Abstract
Background: Bone marrow stromal cells (BMSC) have been successfully employed for movement deficit recovery in spinal cord injury (SCI) rat models. One of the unsettled problems in cell transplantation is the relative high proportion of cell death, specifically after neural differentiation. According to our previous studies, p75 receptor, known as the death receptor, is only expressed in BMSC in a time window of 6-12 hours following neural induction. Moreover, we have recently reported a decreased level of apoptosis in p75-suppressed BMSC in vitro. Therefore, our objective in this research was to explore the functional effects of transplanting p75:siRNA expressing BMSC in SCI rats. Methods: Laminectomy was performed at L1 vertebra level to expose spinal cord for contusion using weight-drop method. PBS-treated SCI rats (group one) were used as negative controls, in which cavitations were observed 10 weeks after SCI. pRNA-U6.1/Hygro- (group two, as a mock) and pRNA-U6.1/Hygro-p75 shRNA- (group three) transfected BMSC were labeled with a fluorescent dye, CM-DiI, and grafted into the lesion site 7 days after surgery. The Basso-Beattie-Bresnehan locomotor rating scale was performed weekly for 10 weeks. Results: There was a significant difference (P≤0.05) between all groups of treated rats regarding functional recovery. Specifically, the discrepancy among p75 siRNA and mock-transfected BMSC was statistically significant. P75 siRNA BMSC also revealed a higher level of in vivo survival compared to the mock BMSC. Conclusion: Our data suggest that genetically modified BMSC that express p75:siRNA could be a more suitable source of cells for treatment of SCI.
Key Words: Spinal cord injury (SCI), Apoptosis, Bone marrow stromal cells (BMSC)
INTRODUCTION
Spinal cord injury (SCI) is one of the major causes of paralysis (quadriplegia or paraplegia) worldwide. The phenomenon greatly influences quality of life of patients, their family, and society. The trauma destroys axonal pathways at the lesion site, and by disrupting communication between the central nervous system and the lower body parts results in the loss of motor, sensory, and autonomic functions below the level of injury. Because of the complex pathophysiology of SCI resulting from the secondary injury cascade [1], none of the current therapies alone provides a complete recovery for the disease [2]. Future therapies need to combine the current partially successful treatment strategies, such as tissue or cell transplantation [3], providing growth factors [4], blocking neural regeneration inhibitory factors (glial scar and myelin/oligodendrocyte inhibitory molecules) [5, 6] and modulation of inflammatory response following SCI [7] to obtain more efficient regeneration.
Stem cell-based therapies [8], one of the most promising treatments for restoration of SCI, exert their effects through various aspects including: 1) offering a permissive cellular substrate to support axonal attachment and growth, 2) producing growth factors, 3) providing a cell-based electrical relay, 4) promoting neovascularization, 5) re-myelinating host axons, and 6) replacing damaged cells following neuronal differentiation. Stem cells can also be applied as appropriate vehicles for delivery of desired genes. Bone marrow stromal cells (BMSC) are capable of differentiating into neural cells and have been widely utilized for cell and gene therapy of neurodegenerative diseases including SCI [9, 10]. There are many other advantages that have motivated researchers to employ BMSC as a source of cells for transplantation. BMSC have immunomodulating properties which result in circumventing rejection problems and immuno-suppressive therapies. They are readily obtained and propagated in vitro and therefore they can be used for autologous transplantation. Finally, the long history of applying BMSC in hematologic malignancies has given BMSC a proven safety record [11].
One of the main obstacles in cell therapy of central nervous system (CNS) is the high proportion of cell death, specifically after neural differentiation of grafted cells. P75 receptor, also known as the death receptor, has been proven to cause apoptosis of neural cells after binding to pro-neurotrophin [12]. According to our previous data, rat BMSC express p75 in a short time window of 6-12 hours after the induction of neural differentiation [13]. We also demonstrated that p75 suppression could significantly reduce the rate of apoptosis and therefore increase the viability of BMSC during the process of neural differentiation [14]. In the continuation of our previous works [13, 14], we have examined here whether p75-siRNA expressing BMSC are able to better survive at the site of implant, and whether they can improve the functional behavior of spinal cord-injured rats.
