Abstract
Collagen is the most abundant protein in mammals, and there has been long-standing interest in understanding and controlling collagen assembly in the design of new materials. Collagen-like peptides (CLP), also known as collagen-mimetic peptides (CMP) or collagen-related peptides (CRP), have thus been widely used to elucidate collagen triple helix structure as well as to produce higher-order structures that mimic natural collagen fibers. This mini-review provides an overview of recent progress on these topics, in three broad topical areas. The first focuses on reported developments in deciphering the chemical basis for collagen triple helix stabilization, which we review not with the intent of describing the basic structure and biological function of collagen, but to summarize different pathways for designing collagen-like peptides with high thermostability. Various approaches for producing higher-order structures via CLP self-assembly, via various types of intermolecular interaction, are then discussed. Finally, recent developments in a new area, the production of polymer-CLP bioconjugates, are summarized. Biological applications of collagen contained hydrogels are also included in this section. The topics may serve as a guide for the design of collagen-like peptides and their bioconjugates for targeted application in the biomedical arena.
Keywords: Collagen like peptide, triple helix, bioconjugate, self-assembly, hydrogel
1. Introduction
Collagen is the main component of the extracellular matrix (ECM) and comprises 25% to 35% of whole body protein content in humans. The collagens are a large family of proteins, comprising 28 different types, which have widespread functions such as mediating cell adhesion, cell migration, tissue scaffolding and repairing. The most prevalent class of collagen – fibrous collagen – includes type I, II, and III collagens and is the main component of tendon, ligament, skin, cartilage and bone. Other types of collagens, such as type IV and type VIII collagen, play a vital role in the formation of network structures such as basement membranes. Fundamentals about the classification and biological functions of collagen will not be discussed in this review, but interested readers are directed to other previous reviews for more detailed information on this topic.[1, 2]
Although the architectures and roles of these collagens vary widely, they all comprise triple helix bundles.[3] The collagen triple helix comprises three polyproline-II type helices held together by periodic interchain hydrogen bonding between the glycine amide in one chain and the carbonyl of the amino acid of an adjacent chain. Each strand of the helix consists of regularly repeated Gly-X-Y tripeptide repeats, with every third residue comprising glycine. Proline (Pro) and (4R)-hydroxyproline (Hyp) occupy the X and Y positions of the triplet repeats at the highest statistical frequency within native collagens.
Due to the excellent gel-forming abilities and biodegradability of the natural collagens, they have been widely used as biomedical materials for tissue support and regeneration.[4, 5] However, some of the limitations of using animal-derived collagens, such as their thermal instability, possible contamination with pathogenic substances,[6] and relative difficulty in the introduction of specific sequence modifications, have motivated the use of synthetic model collagens, also known as collagen-like peptides (CLPs) or collagen-mimetic peptides (CMPs), in these types of applications. These collagen-mimetic sequences have been employed to elucidate the triple helix structure and the stabilization effect of different amino acid residues,[7–11] and more recently, a variety of CLPs with novel sequences and interactions have been developed to mimic collagen fibril formation and to produce other higher-order supramolecular structures.[12–16]
In addition to peptide-based assembly, the self-assembly of amphiphilic block copolymers has long been a very useful tool to fabricate nanostructured materials. Combining advantages of synthetic polymers, such as flexibility in architecture and functionality,[17–19] biocompatibility, and mechanical strength, as well as stimuli responsiveness,[20] with advantages of (poly)peptides including monodispersity, precise primary to tertiary structure, and specified bioactivity,[21] polymer-peptide bioconjugates are of particular recent interest, especially in biomedical applications such as drug and gene delivery.[22–24] Polymer conjugates with α-helical coiled-coil peptide domains,[25–28] β-sheet peptide motifs,[29–31] and elastin mimetic peptides[32–34] have been widely employed. However, compared to these biohybrid materials, polymer-collagen like peptide bioconjugates represent a relatively new area for the development of functional polymeric materials;[35–38] we provide a summary of select areas here and refer readers to the review by He and Theato that is in this special issue [REF].
2. Triple helix stability
The increasing demand for collagen-related peptides, as drug delivery vehicles, labeling agents, and tissue engineering scaffolds with select bioactivity, requires understanding of the thermal stability and specific binding properties with of CLPs with certain biomaterials. Understanding triple helix stability and factors affecting it is thus of particular significance in the design of such sequences. In early work in this area, Brodsky and coworkers[39] described the propensities for all natural amino acids to form stable triple helical structures. Triplets with the form Gly-X-Hyp and Gly-Pro-Y were used as guest sequences in the context of the (Gly-Pro-Hyp)8 host peptide, to form model CLPs with the sequence Ac-(Gly-Pro-Hyp)3-Gly-X-Hyp-(Gly-Pro-Hyp)4-Gly-Gly-CONH2 or Ac-(Gly-Pro-Hyp)3-Gly-Pro-Y-(Gly-Pro-Hyp)4-Gly-Gly-CONH2. The melting temperatures of these model peptides with substitutions of all natural amino acids in the X and Y positions were studied via circular dichroic spectroscopy (CD); the results of these detailed studies are provided in Table 1. These results confirmed that placement of the imino acids Pro at the X position and Hyp at Y position have the best stabilizing effect, while Gly and aromatic residues tend to destabilize the triple helix. Since then, there have been a wide variety of studies aimed at determining the molecular details of stable triple helix assembly.
Table 1.
Melting temperatures of host-guest peptides (Reproduced with permission from Ref. 39 © American Chemical Society)
| Gly-X-Hyp | Tm (°C) | Gly-Pro-Y | Tm (°C) |
|---|---|---|---|
| Pro | 47.3 | Hyp | 47.3 |
| Glu | 42.9 | Arg | 47.2 |
| Ala | 41.7 | Met | 42.6 |
| Lys | 41.5 | Ile | 41.5 |
| Arg | 40.6 | Gln | 41.3 |
| Gln | 40.4 | Ala | 40.9 |
| Asp | 40.1 | Val | 40.0 |
| Leu | 39.0 | Glu | 39.7 |
| Val | 38.9 | Thr | 39.7 |
| Met | 38.6 | Cys | 37.7 |
| Ile | 38.4 | Lys | 36.8 |
| Asn | 38.3 | His | 35.7 |
| Ser | 38.0 | Ser | 35.0 |
| His | 36.5 | Asp | 34.0 |
| Thr | 36.2 | Gly | 32.7 |
| Cys | 36.1 | Leu | 31.7 |
| 2Thr | 34.3 | Asn | 30.3 |
| Phe | 33.5 | Thy | 30.2 |
| Gly | 33.2 | Phe | 28.3 |
| Trp | 31.9 | Trp | 26.1 |
2.1 Role of (4R)-hydroxyproline
From Table 1, it is clear that, when only considering the natural amino acids, any replacement of Pro in the X position and Hyp in the Y position destabilizes the collagen triple helix. The importance of Pro and Hyp in the X and Y residues in the collagen triple helix is further suggested by the fact that 10.5% of Gly-X-Y triplets within human collagens are Gly-Pro-Hyp and 38% of the amino acids in the Y position are (4R)-hydroxyproline.[40] While the glycine residues are buried in the middle, the side chains of amino acids in the X and Y positions are generally exposed to water,[41] and the particular importance of Hyp residue at the Y position in collagen structures was originally attributed to the formation of water-mediated hydrogen bonds between the strands.[42–45]
This long-held hypothesis was challenged by work reported by Raines and coworkers in 1998, [46, 47] in which the hydroxyprolines in (Gly-Pro-Hyp)10 were replaced with (2S, 4R)-4-fluoroproline (Flp) to yield the new sequence (Gly-Pro-Flp)10. The new peptide was able to form a hyperstable triple helix with a melting temperature of 91°C, dramatically higher than that of (Gly-Pro-Pro)10 or (Gly-Pro-Hyp)10. Since fluorine groups do not form strong hydrogen bonds,[48] the stabilization imparted by the inclusion of Flp was indicated to arise from the high electronegativity of the Cγ substitute.[49] Additionally, CLPs with peptide sequences (Gly-Pro-hyp)10 and (Gly-Pro-flp)10 (where hyp stands for (4S)-hydroxyproline and flp stands for (4S)-4-fluoroproline) do not form triple helix,[50, 51] indicating that the stereoelectronic effect of the Cγ substitute is another factor which affects triple helix stability.[51, 52] Through studying the molecular structure of Ac-Flp-OMe, Bretscher et al.[51] demonstrated that the trans prolyl peptide bond isomer and an optimal main-chain dihedral angle (ψ), required for triple helix formation, were stabilized by the strong O0···C1 interaction afforded by Flp (Figure 1, black arrow). The stereoelectronic effect was also observed to stabilize 4-chloroproline containing CLP (Pro-Clp-Gly)10[53]. Furthermore, introduction of an azobenzene-derived chromophore [54] permits control the folding and unfolding of collagen triple helix by photo-isomerization of the “light switch”, thus narrowing the gap between theoretical and experimental investigations on collagen like triple helix.
