Abstract
In insulin dependent diabetes mellitus (T1D), self-reactive T cells infiltrate pancreatic islets and induce beta cell destruction and dysregulation of blood glucose. A goal is to control only the self-reactive T cells, leaving the remainder of the T cell population free to protect the host. One approach is blockade of the second signal for T cell activation while allowing the first (antigen-specific) signal to occur. This work proposes that small peptides that block interaction of second signals delivered through the counter receptors LFA-1:ICAM-1 will induce attacking T cells (receiving the antigen signal) to become anergic or undergo apoptosis. In NOD mice, the peptides eliminated T cell reactivity against pancreatic antigens and reduced cellular infiltration into islets, which retained stronger density of insulin staining at five weeks after cessation of therapy. In in vitro studies the peptides induced nonresponsiveness during activation of T cells from mice and from human peripheral blood.
Keywords: Peptide costimulatory blockade, Diabetes, Autoimmunity, T cell tolerance, ICAM-1:LFA-1, NOD mice
1. Introduction
The autoimmune disease, type I diabetes (T1D), is driven by self reactive T cells that infiltrate the pancreatic islets of Langerhans, and induce destruction of beta cells and loss of insulin production. This gradually renders the pancreas unable to control blood glucose levels [1]. A long sought approach to therapy of autoimmune disease has been to restore self-tolerance by inducing death or anergy in the T cells that are attacking the target organ while leaving the remainder of the uninvolved T cells free to function in defense of the organism. In T1D, it would be optimal if restoration of tolerance and cessation of attack on the beta cells could be effected while a percentage of beta cells remain so that the patient could retain some insulin producing activity. These goals are the subject of the present manuscript.
1.1. Costimulation blockade
One approach for tolerance induction is based on the longstanding concept of second signal blockade. The concept of the two signal requirement for T cell activation was first proposed by Kevin Lafferty and coworkers [2,3] in 1975, and has been under intensive study since. Engagement of the T cell antigen receptor (TCR) by appropriately presented cognate antigen (signal 1) provides specificity of interaction, activates the T cell and prepares it to respond [reviewed in 4,5]. Signal 1 also prepares the cell to become anergic or die in the absence of the second signal, which would normally rescue the cell from induced anergy/death. So second signal blockade can lead to inactivation of attacking T cells. The most studied second signal is received by resident CD28, although several additional T cell surface proteins have been classified as receivers for a second signal [rev, 5]. This includes both of the T cell surface proteins targeted in the present study, ICAM-1 (intercellular adhesion molecule-1) [6] and LFA-1 (leukocyte function associated antigen-1) [7].
Second signal blockade has been employed in association with diabetes and most studies have used antibody to effect the blockade. Examples include blockade of the T cell costimulatory molecules CD28/B7 or CD40/CD40L [8–11] to treat T1D or assist in islet graft acceptance in NOD mice. Also, over nearly two decades, antibody or full protein blockade of LFA-1 and ICAM-1 interactions has shown promise for treating T1D by tolerance induction [12–14] but the promise has not been fully realized. Because second signal blockade has shown such promise in animal models, it seems imperative to continue to explore this approach from different angles to elucidate the factors that might promote success in humans.
1.2. Peptide blockade of LFA-1:ICAM-1 interaction
The present work uses short peptides to block interaction of the counter receptor pair LFA-1 and ICAM-1 in NOD mice, as a therapeutic approach for T1D. LFA-1is a β2 integrin expressed on leukocytes and ICAM-1 is a member of the Ig superfamily and is expressed on many different cell types including leukocytes, endothelial and epithelial cells [15,16]. Both LFA-1 and ICAM-1 are resident on the T cell surface, and both are known to have costimulatory function that can receive a second signal into an activating T cell [5–7,17]. Blockade of LFA-1:ICAM-1 interaction using antibodies has been used in several autoimmune diseases and organ transplantation [12–14,18]. The peptides that we have generated were derived from contact domains in the human sequences of LFA-1 and ICAM-1; they block intercellular adhesion and inhibit human T cell function in a mixed lymphocyte reaction [19]. These human sequences are conserved in mice and work well in mouse assays in vitro.
1.3. Potential for inhibition of cell adhesion and migration
In addition to participating in T cell activation, both LFA-1 and ICAM-1 are important for adhesion, extravasation and migration of leukocytes as well as cytotoxic T cell function [rev [15,16]]. Specifically, LFA-1 on leukocytes interacts with ICAM-1 on endothelial cells lining blood vessels and this facilitates exit from the blood. Thus, a potential second mechanism of action by interfering with interaction of LFA-1:ICAM-1 is transient impairment of leukocyte migration toward the site of attack. The LFA-1:ICAM-1 interaction also plays an important role in intercellular interaction of CTLs with target cells during CTL-mediated killing [20].
1.4. Approach
Here, we explore the hypothesis that use of the LFA-1- and ICAM-1-derived peptides in treatment of T1D will induce tolerance to pancreatic autoantigens. The non-obese diabetic (NOD) mouse is widely used to study T1D [21,22]. Ability of T cells from treated animals to respond to pancreatic Ag in a recall response was determined and effects on integrity of pancreatic islets were assessed. Mechanism of action of the peptides was studied using mouse and human T cells in vitro.
2. Materials and methods
2.1. Mice and diabetes monitoring
Female NOD/ShiLtJ mice (Jackson Laboratories, Bar Harbor, ME) were obtained at 8 weeks of age and housed individually in barrier cages. Animal experiments were performed with approval from the University of Kansas Institutional Animal Care and Use Committee. Mice exhibited an average BGL (blood glucose level) of 80 mg/dl at intake; weight and BGL monitoring began at week 11, and at 13 weeks average BGL was 115 mg/dl. Mice were placed into two equal groups by matching BGL levels, and were injected i.v. with the therapeutic peptide combination (cIE-L + cLAB-L), or saline at 48 h intervals. Peptides were injected at 50 μg (each) per dose for a total of 100 μg/dose. BGL and weight assessments were recorded at the time of injection. Injections were continued for five weeks (17 injections) with the final injection on day 126 (18 weeks). BGL and weight were monitored for an additional five weeks, until day 161 (23 weeks). Weight data were unremarkable across groups throughout the experiment (not shown).
2.2. Peptides
Peptides were prepared and cyclized as we have described previously [23]. Cyclized peptides were purchased from American Peptide Company, Inc. (Sunnyvale, CA). Lyophilized peptides were stored desiccated at −20 °C until use when they were resuspended in normal saline. For i.v. injections, 50 μg c-IE-L + 50 μg cLAB-L were mixed immediately prior to use. For in vitro experiments, peptides were used at 250 μM each in cell culture medium RPMI 1640.
2.3. Monitoring of diabetes and treatments
Blood glucose level (BGL) was monitored weekly before and after treatment and on alternate days during treatment using the OneTouch Ultra 2 glucometer and test strips from LifeScan, Inc. (Milpitas, CA). Mice were fasted 2 h before each BGL reading and weight was determined on the same days as BGL monitoring. Mice were considered diabetic after two consecutive BGL readings >250 mg/dl. When mice were terminated due to severity of disease, BGL of 600 mg/dl was used thereafter in the calculations for those mice. More than five weeks after cessation of injections, mice were sacrificed and pancreata and spleens were harvested for further analysis.