MATERIALS AND METHODS
Animals. Female Sprague-Dawley rats were purchased from the Pasteur Institute of Iran (Tehran, Iran). Animals were stored under standard conditions of constant humidity (55-65%) and temperature (22-24°C), 12 hours dark/light cycle, and with unrestricted access to food and water. Animal housing and surgical procedures were carried out in accordance with the Animal Care and Use Committee Laws of Tarbiat Modares University (Tehran, Iran) to reduce animal suffering and the number of animals.
BMSC extraction and culture. Male Spargue-Dawley rats (4-6 weeks old) were sacrificed according to the principles of the Bioethics Committee of Tarbiat Modares University. BMSC isolation was performed as described previously [13]. Briefly, the marrow was extruded from the central canal of dissected tibias and femurs by flushing using a 26 gauge needle syringe filled with α-MEM (Gibco, USA). The extracted marrow cells were seeded in α-MEM supplemented with 20% (Gibco, USA), 100 units/ml penicillin, 100 µg/ml streptomycin and 25 ng/ml amphotericin B at a density of 5-10 × 105 cells/cm2 and incubated at 37°C with 5% humidified CO2. The medium containing non-adherent cells was removed in the next day to allow propagation of adherent cells. The medium was replaced every other day. Confluent BMSC were subcultured at 1:3 every 4-5 days using 0.25% trypsin and 1 mM EDTA. The cells were kept in liquid nitrogen in 90% FBS and 10% DMSO.
Cell transfection. Cell transfection was performed according to the manufacturer’s protocol with minor modifications. Briefly, 80-90% confluent BMSC in a 24-well plate were transfected with 0.5, 1, and 2 µg of pEGFP-N1 vector by 0.5, 1, and 2 µl of lipofectamine 2000 (Invitrogen, USA). Medium was replaced 4 hours after transfection and transgene expression was examined after 48 hours using a fluorescence microscope. pRNA-U6.1/Hygro and pRNA-U6.1/Hygro-p75 shRNA vectors were introduced to the non-differentiated BMSC by Lipofectamine 2000 as described above. The medium was then replaced by a fresh complete medium containing hygromycin antibiotic (concentration was determined by toxicity curve for BMSC) to select the cells that had taken up the vector. The cells were then grown under antibiotic selection until they were confluent.
Rat model of spinal cord injury. A total of 32 female Sprague-Dawley rats weighting between 200 and 250 g were used in the study. For behavioral analysis, the animals were divided into four groups as follows: sham-operated group (in which the rats underwent laminectomy without contusion, n = 5), control group or group one (in which the rats received PBS at the lesion site, n = 10), mock BMSC-grafted group or group two (in which pRNA-U6.1/Hygro-transfected BMSC were transplanted into the lesion site, n = 10) and p75-siRNA BMSC-grafted group or group three (in which pRNA-U6.1/Hygro-p75 shRNA-transfected BMSC were transplanted into the lesion site, n = 7). Two additional mock BMSC-grafted rats and two additional p75-siRNA BMSC-grafted ones were employed for tissue analysis. To produce moderate SCI model, the animals were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). Efforts were made to reduce animal suffering and minimize the number of animals used. The back of rats were shaved and disinfected with betadine and 75% ethanol to minimize the infection risk. A heating pad was used to maintain body temperature at 37°C during surgery. Dorsal laminectomy was performed to expose the L1 spinal segment. Dorsal-vertebral organs were tightly clamped to stabilize the spinal cord during injury process. A 10-g impact rod (with a 2 mm diameter) was dropped from a height of 25 mm in order to produce contusive SCI. Then, the muscles and skin were closed and 12-25 ml lactated Ringer’s solution (containing 130 mM sodium ion, 109 mM chloride ion, 28 mM lactate, 4 mM potassium ion and 3 mM calcium ion) was administered subcutaneously to assist animal recovery. Cefazolin (50 µg/kg, Jabir Ibn Hayan, Tehran) was injected twice daily for 3 days. The urinary bladders were pressed three times a day until the full bladder function was reestablished.
