24 h. Afterward, the fresh medium supplemented with 35 ng/ml all-trans-retinoic acid was added. After 72 h, medium was changed with the differentiation medium containing 5 ng/ml platelet-derived growth factor, 10 ng/ml basic fibroblast growth factor, 14 μM forskolin and 200 ng/ml β-heregulin (all from Sigma-Aldrich, USA). Cells were then incubated for 8 days under these conditions with the fresh medium added approximately every 72 h [12, 13].
Differentiation confirmation
Immunocytochemistry for Schwann cell markers. After fixation with 4% paraformaldehyde and blocking with goat serum (Sigma-Aldrich, USA), cells were incubated at 4-8°C overnight with FITC conjugated mouse monoclonal antibody against low-affinity nerve growth factor (NGF) p75 receptor (1:500; Abcam, Canada) and rabbit polyclonal antibody against S100 protein (1:500; Abcam, Canada). After rinsing in PBS, Texas Red linked secondary goat anti-rabbit antibody (1:1000; Abcam, Canada) was applied in a dark place at ambient temperature for 1 h. The cells were then stained with 4′,6′-diamidino-2-phenylindole hydro-chloride(DAPI;Sigma-Aldrich,USA).Preparations were photographed with a Nikon DXM1200 digital camera attached to a Nikon TE3000 fluorescence microscope.
RT-PCR for glial markers. The RNeasyTM mini kit (Qiagen Ltd., UK) was used for isolation of total RNA from the cell pellets of MSC. All of the PCR primers were synthesized by Sigma-Aldrich, USA (Table 1). The optimum annealing temperature for each primer pair was determined experimentally. The One-Step RT-PCR Kit (Qiagen Ltd, USA) was used for all RT-PCR based on the manufacturer’s instructions with the addition of RNase-free water (negative control). A Techen Genius thermocycler was used for all reactions.
Table 1.
Primer sequences for RT-PCR
| Rat primer | Accession no. | Sequence | Product size (bp) |
|---|---|---|---|
| S100 | NM_013191.1 | 5´-ATAGCACCTCCGTTGGACAG-3´ 5´-TCGTTTGCACAGAGGACAAG-3´ |
132 |
| B-actin | NM_031144.2 | 5´-CACCCGCGAGTACAACCTTC-3´ 5´-CCCATACCCACCATCACACC-3´ |
207 |
| PMP22 | NM_017037.1 | 5´-TCTCACGGTCGGAGCATCA-3´ 5´-CAGTCCTTGGAGGCACAGAAC-3´ |
97 |
| BDNF | NM_012513.3 | 5´-GTCATTGGTAACCTCGCTCATTC-3´ 5´-ATAGATTTACGCAAACGCCCTC-3´ |
90 |
| NT-3 | NM_031073.2 | 5´-CGGCAACAGAGACGCTACAA-3´ 5´-CCTCCGTGGTGATGTTCTATTG-3´ |
132 |
| NGFr(p75) | NM_012610.2 | 5´-GGTCTATTCTGATGGAGTCAAGCTAAG-3´ 5´-ACCAAGAATGAGCGCACTAACAG-3´ |
123 |
| NGF | XM_003753645.1 | 5´-GGACGCAGCTTTCTATCCTG-3´ 5´-GTCCGTGGCTGTGGTCTTAT-3´ |
496 |
PMP22, peripheral myelin protein 22; BDNF, brain-derived neurotrophic factor; NT-3, neurotrophin-3; NGF, nerve growth factor
Scaffold preparation. In this study, atelocollagen honeycomb (KOKEN Inc., Tokyo, Japan) scaffolds were applied for implantation in lesion sites. Scaffolds had unidirectional porous pattern with pores diameter of 250-400 µm. Twenty hour before implantation, MSC-derived Schwann cells were detached, counted and seeded about 10 × 104 cells on each scaffold. A scanning electron microscope (Model: Tescan, Vega II, Czech Republic) was used for confirmation of presence of cells on scaffold.
Spinal cord injury and transplantation. Male Wistar rats (n = 40, 250-300 g) were anesthetized intraperitoneally by injection of 60 mg/kg of ketamine HCl and 5 mg/kg xylazine HCl (both from Alfasan, Woerden, Holland). Under an operation microscope, laminectomy was performed at T9 and T10 vertebral levels. Left lateral hemisections were performed at these levels, followed by a left lateral incision to excise a segment of spinal cord tissue of 3.0 mm length.
In the first group (laminectomy group, n = 10), rats were subjected only to laminectomy without any lesion. In the second group (lesion-control group, n = 10), lateral hemisection operation was performed without implantation and in the third group (Scaffold group, n = 10), a free cell-collagen scaffold was put in lesion. Finally, in fourth group (scaffold + Schwann cells-BMSC group, n = 10), a collagen scaffold filled with BMSC-derived Schwann cells was implanted into the lesioned site. All animals were given appropriate antibiotics and analgesic: buprenorphine (0.05 mg/kg/ day Schwann cells, Reckitt Benckiser, Berks, UK) for 3 consecutive days and cefazolin (50 mg/kg/day i.p., DaruPakhsh, Tehran, Iran) for 5 days. Food and water were given ad libitum. Sensory-motor recovery of all groups was assessed for eight weeks. The above experimental procedures were treated in accordance with the guidelines approved by the Animal Care and Use Committee of Pasteur Institute of Iran (Tehran).