Figure 1.
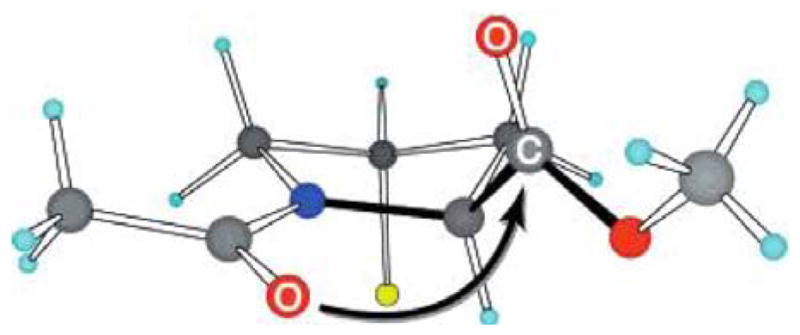
Structure of Ac-Flp-OMe. (Reprint with permission from Ref. 51 © American Chemical Society)
2.2 Electrostatic interactions
In addition to the imino acids Pro and Hyp, non-imino residues, especially charged amino acids such as Lys, Arg, Glu and Asp, comprise up to 15–20% of all residues in collagen and more than 40% of Gly-X-Y triplets contain at least one of these ionizable residues.[40] These charged amino acids are crucial for self-association of collagen fibers and ligand binding to certain biomolecules, as well as contributions to collagen triple helix stability. The importance of these interactions arises from the one-residue staggering between chains of the triple helix structure and the right-handed rotation of the helix bundle, which brings the residues into very close proximity and gives rise to possible interchain electrostatic interaction and hydrogen bonding between charged residues located at these positions (Figure 2).[55, 56]
Figure 2.
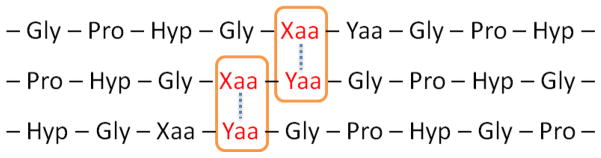
Potential interchain interaction between residue at X position of one chain and residue at Y position of another.
Such pairwise interactions have been widely studied using model peptides. Investigations of the melting temperatures of host-guest CLPs ((GPO)3GXY(GPO)4GG) [55] illustrated that triplets with opposite charged residues at X and Y position (such as Gly-Lys-Asp and Gly-Arg-Asp) showed a major stabilizing electrostatic interaction; while triplets with like-charged residues (e.g., Gly-Lys-Arg and Gly-Arg-Lys) showed electrostatic repulsion and destabilization of the collagen helix. In addition to interactions within the same triplets, similar stabilizing electrostatic interactions were also observed between adjacent triplets. A study of the thermal stability of peptides in the form (GPO)3-G-X-Y-G-X′-Y′-(GPO)3[57] suggested a strong stabilization effect in the presence of GPKGEO or GPKGDO sequences, leading to Tm values of the two peptides equal to that in unsubstituted (GPO)8. Molecular modeling studies suggested that the stabilization may be attributed to interstrand pairwise interaction between positively charged Lys and negatively charged Glu/Asp in the adjacent triplet (Figure 3a). Hartgerink and coworkers further divided this interaction into two categories: one called axial contact, involving interactions from the leading chain to the middle chain as well as from the middle chain to the lagging chain; another called lateral contacts involving interactions from the lagging chain to the leading chain. They further proposed that the stabilization mainly originates from the two axial interactions (Figure 3b).[7, 58] Deciphering such interchain electrostatic interactions are crucial to understand the stability and the registration process of natural collagens as well as to more effectively design collagen related peptides.
Figure 3.
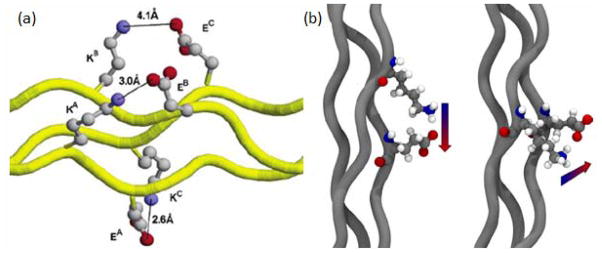
Pairwise interactions between Glu and Lys in GPKGEO containing CLPs. (a) computer model proposed by Persikov et al; (b) axial (left) and lateral (right) interactions proposed by Fallas et al. (Reprinted with permission from Ref. 57 © American Chemical Society and Ref. 7 ©American Society for Biochemistry and Molecular Biology)
2.3 Cystine knots
The homotrimeric collagen type III contains two adjacent cysteine residues at the C-terminus, which form three disulfide bonds between the chains at the non-collagenous domains (Figure 4).[59] This cystine knot was found to provide an efficient nucleus for triple helix growth from C-terminus in a zipper-like mechanism[60]. Moroder and coworkers[10] studied the role of the cystine knot on collagen folding and stability and found that the thermostability of cystine knot-linked (POG)5 was dramatically improved (compared with (POG)5 without a cystine knot), with an increase of melting temperature from 20.3 °C to 68.0 °C. Similar stabilization was also observed in hydroxyproline-lacking collagen-like peptides.[16, 61]
Figure 4.
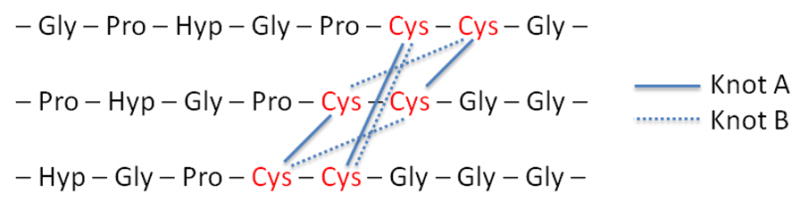
Two types of cystine knot connectivities.
2.4 Template stabilization
In addition to natural templates such as the cystine knot, another well-known method to stabilize the collagen helix is to link three α-chains covalently at the C-terminus. When conjugated to templates such as cis,cis-1,3,5-trimethylcyclohexane-1,3,5-tricarboxylic acid (Kemp triacid),[62, 63] tris(2-aminoethyl)amine-(suc-OH)3 (TREN-(suc-OH)3)[63] and the N-terminus of the bacteriophage T4 fibritin foldon domain,[64] the thermostablility of various collagen-like peptides has been dramatically improved (Table 2), and the triple helix folding process also significantly accelerated.[62] The stabilization from these templates arises from the reduction of the entropy difference between the unfolded and folded states of the CLP domain, as well as the presence of a higher concentration of collagen strand at the C-terminus.
Table 2.
Comparison of melting temperature of CLP with/without the template. (Data taken in part from Ref. 62, 63 © American Chemical Society and Ref. 64 © Academic Press.)
| Collagen like peptides | Melting temperature (°C) | |||
|---|---|---|---|---|
| n=3 | n=5 | n=6 | n=10 | |
| Ac-(Gly-Pro-Hyp)n-NH2 | NTa | 18 | 36 | - |
| KTA-[Gly-(Gly-Pro-Hyp)n-NH2]3 | 30 | 70 | 81 | - |
| Ac-(Gly-Nleu-Pro)n-NH2 | NT | - | 26 | - |
| KTA-[Gly-(Gly-Nleu-Pro)n-NH2]3 | NT | - | 36 | - |
| TREN-[suc-(Gly-Nleu-Pro)n-NH2]3 | NT | 38 | 46 | - |
| (Gly-Pro-Hyp)n | - | - | - | 23 |
| T4 fibritin foldon (Gly-Pro-Hyp)n | - | - | - | 65 |
NT denotes no transition observed.
