Abstract
Background
Lack of Gal expression on pig cells is associated with a reduced primate humoral immune response as well as a reduction in cytokine production by human cells in vitro. We investigated whether lack of Gal expression is associated with reduced human T-cell response in vitro.
Methods
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy humans and naïve baboons. Human CD4+ and CD8+ T cells were isolated. Porcine aortic endothelial cells (pAECs) were isolated from wild-type (WT) and α1,3-galactosyltransferase gene-knockout (GTKO) pigs. WT pAECs were treated with α-galactosidase, reducing Gal expression. Swine leukocyte antigen (SLA) class I and II expression on pAECs was measured, as was T-cell proliferation and cytokine production in response to pAECs.
Results
Reduced Gal expression on WT pAECs after α-galactosidase treatment was associated with reduced human PBMC proliferation (P < 0.005). SLA class I and II expression on WT and GTKO pAECs was comparable. Human CD4+ and CD8+ T-cell proliferation was less against GTKO pAECs before (P < 0.001) and after (P < 0.01 and P < 0.05, respectively) activation. Human and baboon PBMC proliferation was less against GTKO pAECs before (P < 0.05) and after (P < 0.01 and P < 0.05, respectively) activation. Human PBMCs produced a comparable cytokine/chemokine response to WT and GTKO pAECs. However, there was less production of IFN-γ/TNF-α by CD4+ and IFN-γ/granzyme B/IP-10 by CD8+ T cells in response to GTKO pAECs.
Conclusions
The absence of Gal on pig cells is associated with reduced human T-cell proliferation (and possibly selected cytokine production). Adaptive primate T-cell responses are likely to be reduced in GTKO xenograft recipients.
Keywords: cellular; Galα1,3Gal; immune response; oligosaccharides; pig; xenotransplantation; α1,3-galactosyltransferase
Introduction
Pig-to-human xenotransplantation provides a possible solution to the shortage of human donor organs available for transplantation. Until recently, pig xenografts were subject to hyperacute rejection because of the binding of primate antibodies directed toward the galactose-α1,3-galactose (Gal) oligosaccharide antigen, which is expressed on wild-type (WT) porcine tissues [1–4]. However, the development of genetically engineered pigs deficient in α1,3-galactosyltransferase (GTKO), which consequently do not express Gal antigens [5,6], has provided a source of xenografts that largely evade primate antibody-mediated hyperacute rejection [7,8]. However, other means of graft injury, such as the cellular responses, continue to provide barriers to successful xenotransplantation [9].
It has been shown that the human T-cell responses to xenogeneic pig cells and allogeneic human cells are comparable [10]. In addition, calcineurin inhibitors have been demonstrated to suppress these in vitro proliferative responses similarly [11,12].
Immunosuppressive therapy aimed at reducing primate T-cell activity can therefore help achieve prolonged xenograft survival in vivo [13–15]. Accordingly, reduced T-cell responses in pig xenograft recipients would curtail the need for intense immunosuppression and allow the use of more clinically applicable regimens.
Previous data from our group have suggested that the human T-cell response is stronger to WT than GTKO cells [16–18]. It has been shown that different cytokine profiles are produced in human whole blood in response to WT and GTKO pAECs in vitro, which were Gal dependent [19]. Others have hypothesized that Gal reduction is associated with reduced primate cellular proliferation [20]. In this study, we sought to explore the correlation between Gal expression and the human T-cell response to pig cells in vitro. We observed less human T-cell proliferation and selected cytokine production in response to cells lacking the Gal oligosaccharide.
Materials and methods
Reagents
Porcine interferon gamma (pIFN-γ) and mouse anti-SLA-1:FITC were purchased from AbD-Serotec (Raleigh, NC, USA), mouse anti-SLA-DR and FITC anti-mouse IgG2a/2b antibodies from BD-Pharmingen (San Diego, CA, USA). Lipopolysaccharide (LPS) and FITC-conjugated isolectin B4 (BSI-B4) were obtained from Sigma-Aldrich (St Louis, MO, USA).