Sensory-motor recovery evaluation
Locomotor activity assessment. The locomotor capacity of the hind limb was assessed using Basso, Beattie and Bresnahan (BBB) scale [14]. Animals were tested before injury and 1, 2, 4, 6, 8 weeks after injury.
Sensory-motor test. An additional measurement for evaluating recovery was obtained using narrow beam test [15]. Prior to test, rats were trained to traverse a beam (4 cm wide and 80 cm high) for 5 days (5 min per day). A scoring system was used to assess the ability of the rats to traverse the beam: 0 was counted as complete inability to walk (fall down immediately), 0.5 was scored if they traverse half of the beam, 1 point was given for traversing the whole
length and 2 points were noted for normal weight support and accurate foot placement. The test for each rat was repeated 3 times.
Sensory test. The level of nociception after SCI was evaluated by performing a standard tail flick test [16]. An infrared source producing a calibrated heating beam (1 mm diameter) was placed under the tail base and triggered together with a timer (Plantar tester, Ugo Basile, Comerio, Italy). The time for the first movement of the tail was noted. Each measurement was repeated three times with at least a 5-min interval. A cut-off time of 20 s was used to prevent tissue damage.
Tissue preparation. Eight weeks after trans-plantation, all groups were perfused. Isolated spinal cords with or without scaffolds were processed for histology and immunohistochemistry. Spinal cord segments were dehydrated and embedded in paraffin. Tissue blocks were cut into 5-µm thick sections (Leica Microsystems) and stained with rabbit polyclonal antibody against neurofilament 200 kDa (1:1000, Abcam, Canada). After that, sections were treated with biotinylated goat polyclonal secondary antibody to rabbit IgG (1:200, Abcam, Canada) and then incubated with streptavidin-horseradish peroxidase complex. For horseradish peroxidase reaction, we used Diamino-benzidine Histochemistry Kits (molecular probes, Invitrogen, USA) according to manufacturer's instructions. Some paraffin blocks were also cut into 15-μm thick sections and stained for myelin with luxol fast blue (Sigma-Aldrich, USA). In addition, some sections were stained with H & E.
Statistical analysis. One-way analysis of variance (ANOVA) followed by post hoc Scheffe test was used to determine statistical differences between the experimental groups. Data were expressed as the mean ± standard deviation.
RESULTS
Animal observation. Immediately after surgery, all injured rats were incapable of ipsilateral hind limb movement. The majority (≈70%) of rats survived after injury. There was a loss of about 10-30% in rat body weight during the first month, but the animals recovered. Cell graft was performed immediately after injury. No teratoma formation was observed 60 days after transplantation.
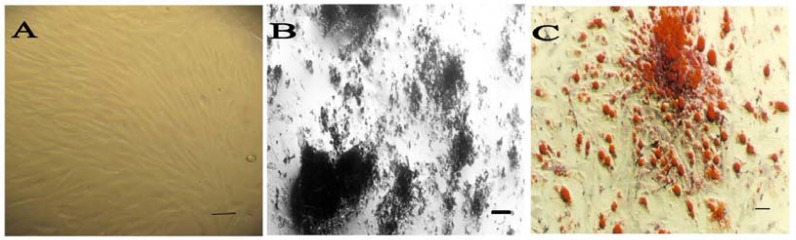
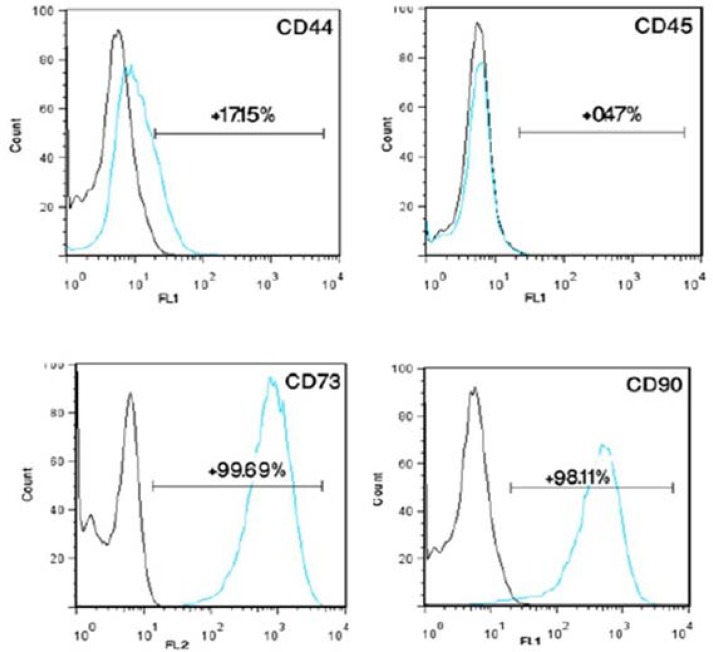
Characterization of stem cells. Cells from the stromal fraction formed confluent fibroblast-like monolayers on 75 cm2 flasks (Fig. 1A). To characterize the rat-derived MSC, multilineage differentiation was evaluated. Osteogenic differentiation was confirmed by production of calcium deposits detected with von Kossa (Fig. 1B) and adipogenic differentiation by Oil Red O staining of lipid droplet (Fig. 1C). In addition, we performed flow cytometric analysis (Fig. 2) and isolated MSC showed strong CD90 and CD73 expressions. They also were positive to CD44. MSC had a negative tendency to express CD45 (lympho-hematopoietic stem cell marker). These results indicate that the isolated stem cells, characterized by surface markers and multilineage differentiation, were multipotent MSC.