2.4. Splenocyte isolation, cell culture and pancreatic lysates
Spleens were harvested, minced and repeatedly pressed through a sterile 70 μm nylon mesh cell strainer. Erythrocytes were cleared using ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2) at 37 °C. Human T cells were isolated as we have described [6,17], from blood of healthy donors by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) centrifugation followed by E-rosetting. T cell purity was verified by flow cytometric analysis. Total splenocytes and human T cells were cultured in complete RPMI 1640 (Mediatech, Herndon, VA) supplemented with 10% FBS (Atlanta Biologicals, Norcross, GA) as described [6]. Mouse cells were cultured with addition of beta mercaptoethanol. Pancreatic lysates used for antigenic stimulation of splenocytes were made from healthy NOD mice by mincing and digestion in 1.1 mg/ml Collagenase P (Roche Diagnostics, Morristown, NJ) in HBSS containing 10 mM HEPES for 15 min at 37 °C, followed by three cycles of freeze–thaw.
2.5. Cell stimulation, proliferation assay and flow cytometry
Mouse splenocytes were stimulated via plate bound 0.5 μg/ml anti-CD3ε (clone 500A2) as we have described [6,17] or with 25 μg/ml pancreatic lysates. Human T cell stimulations were similar using 1 μg/ml anti-CD3 (clone OKT3), 10 μg/ml anti-ICAM-1 (clone R6.5) and a concentration of 2 × 106 cells/ml. Cells were incubated with 250 μM of each peptide for 30 min prior to in vitro stimulation. Cell proliferation was measured with CFSE dilution as we have described [6,17]. T cells were labeled with 2.5 μM carboxyfluorescein succinimidyl ester (CFSE) for 7 min at 37 °C in serum-free RPMI 1640. Cells were labeled for flow cytometry according to the appropriate manufacturer's instructions for the antibody or reagent used. For splenocyte surface protein staining, cells were incubated with anti-CD4-PE (clone RM4-5) and anti-CD8-PE-Cy5 (clone 53-6.7) from BD Pharmingen. Human T cells were stained with anti-CD4-APC (clone OKT4) purchased from BioLegend, Inc. (San Diego, CA). Flow cytometry was performed using the Accuri C6 Flow Cytometer and data were analyzed with CFlow (Accuri Cytometers, Inc., Ann Arbor, MI).
2.6. Pancreas imaging
Pancreata were harvested and frozen for sectioning. Planar slices (10 μm) were stained with hematoxylin and eosin (H&E), and photographed at 20× using a Zeiss Axioscop photomicroscope (Carl Zeiss MicroImaging, LLC, Thornwood, NY). Infiltration was scored in the traditional manner [1,21]. Islets were scored as pre-insulitis when no infiltration was observed, peri-insulitis when infiltration had begun with peripherally observed immune cells, intra-insular insulitis when immune cells had clearly infiltrated the islet and complete islet destruction when the islet appeared totally infiltrated by immune cells.
Insulin immunohistochemistry was conducted using anti-insulin Ab (Santa Cruz Biotechnology, Santa Cruz, CA) and a Histostain-Plus Kit (Invitrogen, Frederick, MD) as we have published previously [24,25]. Slides were counterstained with hematoxylin to identify cell nuclei. For fluorescence imaging, samples were prepared and stained according to published procedures [24,25]. Images were captured on a Nikon C1Si or C1 Plus confocal microscope and analyzed using EZ-C1 3.90 Free viewer or Adobe Photoshop CS5 software. We estimated the cellular composition of islets by counting the individual types of cells [α cells (anti-glucagon, Abcam, Cambridge, MA), β cells (anti-insulin, Abcam or Santa Cruz Biotechnology), or δ cells (anti-somatostatin, Abcam)] in each islet, and dividing each by the total number of endocrine cells per islet.
2.7. Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA.) or SigmaStat (Systat Software Inc. San Jose, CA). Statistical tests and significance for individual figures are indicated in figure legends.
3. Results
3.1. Peptides and diabetes
Others have used peptides designed from ICAM-1 to cause inhibition of LFA-1/ICAM-1 interaction [26,27]. Our group designed a series of peptides [17,19,23] from contact domains of human ICAM-1 and LFA-1. These peptides inhibited the human T cell response in a mixed lymphocyte reaction (MLR) in a dose dependent manner, were not agonistic in a T cell activation response, and were not toxic to the cells. For use here, peptides were synthesized in a cyclic form [23]. cIE-L (DQPKLLGIET) was derived from the first Ig domain of ICAM-1; cLAB-L (ITDGEATDSG) was from the I-domain of the LFA-1 α-subunit (CD11a).
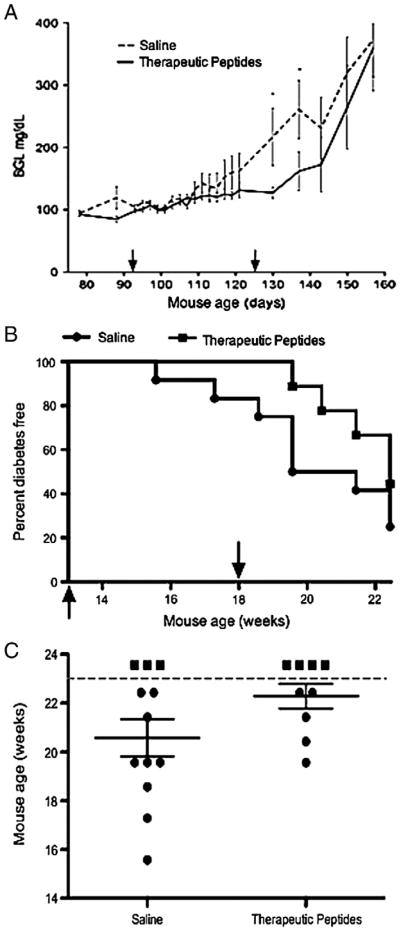
NOD mice were injected on alternate days for five weeks with saline or the therapeutic peptide combination beginning in week 13 (91 days) of life and ending in week 18 after 17 doses had been administered. Blood glucose level (BGL) was monitored weekly (and on injection days) from 11 weeks of age until 23 weeks when the mice were harvested.
The experiment showed a trend toward slight delay of diabetes onset in therapeutically treated mice. Mice receiving saline exhibited a sharp increase in the rate of BGL elevation beginning at 17 weeks, just before cessation of injections (Fig. 1A) whereas treated mice appeared to experience a slightly later onset. However, the difference between the two curves was not statistically significant (p = 0.06) over two weeks and the data only suggest a trend of slight delay. Elevated BGL was accompanied by onset of diabetes (Fig. 1B). The mice receiving therapeutic peptides showed a trend suggesting only a barely modest delay in onset of diabetes until 20 weeks (140 days), three weeks after disease onset in the controls and 2 weeks after termination of the peptide injections. The apparent delay was not statistically supported using the log rank test. The day of overt diabetes onset (BGL >250 mg/dl) for each mouse was plotted (Fig. 1C) using mice shown in panel B. The dotted line indicates the end of the study and mice not scored as diabetic at that point are indicated above the line as square symbols. Statistical significance was determined to be less than 0.05 using one-tailed Student's t test to compare results from saline and therapeutic peptide injection of the mice. We conclude from this experiment that when therapy was begun at 13 weeks, its effects in NOD mice on BGL, the primary index of diabetes, were modest at best. However, administration of therapy was initiated later than is customary with these mice and reports exist indicating that in the NOD mouse, BGL readings are not always coincident with other measures of disease [28].