DiI staining and cell transplantation. Mock and p75-siRNA-transfected BMSC were stained with 3 µM CM-DiI (Invitrogen, USA) solution at 37°C for 30 min and at 4°C for 15 min. Then, the cells were washed out with PBS three times. Trypan blue staining was performed in order to determine the number of viable cells before grafting. Briefly, 100 µl of 0.4% (w/v) trypan blue was added to the cell suspension. Cells were counted using hematocytometer, and cell viability rate was calculated. Cell transplantation was carried out on day 7 after injury. Cells (5 × 105) were resuspended in 10 µl PBS and injected directly into the spinal cord at 4 directions of the lesion site (2.5 µl in each direction with duration of 5 min) using a 10-µl Hamilton syringe. The control group rats were injected with 10 µl PBS in the same way.
Tissue harvesting, spinal cord cryosection, and fluorescence microscopic observation. 3 weeks after transplantation, the animals were deeply anesthetized by i.p. injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). A cannula was inserted into the left ventricle, and then the right atrium was pierced to allow the blood and fixative to leave the heart. After washing with 100 ml of normal saline containing 0.1% heparin, 200 cc of 4% paraformaldehyde was administered. Then, the spinal cord was dissected at T13-L2 levels and placed overnight in 4% paraformaldehyde. The separated spinal cord tissue was then placed for another 24 h in 30% sucrose at 4°C, for cryosectioning preparation. Spinal cord tissues were cut five times in 20-µm sections at 200-µm intervals at the center of lesion region. Observation of DiI-labeled cells in vitro and tracking of the labeled cells after transplantation were carried out using a fluorescence microscope (Nikon, model TE 2000-S, Germany). The amount of red color density in cross sections was quantified using Image-J 1.41a software, as a measure of survival rate of transplanted cells.
Functional behavior evaluation. The hind limb motor function was analyzed using Basso-Beattie-Bresnehan (BBB) behavioral score test. This score was given to each rat according to its movement ability so that a 0 score represented a total paralyzed rat with no hind limb locomotion and a 21 score was given to a rat with completely normal gait [15]. The test was performed by 2 researchers weekly for 10 weeks in an open field (75 × 120 cm) as a 4-minute double-blind test.
Statistical analysis. To evaluate functional outcomes and tissue analysis, multiple group comparisons were made by one-way analysis of variance (ANOVA) using a significance level of 5%. Post hoc differences were tested by Tukey. All applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research.
RESULTS
BMSC transfection optimization. pEGFP-N1 was used to optimize the efficiency of transfection of BMSC with Lipofectamine 2000. We determined that 1 µl of Lipofectamine and 1 µg of plasmid DNA would yield the highest rate of green fluorescently labeled cells in an 80-90% confluency of a 24-well plate. These conditions were employed in the following transfection experiments with pRNA-U6.1/Hygro and pRNA-U6.1/Hygro-p75-shRNA. After 48 h, 40-50% of the cells were estimated to be GFP positive. Lipofection and GFP expression caused no morphological changes in the BMSC. Furthermore, freezing and thawing of the transfected cells had no obvious effects on the survival of labeled cells (data not shown).
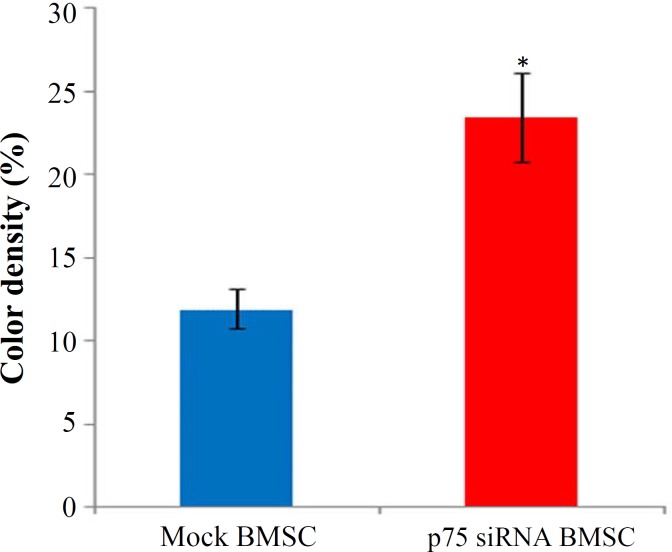
DiI labeling and Tracing the fate of grafted cells. Intact and p75-suppressed cells were labeled with fluorescent carbocyanine dye DiI. Fluorescently labeled cells were completely normal in survival, propagation and in vitro differentiation for at least 3 weeks (data not shown). Cross sections of the spinal cord were examined histologically after injury. Contused spinal cords represented an area of cavity in the dorsal zone of the spinal cord (Fig. 1). Fluorescently labeled p75-siRNA BMSC were well survived and integrated into the host tissue for at least 3 weeks after transplantation. The migration of the genetically modified cells from the initial transplantation sites could be easily noticed (Figs. 2A-2F). A similar distribution pattern was observed in cross sections obtained from injured spinal cords after transplantation of mock BMSC (data not shown). In vivo evaluation of signal intensity, as a rough estimation of number of labeled survived cells by ImageJ program (version 1.41a), revealed a significant increase (about 11%) in the sections of p75-siRNA BMSC-grafted rats, compared to the mock BMSC-grafted ones (Fig. 3).