Similar stabilization has been observed for CLPs in which the C-terminus is conjugated to hydrophobic groups or synthetic polymers. Instead of a template that covalently links the three strands, the hydrophobic interactions of the attached moieties bring the strands into close proximity in aqueous solution, thus accelerating triple helix formation. For example, the thermostability of collagen triple helices from peptide-amphiphiles with collagen-peptide head groups and alkyl chain tails is substantially higher than that of peptides without lipidation and the stability increases with the length of the lipid tail.[35, 36, 65] Most recently, our group, in collaboration with the Theato group,[38, 66] designed a polymer-CLP-polymer triblock system, in which both C- and N-termini of the collagen-like peptide were conjugated to a thermoresponsive polymer, to investigate the influence of conjugated macromolecules on the conformational behavior of the collagen domain. The denaturing profile of the triblock, assessed via circular dichroic spectroscopy, suggested that the polymer-conjugated peptide domain was still capable of assembly into a stable collagen triple helix. More interestingly, in contrast to the standard sigmoidal unfolding curve for the CLP alone, the triblock showed a more gradual unfolding with two potential transitions, indicating that above its LCST, the collapsed thermoresponsive polymer constricted the unfolding of the peptide and stabilized the collagen-like triple helical domain.
3. Higher-order assembly
In vivo, natural collagens self-assemble into higher order hierarchical structures with precise chemical compositions and molecular structures, which are of essential importance in ECM pathology and development.[2] The wealth of knowledge in understanding collagen structure accumulated in the past few decades has given rise to a surge in research activities focused on supramolecular assembly of collagen-like peptides. Although much of the research is still in early stages, the results are of significant importance in developing collagen-like materials in biomedical applications. Collagen-like peptides as simple as (Pro-Hyp-Gly)10 are indicated to form higher order structures[67] via a nucleation and growth mechanism, similar to natural collagen, although form branched filamentous structures rather than the long fibrous structures of native collagen. Recently, synthetic collagen fibers which bear more similarity to natural collagen fibers have been produced successfully by various well-designed CLP systems and assembly strategies.
3.1 π-π stacking interaction
Maryanoff and coworkers reported a novel collagen like peptide that is able to self-assemble into triple helical, micrometer-scale fibrillar structure through noncovalent end-to-end interactions.[68, 69] In this work, phenylalanine, which has an electron-rich benzyl ring, and pentafluorophenylalanine, which has an electron-poor benzyl ring, were introduced at the C- and N-terminus of the collagen-like peptide (Gly-Pro-Hyp)10 (Figure 5a). When the phenyl/pentafluorophenyl pairs from two adjacent triple helices approached face to face, the overall interface energy decreased about 55.2kcal/mol, leading to the formation of micrometer-long fibrils with an average diameter of 0.26 μm (Figure 5b, c). In addition to end-to-end (linear) interactions, the dimensions of these fibrils also require side-to-side (lateral) interaction. Most importantly, this CLP stimulated aggregation of human blood platelets, demonstrating the potential use of the peptide as a hemostatic material.
Figure 5.
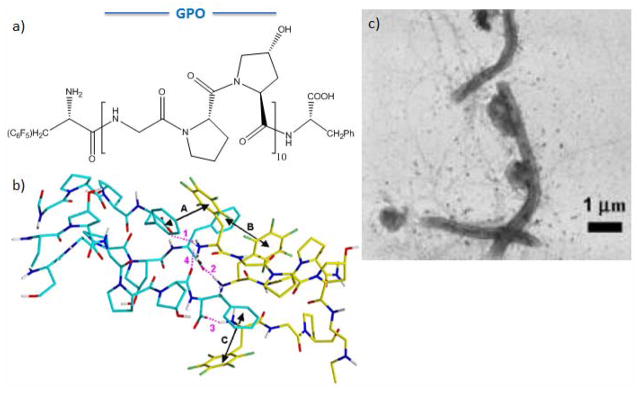
CLP self-assembly via π-π stacking interaction. (a) (GPO)10 end-functionalized with pentafluorophenylalanine and phenylalanine. (b) Interface between two triple helices (one blue, one yellow) showing three aromatic stacking interactions (black double-headed arrows; A-C). (c) TEM image of self-assembled CLP. (Reproduced with permission from Ref. 68 © The National Academy of Sciences of the USA and Ref. 69 © American Chemical Society.)
In addition to π-π stacking, similar interactions, such as CH···π interaction between imino acids (Pro and Hyp) and aromatic residues (Phe and Tyr);[70] as well as cation···π interaction between positive charged N-terminal Arg and C-terminal Phe[71] were also introduced for the fabrication of collagen like fibrillar structures.
3.2 Lateral electrostatic interactions
Natural collagen type I, II, III, V and XI self-assemble into 67nm-periodic cross-striated fibrils.[2] This D-periodicity arises from the differential inter-procollagen interactions, including hydrophobic interaction and electrostatic interactions between oppositely charged side chains. These interactions are optimized when the protomers are placed parallel to the nearest neighbor, with a pre-defined stagger distance.[72] Partially due to the difficulty in precise control of these interactions between the protomers at staggered positions, artificial collagen-like fibrils with periodic bands have not been produced until recently.
Work by Rele et al. [73] first reported a fibrous structure with well-defined D-periodicity from collagen-like peptide self-assembly. The peptide (PRG)4(POG)4(EOG)4 contains three different domains, with a central POG repeat sequence flanked by a positively charged domain at N-terminus and a negatively charged domain at the C-terminus (Figure 6a). After 9-day incubation at room temperature, micron-length fibers with well-defined transverse bands were observed via transmission electron microscopy (TEM) (Figure 6c). These fibers were self-associated following a nucleation-growth mechanism, driven by hydrophobic interaction between the central domain and lateral electrostatic interaction between oppositely charged residues from two neighboring triple helices (Figure 6b).
Figure 6.
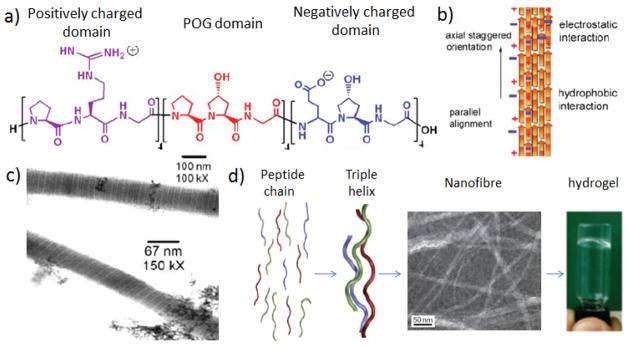
Self-assembly of CLP via lateral electrostatic interaction. (a) Amino acid sequence of CLP, indicating three different domains. (b) Axial staggered interchain electrostatic interactions. (c) Well-defined D-periodicity observed via high resolution transmission electron microscopy. (d) Multi-hierarchical self-assembly of related collagen-mimetic peptides. (Figure a, b, c are reproduced with permission from Ref. 73 © American Chemical Society, Figure d is reproduced with permission from Ref. 14 © Macmillan Publishers Limited.)
More recently, O’Leary et al.[14] demonstrated that due to the very tight hydrogen bonding between the arginine side chain and the backbone carbonyl of an adjacent strand, the arginine residue in the CLP mentioned above was unable to interact with the glutamic acid efficiently. By replacing all the Arg residues with Lys residues, and Glu residues with Asp, a new peptide with sequence (PKG)4(POG)4(DOG)4 was produced, which had stronger interaction between intermolecular, oppositely charged Lys and Asp residues. As predicted, the new peptide formed homogeneous collagen mimetic nanofibers. More interestingly, these nanofibers were also able to form high quality hydrogels, which degrades at a similar rate to rat-tail collagen (Figure 6d).
3.3 Metal-triggered assembly
In addition to lateral electrostatic interactions, coordination bonds between metal ions and specific ligands can also be introduced for controlled self-assembly of collagen-like peptides. Chmielewski and coworkers designed a stimuli-responsive coassembly system, which involves two collagen-like peptides, each individually containing a (Pro-Hyp-Gly)9 core and identical metal binding units at both termini (Figure 7a).[13] One peptide, HisCol, contains two histidines at both termini; the other peptide, IdaCol, contains an iminodiacetic acid (Ida) moiety incorporated onto the side chain of lysine at both termini. By adding divalent metal ions such as Ni2+, Zn2+ and Cu2+, the CLPs co-assemble into petal-like microstructures with a periodic banding at the nanometer scale. The distance between the banding gaps was found to be in the range of 9- 12 nm, which approximately corresponds to the length of (POG)9 collagen triple helix with termini modifications (Figure 7b). Subsequent results from the same group demonstrated that by varying the length of the (POG)n core of the peptides, a range of distinct structures including microflorettes, stacked sheet microsaddles, and fiberlike meshes, can be generated (Figure 7c).[12] Similar metal-triggered assembly of supramolecular structures was also obtained from hydroxyproline-lacking CLPs designed by Hsu and coworkers.[74] In this work, histidine residues were not only incorporated at the termini of the peptide domain, but also incorporated in the center of the peptide domain. Two distinct CLPs, with sequences HG(PPG)9GH (X9) and HG(PPG)4(PHG)(PPG)4GH (PHG) were synthesized respectively. A variety of higher-order structures were obtained from incubation with metal ions, ranging from nanofibrils to microscale spherical, laminated and granulated assemblies (Figure 7d).