Figure 1.
Therapeutic peptides exerted modest effect on onset of diabetes in NOD mice. (A) NOD mice were injected on alternate days for 5 weeks with saline (dashed line) or therapeutic peptides (solid line). Injections began in week 13 (91 days of life) indicated by the first arrow and ended in week 18 (second arrow) after 17 doses had been administered. Blood glucose level (BGL) was monitored weekly (and also on injection days) from 11 to 23 weeks of age and the means ± SEM are presented. Student's t test showed a very modest difference (p < 0.06) between saline control and therapeutically treated animals with only two time points (*). (B) Percentages of mice remaining diabetes-free (where diabetes was scored as >250 mg/dl) are shown for each group (n = 9 to 12 mice per group). Saline treated mice are indicated by circles and therapeutically treated mice by squares. Apparent differences between groups were not statistically significant as determined by the log rank test. (C) The day each mouse became diabetic was plotted for the control and therapeutically treated groups used for panels A and B. The dotted line indicates the end of the study and any mice not diabetic by that time were placed above the line as square symbols. Four of the 9 mice that received therapy remained healthy and 3 of the 12 saline mice did not develop disease. Statistical significance was determined to be less than 0.05 using one-tailed Student's t test.
3.2. T cells from peptide treated mice did not respond to islet antigen in a recall response
The basic hypothesis under investigation was that peptide inhibition of the costimulatory signals delivered by interaction of LFA-1 and ICAM-1 would lead to anergy or death of the antigen reactive T cells in vivo. If the hypothesis were supported, T cells reactive to pancreatic antigens and therefore capable of attacking the pancreas would be detectable in the control mice but not in therapeutically treated animals.
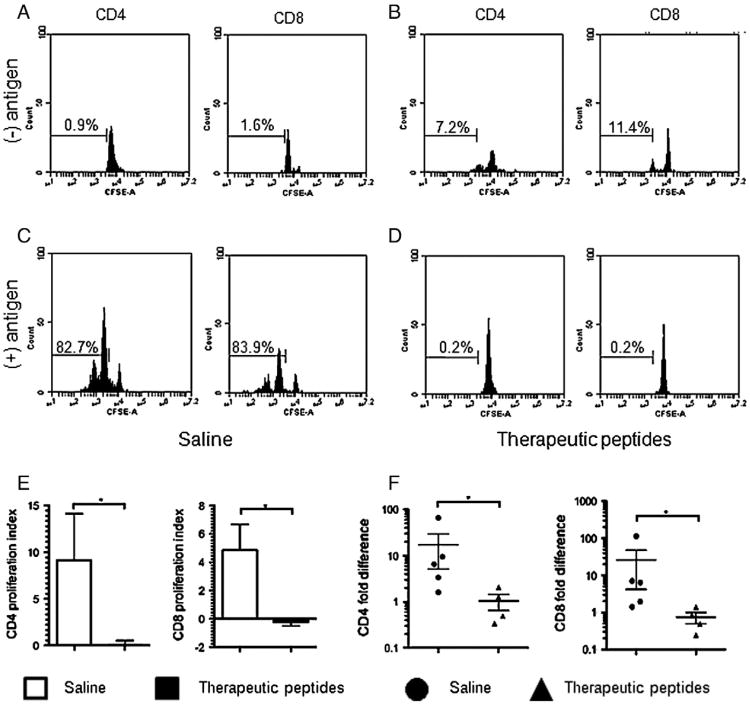
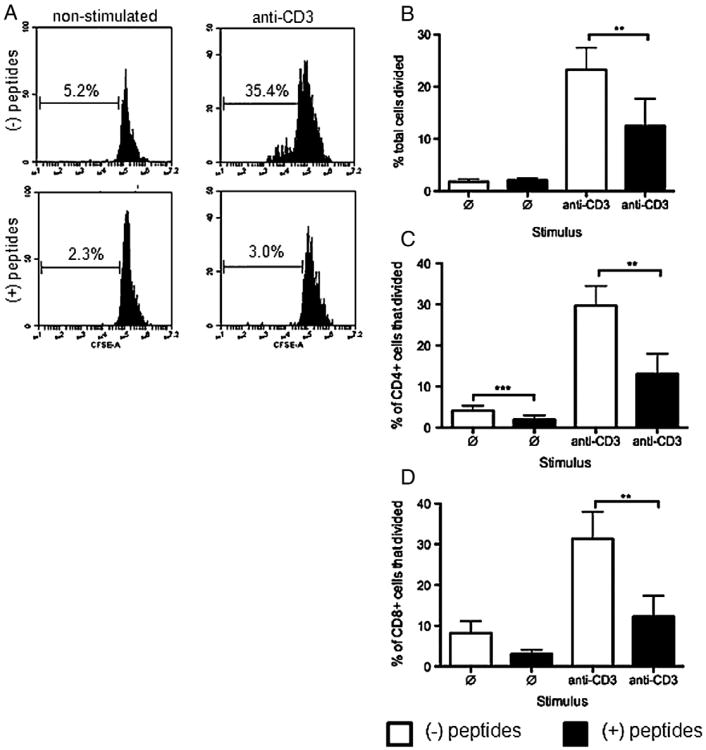
Splenocytes were harvested at 23 weeks, five weeks after cessation of injections, and proliferative response to pancreatic antigen by the splenic T cells was measured over five days in culture. Mice used here were also used in later experiments. BGL in saline control animals shown in Fig. 2 were: 600, 600, 445, 251 and 113. BGL in the peptide treated animals at time of harvest was: 600, 251, 147, and 137. Representative experiments are shown in Figs. 2A–D, and results are summarized in panels E and F. Cell division was gated based on the undivided peak from the negative control in Fig. 2A. The same gate was used for Figs. 2B–D for consistency. CD4+ or CD8+ splenocytes from saline control mice (panel A) or therapeutically treated mice (panel B) incubated in the absence of specific antigen underwent minimal spontaneous cell division. Approximately 80% of both CD4+ and CD8+ T cells from saline injected control mice responded to pancreatic antigen by proliferating (Fig. 2C), indicating the presence of Ag-specific T cells in both the CD4 and CD8 populations. In contrast, no Ag-induced cell division was observed in either the CD4+ or CD8+ T cells taken from therapeutically treated mice (Fig. 2D) suggesting that the pancreas-reactive T cell response had been eliminated by in vivo treatment with the therapeutic peptides.
Figure 2.