Fig. 1.
Multilineage differentiation of mesenchymal stem cells. (A) Undifferentiated bone marrow stem cells under phase contrast microscopy display a flattened fibroblast-like morphology (Scale bar 100 µm). (B) von Kossa staining of mineralized tissue (scale bar 10 µm) and (C) Oil red O positive intracellular lipid droplets indicates these cells can differentiate to osteoblasts and adipocytes (scale bar 100 µm).
Fig. 2.
Flow cytometric characterization of bone marrow stem cells. CD marker analysis of mesenchymal stem cells (MSC) at passage 3 revealed a similar positivity of indicated surface markers between isolated cells from bone marrow and MSC
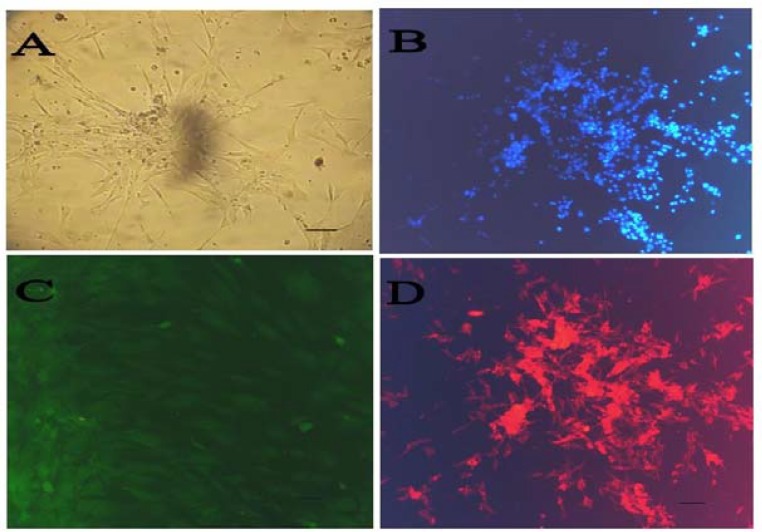
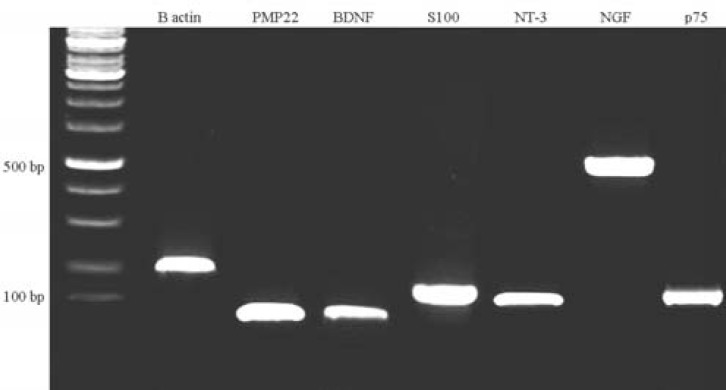
Differentiation to a Schwann cell phenotype. After induction, cells showing a bipolar shape with two or three processes were identified (Fig. 3A and 3B). To determine the molecular features of these Schwann-like cells, immunofluorescence staining was performed by using antibodies against p75 and S100; molecular markers for Schwann cells. RT-PCR analysis was also performed for expression of some glial genes, such as p75, S100, NGF, brain-derived neurotrophic factor (BDNF), neurotrophin-3 and peripheral myelin protein 22. Immunocytochemistry showed that induced MSC were positive for S100 and p75, widely-known markers for Schwann cells (Fig. 3C and 3D). The percentage of cells positive for S100 markers after Schwann cell induction in vitro were all approximately 70-75%. Several neural and glial genes, such as p75, S100β, NGF, BDNF, neurotrophin-3 and peripheral myelin protein 22 were constitutively expressed in Schwann-like cells (Fig. 4). After differentiation,
Fig. 3.
Transdifferentiation of mesenchymal stem cells (MSC) to Schwann cells and characterization. Bone marrow stem cells (BMSC) post differentiation showing a bipolar, spindle-shaped morphology with 2-3 processes. (A) Confluent differentiated MSC; (B) DAPI staining of Schwann cell-BMSC; (C) immunofluorescence staining of differentiated MSC: anti-p75-FITC staining and (D) anti-S100-Texas red staining. Scale bar 100 µm
Fig. 4.