Figure 7.
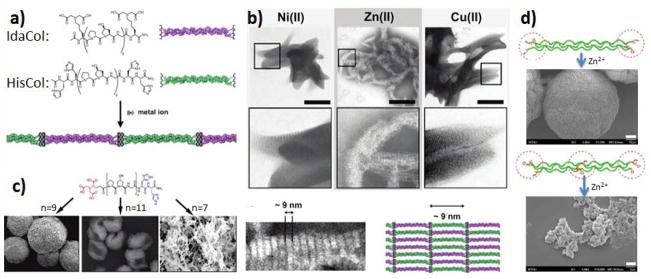
(a) Scheme showing the structure of IdaCol and HisCol and subsequent tandem assembly. (b) TEM images of petal like structures with periodic bands observed from metal triggered CLP self-assembly. (c) Assembled structures depend on length of CLP domain. (d) SEM images of large aggregates of X9 (top) and PHG (bottom) after incubating with Zn2+. (Reproduced with permission from Ref. 12, 13 and 74 © American Chemical Society)
In addition to end-to-end metal-ligand coordination, metal-triggered radial growth of collagen-like peptide triple helices into fibrous structures can also be achieved via these methods.[15] By replacing the hydroxyproline residue in the central tripeptide of (Pro-Hyp-Gly)9 with a bipyridyl-modified lysine, the new peptide can be triggered to form branched collagen fibers by addition of metal ions (Fe2+) (Figure 8). Although the assembled fibers lack the periodic bands of natural collagen, these data provide proof that collagen-like fibers can be generated via nonlinear assembly.
Figure 8.
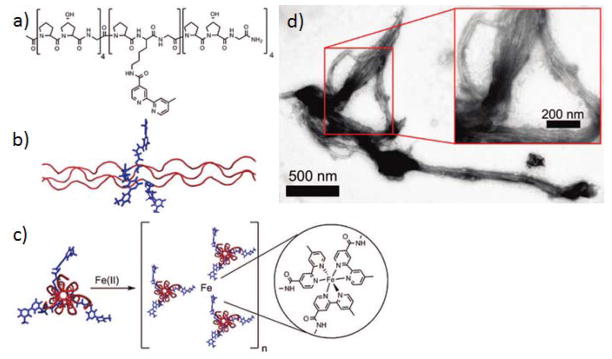
Metal-triggered radial assembly of collagen peptide fibers. (a) amino acid sequence of the CLP. (b) Side view of the triple helix. (c) Hypothesized interaction between bipyridyl-modified lysine and Fe2+. (d) TEM image of the assembled structure. (Reproduced with permission from Ref. 15 © American Chemical Society)
4. Synthetic polymer – CLP bioconjugates
Polymer–peptide bioconjugates are hybrid materials, in which one or more traditional synthetic polymeric domains are covalently bound to peptide domains with specific sequences and functionalities.[75] The combination of peptides with synthetic polymers in a single hybrid molecule offers unique opportunities to combine the advantages of the two. Although peptide-based materials exhibit a variety of well-defined hierarchical structures and important biological functions such as cell recognition and adhesion,[76–78] their use in therapeutic applications is limited since they can cause immune response and are easily degraded by enzymes and thus have short circulation times in vivo.[79, 80] On the other hand, while biocompatibility and high resistance to enzymatic degradation have been observed for well controlled synthetic polymers, they are biologically inactive due to the lack of precisely controlled molecular structures. The bioconjugation of peptide and synthetic polymers has thus become a useful tool to overcome these limitations of the two components. For example, conjugation of poly(ethylene glycol) (PEG) to various protein-based drugs, enzymes and antibodies endows the conjugates with certain beneficial properties such as improved solubility, reduced immunogenicity, as well as increased blood circulation time.[79] Compared to the recent surge of research activities on polymer-peptide conjugates involving peptides with α-helix and β-sheet secondary structure,[29] such conjugates with collagen like-peptides have not been as intensively studied.[37, 38]
The most convenient way to synthesize polymer-peptide conjugates is to couple a functionalized peptide, either at the termini or side chain, with a complementary functionalized synthetic polymer. Although there are some difficulties in this approach, such as low accessibility of functional groups on macromolecules and separation of the desired conjugate from the starting materials, a variety of chemical reactions have been employed for efficient conjugation, including traditional solid phase peptide coupling chemistry,[81–83] Staudinger ligation,[84] Schiff base formation,[85–88] click reaction[89–92] and Michael-type addition.[93–96] Readers interested in more details on polymer-peptide conjugation strategies are directed to a complementary review published by He and Theato, in this special issue (REF).
4.1 Assembly of polymer–CLP conjugates
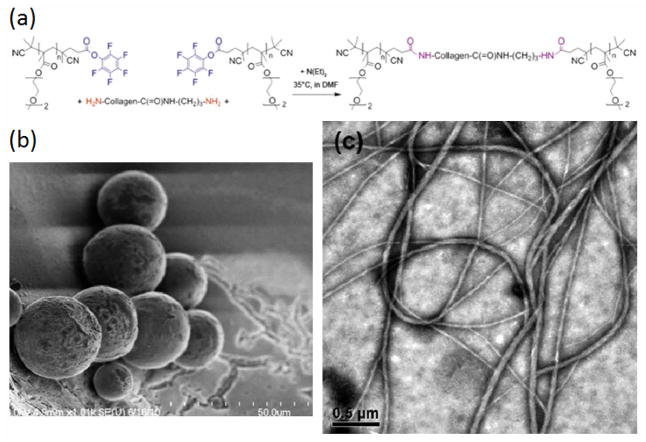
The utilization of the efficient conjugation reactions discovered in the past few decades has given rise to an expansion in the design of novel self-assembled structures based on polymer-peptide biohybrid materials with promising chemical and biological functions.[97, 98] Nonetheless, self-assembly of polymer conjugates with collagen-like peptides remains a fresh research area, and in 2011, Tong and coworkers[37] reported the self-assembly of a collagen-like peptide amphiphile. With a C16 lipophilic tail attached to the C-terminus of a CLP with the sequence (GPO)3GFOGER(GPO)3, the amphiphile self-assembled into micrometer-long nanofibers with a diameter of approximately 16nm under aqueous conditions. More importantly, given the inclusion of the integrin-specific binding sequence GFOGER, the amphiphile was shown to promote the adhesion and spreading of HepG2 cells.
More recently, we have reported the conformational and assembly behavior of a thermoresponsive polymer-CLP-polymer triblock.[38, 66] The triblock comprises a thermoresponsive polymer, namely poly(diethylene glycol methyl ether methacrylate) (PDEGMEMA), conjugated to both termini of a triple-helix forming hydroxyproline-lacking collagen-like peptide (Figure 9a). Morphology study at 37 °C suggested that the collapse of polymeric domain above its LCST caused the formation of large hollow spherical structures (Figure 9b). More interestingly, a morphological transformation into fibrils (Figure 9c) was observed at 75 °C, likely due to the unfolding of the collagen triple helix domain. As a negative control, PEG-CLP conjugates were also synthesized, but did not assemble into such structures. This work provides further evidence that higher-order structures, including fibrils, can be obtained from appropriately designed polymer-conjugated collagen-like peptides.
Figure 9.
(a) Synthesis of hybrid triblock copolymer. Collagen like peptide sequence: G(GPP)3GPRGEKGERGPR(GPP)3GPCCG. (b) Cryo-TEM image of spherical structures at 37 °C. Scale bar = 50 μm (c) TEM image of collagen like fibrils at 75 °C. Scale bar = 0.5 μm (Reproduced with permission from Ref.38 © The Royal Society of Chemistry and Ref.66 © American Chemical Society)
In addition to copolymers containing CLP and traditional synthetic polymers, CLP-related peptide-peptide conjugates have also been studied very recently. Yu and coworkers [99–101] reported that unfolded collagen-like peptides had a strong propensity to bind to denatured collagen strands via stereo-selective triple-helical hybridization. Based on this physical hybridization binding effect, they further designed a conjugate peptide including eight negative charged glutamic acid at the N-terminal of (Pro-Hyp-Gly)9.[102] The new peptide is not only able to bind collagen scaffolds via strand-strand hybridization, but also able to immobilize vascular endothelial growth factor (VEGF) through electrostatic interactions. These results show that polymer-CLP bioconjugates may have potential in targeted drug delivery, especially for cartilage- and bone-related diseases.