Splenic T cells from therapeutically treated mice were not responsive in vitro to pancreatic islet antigen in a recall response. Splenocytes were isolated at the end of the experiment (23 weeks) from mice treated with saline or therapeutic peptides and cultured in medium alone or medium plus 25 μg/ml pancreatic lysate antigen. The cellular proliferative recall response was determined using a CFSE dilution proliferation assay. Representative flow cytometry histogram plots are shown for CD4+ and CD8+ T cells. Cell division was assessed without pancreatic lysate antigen (A and B) or with antigen (C and D) in cells from saline control (A and C) or therapeutic peptide treated (B and D) mice. (E) CD4 and CD8 splenocytes from mice injected with saline control (open bars) or therapeutic peptides (filled bars) were cultured without or with antigen, proliferation was measured using CFSE and a proliferation index was calculated as described in the text and plotted. (F) The cell division response was measured for CD4+ or CD8+ T cells according to individual mice. Cells from each mouse were stimulated in vitro or not stimulated with pancreatic antigen as described for panels A–D, and fold differences in proliferation were calculated per mouse. Circles represent saline treated and triangles represent therapeutically treated animals. Data are shown as mean ± SEM (n = 4 or 5 animals per group). Statistical significance was determined using a Mann–Whitney test. *p < 0.05.
In individual experiments, the average increase in the number of dividing cells stimulated with pancreatic antigen when compared to cells with no stimulation was between 15 and 30 fold with the saline control mice (not shown). This was in contrast to no increase with the therapeutic peptide-treated mice and this difference held for both CD4+ and CD8+ T cells (individual data not shown, but see below). Proliferation indices were calculated for each mouse by dividing the number of proliferating cells without antigen stimulation into the number of proliferating cells with antigen stimulation minus the number of proliferating cells without antigen; these were plotted for both CD4 and CD8 cells (Fig. 2E). The proliferation index for cells from saline control mice was ∼9 for CD4+ T cells and almost 5 for CD8+ T cells while both indices were 0 for T cells from therapeutically treated animals. Fold increases of response by individual mice for CD4+ and CD8+ cells are shown in Fig. 2F. A value of 1 indicates no response. This approach exhibited the same trend of difference between treatment groups as did quantifying the average number of dividing cells and the proliferation index. The inability of T cells from therapeutically treated mice to respond to pancreatic antigen suggests that the basic hypothesis was upheld and the Agresponsive T cells were either rendered anergic or induced to die in vivo in response to the therapeutic peptides.
3.3. Islets in mice treated with therapeutic peptides showed greatly reduced infiltration
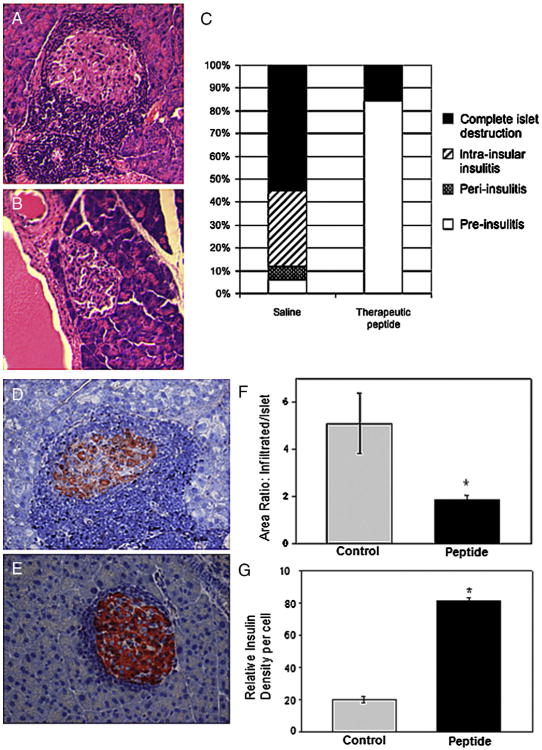
Blockade of T cell second signal is known to induce anergy or apoptosis in T cells that are receiving signal 1. This indirectly should lead to diminished infiltration of islets by T cells. In addition, the counter receptors ICAM-1 and LFA-1 interact during leukocyte extravasation and potentially play a direct role in infiltration of islets. Five weeks after cessation of treatment, pancreata were harvested, and islets were examined for level of infiltration (Fig. 3). Using conventional hematoxylin and eosin preparations, pancreata from saline injected mice exhibited a high level of leukocyte infiltration (Fig. 3A) compared with therapeutically treated mice (Fig. 3B). Eighteen islets in the tail section of the pancreas were examined in 3 saline control mice (BGL levels, 251, 224, 600 mg/dl), and 17 islets were observed in 4 therapeutically treated mice (BGL levels, 137, 147, 226, 600 mg/dl). Infiltration was almost exclusively by mononuclear cells. Using a conventional scoring system (Fig. 3C), over half the islets from saline treated mice were completely infiltrated and another third were classified in the intra-insular infiltration category while only 5% scored as pre-insulitis indicating no infiltration. In contrast, the majority (almost 85%) of islets from therapeutically treated mice showed no infiltration.
Figure 3.
Therapeutic peptides reduced leukocyte infiltration of islets. Pancreata were isolated from saline control or therapeutic peptide treated mice, and pancreas sections were stained with hematoxylin and eosin (A and B) or hematoxylin and anti-insulin(D and E). Representative images of islets are shown from a saline treated mouse(A and D) and a therapeutically treated mouse (Band E). The levels of islet infiltration were quantified from control and treated mice using H&E staining and severity was assigned according to the characteristics listed in Materials and methods, and plotted (C). Relative areas of islets undergoing infiltration were quantified in slides stained with anti-insulin and hematoxylin (D, E). Total area of each islet as well as infiltrated area were quantified and presented (F) as a ratio of infiltrated to total area. For peptide treated mice, n = 13 islets, and n = 17 islets for saline injected animals. Intensity of staining for insulin (G) was quantified in islets from 3 saline control or 4 therapeutically treated animals by assessing the brightness of insulin staining per cell and normalizing to the intensity of hematoxylin stained cells for that slide. Results are presented as relative insulin density (G). Numbers of islets or cells were taken as described in the text. Statistical significance was determined by hierarchical ANOVA and * indicates p < 0.001.
For a more detailed analysis, we stained for insulin in conjunction with hematoxylin. In Fig. 3D a high degree of cellular infiltration surrounded the (representative) insulin-containing islet from a saline treated mouse, in contrast with the (representative) islet from a therapeutically treated mouse (Fig. 3E) where infiltration was considerably weaker. The cellular infiltrates of 17 islets from saline treated and 13 islets from therapeutic peptide treated mice were quantified (Fig. 3F), and presented as the ratio of infiltrated area to remaining islet area. The ratio was approximately 3 fold greater in islets from saline treated mice. Thus, mice treated with the therapeutic peptide combination were experiencing greatly reduced leukocyte infiltration compared with controls, at the time the experiment was terminated.
3.4. Peptide therapy preserved the insulin response
Islets stained for insulin and hematoxylin (such as the ones shown in Figs. 3D, E) were evaluated for intensity of staining for insulin using our previously published protocols [24,25]. Seven control islets and five islets from therapeutically treated animals were scored for pixel intensity associated with individual insulin-stained beta cells. Insulin data from each microscopic field were normalized to the intensity of staining by hematoxylin in individual cells to avoid differences among slides. Insulin in 450 β cells from the control animals was compared with 157 β cells from therapeutically treated animals. In Fig. 3G, β cells from therapeutically treated animals stained 4-fold brighter for insulin than beta cells from the control animals. Thus it appeared that the therapeutic peptides preserved some insulin production for at least 5 weeks after cessation of therapy. Results were uniform among peptide treated as compared with saline treated mice regardless of BGL values at time of harvest.