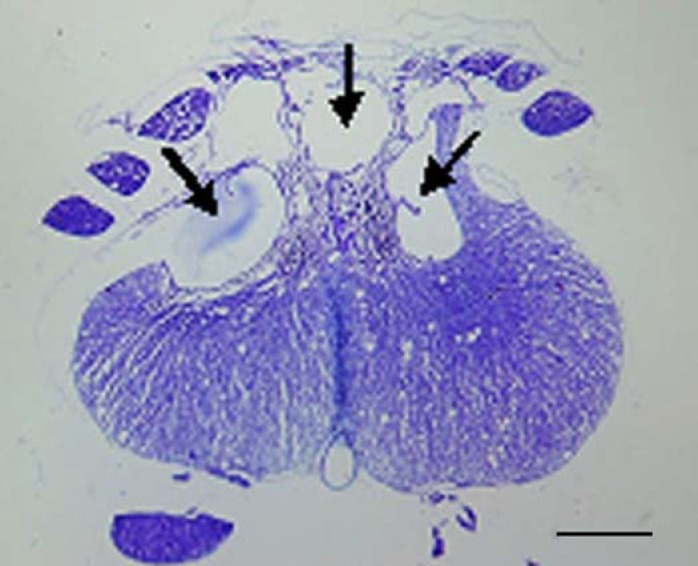
Fig. 1.
A light micrograph of a section of contused spinal cord (stained with cresyl violet) 3 weeks after injury. The arrows show the cavitations in the posterior region of the spinal cord. Scale bar, 300 µm
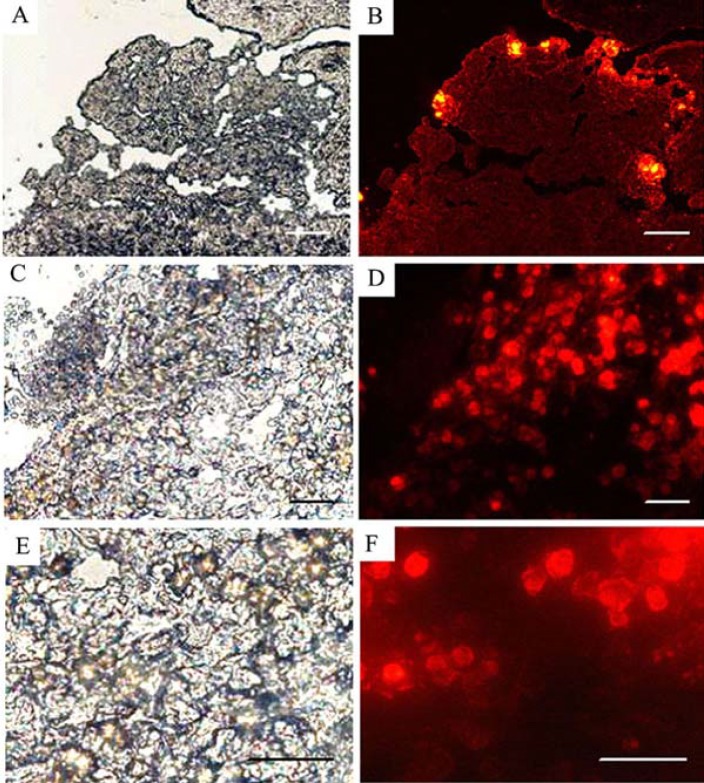
Fig. 2.