Cells
Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of blood type O (Institute for Transfusion Medicine, Pittsburgh, PA, USA). CD4+ and CD8+ T cells were isolated using T Cell Isolation kits (Miltenyi Biotec, Auburn, CA, USA). Baboon PBMCs were obtained from healthy baboons (Oklahoma University Health Sciences Center, Oklahoma City, OK, USA). WT (n = 5) and GTKO (n = 6) pigs were unique individuals of the same genetic background, obtained from Revivicor, Inc. (Blacksburg, VA, USA), and were not necessarily MHC identical. Porcine aortic endothelial cells (pAECs) were collected from freshly harvested porcine aortas and were used from passages two to eight.
Human blood samples were collected after informed consent under University of Pittsburgh IRB approval. All animal care was in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals.
Reducing Gal expression
Gal expression on WT pAECs was reduced by incubation with green coffee bean α-galactosidase (Sigma-Aldrich). PAECs (2.5 × 106) were washed, reconstituted in warm PBS, and incubated with either 10 or 20 μl of α-galactosidase (i.e., concentrations of 4 and 8 μl/106 cells) for 1 h at 37 °C.
Mixed lymphocyte reaction
Co-cultures were carried out in round-bottom, 96-well plates with serum-free AIM-V medium (Invitrogen, Carlsbad, CA, USA). Human PBMCs, CD4+ and CD8+ T cells, and baboon PBMCs were used as responders at 0.2 × 106 cells/well. Irradiated pAECs (with or without activation) were used as stimulators at stimulator–responder ratios of 1 : 10. To induce activation, pAECs were stimulated with pIFN-γ (500 U/ml for 24 h). Dead cells were excluded from counting by staining with trypan blue. Similar numbers of washed viable cells were used in all MLR co-cultures. Co-cultures were carried out for 4 to 6 days in all MLR assays. 3H-thymidine (1 μCi/well) was added to each well during the last 16 h of incubation. Cells were harvested on glass-fiber filter mats. Next, they were analyzed by beta-scintillation counting on a liquid scintillation counter (PerkinElmer, Waltham, MA, USA). The mean of triplicate results was expressed as 3H-thymidine incorporation values. These were presented either in units of counts per minute (CPM) or as stimulation index (SI). SI was calculated by dividing the magnitude of the xenogeneic proliferative response by the magnitude of the autologous proliferative response.
Luminex multiplex immunoassays
Using a custom, LEGENDplex Luminex kit (Bio-Legend, San Diego, CA, USA), human cytokines/chemokines were tested. Supernatants were collected from previous MLR on day 3 for analysis (PBMC n = 7; CD4+ n = 2; CD8+ n = 2). Additionally, supernatants from quiescent and LPS-activated (10 μg/ml) WT and GTKO pAECs (n = 2 for each) were used as controls for pig cytokine production.
Flow cytometry
Using LSR II flow, pAECs were assessed for Gal expression and SLA class I and II expression. All data were analyzed using WinMDI software.
Statistical analyses
Significance was determined by paired, two-tailed Student's t-tests. Analyses were carried out using GraphPad Prism version 4 (GraphPad Software, San Diego, CA, USA). P < 0.05 was considered significant.