3.5. Islet integrity was preserved by peptide treatment
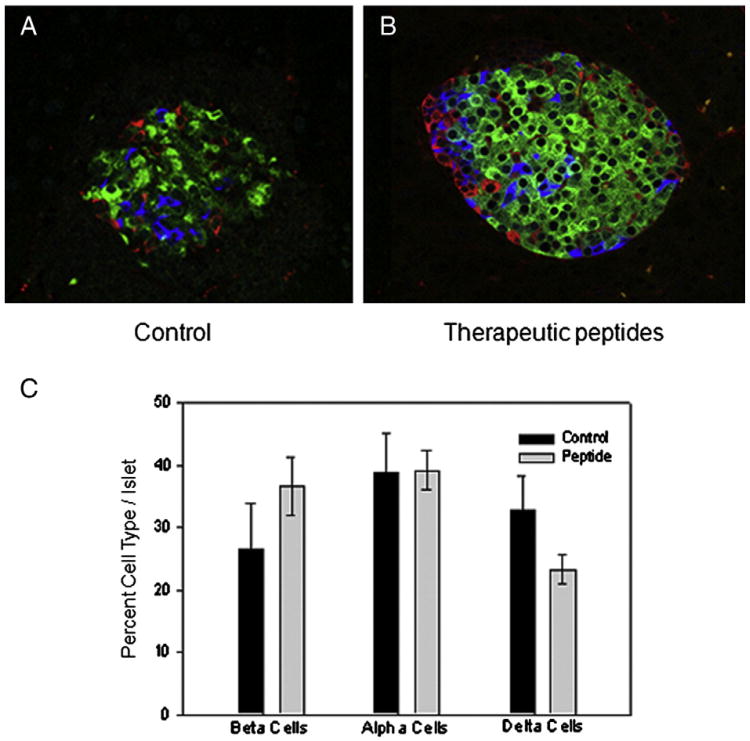
As just noted, treatment with therapeutic peptides preserved some insulin production (Fig. 3G). To explore this further, islets were compared for morphology and cellular constitution (Fig. 4). In the representative confocal images (A and B), insulin positive cells (β cells) are green, glucagon positive cells (α cells) are red and somatostatin positive cells (δ cells) appear blue. Consistent with results in Fig. 3, the overall intensity of insulin staining was stronger in 47 islets from therapeutically treated mice than in 15 islets from saline treated controls (not shown). As exemplified in the representative images (A and B), islets in saline treated mice were in an advanced state of destruction at time of harvest compared with islets in therapeutically treated animals. Interestingly, overall cellular composition of the islets suggested that no difference existed in the relative percentages of α, β and δ cells between islets from saline treated and therapeutically treated mice (Fig. 4C), although a trend occurred toward more β cells in therapeutically treated animals and more δ cells in the control animals. Since healthy mouse islets contain ∼60% β cells, we calculated the percentage of islets with 60% or greater β cells. In the saline control group, only 12% of the islets reached this threshold, whereas in the peptide-treated group, 31% reached the mark (∼2.5 times greater). Finally, the density of islets per unit area was higher (1.7 ± 0.2 islets per field) in the therapeutically treated mice compared with saline treated mice at 1.05 ± 0.2 islets per field (not shown). Thus, at termination of the experiment, therapeutically treated mice had retained more islets with greater intensity of insulin than were present in the saline control mice.
Figure 4.
Mice treated therapeutically retained islets with near normal integrity. Pancreatic slices were stained (A and B) for insulin (β cells) seen as green, glucagon (α cells) seen as red and somatostatin (δ cells) which appear as blue. Relative cellular composition of islets was determined (panel C) and plotted as percentage of total. There was no statistical difference in relative cellular composition between the groups as determined by hierarchical test.
3.6. Mouse T cells treated in vitro with peptides did not proliferate in response to a mitogenic stimulus
Mice treated in vivo with therapeutic peptides, were found to contain no T cells that responded to specific antigen (Fig. 2) suggesting that the peptides induced death or anergy in vivo in an antigen-selective manner. We attempted to mimic this in an in vitro T cell activation assay by exposure of cells to therapeutic peptides immediately before general stimulation through the TCR using anti-CD3 Ab. Nontreated NOD mice were used for consistency in the study, although Ag specificity was not an issue here. The mice exhibited BGL of 93, 87, 90, 138, 168, 179, and one was frankly diabetic with BGL of 437. Splenocytes from these nontreated NOD mice ranging from 10 to 32 weeks of age were tested for effects of the peptides in an in vitro cell proliferation assay. Cells were cultured in plastic wells coated with anti-CD3 to stimulate through the TCR complex and activate effector/memory T cells. Activation was determined by measuring CFSE dilution and cells were co-stained for CD4 or CD8 (Fig. 5A). As expected, in these representative panels, total splenocytes left nonstimulated, with (2.3%) or without (5.2%) therapeutic peptides did not proliferate. Splenoctyes stimulated through CD3 in medium alone for 7 days proliferated (35.4%) whereas splenocytes stimulated through CD3 in the presence of added therapeutic peptides did not proliferate (3.0%). Data from all seven mice were combined and percent of cells responding was calculated for each group (Fig. 5B). Percent of total splenocytes that were dividing in response to stimulation through CD3 in the presence of therapeutic peptides (∼12%) was significantly lower than percent of splenocytes (22%) dividing in response to stimulation through CD3 without peptide inhibition (Fig. 5B). CD4+ and CD8+ T cells followed the same proliferation trend as did the total splenocytes in response to peptides (Fig. 5C). Notably, there was little difference in proliferation or lack thereof associated with individual BGL levels or disease status of the mice used. Thus, the therapeutic peptides inhibited proliferation in an in vitro assay as NOD mouse T cells were being stimulated through CD3.
Figure 5.
T cells from nontreated NOD mice stimulated mitogenically in vitro in the presence of therapeutic peptides did not proliferate. Total NOD splenocytes were isolated from nontreated animals and stained with CFSE. Cells were preincubated with or without peptides for 30 min, and left nonstimulated or stimulated with plate bound anti-CD3 for 7 days, with or without therapeutic peptides. Each peptide was used at 250 μM. (A) Representative histogram plots of total splenic T cell CFSE dilution by nonstimulated or stimulated cells in the absence (upper panels) or presence (lower panels) of the therapeutic peptides. (B) Percentages of total splenic T cells that divided in the absence (open bars) or presence (closed bars) of peptides. (C) Percentage of CD4+ or CD8+ T cells that divided in the absence (open bars) or presence (closed bars) of peptides. Values are presented as mean ± SEM (error bars) and represent data from 7 mice, each done in triplicate. Statistical significance was determined using two-tailed Student's paired t test. **p < 0.01, ***p < 0.001.