Survival, migration and integration of genetically modified cells was traced in the graft region via DiI labeling of the transplanted cells. The fluorescent signal of the p75-suppressed BMSC is localized at 3 hours after grafting (A, B) and distributed inside the injured area at 3 weeks post transplantation (C-F). Scale bars, 10 µm
Fig. 3.
In vivo calculation of DiI florescent signal density at graft site. As it shows, the p75-siRNA-transfected cells generated more signal intensity compared to the mock-transfected cells; suggesting a better cell survival following p75 suppression. Error bars represent standard errors of the mean (*P<0.05
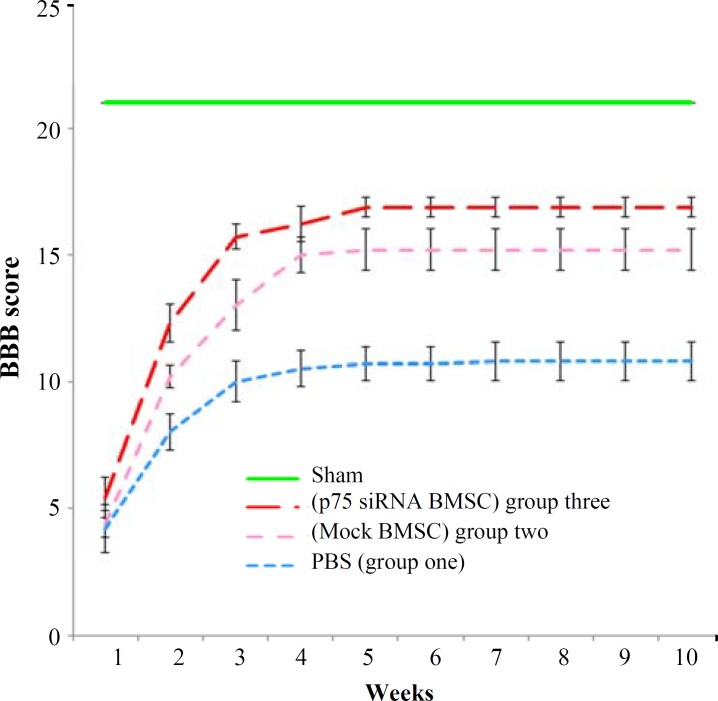
Evaluation of behavioral and functional outcomes of treated rat models. To measure functional recovery in treated animals, BBB scores were determined weekly for 10 weeks. Statistical analyses of BBB scores revealed a significant difference among all examined groups (Fig. 4). After 10 weeks, the final BBB score for the control group (group one) was 10.8 ± 0.76. The score was 15.2 ± 0.84 for the BMSC-treated rats (group two), and 16.8 ± 0.38 for p75-siRNA BMSC-treated ones (group three). The sham-operated group showed the BBB score of 21 at all time points.
Fig. 4.
Analysis of locomotor recovery in the SCI rat models by BBB test. Graphic representation of weekly BBB scores of rats that received PBS (group one), BMSC (group two), and p75-siRNA BMSC (group three) at 1 week to 10 weeks after grafting. Transplantation of the p75-siRNA expressing BMSC in SCI rats demonstrated a significant improvement in locomotor recovery, compared to the mock-transfected BMSC-treated ones
DISCUSSION
Previous studies have demonstrated the effectiveness of mesenchymal stem cells (MSC) in promoting functional recovery after SCI [16, 17-19]. In the view of clinical perspectives, BMSC are more advantageous than any other kinds of stem cells. The most important advantage of BMSC, as a source of cell therapy, is their accessibility. BMSC could be engrafted functionally into the host tissue and have immunomodulatory properties and proven safety record. They do not have the same tumor formation problem attributed to the embryonic stem cells [20]. Various reports have confirmed BMSC as a reliable source of producing neural-like cells after specific treatments in vitro [21-23]. Moreover, in a previous report, we demonstrated that BMSC are more similar to neural stem cells than embryonic stem cells [13]. These observations provided an additional advantage for BMSC that motivated researchers to employ them for restoration of neurodegenerative disorders.