Expression pattern of several genes in trans-differentiated MSC at mRNA level. For product sizes, see Table 1
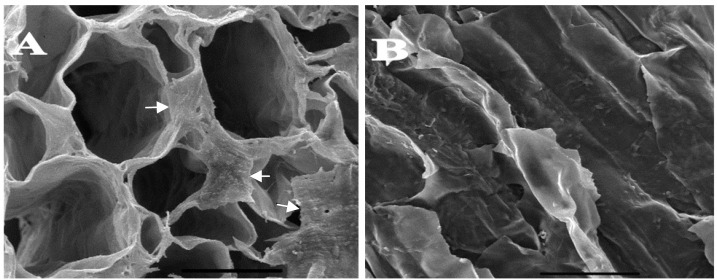
Schwann cells-BMSC were seeded in scaffolds 24 before implantation. Images from the scanning electron microscope showed the existence of cells inside the scaffolds (Fig. 5).
Fig. 5.
Scanning electron microscopy of scaffold showing presence of Schwann cell derived bone marrow stem cells in scaffolds before implantation. The upper surface (the cells has been indicated by arrows) (A) and inside the scaffold pores (B). Scale bar 200 µm
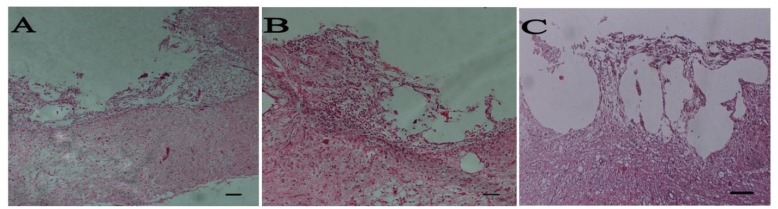
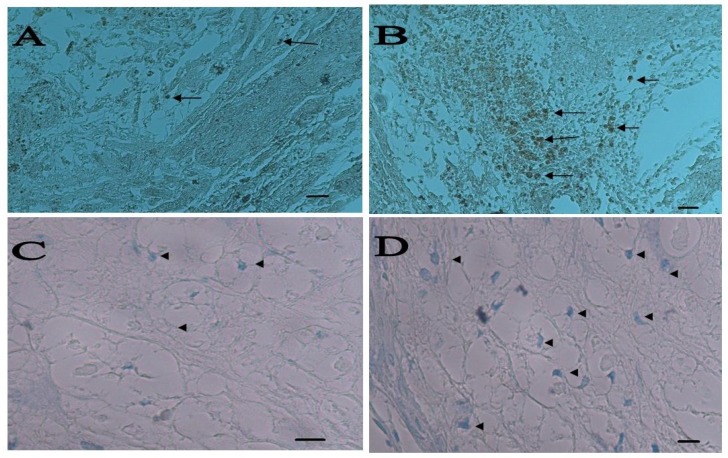
Histological findings. After 8 weeks, rats were evaluated histologically (Fig. 6). The spinal cord appeared to have structural continuity with the implant in the entire implant group at 8 weeks postoperatively. Regenerated axons were seen to cross the implant-spinal cord in the implant group at 8 weeks postoperatively. The central portion of the implant was occupied by a cystic cavity in the cell-implanted groups at 8 weeks postoperatively. Sever scar formation was not seen at the implant-spinal cord interface (Fig. 6). Axons were found to run parallel to the axis of the implant. A number of regenerated nerve axons were found to run parallel to the axis of the implant at the middle of the implant, mainly at its periphery (Fig. 7A and 7B). Many myelinated axons were found to run through the implant in the implant groups, especially in cell treated group (Fig.7C and 7D). Nerve-free cysts occupied the central portion of the implants.
Fig. 6.
Spinal cord sections at 8 weeks after injury and following implantation of graft. H&E staining of longitudinal sections: control (A), bare scaffold (B), Schwann cell-bone marrow stem cells (C) groups. Scale bar 200 µm
Fig. 7.
Staining for axon regeneration and remyelination. Positive axons for NF200 were detected in lesion site as black spots, (A) bare scaffold and (B) bone marrow stem cells (BMSC) (scale bar 100 µm). Myelination of some axons were shown by luxol fast blue staining, (C) bare scaffold and (D) Schwann cell-BMSC have been indicated by arrows (A and B) and arrow heads (C and D) (Scale bar 10 µm).