4.2 Collagen/CLP containing hydrogels
Due to the excellent mechanical and unique biological properties of collagen, collagen containing 3-dimentional porous hybrid hydrogels has become a very useful tool to mimic the structure and functions of microenvironment in extracellular matrix (ECM) and has been widely used in articular cartilage tissue engineering and regeneration. In order to obtain desired mechanical and physiological properties of the scaffolds, a variety of other components rather than collagen have been incorporated to form the hydrogels. These components includes not only synthetic polymers such as poly(ethylene glycol) (PEG),[103–105] poly(ethylene oxide) diacrylate (PEODA),[106] poly(lactic-co-glycolic acid) (PLGA),[107, 108] and poly(ethylene terephthalate) (PET),[109] but also naturally occurring biomacromolecules such as hyaluronic acid,[104, 110–112] and chitosan.[105, 111]
In addition to natural collagen, hydrogels consisting of chemically synthesized short collagen model peptide has also been produced recently. By photo-copolymerization of CLP conjugated PEG (Acry-PEG-(Pro-Hyp-Gly)7 -Tyr-OH) and PEG diacrylate (PEGDA), Lee et al[113] were able to produce PEG hydrogels with controlled amount of CLP dangling chains, which showed great potential to be used as tissue engineering scaffolds. Through histology studies, the CLP conjugated hydrogels were observed to encapsulate chondrocytes homogeneously. More importantly, these hydrogels stimulated the production of extracellular matrix (ECM) components including glycosaminoglycan (GAG) and type II collagen. The authors hypothesized such stimulation originated from the affinity between collagen-like peptide domain of the hydrogel and chondrocyte-secreted collagen, which has also been discussed in the last section.
Instead of improving cell adhesion and ECM production of PEG hydrogels, CLP triple helix was also exploited for physical cross-linkers. By conjugating CLP single strand to multi-armed PEG at elevated temperature above the melting temperature of CLP triple helix and incubating the PEG-CLP copolymer at a lower temperature, Yu and coworkers[114] reported a novel type of PEG-hydrogel, which was cross linked by intermolecular collagen triple helix formation. Due to the temperature mediated folding and unfolding of the triple helix domain, the elasticity of the hydrogel was temperature dependent. More importantly, the mechanical stiffness of the hydrogel could be easily manipulated by adding free CLPs for competing triple helix formation, which endowed the hydrogel with potential to detect physicochemical signals for tissue formation.
Conclusions
Collagen-like peptide-based biomaterials have emerged as a new class of biomaterials that possesses unique structure and properties. Abundant research has been conducted in the past few decades in deciphering the role of different amino acid residues and tripeptide motifs in stabilizing the collagen triple helix, as well as elucidating intermolecular interactions and other factors affecting collagen fibrogenesis. A variety of artificial collagen-like peptides with well-defined sequences have been designed to create higher order assemblies with specific biological functions. However, compared with polymer conjugates with α-helix and β-sheet peptides that have already been used in clinical trials, synthetic conjugates of polymers with collagen-like peptides are relatively unexplored. Considering the critical role of collagen in extracellular matrix (ECM) biology, collagen-like peptides and their bioconjugates may eventually become an indispensable component in artificial tissue scaffolds and targeted drug delivery systems.
Highlights.
Physical and chemical basis for collagen triple helix stabilization
Higher-order assembly of collagen like peptides
Self-assembly of CLP-polymer conjugates for drug delivery
CLP containing hydrogels for tissue engineering
Acknowledgments
Related work in the authors’ laboratories has been funded in part by the National Science Foundation (DMR 0907478 to KLK) and the National Center for Research Resources (NCRR), a component of the National Institutes of Health (P30-RR031160 for instrument resources). This work was also supported in part by the Center for Neutron Science at UD under award (U.S. Department of Commerce) #70NANB7H6178. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR, NIH, or Department of Commerce.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shoulders MD, Raines RT. Annu Rev Biochem. Palo Alto: Annual Reviews; 2009. Collagen Structure and Stability; pp. 929–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci. 2007;120(12):1955–1958. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran GN, Kartha G. STRUCTURE OF COLLAGEN. Nature. 1955;176(4482):593–595. doi: 10.1038/176593a0. [DOI] [PubMed] [Google Scholar]
- 4.von Arx T, Buser D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: a clinical study with 42 patients. Clin Oral Implant Res. 2006;17(4):359–366. doi: 10.1111/j.1600-0501.2005.01234.x. [DOI] [PubMed] [Google Scholar]
- 5.Haslik W, Kamolz LP, Nathschlager G, Andel H, Meissl G, Frey M. First experiences with the collagen-elastin matrix Matriderm((R)) as a dermal substitute in severe burn injuries of the hand. Burns. 2007;33(3):364–368. doi: 10.1016/j.burns.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Nemoto T, Horiuchi M, Ishiguro N, Shinagawa M. Detection methods of possible prion contaminants in collagen and gelatin. Arch Virol. 1999;144(1):177–184. doi: 10.1007/s007050050494. [DOI] [PubMed] [Google Scholar]
- 7.Fallas JA, Dong JH, Tao YZJ, Hartgerink JD. Structural Insights into Charge Pair Interactions in Triple Helical Collagen-like Proteins. J Biol Chem. 2012;287(11):8039–8047. doi: 10.1074/jbc.M111.296574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persikov AV, Ramshaw JAM, Brodsky B. Prediction of collagen stability from amino acid sequence. J Biol Chem. 2005;280(19):19343–19349. doi: 10.1074/jbc.M501657200. [DOI] [PubMed] [Google Scholar]
- 9.Boudko SP, Engel J. Structure formation in the C terminus of type III collagen guides disulfide cross-linking. J Mol Biol. 2004;335(5):1289–1297. doi: 10.1016/j.jmb.2003.11.054. [DOI] [PubMed] [Google Scholar]
- 10.Barth D, Kyrieleis O, Frank S, Renner C, Moroder L. The role of cystine knots in collagen folding and stability, part II. Conformational properties of (Pro-Hyp-Gly)(n) model trimers with N- and C-terminal collagen type III cystine knots. Chem-Eur J. 2003;9(15):3703–3714. doi: 10.1002/chem.200304918. [DOI] [PubMed] [Google Scholar]
- 11.Yang W, Chan VC, Kirkpatrick A, Ramshaw JAM, Brodsky B. Gly-Pro-Arg confers stability similar to Gly-Pro-Hyp in the collagen triple-helix of host-guest peptides. J Biol Chem. 1997;272(46):28837–28840. doi: 10.1074/jbc.272.46.28837. [DOI] [PubMed] [Google Scholar]
- 12.Pires MM, Lee J, Ernenwein D, Chmielewski J. Controlling the Morphology of Metal-Promoted Higher Ordered Assemblies of Collagen Peptides with Varied Core Lengths. Langmuir. 2012;28(4):1993–1997. doi: 10.1021/la203848r. [DOI] [PubMed] [Google Scholar]
- 13.Pires MM, Przybyla DE, Perez CMR, Chmielewski J. Metal-Mediated Tandem Coassembly of Collagen Peptides into Banded Microstructures. J Am Chem Soc. 2011;133(37):14469–14471. doi: 10.1021/ja2042645. [DOI] [PubMed] [Google Scholar]
- 14.O’Leary LER, Fallas JA, Bakota EL, Kang MK, Hartgerink JD. Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat Chem. 2011;3(10):821–828. doi: 10.1038/nchem.1123. [DOI] [PubMed] [Google Scholar]