Although some researchers have completely denied the presence of any evidence for neural differentiation of transplanted MSC [16], others have detected this phenomenon at a low rate [24]. It is probable that BMSC are indeed differentiated into neural-like phenotype in vivo, but most of them are eliminated by means of apoptosis induction, as we reported previously [13,14].
p75 is known as a death-related receptor, which promotes apoptosis of neurons, specifically in the absence of Trk receptors [12]. Our previous in vitro study has shown that the expression of p75 could be detected in BMSC after initiation of neural induction in a time window of 6-12 h [13], which coincides with the in vitro occurrence of neuronal death. The observation also mimics the in vivo phenomenon of neuronal death that happens when neurons fail to receive their survival factors.
We have also reported that interfering with p75 expression (synchronous with its transient in vitro expression after neural induction) is able to reduce the rate of apoptosis of neural-like cells derived from BMSC [14]. Therefore, the p75 down-regulation in BMSC may be sufficient to reduce the in vivo death rate of neurally differentiated cells obtained from the grafted BMSC. Accordingly, interfering with p75 expression in transplanted BMSC may enhance other potential regenerative capacities of BMSC. Other researchers have also reported the impact of p75 suppression on increasing cell survival [25].
The present study has provided an approach toward combination therapy by integration of cell and gene therapies. Moreover, combination of the current method with neurotrophic factor supply may provide a more suitable microenvironment for differentiation of BMSC and also for survival of newly formed neural-like cells [4].
There are, however, some reports emphasizing on the use of genetically engineered cells to increase their survival rate either in vitro or in vivo [26], e.g. introduction of tumor necrosis factor receptor [27, 28], heme oxygenase-1 [29] and Akt genes [30].
Here, we transplanted genetically modified cells without any pretreatment for neural differentiation, as this procedure has proved to be more effective in recovering the SCI rat models. Pre-induction of BMSC by applying bFGF before transplantation is one of the procedures that should be examined in future transplantation experiments. Using undifferentiated BMSC following neural pre-induction, instead of totally differentiated neural-like BMSC, may have some practical benefits. The BMSC that have not totally entered the differentiation process would retain some of their stem cell characteristics such as self-renewal and higher survival rate after transplantation. They could be differentiated after the grafting, according to the microenvironmental clues present at the site of transplantation. In this way, the acquired neural-like phenotype of the cells would be more compatible with the survival of the cells and the needed functional cells at the damaged area.
The current in vivo data are in line with our previous in vitro results, which demonstrated a diminished rate of apoptosis in neurally differentiating BMSC after p75-siRNA transfection [14]. Based on our data, the more potent regenerative effect of p75-suppressed BMSC compared to the untreated transplanted mock BMSC might be deduced from its higher survival rate after grafting. In summary, this study highlights the potential usefulness of applying p75-suppressed BMSC for promoting functional recovery after SCI.
ACKNOWLEDGEMENTS
This research was supported by a grant from Basij Elmi Organization (grant no. 3657). The authors are grateful to Dr. Gholam Reza Kaka (Baqiyatallah Medical Sciences University) for his significant assistance to the study.