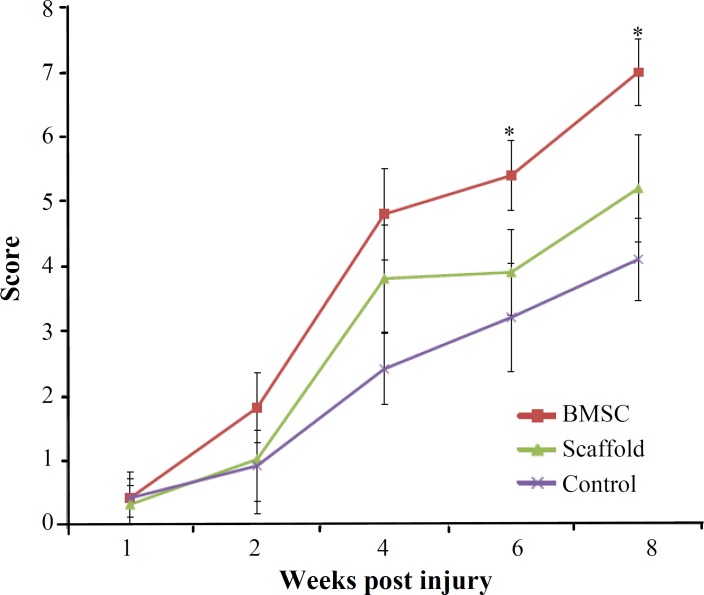
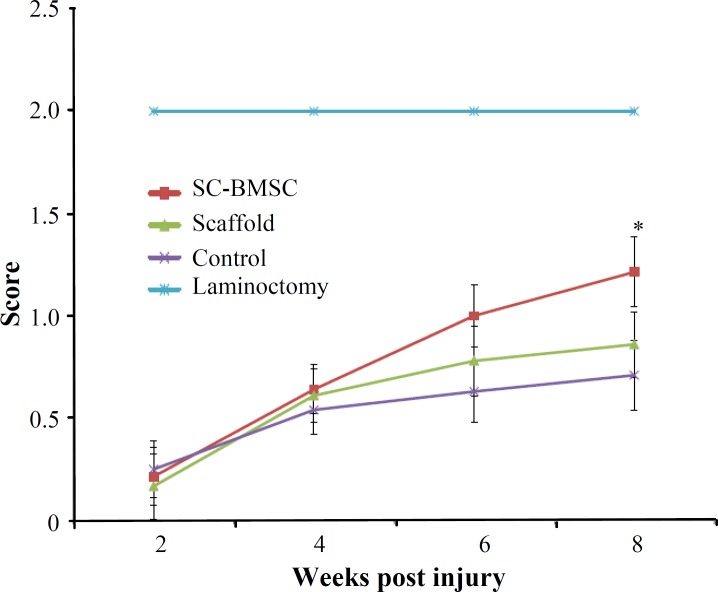
Functional recovery. Hind limb motor function was assessed using the BBB locomotor rating scale. The behavioral results were collected every two weeks for 8 weeks (Fig. 8). Before the injury, all animals showed normal locomotor activity, scored as 21 on the BBB scale, although all injured rats manifested complete hind limb paralysis 7 days after injury, resulting in a score of 0. The BBB scores were in the range of 4.1 ± 0.64 in the control animals 2 months after SCI. In contrast, locomotor recovery in implanted groups was increased gradually after 3 weeks of transplantation. Two months after transplantation all transplanted animals displayed BBB scores significantly (P<0.05) higher than those achieved by the control group. Schwann cells-BMSC animals reached a final average BBB score of 7.9 ± 0.55, which is significantly (P<0.05) higher than the control group (4.1 ± 0.64). In addition, the plain scaffold group (5.2 ± 0.84) regained significantly (P<0.04) more functional recovery than the control group (Fig. 8). Also, average BBB score of cell transplanted group was significantly (P<0.05) higher than control and plain scaffold.
Fig. 8.
Hind limb functional recovery. The Basso, Beattie and Bresnahan scale scores of the implanted group were significantly better than those of the control group at week 8 postoperatively. Lminectomy group is omitted. The data represent mean ± SD.*P<0.05
Narrow beam test. Before the injury, rats were able to cross the beam with no errors in foot placement. Narrow beam performance after the initial injury was too poor (Fig. 9). Although some of the animals could cross the beam 6 weeks after the surgery, gradual improvements in performance were observed. However, cell transplanted animals showed significantly higher score (1.21 ± 0.18) in comparison with control (0.71 ± 0.17) and plain scaffold (0.86 ± 0.16).
Fig. 9.
Narrow beam test. Sensorimotor test showed higher recovery of function in implanted group in comparison with control group at week 8 (*P<0.05).
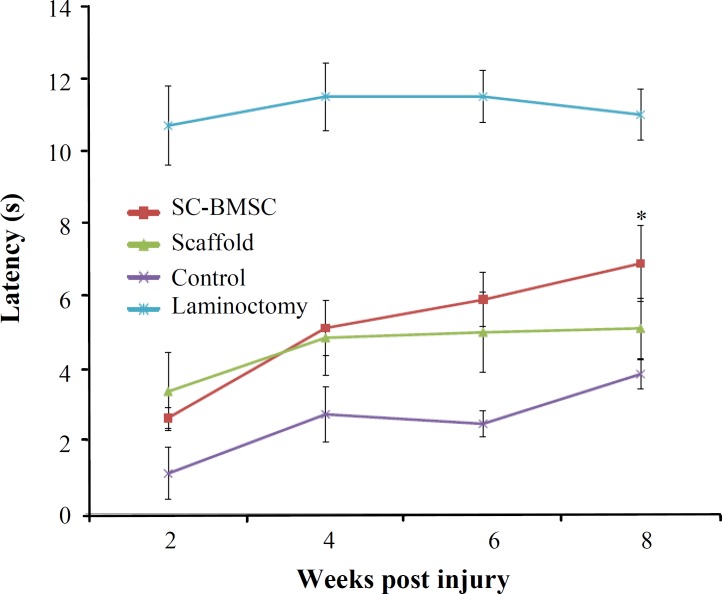
Tail flick test. After surgery, tail flick test showed a sharp decreased latency of withdrawal, which was developed by 2 weeks post transplant (Fig. 10), indicating thermal hypersensitivity. Four weeks after the surgery, deficits had partially recovered in treated groups to about 4.5-5 s. Partial recovery occurred in all groups during the next 4 weeks (P≤0.05). After 8 weeks, animals that were received cell grafts recovered almost similar to laminectomy group levels (6-7 s). Animals that had received cells transplants showed significant improvement in comparison to control after 8 weeks.