- 15.Przybyla DE, Chmielewski J. Metal-triggered radial self-assembly of collagen peptide fibers. J Am Chem Soc. 2008;130(38):12610. doi: 10.1021/ja804942w. [DOI] [PubMed] [Google Scholar]
- 16.Krishna OD, Kiick KL. Supramolecular Assembly of Electrostatically Stabilized, Hydroxyproline-Lacking Collagen-Mimetic Peptides. Biomacromolecules. 2009;10(9):262–2631. doi: 10.1021/bm900551c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathfon JM, Tew GN. Synthesis of thermoresponsive poly (N-isopropylmethacrylamide) and poly(acrylic acid) block copolymers via post-functionalization of poly(N-methacryloxysuccinimide) Polymer. 2008;49(7):1761–1769. [Google Scholar]
- 18.McCormick CL, Sumerlin BS, Lokitz BS, Stempka JE. RAFT-synthesized diblock and triblock copolymers: thermally-induced supramolecular assembly in aqueous media. Soft Matter. 2008;4(9):1760–1773. [Google Scholar]
- 19.Shunmugam R, Tew GN. Efficient route to well-characterized homo, block, and statistical polymers containing terpyridine in the side chain. J Polym Sci Pol Chem. 2005;43(23):5831–5843. [Google Scholar]
- 20.Lutz JF, Borner HG. Modern trends in polymer bioconjugates design. Prog Polym Sci. 2008;33(1):1–39. [Google Scholar]
- 21.Krishna OD, Kiick KL. Protein- and Peptide-Modified Synthetic Polymeric Biomaterials. Biopolymers. 2010;94(1):32–48. doi: 10.1002/bip.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothenfluh DA, Bermudez H, O’Neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7(3):248–254. doi: 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- 23.Osada K, Kataoka K. Drug and gene delivery based on supramolecular assembly of PEG-polypeptide hybrid block copolymers. In: Klok HA, Schlaad H, editors. Peptide Hybrid Polymers. Berlin: Springer-Verlag Berlin; 2006. pp. 113–153. [Google Scholar]
- 24.Mitra A, Nan A, Papadimitriou JC, Ghandehari H, Line BR. Polymer-peptide conjugates for angiogenesis targeted tumor radiotherapy. Nucl Med Biol. 2006;33(1):43–52. doi: 10.1016/j.nucmedbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Ding JX, Xiao CS, Tang ZH, Zhuang XL, Chen XS. Highly Efficient “Grafting From” an alpha-Helical Polypeptide Backbone by Atom Transfer Radical Polymerization. Macromol Biosci. 2011;11(2):192–198. doi: 10.1002/mabi.201000238. [DOI] [PubMed] [Google Scholar]
- 26.Wigenius J, Bjork P, Hamedi M, Aili D. Supramolecular Assembly of Designed alpha-Helical Polypeptide-Based Nanostructures and Luminescent Conjugated Polyelectrolytes. Macromol Biosci. 2010;10(8):836–841. doi: 10.1002/mabi.200900463. [DOI] [PubMed] [Google Scholar]
- 27.Shu JY, Tan C, DeGrado WF, Xu T. New design of helix bundle peptide-polymer conjugates. Biomacromolecules. 2008;9(8):2111–2117. doi: 10.1021/bm800113g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahin E, Kiick KL. Macromolecule-Induced Assembly of Coiled-Coils in Alternating Multiblock Polymers. Biomacromolecules. 2009;10(10):2740–2749. doi: 10.1021/bm900474k. [DOI] [PubMed] [Google Scholar]
- 29.Kopecek J, Yang JY. Self-assembly of hybrid copolymers grafted with beta-sheet and alpha-helix peptides. Abstr Pap Am Chem Soc. 2011:241. [Google Scholar]
- 30.Elder AN, Dangelo NM, Kim SC, Washburn NR. Conjugation of beta-Sheet Peptides to Modify the Rheological Properties of Hyaluronic Acid. Biomacromolecules. 2011;12(7):2610–2616. doi: 10.1021/bm200393k. [DOI] [PubMed] [Google Scholar]
- 31.Lee OS, Liu YM, Schatz GC. Molecular dynamics simulation of beta-sheet formation in self-assembled peptide amphiphile fibers. J Nanopart Res. 2012;14(8) [Google Scholar]
- 32.Aluri S, Pastuszka MK, Moses AS, MacKay JA. Elastin-Like Peptide Amphiphiles Form Nanofibers with Tunable Length. Biomacromolecules. 2012;13(9):2645–2654. doi: 10.1021/bm300472y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grieshaber SE, Farran AJE, Lin-Gibson S, Kiick KL, Jia XQ. Synthesis and Characterization of Elastin-Mimetic Hybrid Polymers with Multiblock, Alternating Molecular Architecture and Elastomeric Properties. Macromolecules. 2009;42(7):2532–2541. doi: 10.1021/ma802791z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grieshaber SE, Farran AJE, Bai S, Kiick KL, Jia XQ. Tuning the Properties of Elastin Mimetic Hybrid Copolymers via a Modular Polymerization Method. Biomacromolecules. 2012;13(6):1774–1786. doi: 10.1021/bm3002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu YC, Berndt P, Tirrell M, Fields GB. Self-assembling amphiphiles for construction of protein molecular architecture. J Am Chem Soc. 1996;118(50):12515–12520. [Google Scholar]
- 36.Gore T, Dori Y, Talmon Y, Tirrell M, Bianco-Peled H. Self-assembly of model collagen peptide amphiphiles. Langmuir. 2001;17(17):5352–5360. [Google Scholar]
- 37.Luo JN, Tong YW. Self-Assembly of Collagen-Mimetic Peptide Amphiphiles into Biofunctional Nanofiber. Acs Nano. 2011;5(10):7739–7747. doi: 10.1021/nn202822f. [DOI] [PubMed] [Google Scholar]
- 38.Krishna OD, Wiss KT, Luo TZ, Pochan DJ, Theato P, Kiick KL. Morphological transformations in a dually thermoresponsive coil-rod-coil bioconjugate. Soft Matter. 2012;8(14):3832–3840. doi: 10.1039/C2SM07025A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persikov AV, Ramshaw JAM, Kirkpatrick A, Brodsky B. Amino acid propensities for the collagen triple-helix. Biochemistry-Us. 2000;39(48):14960–14967. doi: 10.1021/bi001560d. [DOI] [PubMed] [Google Scholar]
- 40.Ramshaw JAM, Shah NK, Brodsky B. Gly-X-Y tripeptide frequencies in collagen: A context for host-guest triple-helical peptides. J Struct Biol. 1998;122(1–2):86–91. doi: 10.1006/jsbi.1998.3977. [DOI] [PubMed] [Google Scholar]
- 41.Jones EY, Miller A. ANALYSIS OF STRUCTURAL DESIGN-FEATURES IN COLLAGEN. J Mol Biol. 1991;218(1):209–219. doi: 10.1016/0022-2836(91)90885-a. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki E, Fraser RDB, Macrae TP. ROLE OF HYDROXYPROLINE IN THE STABILIZATION OF THE COLLAGEN MOLECULE VIA WATER-MOLECULES. Int J Biol Macromol. 1980;2(1):54–56. [Google Scholar]
- 43.Traub W. SOME STEREOCHEMICAL IMPLICATIONS OF MOLECULAR-CONFORMATION OF COLLAGEN. Isr J Chem. 1974;12(1–2):435–439. [Google Scholar]
- 44.Ramachan Gn, Bansal M, Bhatnaga Rs. HYPOTHESIS ON ROLE OF HYDROXYPROLINE IN STABILIZING COLLAGEN STRUCTURE. Biochimica Et Biophysica Acta. 1973;322(1):166–171. doi: 10.1016/0005-2795(73)90187-6. [DOI] [PubMed] [Google Scholar]
- 45.Bansal M, Ramakrishnan C, Ramachandran GN. STABILIZATION OF COLLAGEN STRUCTURE BY HYDROXYPROLINE RESIDUES. Proceedings of the Indian Academy of Sciences Section A. 1975;82(4):152–164. [Google Scholar]
- 46.Holmgren SK, Taylor KM, Bretscher LE, Raines RT. Code for collagen’s stability deciphered. Nature. 1998;392(6677):666–667. doi: 10.1038/33573. [DOI] [PubMed] [Google Scholar]
- 47.Holmgren SK, Bretscher LE, Taylor KM, Raines RT. A hyperstable collagen mimic. Chem Biol. 1999;6(2):63–70. doi: 10.1016/S1074-5521(99)80003-9. [DOI] [PubMed] [Google Scholar]
- 48.Dunitz JD, Taylor R. Organic fluorine hardly ever accepts hydrogen bonds. Chem-Eur J. 1997;3(1):89–98. [Google Scholar]
- 49.Eberhardt ES, Panasik N, Raines RT. Inductive effects on the energetics of prolyl peptide bond isomerization: Implications for collagen folding and stability. J Am Chem Soc. 1996;118(49):12261–12266. doi: 10.1021/ja9623119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inouye K, Sakakibara S, Prockop DJ. EFFECTS OF STEREO-CONFIGURATION OF HYDROXYL GROUP IN 4-HYDROXYPROLINE ON TRIPLE-HELICAL STRUCTURES FORMED BY HOMOGENEOUS PEPTIDES RESEMBLING COLLAGEN. Biochimica Et Biophysica Acta. 1976;420(1):133–141. doi: 10.1016/0005-2795(76)90352-4. [DOI] [PubMed] [Google Scholar]