3.7. Peptide treatment diminished TCR-induced T cell proliferation in vitro by human T cells
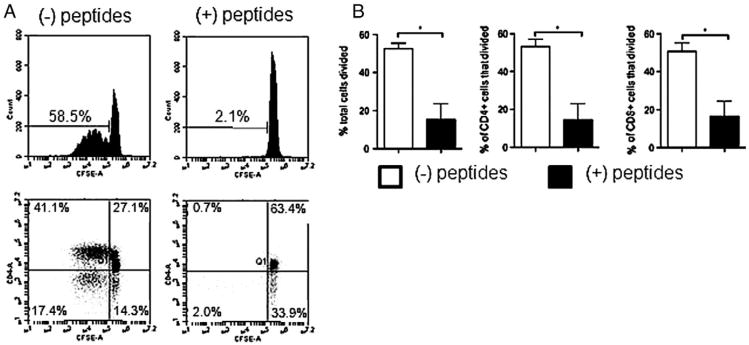
We investigated whether the therapeutic peptides also inhibited the ability of human T cells (Fig. 6) to proliferate in response to a general mitogenic stimulus in the same manner as we observed in the mouse. As mentioned, the amino acid sequences of the peptides are conserved between human and mouse. Proliferation in the presence or absence of peptides was determined by CFSE dilution as we have done many times [e.g. [6,17,29]]. Representative results of three separate experiments are presented in dot plots and a histogram (Fig. 6A) and summarized in Fig. 6B. When cultured in medium alone, total human peripheral T cells stimulated through CD3 + ICAM-1 proliferated robustly (59%), undergoing multiple rounds of division (Fig. 6A upper left panel), and this proliferation was reduced to 2% when cells were stimulated in the presence of the therapeutic peptide combination (Fig. 6A, upper right panel). The CD4+ cells (Fig. 6A, lower panels), proliferated robustly (41%) as did the CD4(−) (putative CD8+) T cells (17%) in response to the stimuli (left panel). In the presence of therapeutic peptides (right panel) neither the CD4+ nor CD8+ cells proliferated. Over the three experiments, the average percent (Fig. 6B) of total (left panel), CD4+ (center panel) and putative CD8+ (right panel) T cells that had undergone division when stimulated in the presence of therapeutic peptides was dramatically lower than in cells stimulated in the absence of peptides.
Figure 6.
Human T cells stimulated mitogenically in vitro in the presence of therapeutic peptides did not proliferate. Human T cells were isolated from peripheral blood and stimulated for five days with plate bound anti-CD3 + anti-ICAM-1. (A) Representative histogram plots of CFSE dilution by total T cells (upper panels) and dot plots of CFSE dilution (lower panels) of CD4+ and putative CD8+ [CD4(−)] T cells in the absence or presence of 250 μM each therapeutic peptide. (B) Percentage of total T cells (left panel), CD4+ cells (center panel), or putative CD8+ cells (right panel) that divided in the absence (open bars) or in the presence (closed bars) of therapeutic peptides. Statistical significance was determined using two-tailed Student's paired t test. *p < 0.05, n = three independent experiments, each done in triplicate.
Human-derived peptides representing contact domains of LFA-1 and ICAM-1, and inhibiting interaction of these two counter receptors, were used to study T1D. The sequences are conserved in mice, and in the NOD mouse model, the human-derived peptides reduced the presence of T cells capable of specifically attacking the pancreas in vivo, prevented infiltration of pancreatic islets and inhibited islet degradation. In cell culture, the human-derived peptides reduced mitogen-induced proliferation of mouse and human T cells.
4. Discussion
4.1. Overview
Interaction of the adhesion molecule, ICAM-1 with the integrin, LFA-1 is likely to play a role in both initiation and progression of T1D since ICAM-1 is expressed at high levels on vascular endothelium, ductal epithelial cells, and endothelial cells in NOD mouse pancreas and both LFA-1 and ICAM-1 are expressed on infiltrating lymphocytes [14,30–32]. Our previous findings identified short, cyclic peptides that inhibit ICAM-1:LFA-1 interactions, thereby putatively arresting T cell activation [19,23]. In the present study, we examined the ability of peptide blockade of ICAM-1:LFA-1 interaction to affect the function of T cells in the NOD mouse model of T1D.
4.2. Reduction of pancreas-specific T cell activity and islet preservation
Blockade of LFA-1:ICAM-1 interactions beginning at 13 weeks induced inactivity of pancreas-specific T cells and this influence remained until at least 23 weeks (5 weeks after cessation of therapy) at which time the experiment was terminated. This lack of recall response was similar to that observed by others [9,11] when the therapy was effected by blockade of CD40:CD40L, or B7-H4. Islet specific T cell clones have been established from spleens of NOD mice [33], suggesting that antigen-specific responses in the spleen are representative of the T cell response in T1D. Splenocytes from saline treated control mice specifically responded to β cell antigen when challenged in vitro, whereas splenocytes from therapeutically treated mice did not. This suggests that the therapeutic peptides rendered the mixed T cell population unable to respond to pancreatic antigens. Secondly, in vitro incubation of splenocytes from untreated NOD mice, as well as human PBL-T cells with therapeutic peptides prevented the response to TCR stimulation. These cells also did not demonstrate an elevated degree of apoptosis (not shown). Based on the in vitro data, it seems likely that in mice and humans, the peptides might not induce death of the T cells in vivo, but instead may render them unresponsive. This remains to be tested. The length of time that such putatively anergic cells will remain nonresponsive also remains to be determined. However, based on the in vivo mouse experiment, cells were nonresponsive at least 5 weeks after cessation of therapy (time of termination of the experiment) suggesting the beginning of a reasonable level of durability.
In other studies using costimulatory blockade, the islets of Langerhans typically experienced massive insulitis when the blockade was initiated as late as 9 weeks [e.g. [8,9]]. In the work described here, blockade of LFA-1:ICAM-1 interactions initiated at 13 weeks led to markedly reduced insulitis at 23 weeks (5 weeks after cessation of therapy) accompanied by comparatively greater insulin content in each islet. In contrast, islets of control saline-treated mice were heavily infiltrated at the same point in the experiment and had undergone considerable destruction.
4.3. In vivo blockade of two sets of T cell signals compared favorably with other such approaches
Blocking multiple costimulatory pathways does seem to provide more efficient treatment of T1D than blockade of a single costimulatory pathway. The combination of monoclonal antibodies against ICOS and CD40L significantly diminished disease in NOD mice compared with blockade of either pathway alone when the treatment was given at 10 weeks of age [10]. The present work effectively blockades two different T cell signaling pathways since both LFA-1 and ICAM-1 are resident on T cells and each sends its own set of signals into the cell [6,7].
Costimulatory blockade of T cell signaling has been examined by others as treatment for diabetes and other autoimmune diseases [rev in [34]]. In one example, use of CTLA4Ig and anti-B7.2 to interfere with the CD28:B7 pathway during the development of diabetes, inhibited the disease when administered at 2–4 weeks of age but not after 10 weeks [8]. Also, this therapy did not inhibit insulitis at any stage of administration; and treatment with anti-B7.1 accelerated the disease [8]. Inhibiting the CD40:CD40L interaction also prevents onset of the disease in NOD mice when administered at 3 weeks of age but not after 9 weeks and the mechanism i.s not via a regulatory T cell pathway [9]. Coincidentally, the CD4lo CD40+ (Th40) population, known to participate in autoimmunity and in diabetes in the NOD mouse [35,36] undergoes strong expansion over this time interval and may have the ability to overcome the CD40:CD40L blockade when it is administered later. The balance between Treg and Th40 seems to be crucial in regulation of onset. Previous work with ICAM-1:LFA-1 blockade was conducted with monoclonal antibodies and did cause reduced symptoms of T1D [13]. This was attributed to the inability of lymphocytes to enter islets, and at the time (1994), was not considered to be due to functional impairment of lymphocytes. Our present data are consistent with the hypothesis that blocking the LFA-1:ICAM-1 interaction with these peptides inhibits both T cell localization and T cell function.