Fig. 10.
Sensory test. Responses to thermal stimuli in tail flick showed hypersensitivity at 2 weeks post transplantation. In cell-implanted group, further recovery occurred toward normal values in comparison with operated controls (*P<0.05).
DISCUSSION
We showed that Schwann cells were efficiently differentiated from MSC (approximately 70-75% of adherent cells) in vitro. To evaluate the ability of such cells to repair CNS injury, induced Schwann cells were transplanted to the lesion site of a hemisection SCI along with collagen scaffolds and successfully engrafted. Results showed Schwann cells-BMSC contributed to axonal regeneration and functional recovery. We tested Schwann cells derived from the most common MSC source to repair damaged spinal cords and found that these cells have the potential to regenerate spinal cord and motor-sensory recovery. Transplantation of Schwann cells-BMSC showed stronger functional recovery compared with bare scaffold and control group. Furthermore, we showed that they have almost the same potential for healing the injured spinal cord.
Schwann cells make and secrete a variety of neurotrophic factors and extracellular matrix to guide axon growth and establish a permissive micro-environment for specific innervations [17]. However, the limitations associated with the direct use of Schwann cells, especially their limited accessibility, encourage the efforts to look for alternatives to Schwann cells. Earlier studies have reported that MSC derived from several mammalian species can be transdifferentiated into cells with Schwann cell phenotype in the presence of inducing agents or growth factor mixtures [18]. However, there are still many questions about their functional capabilities. MSC have been considered as an important candidate as alternative of Schwann cells for cell-based therapies. To understand that if Schwann cells-MSC will involve in axon regeneration and consequently in functional recovery in hemisection model and act like genuine Schwann cells, we focused our efforts on investigating of cellular transplantation effects and functional recovery.
Lack of extracellular matrix conducting regenerating axons is one of the main mechanisms that interrupt spinal cord regeneration [19]. The axon regeneration in the central nervous system is influenced by their path- and target-finding that needs environmental signals [20]. An important point is that contact guidance for transected axons to reconnect with the ends of the spinal cord is necessary [21]. Therefore, regeneration of damaged pathways requires a conduit bridging the trauma zone to conduct the regenerating axons and also to rebuild spinal cord structure and to prevent the invasion of scar tissue [22].
Several synthetic extracellular matrixes with various 3D patterns have been previously studied in the SCI model [23]. Kuboki et al. [24] showed dissimilarities in regenerated tissue depending upon the 3D pattern of the artificial extracellular matrix used. Therefore, we provided honeycomb collagen scaffold with various pore sizes, and assumed that the serial tunnel structure could guide regenerated axons in the injured spinal cord in a specific and correct direction. To evaluate regenerated neurites or axons in implanted honeycomb, we used anti-neurofilament 200 antibodies. The current results showed that cell transplantation increased the number of positive fibers at lesioned site and adjacent sites. The honeycomb-implanted spinal cords have shown that a greater number of NF-positive fibers entered the scaffold. We observed that regenerated axons mostly accumulated around the injured area and the center of lesion occupied with cysts which created an axon free area zone.
Schwann cells-BMSC transplantation was shown to help SCI repair, that demonstrated by reformation of repaired tissue in the damaged site and an increase in locomotor activity [25]. Our data also indicate that Schwann cells-like cells derived from MSC have myelin-forming ability. The results in our experiment were consistent with previous report [26]. This might be achieved either by direct remyelination of surviving neurons by Schwann cells-MSC or by stimulation of endogenous precursor cells and protection from more cell loss resulting from the action of neurotrophic factors released from Schwann cells-BMSC [27]. In Schwann cells-BMSC transplantation, both mechanisms may be involved in facilitation of repair because it was shown that Schwann cells-BMSC were promoted myelin sheath formation of the peripheral nervous system type, and Schwann cells-BMSC also secreted neurotrophic factors such as NGF, BDNF, CTNF, and GDNF, as did native Schwann cells. However, to understand which and how many percent of Schwann cells (endogenous or transplanted Schwann cells) contribute to SCI repair that needs to be examined.
It has been shown that Schwann cells secrete a variety of growth factors, including NGF, BDNF, and NT3 [28], which were consistent with our RT-PCR analysis data. They have a potential to promote regeneration of the injured axons and functional recovery [29]. Therefore, it is possible that Schwann cells-BMSC with secretion of some growth factors after the transplantation promote axonal regrowth. These growth factors may contribute to the axonal regeneration and the migration of endogenous Schwann cells [30]. Schwann cells derived from MSC were shown to produce NGF and BDNF [31]. A recent study revealed that human Schwann cells-BMSC cells also secrete abundant amount of vascular endothelial growth factor and hepatocyte growth factor in addition of producing NGF [32]. Furthermore, it has also revealed that the secretion of BDNF by Schwann cells-BMSC was greater than that by Schwann cells [31]. BDNF has been reported to have a role in inducing neuritogenesis and axonal outgrowth and involve in axon myelination and regeneration, neuronal survival, differentiation and glial cell proliferation [33].