- 51.Bretscher LE, Jenkins CL, Taylor KM, DeRider ML, Raines RT. Conformational stability of collagen relies on a stereoelectronic effect. J Am Chem Soc. 2001;123(4):777–778. doi: 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]
- 52.DeRider ML, Wilkens SJ, Waddell MJ, Bretscher LE, Weinhold F, Raines RT, et al. Collagen stability: Insights from NMR spectroscopic and hybrid density functional computational investigations of the effect of electronegative substituents on prolyl ring conformations. J Am Chem Soc. 2002;124(11):2497–2505. doi: 10.1021/ja0166904. [DOI] [PubMed] [Google Scholar]
- 53.Shoulders MD, Guzei IA, Raines RT. 4-chloroprolines: Synthesis, conformational analysis, and effect on the collagen triple helix. Biopolymers. 2008;89(5):443–454. doi: 10.1002/bip.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kusebauch U, Cadamuro SA, Musiol HJ, Lenz MO, Wachtveitl J, Moroder L, et al. Photocontrolled folding and unfolding of a collagen triple helix. Angew Chem-Int Edit. 2006;45(42):7015–7018. doi: 10.1002/anie.200601432. [DOI] [PubMed] [Google Scholar]
- 55.Persikov AV, Ramshaw JAM, Kirkpatrick A, Brodsky B. Peptide investigations of pairwise interactions in the collagen triple-helix. J Mol Biol. 2002;316(2):385–394. doi: 10.1006/jmbi.2001.5342. [DOI] [PubMed] [Google Scholar]
- 56.Kramer RZ, Venugopal MG, Bella J, Mayville P, Brodsky B, Berman HM. Staggered molecular packing in crystals of a collagen-like peptide with a single charged pair. J Mol Biol. 2000;301(5):1191–1205. doi: 10.1006/jmbi.2000.4017. [DOI] [PubMed] [Google Scholar]
- 57.Persikov AV, Ramshaw JAM, Kirkpatrick A, Brodsky B. Electrostatic interactions involving lysine make major contributions to collagen triple-helix stability. Biochemistry-Us. 2005;44(5):1414–1422. doi: 10.1021/bi048216r. [DOI] [PubMed] [Google Scholar]
- 58.Fallas JA, O’Leary LER, Hartgerink JD. Synthetic collagen mimics: self-assembly of homotrimers, heterotrimers and higher order structures. Chem Soc Rev. 2010;39(9):3510–3527. doi: 10.1039/b919455j. [DOI] [PubMed] [Google Scholar]
- 59.Chung E, Keele EM, Miller EJ. ISOLATION AND CHARACTERIZATION OF CYANOGEN-BROMIDE PEPTIDES FROM ALPHA 1 (III) CHAIN OF HUMAN COLLAGEN. Biochemistry-Us. 1974;13(17):3459–3464. doi: 10.1021/bi00714a006. [DOI] [PubMed] [Google Scholar]
- 60.Bachinger HP, Bruckner P, Timpl R, Prockop DJ, Engel J. FOLDING MECHANISM OF THE TRIPLE HELIX IN TYPE-III COLAGEN AND TYPE-III PN-COLLAGEN - ROLE OF DISULFIDE BRIDGES AND PEPTIDE-BOND ISOMERIZATION. Eur J Biochem. 1980;106(2):619–632. doi: 10.1111/j.1432-1033.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- 61.Mechling DE, Bachinger HP. The collagen-like peptide (GER)(15)GPCCG forms pH-dependent covalently linked triple helical trimers. J Biol Chem. 2000;275(19):14532–14536. doi: 10.1074/jbc.275.19.14532. [DOI] [PubMed] [Google Scholar]
- 62.Feng YB, Melacini G, Taulane JP, Goodman M. Acetyl-terminated and template-assembled collagen-based polypeptides composed of Gly-Pro-Hyp sequences .2. Synthesis and conformational analysis by circular dichroism, ultraviolet absorbance, and optical rotation. J Am Chem Soc. 1996;118(43):10351–10358. [Google Scholar]
- 63.Kwak J, De Capua A, Locardi E, Goodman M. TREN (tris(2-aminoethyl)amine): An effective scaffold for the assembly of triple helical collagen mimetic structures. J Am Chem Soc. 2002;124(47):14085–14091. doi: 10.1021/ja0209621. [DOI] [PubMed] [Google Scholar]
- 64.Frank S, Kammerer RA, Mechling D, Schulthess T, Landwehr R, Bann J, et al. Stabilization of short collagen-like triple helices by protein engineering. J Mol Biol. 2001;308(5):1081–1089. doi: 10.1006/jmbi.2001.4644. [DOI] [PubMed] [Google Scholar]
- 65.Fields GB, Lauer JL, Dori Y, Forns P, Yu YC, Tirrell M. Proteinlike molecular architecture: Biomaterial applications for inducing cellular receptor binding and signal transduction. Biopolymers. 1998;47(2):143–151. doi: 10.1002/(SICI)1097-0282(1998)47:2<143::AID-BIP3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 66.Wiss KT, Krishna OD, Roth PJ, Kiick KL, Theato P. A Versatile Grafting-to Approach for the Bioconjugation of Polymers to Collagen-like Peptides Using an Activated Ester Chain Transfer Agent. Macromolecules. 2009;42(12):3860–3863. [Google Scholar]
- 67.Kar K, Amin P, Bryan MA, Persikov AV, Mohs A, Wang YH, et al. Self-association of collagen triple helix peptides into higher order structures. J Biol Chem. 2006;281(44):33283–33290. doi: 10.1074/jbc.M605747200. [DOI] [PubMed] [Google Scholar]
- 68.Cejas MA, Kinnney WA, Chen C, Vinter JG, Almond HR, Balss KM, et al. Thrombogenic collagen-mimetic peptides: Self-assembly of triple helix-based fibrils driven by hydrophobic interactions. P Natl Acad Sci USA. 2008;105(25):8513–8518. doi: 10.1073/pnas.0800291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cejas MA, Kinney WA, Chen C, Leo GC, Tounge BA, Vinter JG, et al. Collagen-related peptides: Self-assembly of short, single strands into a functional biomaterial of micrometer scale. J Am Chem Soc. 2007;129(8):2202. doi: 10.1021/ja066986f. [DOI] [PubMed] [Google Scholar]
- 70.Kar K, Ibrar S, Nanda V, Getz TM, Kunapuli SP, Brodsky B. Aromatic Interactions Promote Self-Association of Collagen Triple-Helical Peptides to Higher-Order Structures. Biochemistry-Us. 2009;48(33):7959–7968. doi: 10.1021/bi900496m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen CC, Hsu W, Kao TC, Horng JC. Self-Assembly of Short Collagen-Related Peptides into Fibrils via Cation-pi Interactions. Biochemistry-Us. 2011;50(13):2381–2383. doi: 10.1021/bi1018573. [DOI] [PubMed] [Google Scholar]
- 72.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316:1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rele S, Song YH, Apkarian RP, Qu Z, Conticello VP, Chaikof EL. D-periodic collagen-mimetic microfibers. J Am Chem Soc. 2007;129(47):14780–14787. doi: 10.1021/ja0758990. [DOI] [PubMed] [Google Scholar]
- 74.Hsu W, Chen YL, Horng JC. Promoting Self-Assembly of Collagen-Related Peptides into Various Higher-Order Structures by Metal-Histidine Coordination. Langmuir. 2012;28(6):3194–3199. doi: 10.1021/la204351w. [DOI] [PubMed] [Google Scholar]
- 75.Rabotyagova OS, Cebe P, Kaplan DL. Protein-Based Block Copolymers. Biomacromolecules. 2011;12(2):269–289. doi: 10.1021/bm100928x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krishna OD, Jha AK, Jia XQ, Kiick KL. Integrin-mediated adhesion and proliferation of human MSCs elicited by a hydroxyproline-lacking, collagen-like peptide. Biomaterials. 2011;32(27):6412–6424. doi: 10.1016/j.biomaterials.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Z, Lai YX, Yu L, Ding JD. Effects of immobilizing sites of RGD peptides in amphiphilic block copolymers on efficacy of cell adhesion. Biomaterials. 2010;31(31):7873–7882. doi: 10.1016/j.biomaterials.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 78.Humtsoe JO, Kim JK, Xu Y, Keene DR, Hook M, Lukomski S, et al. A streptococcal collagen-like protein interacts with the alpha(2)beta(1) integrin and induces intracellular signaling. J Biol Chem. 2005;280(14):13848–13857. doi: 10.1074/jbc.M410605200. [DOI] [PubMed] [Google Scholar]
- 79.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 80.Klok HA. Biological-synthetic hybrid block copolymers: Combining the best from two worlds. J Polym Sci Pol Chem. 2005;43(1):1–17. [Google Scholar]