References
- 1.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991 Jul;75(1):15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 2.Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma. 2006 Mar;23(3-4):318–34. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- 3.Nayak MS, Kim YS, Goldman M, Keirstead HS, Kerr DA. Cellular therapies in motor neuron diseases. Biochim Biophys Acta. 2006 Nov-Dec;1762(11-12):1128–38. doi: 10.1016/j.bbadis.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Kirstein M, Farinas I. Sensing life: regulation of sensory neuron survival by neurotrophins. Cell Mol Life Sci. 2002 Nov;59(11):1787–802. doi: 10.1007/PL00012506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000 Jan;403(6768):434–9. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 6.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006 Aug;7(8):617–27. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice T, Larsen J, Rivest S, Yong VW. Characterization of early inflammation after spinal cord injury in mice. J Neuropathol Exp Neurol. 2007 Mar;66(3):184–95. doi: 10.1097/01.jnen.0000248552.07338.7f. [DOI] [PubMed] [Google Scholar]
- 8.Coutts M, Keirstead HS. Stem cells for the treatment of spinal cord injury. Exp Neurol. 2008 Feb;209(2):368–77. doi: 10.1016/j.expneurol.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Rismanchi N, Floyd CL, Berman RF, Lyeth BG. Cell death and long-term maintenance of neuron-like state after differentiation of rat bone marrow stromal cells: a comparison of protocols. Brain Res. 2003 Nov;991(1-2):46–55. doi: 10.1016/j.brainres.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003 Sep;53(3):697–702. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- 11.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair-current views. Stem Cells. 2007 Nov;25(11):2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 12.Florez-McClure ML, Linseman DA, Chu CT, Barker PA, Bouchard RJ, Le SS, et al. The p75 neurotrophin receptor can induce autophagy and death of cerebellar purkinje neurons. J Neurosci Res. 2004 May;24(19):4498–509. doi: 10.1523/JNEUROSCI.5744-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaghoobi MM, Mowla SJ. Differential gene expression pattern of neurotrophins and their receptors during neuronal differentiation of rat bone marrow stromal cells. Neurosci Lett. 2006 Apr;397(1-2):149–54. doi: 10.1016/j.neulet.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Edalat H, Hajebrahimi Z, Movahedin M, Tavallaei M, Amiri S, Mowla SJ. p75NTR suppression in rat bone marrow stromal stem cells significantly reduced their rate of apoptosis during neural differentiation. Neurosci Lett. 2011 Jul;498(1):15–9. doi: 10.1016/j.neulet.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 15.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995 Feb;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 16.Parr AM, Kulbatski I, Wang XH, Keating A, Tator CH. Fate of transplanted adult neural stem/progenitor cells and bone marrow–derived mesenchymal stromal cells in the injured adult rat spinal cord and impact on functional recovery. Surg Neurol. 2008 Dec;70(6):600–7. doi: 10.1016/j.surneu.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama Y, Radtke C, Honmou O, Kocsis JD. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002 Sep;39(3):229–36. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koda M, Okada S, Nakayama T, Koshizuka S, Kamada T, Nishio Y, et al. Hematopoietic stem cell and marrow stromal cell for spinal cord injury in mice. Neuroreport. 2005 Nov;16(16):1763–7. doi: 10.1097/01.wnr.0000183329.05994.d7. [DOI] [PubMed] [Google Scholar]
- 19.Enzmann GU, Benton RL, Talbott JF, Cao Q, Whittemore SR. Functional considerations of stem cell transplantation therapy for spinal cord repair. J Neurotrauma. 2006 Mar-Apr;23(3-4):479–95. doi: 10.1089/neu.2006.23.479. [DOI] [PubMed] [Google Scholar]
- 20.Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010 Nov;5(6):933–46. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000 Aug;61(4):364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 22.Choong PF, Mok PL, Cheong SK, Leong CF, Then KY. HYPERLINK Generating neuron-like cells from BM-derived mesenchymal stromal cells in vitro. Cytotherapy. 2007;9(2):170–83. doi: 10.1080/14653240701196829. [DOI] [PubMed] [Google Scholar]
- 23.Bossolasco P, Cova L, Calzarossa C, Rimoldi SG, Borsotti C, Deliliers GL, et al. Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp Neurol. 2005 Jun;193(2):312–25. doi: 10.1016/j.expneurol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DI, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002 Feb;99(4):2199–204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Firouzi M, Sabouni F, Deezagi A, Hassannejad Pirbasti Z, Poorrajab F, Rahimi-Movaghar V. Schwann cell apoptosis and p75NTR siRNA. Iran J Allergy Asthma Immunol. 2011;10(1):53–9. [PubMed] [Google Scholar]
- 26.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001 May;33(5):907–21. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 27.Bao C, Guo J, Zheng M, Chen Y, Lin G, Hu M. Enhancement of the survival of engrafted mesenchymal stem cells in the ischemic heart by TNFR gene transfection. Biochem Cell Biol. 2010 Aug;88(4):629–34. doi: 10.1139/O10-018. [DOI] [PubMed] [Google Scholar]
- 28.Rayner SA, Larkin DF, George AJ. TNF receptor secretion after ex vivo adenoviral gene transfer to cornea and effect on in vivo graft survival. Invest Ophthalmol Vis Sci. 2001 Jun;42(7):1568–73. [PubMed] [Google Scholar]
- 29.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia- regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005 Oct;46(7):1339–50. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 30.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003 Sep;9(9):1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]