The poor recovery of motor tasks shows that behavioral compensation from contralateral sources was insufficient to restore function. On the contrary, some recovery was observed in tasks that depend more on sensory information (narrow beam and responses to thermal stimuli and tail flick). Both operated control and experimental groups showed recovery on these tests. Recovery in tests with sensory factors would be well-matched with lesion-induced growth of primary afferents. It has been shown that after spinal injury dorsal root sprouting occurs and dorsal root axons regenerate in reaction to NGF secretion [34]. Collateral and/or compensatory sprouting of descending tracts also occurs after spinal injuries [35]. These mechanisms may cause the spontaneous recovery in narrow beam observed in the hind limb after hemisection and for the greater recovery observed after transplantation.
The heat test has drawn attention since the increased sensitivity that typically develops after thoracic hemisection is consistent with development of a central pain syndrome [36]. Nociceptive dorsal root axons are sensitive to neurotrophic factors and grow strongly in response to injury and to injury plus neurotrophins [37]. Indeed in our study, all operated animals became hypersensitive to a modest heat stimulus, exhibiting a sharply decreased latency of withdrawal. Therefore, thermal hyperalgesia appears to have developed by 2 weeks postoperatively, but later a slight recovery occurred. Our experiments were not designed to determine the contribution of these ascending and descending pain pathways to recovery in the thermal sensitivity test, but the potential for recovery mediated by these tracts in the presence of neurotrophins necessitates further investigation.
Therefore, more extensive, more controlled, and more long-term period investigations are needed to validate the conclusion of the present study. In addition, more studies are required to better understand of axon regeneration mechanisms and find optimal strategy for treatment of SCI. Acceptable results have not been achieved to date in treating SCI by means of a single approach.
In summary, scaffold implantation may be useful in improvement of functional recovery following hemisection in rat. This functional recovery improved when seeded with Schwann cells-BMSC. Therefore, Schwann cells-MSC could possibly introduce as a source for Schwann cells in future studies and clinical trials and with additional studies, the idea of new scaffolds seeded with Schwann cells-BMSC may accompany with other cells, contribute toward a new comprehensive approach for treatment of SCI.
ACKNOWLEDGEMENTS
We would like to thank Martin Oudega (Pittsburg, USA) and Ales Hejcl (Czech Rep) for their generous scientific assistance. Also, we would like to take this opportunity to express our gratitude to Dr. Jalal Izadi Mobarakeh and Ms. Samira Choopani (Dep. of Physiology, Pasteur Institute of Iran) for their valuable help.
References
- 1.Yoshii S, M Oka, M Shima M, Akagi M, Taniguchi A. Bridging a spinal cord defect using collagen filament. Spine (Phila Pa 1976) 2003 Oct;28(20):2346–51. doi: 10.1097/01.BRS.0000085302.95413.16. [DOI] [PubMed] [Google Scholar]
- 2.Oudega M, Moon LDF, Almeida Leme RJ. Schwann cells for spinal cord repair. Braz J Med Biol Res. 2005;38(6):825–35. doi: 10.1590/s0100-879x2005000600003. [DOI] [PubMed] [Google Scholar]
- 3.Bunge MB. Bridging areas of injury in the spinal cord. Neuroscientist. 2001 Aug;7(4):325–39. doi: 10.1177/107385840100700409. [DOI] [PubMed] [Google Scholar]
- 4.Bunge MB, Pearse DD. Transplantation strategies to promote repair of the injured spinal cord. J Rehabil Res Dev. 2003 Jul-Aug;40(4 Suppl 1):55–62. doi: 10.1682/jrrd.2003.08.0055. [DOI] [PubMed] [Google Scholar]
- 5.Trzaska KA, Kuzhikandathil EV, Rameshwar P. Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells. 2007;25(11):2797–808. doi: 10.1634/stemcells.2007-0212. [DOI] [PubMed] [Google Scholar]
- 6.Thuret S, LD Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7(8):628–43. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 7.Brohlin M, Mahay D, Novikov LN, Terenghi G, Wiberg M, Shawcross SG, et al. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci Res. 2009 May;64(1):41–9. doi: 10.1016/j.neures.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Biernaskie J, Sparling JS, Liu J, Shannon CP, Plemel JR, Xie Y, et al. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci. 2007 Sep;27(36):9545–59. doi: 10.1523/JNEUROSCI.1930-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu S, Kitada M, Ishikawa H, Itokazu Y, Wakao S, Dezawa M. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem Biophys Res Commun. 2007 Aug;359(4):915–20. doi: 10.1016/j.bbrc.2007.05.212. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999 Apr;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Caddick J, Kingham PJ, Gardiner NJ, Wiberg M, Terenghi G. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia. 2006 Dec;54(8):840–9. doi: 10.1002/glia.20421. [DOI] [PubMed] [Google Scholar]
- 12.Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci. 2001 Dec;14(11):1771–6. doi: 10.1046/j.0953-816x.2001.01814.x. [DOI] [PubMed] [Google Scholar]