4.4. Lack of insulitis plus ablation of the T cell response failed to stop increased BGL
The present therapy was initiated at 13 weeks, a time when insulitis is reported to be well under way, and the trend of the BGL data was that the therapeutic peptides at best only modestly delayed onset of diabetes compared to saline injection. Our data suggest that the therapeutic peptides inactivated the T cell component of T1D, and possibly stopped insulitis. However, for both treated and control groups, ∼75% of the mice still eventually did develop elevated BGL. This is not uncommon since delay in onset accompanied by diabetes later was noted in at least 15 other studies [34].
One possibility to explain delay without prevention is that the therapy was initiated too late after onset of insulitis. In these mice, insulitis begins around five weeks of age [21,22]. As early as three weeks of age, dendritic cells and macrophages are found in pancreatic infiltrates and these cells are thought to be participating in initiation of self-reactive responses [37–39]. It is conceivable that some crucial event was too advanced for recovery from symptoms even though the therapy greatly reduced the pancreatic infiltration and diminished the T cell response. In a comparable NOD study [40] anti-CD3 Ab caused a delaying effect on disease onset only when administered at 12 weeks (not at 4 or 8 weeks) and all animals eventually developed diabetes. So timing of therapy indeed is important for outcome in this model. Also, insulin autoantibodies can reach peak levels in prediabetic mice [41,42]. Although T1D is a T cell driven disease, insulin autoantibodies are a contributing factor to disease progression and would have begun to be produced at elevated levels by the time of administration of the therapeutic peptides. Such Ab would theoretically not be influenced by the LFA-1:ICAM-1 blockade. So even if some of the immune components can be diminished in the NOD mouse, disease progression may continue based on other predisposing genetic factors. In a comprehensive review of all NOD studies up to the year 2005, [34] it was noted that some therapeutic approaches are more successful when administered early and others are better when applied later in the disease. The timing of 13 weeks is likely to be late in the prediabetic process and additional studies at both earlier and later times would be fruitful.
The use of the NOD mouse model for T1D has many advantages when examining the immune reaction due to its similarity to the human immune process of disease [43]. In the present work, it seems that the NOD mouse modeled T cell responses reasonably well. However, the universal utility of the model has come into some question [44–46] and an alternative animal model that more faithfully represents T1D has been deemed appropriate. In the aforementioned comprehensive review [34] it was pointed out that as long as the NOD mouse model is not expected to be completely faithful to the human disease, much can be learned.
4.5. Summary
Considered together, data presented here suggest that peptide blockade of LFA-1:ICAM-1 interactions in NOD mice inhibited or somehow reversed infiltration into the pancreas and also inhibited activation of T cells with reactivity to pancreatic antigens. In vitro, the peptides inhibited activation of both murine and human T cells that were under a general mitogenic stimulus. The accumulated data suggest that this combination of LFA-1 and ICAM-1 blocking peptides may help further elucidate the mechanisms of T1D, and could potentially provide a useful therapeutic model for autoimmune disease and organ transplantation.
Acknowledgments
We thank T. Yankee, M. Chan and C. Rockwell for helpful discussion during the course of the work and Nancy Schwarting and William McGuiness for expert assistance with animal work. This project was funded by a grant-in-aid from the Kansas University Diabetes Institute and in part by DK073119 from the National Institute of Diabetes and Digestive and Kidney Disease.
Footnotes
Disclosure: The University of Kansas owns several patents related to the peptides used in this manuscript
Conflict of interest statement: The author(s) declare that there are no conflicts of interest
References
- 1.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafferty K, Cunningham A. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 3.Lafferty K, Warren H, Woolnough I, Talmage D. Immunological induction of T lymphocytes: role of antigen and the lymphocyte costimulator. Blood Cells. 1978;4:395–406. [PubMed] [Google Scholar]
- 4.Kwon E, Hurwitz A, Foster B, Madias C, Feldhaus A, Breenberg N, Burg M, Allison J. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohlmeier JE, Benedict SH. Alternate costimulatory molecules in T cell activation: differential mechanisms for directing the immune response. Histol Histopathol. 2003;18:1195–1204. doi: 10.14670/HH-18.1195. [DOI] [PubMed] [Google Scholar]
- 6.Chirathaworn C, Kohlmeier JE, Tibbetts SA, Rumsey LM, Chan MA, Benedict SH. Stimulation through intercellular adhesion molecule-1 provides a second signal for T cell activation. J Immunol. 2002;168:5530–5537. doi: 10.4049/jimmunol.168.11.5530. [DOI] [PubMed] [Google Scholar]
- 7.Ni HT, Deeths MJ, Li W, Mueller DL, Mescher MF. Signaling pathways activated by leukocyte function-associated Ag-1-dependent costimulation. J Immunol. 1999;162:5183–5189. [PubMed] [Google Scholar]
- 8.Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, Herold KC, Bluestone JA. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balasa B, Krahl T, Patstone G, Lee J, Tisch R, McDevitt HO, Sarvetnick N. CD40 ligand-CD40 interactions are necessary for the initiation of insulitis and diabetes in nonobese diabetic mice. J Immunol. 1997;159:4620–4627. [PubMed] [Google Scholar]
- 10.Nanji SA, Hancock WW, Luo B, Schur CD, Pawlick RL, Zhu LF, Anderson CC, Shapiro AM. Costimulation blockade of both inducible costimulator and CD40 lig and induces dominant tolerance to islet allografts and prevents spontaneous autoimmune diabetes in the NOD mouse. Diabetes. 2006;55:27–33. [PubMed] [Google Scholar]
- 11.Rigby MR, Trexler AM, Pearson TC, Larsen CP. CD28/CD154 blockade prevents autoimmune diabetes by inducing nondeletional tolerance after effector T-cell inhibition and regulatory T-cell expansion. Diabetes. 2008;57:2672–2683. doi: 10.2337/db07-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin S, Heidenthal E, Schulte B, Rothe H, Kolb H. Soluble forms of intercellular adhesion molecule-1 inhibit insulitis and onset of autoimmune diabetes. Diabetologia. 1998;41:1298–1303. doi: 10.1007/s001250051068. [DOI] [PubMed] [Google Scholar]
- 13.Herold KC, Vezys V, Gage A, Montag AG. Prevention of autoimmune diabetes by treatment with anti-LFA-1 and anti-ICAM-1 monoclonal antibodies. Cell Immunol. 1994;157:489–500. doi: 10.1006/cimm.1994.1244. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa Y, Yokono K, Taki T, Amano K, Tominaga Y, Yoneda R, Yagi N, Maeda S, Yagita H, Okumura K, et al. Prevention of autoimmune insulin-dependent diabetes in non-obese diabetic mice by anti-LFA-1 and anti-ICAM-1 mAb. Int Immunol. 1994;6:831–838. doi: 10.1093/intimm/6.6.831. [DOI] [PubMed] [Google Scholar]