- 81.Pechar M, Brus J, Kostka L, Konak C, Urbanova M, Slouf M. Thermoresponsive self-assembly of short elastin-like polypentapeptides and their poly(ethylene glycol) derivatives. Macromol Biosci. 2007;7(1):56–69. doi: 10.1002/mabi.200600196. [DOI] [PubMed] [Google Scholar]
- 82.Vandermeulen GWM, Tziatzios C, Klok HA. Reversible self-organization of poly(ethylene glycol)-based hybrid block copolymers mediated by a De Novo four-stranded alpha-helical coiled coil motif. Macromolecules. 2003;36(11):4107–4114. [Google Scholar]
- 83.Rosler A, Klok HA, Hamley IW, Castelletto V, Mykhaylyk OO. Nanoscale structure of poly(ethylene glycol) hybrid block copolymers containing amphiphilic beta-strand peptide sequences. Biomacromolecules. 2003;4(4):859–863. doi: 10.1021/bm034058s. [DOI] [PubMed] [Google Scholar]
- 84.Nilsson BL, Kiessling LL, Raines RT. High-yielding Staudinger ligation of a phosphinothioester and azide to form a peptide. Org Lett. 2001;3(1):9–12. doi: 10.1021/ol006739v. [DOI] [PubMed] [Google Scholar]
- 85.Top A, Roberts CJ, Kiick KL. Conformational and Aggregation Properties of a PEGylated Alanine-Rich Polypeptide. Biomacromolecules. 2011;12(6):2184–2192. doi: 10.1021/bm200272w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Top A. Controlling assembly of helical peptides via PEGylation strategies. Soft Matter. 2011 doi: 10.1039/C1SM05686G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv Drug Deliver Rev. 2002;54(4):459–476. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 88.Kinstler O, Molineux G, Treuheit M, Ladd D, Gegg C. Mono-N-terminal poly(ethylene glycol)-protein conjugates. Adv Drug Deliver Rev. 2002;54(4):477–485. doi: 10.1016/s0169-409x(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 89.Huisgen R. 1.3-DIPOLARE CYCLOADDITIONEN - RUCKSCHAU UND AUSBLICK. Angew Chem-Int Edit. 1963;75(13):604. [Google Scholar]
- 90.Tornoe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: 1,2,3 -triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67(9):3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 91.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem-Int Edit. 2002;41(14):2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 92.Dirks AJT, van Berkel SS, Hatzakis NS, Opsteen JA, van Delft FL, Cornelissen J, et al. Preparation of biohybrid amphiphiles via the copper catalysed Huisgen 3+2 dipolar cycloaddition reaction. Chem Commun. 2005;(33):4172–4174. doi: 10.1039/b508428h. [DOI] [PubMed] [Google Scholar]
- 93.Lowe AB. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym Chem. 2010;1(1):17–36. [Google Scholar]
- 94.Mather BD, Viswanathan K, Miller KM, Long TE. Michael addition reactions in macromolecular design for emerging technologies. Prog Polym Sci. 2006;31(5):487–531. [Google Scholar]
- 95.Friedman M, Cavins JF, Wall JS. RELATIVE NUCLEOPHILIC REACTIVITIES OF AMINO GROUPS AND MERCAPTIDE IONS IN ADDITION REACTIONS WITH ALPHA BETA-UNSATURATED COMPOUNDS. J Am Chem Soc. 1965;87(16):3672. [Google Scholar]
- 96.Baldwin AD, Kiick KL. Tunable Degradation of Maleimide-Thiol Adducts in Reducing Environments. Bioconjugate Chem. 2011;22(10):1946–1953. doi: 10.1021/bc200148v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carlsen A, Lecommandoux S. Self-assembly of polypeptide-based block copolymer amphiphiles. Curr Opin Colloid Interface Sci. 2009;14(5):329–339. [Google Scholar]
- 98.Chow D. Peptide-based biopolymers in biomedicine and biotechnology. 2008 doi: 10.1016/j.mser.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y, Foss CA, Summerfield DD, Doyle JJ, Torok CM, Dietz HC, et al. Targeting collagen strands by photo-triggered triple-helix hybridization. P Natl Acad Sci USA. 2012;109(37):14767–14772. doi: 10.1073/pnas.1209721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mo X, An YJ, Yun CS, Yu SM. Nanoparticle-assisted visualization of binding interactions between collagen mimetic peptide and collagen fibers. Angew Chem-Int Edit. 2006;45(14):2267–2270. doi: 10.1002/anie.200504529. [DOI] [PubMed] [Google Scholar]
- 101.Wang AY, Mo X, Chen CS, Yu SM. Facile modification of collagen directed by collagen mimetic peptides. J Am Chem Soc. 2005;127(12):4130–4131. doi: 10.1021/ja0431915. [DOI] [PubMed] [Google Scholar]
- 102.Wang AY, Leong S, Liang YC, Huang RCC, Chen CS, Yu SM. Immobilization of Growth Factors on Collagen Scaffolds Mediated by Polyanionic Collagen Mimetic Peptides and Its Effect on Endothelial Cell Morphogenesis. Biomacromolecules. 2008;9(10):2929–2936. doi: 10.1021/bm800727z. [DOI] [PubMed] [Google Scholar]
- 103.Stahl PJ, Yu SM. Encoding cell-instructive cues to PEG-based hydrogels via triple helical peptide assembly. Soft Matter. 2012;8(40):10409–10418. doi: 10.1039/C2SM25903F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Callahan LAS, Ganios AM, McBurney DL, Dilisio MF, Weiner SD, Horton WE, et al. ECM Production of Primary Human and Bovine Chondrocytes in Hybrid PEG Hydrogels Containing Type I Collagen and Hyaluronic Acid. Biomacromolecules. 2012;13(5):1625–1631. doi: 10.1021/bm3003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rafat M, Li FF, Fagerholm P, Lagali NS, Watsky MA, Munger R, et al. PEG-stabilized carbodiimide crosslinked collagen-chitosan hydrogels for corneal tissue engineering. Biomaterials. 2008;29(29):3960–3972. doi: 10.1016/j.biomaterials.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 106.Lee HJ, Yu C, Chansakul T, Hwang NS, Varghese S, Yu SM, et al. Enhanced Chondrogenesis of Mesenchymal Stem Cells in Collagen Mimetic Peptide-Mediated Microenvironment. Tissue Eng Part A. 2008;14(11):1843–1851. doi: 10.1089/ten.tea.2007.0204. [DOI] [PubMed] [Google Scholar]
- 107.Lu HX, Kawazoe N, Kitajima T, Myoken Y, Tomita M, Umezawa A, et al. Spatial immobilization of bone morphogenetic protein-4 in a collagen-PLGA hybrid scaffold for enhanced osteoinductivity. Biomaterials. 2012;33(26):6140–6146. doi: 10.1016/j.biomaterials.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 108.Dai WD, Kawazoe N, Lin XT, Dong J, Chen GP. The influence of structural design of PLGA/collagen hybrid scaffolds in cartilage tissue engineering. Biomaterials. 2010;31(8):2141–2152. doi: 10.1016/j.biomaterials.2009.11.070. [DOI] [PubMed] [Google Scholar]
- 109.Burrows MC, Zamarion VM, Filippin-Monteiro FB, Schuck DC, Toma HE, Campa A, et al. Hybrid Scaffolds Built From PET and Collagen as a Model For Vascular Graft Architecture. Macromol Biosci. 2012;12(12):1660–1670. doi: 10.1002/mabi.201200154. [DOI] [PubMed] [Google Scholar]
- 110.Kim HJ, Kim KK, Park IK, Choi BS, Kim JH, Kim MS. Hybrid Scaffolds Composed of Hyaluronic Acid and Collagen for Cartilage Regeneration. Tissue Eng Regen Med. 2012;9(2):57–62. [Google Scholar]
- 111.Lin YC, Tan FJ, Marra KG, Jan SS, Liu DC. Synthesis and characterization of collagen/hyaluronan/chitosan composite sponges for potential biomedical applications. Acta Biomater. 2009;5(7):2591–2600. doi: 10.1016/j.actbio.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 112.Fu SZ, Ni PY, Wang BY, Chu BY, Zheng L, Luo F, et al. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials. 2012;33(19):4801–4809. doi: 10.1016/j.biomaterials.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 113.Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials. 2006;27(30):5268–5276. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 114.Stahl PJ, Romano NH, Wirtz D, Yu SM. PEG-Based Hydrogels with Collagen Mimetic Peptide-Mediated and Tunable Physical Cross-Links. Biomacromolecules. 2010;11(9):2336–2344. doi: 10.1021/bm100465q. [DOI] [PMC free article] [PubMed] [Google Scholar]