- 13.Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007 Oct;207(2):267–74. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995 Feb;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 15.Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000 Nov;883(2):165–77. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- 16.Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001 May;21(10):3665–73. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krekoski CA, Neubauer D, Zuo J, Muir D. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J Neurosci. 2001 Aug;21(16):6206–13. doi: 10.1523/JNEUROSCI.21-16-06206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu L, Chen X, Zhang CW, Yang WL, Wu YJ, Sun L, et al. Morphological and functional characterization of predifferentiation of myelinating glia-like cells from human bone marrow stromal cells through activation of F3/Notch signaling in mouse retina. Stem Cells. 2008 Feb;26(2):580–90. doi: 10.1634/stemcells.2007-0106. [DOI] [PubMed] [Google Scholar]
- 19.Schwab ME. Repairing the injured spinal cord. Science. 2002 Feb;295(5557):1029–31. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- 20.Bolz J, Gotz M, Hubener M, Novak N. Reconstructing cortical connections in a dish. Trends Neurosci. 1993;16(8):310–6. doi: 10.1016/0166-2236(93)90107-w. [DOI] [PubMed] [Google Scholar]
- 21.de la Torre JC. Catecholamine fiber regeneration across a collagen bioimplant after spinal cord transection. Brain Res Bull. 1982 Jul-Dec;9(1-6):545–52. doi: 10.1016/0361-9230(82)90162-9. [DOI] [PubMed] [Google Scholar]
- 22.Moore MJ, Friedman JA, Lewellyn EB, Mantila SM, Krych AJ, Ameenuddin S, et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006 Jan;27(3):419–29. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 23.Hurtado A, Moon LD, Maquet V, Blits B, Jerome R, Oudega M. Poly (D,L-lactic acid) macroporous guidance scaffolds seeded with Schwann cells genetically modified to secrete a bi-functional neurotrophin implanted in the completely transected adult rat thoracic spinal cord. Biomaterials. 2006 Jan;27(3):430–42. doi: 10.1016/j.biomaterials.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Kuboki Y, Jin Q, Takita H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 2):S105–15. [PubMed] [Google Scholar]
- 25.Chi GF, Kim MR, Kim DW, Jiang MH, Son Y. Schwann cells differentiated from spheroid-forming cells of rat subcutaneous fat tissue myelinate axons in the spinal cord injury. Exp Neurol. 2010 Apr;222(2):304–17. doi: 10.1016/j.expneurol.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 26.McKenzie IA, Biernaskie J, Toma JG, Midha R, Miller FD. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci. 2006 Jun;26(24):6651–60. doi: 10.1523/JNEUROSCI.1007-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006 Oct;498(4):525–38. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guest JD, Rao A, Olson L, Bunge MB, Bunge RP. The ability of human Schwann cell grafts to promote regeneration in the transected nude rat spinal cord. Exp Neurol. 1997 Dec;148(2):502–22. doi: 10.1006/exnr.1997.6693. [DOI] [PubMed] [Google Scholar]
- 29.Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci. 2002 Aug;22(15):6670–81. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamada T, Koda M, Dezawa M, Yoshinaga K, Hashimoto M, Koshizuka S, et al. Transplantation of bone marrow stromal cell-derived Schwann cells promotes axonal regeneration and functional recovery after complete transection of adult rat spinal cord. J Neuropathol Exp Neurol. 2005 Jan;64(1):37–45. doi: 10.1093/jnen/64.1.37. [DOI] [PubMed] [Google Scholar]
- 31.Mahay D, Terenghi G, Shawcross SG. Schwann cell mediated trophic effects by differentiated mesenchymal stem cells. Exp Cell Res. 2008 Aug;314(14):2692–701. doi: 10.1016/j.yexcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Park HW, Lim MJ, Jung H, Lee SP, Paik KS, Chang MS. Human mesenchymal stem cell-derived Schwann cell-like cells exhibit neurotrophic effects, via distinct growth factor production, in a model of spinal cord injury. Glia. 2010 Jul;58(9):1118–32. doi: 10.1002/glia.20992. [DOI] [PubMed] [Google Scholar]
- 33.Zhao LX, Zhang J, Cao F, Meng L, Wang DM, Li YH, et al. Modification of the brain-derived neurotrophic factor gene: a portal to transform mesenchymal stem cells into advantageous engineering cells for neuroregeneration and neuroprotection. Exp Neurol. 2004 Dec;190(2):396–406. doi: 10.1016/j.expneurol.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Ramer MS, Bishop T, Dockery P, Mobarak MS, O'Leary D, Fraher JP, et al. Neurotrophin-3-mediated regeneration and recovery of proprioception following dorsal rhizotomy. Mol Cell Neurosci. 2002 Feb;19(2):239–49. doi: 10.1006/mcne.2001.1067. [DOI] [PubMed] [Google Scholar]
- 35.Fouad K, Dietz V, Schwab ME. Improving axonal growth and functional recovery after experimental spinal cord injury by neutralizing myelin associated inhibitors. Brain Res Brain Res Rev. 2001 Oct;36(2-3):204–12. doi: 10.1016/s0165-0173(01)00096-0. [DOI] [PubMed] [Google Scholar]
- 36.Yezierski RP, Liu S, Ruenes GL, Kajander KJ, Brewer KL. Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain. 1998 Mar;75(1):141–55. doi: 10.1016/S0304-3959(97)00216-9. [DOI] [PubMed] [Google Scholar]
- 37.Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000 Jan;403(6767):312–6. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]