- 15.Larson RS, Springer TA. Structure and function of leukocyte integrins. Immunol Rev. 1990;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 16.van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med. 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 17.Kohlmeier JE, Rumsey LM, Chan MA, Benedict SH. The outcome of T-cell costimulation through intercellular adhesion molecule-1 differs from costimulation through leukocyte function-associated antigen-1. Immunology. 2003;108:152–157. doi: 10.1046/j.1365-2567.2003.01578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isobe M, Yagita H, Okumura K, Ihara A. Specific acceptance of cardiac allograft after treatment with anti-ICAM-1 and anti-LFA-1. Science. 1992;255:1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- 19.Tibbetts SA, Chirathaworn C, Nakashima M, Jois DS, Siahaan TJ, Chan MA, Benedict SH. Peptides derived from ICAM-1 and LFA-1 modulate T cell adhesion and immune function in a mixed lymphocyte culture. Transplantation. 1999;68:685–692. doi: 10.1097/00007890-199909150-00015. [DOI] [PubMed] [Google Scholar]
- 20.Davignon D, Martz E, Reynolds T, Kürzinger K, Springer T. Lymphocyte function-associated antigen 1 (LFA-1): a surface antigen distinct from Lyt-2,3 that participates in T lymphocyte-mediated killing. Proc Natl Acad Sci U S A. 1981;78:4535–4539. doi: 10.1073/pnas.78.7.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon M, Sarvetnick N. The pathogenesis of diabetes in the NOD mouse. Adv Immunol. 2004;84:239–264. doi: 10.1016/S0065-2776(04)84007-0. [DOI] [PubMed] [Google Scholar]
- 22.Van Belle TL, Taylor P, von Herrath MG. Mouse models for type 1 diabetes. Drug Discov Today Dis Models. 2009;6:41–45. doi: 10.1016/j.ddmod.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tibbetts SA, Seetharama Jois D, Siahaan TJ, Benedict SH, Chan MA. Linear and cyclic LFA-1 and ICAM-1 peptides inhibit T cell adhesion and function. Peptides. 2000;21:1161–1167. doi: 10.1016/s0196-9781(00)00255-2. [DOI] [PubMed] [Google Scholar]
- 24.Huang HH, Novikova L, Williams SJ, Smirnova IV, Stehno-Bittel L. Low insulin content of large islet population is present in situ and in isolated islets. Islets. 2011;3:6–13. doi: 10.4161/isl.3.1.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang HH, Farmer K, Windscheffel J, Yost K, Power M, Wright DE, Stehno-Bittel L. Exercise increases insulin content and basal secretion in pancreatic islets in type 1 diabetic mice. Exp Diabetes Res. 2011;2011:481427. doi: 10.1155/2011/481427. Epub 2011 Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fecondo JV, Kent SB, Boyd AW. Inhibition of intercellular adhesion molecule 1-dependent biological activities by a synthetic peptide analog. Proc Natl Acad Sci U S A. 1991;88:2879–2882. doi: 10.1073/pnas.88.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross L, Hassman F, Molony L. Inhibition of Molt-4-endothelial adherence by synthetic peptides from the sequence of ICAM-1. J Biol Chem. 1992;267:8537–8543. [PubMed] [Google Scholar]
- 28.Wang W, Hao J, Metzger D, Mui A, Ao Z, Akhoundsadegh N, Langermann S, Liu L, Chen L, Ou D, Berchere C, Warnock G. Early treatment of NOD mice with B7-H4 reduces the incidence of autoimmune diabetes. Diabetes. 2011;60:3246–3255. doi: 10.2337/db11-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohlmeier JE, Chan MA, Benedict SH. Costimulation of naive human CD4 T cells through intercellular adhesion molecule-1 promotes differentiation to a memory phenotype that is not strictly the result of multiple rounds of cell division. Immunology. 2006;118:549–558. doi: 10.1111/j.1365-2567.2006.02396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MS, Sarvetnick N. Induction of vascular addressins and adhesion molecules in the pancreas of IFN-gamma transgenic mice. J Immunol. 1994;152:4597–4603. [PubMed] [Google Scholar]
- 31.McMurray RW. Adhesion molecules in autoimmune disease. Semin Arthritis Rheum. 1996;25:215–233. doi: 10.1016/s0049-0172(96)80034-5. [DOI] [PubMed] [Google Scholar]
- 32.Faveeuw C, Gagnerault MC, Lepault F. Expression of homing and adhesion molecules in infiltrated islets of Langerhans and salivary glands of nonobese diabetic mice. J Immunol. 1994;152:5969–5978. [PubMed] [Google Scholar]
- 33.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988;37:1444–1448. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 34.Shoda L, Young D, Ramanujan S, Whiting C, Atkinson M, Bluestone J, Eisenbarth G, Mathis D, Rossini G, Campbell S, Kahn R, Kreuwel H. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Baker R, Wagner D, Haskins K. CD40 on NOD CD4 T cells contributes to their activation and pathogenicity. J Autoimmun. 2008;31(4):385–392. doi: 10.1016/j.jaut.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Waid D, Vaitaitis G, Pennock N, Wagner D. Disruption of the homeostatic balance between autoaggressive (CD4 + CD40+) and regulatory (CD4 + CD25 + FoxP3+) T cells promotes diabetes. J Leukoc Biol. 2008;84(2):431–439. doi: 10.1189/jlb.1207857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, Santamaria P. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186:1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA. Immunohistochemical characterization of monocytes–macrophages and dendritic cells involved in the initiation of the insulitis and beta-cell destruction in NOD mice. Diabetes. 1994;43:667–675. doi: 10.2337/diab.43.5.667. [DOI] [PubMed] [Google Scholar]
- 39.Bouma G, Coppens JM, Mourits S, Nikolic T, Sozzani S, Drexhage HA, Versnel MA. Evidence for an enhanced adhesion of DC to fibronectin and a role of CCL19 and CCL21 in the accumulation of DC around the pre-diabetic islets in NOD mice. Eur J Immunol. 2005;35:2386–2396. doi: 10.1002/eji.200526251. [DOI] [PubMed] [Google Scholar]
- 40.Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- 41.Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity. 1997;7:727–738. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 42.Greeley SA, Katsumata M, Yu L, Eisenbarth GS, Moore DJ, Goodarzi H, Barker CF, Naji A, Noorchashm H. Elimination of maternally transmitted autoantibodies prevents diabetes in nonobese diabetic mice. Nat Med. 2002;8:399–402. doi: 10.1038/nm0402-399. [DOI] [PubMed] [Google Scholar]
- 43.Thayer TC, Wilson SB, Mathews CE. Use of nonobese diabetic mice to understand human type 1 diabetes. Endocrinol Metab Clin N Am. 2010;39:541–561. doi: 10.1016/j.ecl.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 45.Roep BO, Atkinson M. Animal models have little to teach us about type 1 diabetes: 1. In support of this proposal. Diabetologia. 2004;47:1650–1656. doi: 10.1007/s00125-004-1517-1. [DOI] [PubMed] [Google Scholar]
- 46.Roep BO, Atkinson M, von Herrath M. Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat Rev Immunol. 2004;4:989–997. doi: 10.1038/nri1502. [DOI] [PubMed] [Google Scholar]