Results
Human T-cell proliferative response to WT pAECs treated with α-galactosidase
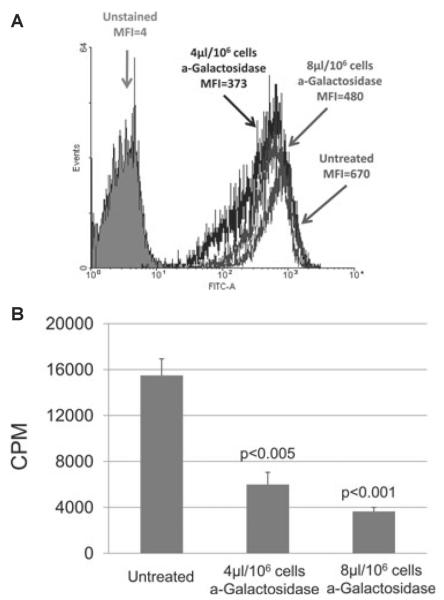
Initially, we needed to confirm previous observations that reduction in Gal expression on WT pAECs is associated with reduced human cellular proliferation in MLR [20]. WT pAECs were incubated with α-galactosidase to reduce Gal expression. For flow cytometry, pAECs were stained for Gal expression with GSI-B4. After α-galactosidase treatment at 4 and 8 μl, Gal expression on WT pAECs was reduced in a dose-dependent manner. Mean fluorescence (MFI) was reduced from 670 (untreated pAECs) to 480 and 373 (Fig. 1A). In MLR, the proliferation of human PBMCs was significantly reduced in response to WT pAECs treated with α-galactosidase at 4 and 8 μl by 61 and 77%, respectively (P < 0.005 and P < 0.001), in comparison with untreated cells (Fig. 1B).
Fig. 1.
(A) Gal expression on untreated and α-galactosidase-treated WT porcine aortic endothelial cells (pAECs) was measured by flow cytometry. The mean fluorescence (MFI) was reduced from 670 (untreated) by 28% to 480 by a treatment of 4 μl/106 cells α-galactosidase and by 44% to 373 at 8 μl/106 cells. For reference, the average MFI from all GTKO pAECs was five (not shown), and the MFI of unstained cells was four. (B) Human peripheral blood mononuclear cell (PBMC) proliferation was reduced following treatments of WT pAECs with α-galactosidase. In MLR, WT pAECs treated with α-galactosidase at 4 and 8 μl/106 reduced Gal expression by 28 and 44% and stimulated 61 and 77% less human PBMC proliferation, respectively, than those untreated. PAEC viability was confirmed before MLR and equal numbers of stimulators and responders were used in each study. 3H incorporation values are presented as counts per minute. Data represent the mean plus or minus standard error of the mean (±SEM) and are representative of three different experiments.
SLA expression on non-activated and pIFN-γ-activated WT and GTKO pAECs
Before activation, MFI for SLA class II-DR expression on WT (5.8 ± 1.0) and GTKO (5.6 ± 1.3) pAECs (n = 7 in each group) was comparable (not shown). After activation with pIFN-γ, there was a marked rise in SLA class II-DR expression on both WT (MFI = 689 ± 228) and GTKO (MFI = 624 ± 208) pAECs; with comparable percentage increases (99% for WT vs. 98% for GTKO). Quiescent SLA class I expression on WT pAECs (MFI = 288 ± 18) was also not significantly different from that on GTKO pAECs (MFI = 253 ± 35) (n = 4 in each group; not shown). After pIFN-γ activation, there remained no significant difference in the level of expression (WT 333 ± 29 vs. GTKO 357 ± 50) nor in the percentage increases (WT 11% vs. GTKO 27%).
Human CD4+ and CD8+ T-cell proliferative responses to non-activated and pIFN-γ-activated WT and GTKO pAECs
In MLR, human CD4+ T-cell (n = 3) proliferation in response to WT pAECs was significantly greater than to GTKO pAECs, before (P < 0.001) and after (P < 0.01) activation by pIFN-γ (Fig. 2A left). Likewise, isolated human CD8+ T-cell (n = 3) proliferation in response to WT pAECs was significantly greater than to GTKO pAECs, before (P < 0.001) and after (P < 0.05) activation (Fig. 2B left). To further assess the pattern of T-cell proliferation over time, MLRs were performed on three consecutive days (4, 5 and 6). The proliferation of CD4+ (Fig. 2A right) and CD8+ (Fig. 2B right) T cells was consistently higher in response to WT pAECs than GTKO pAECs, before and after activation at all three time points.
Fig. 2.
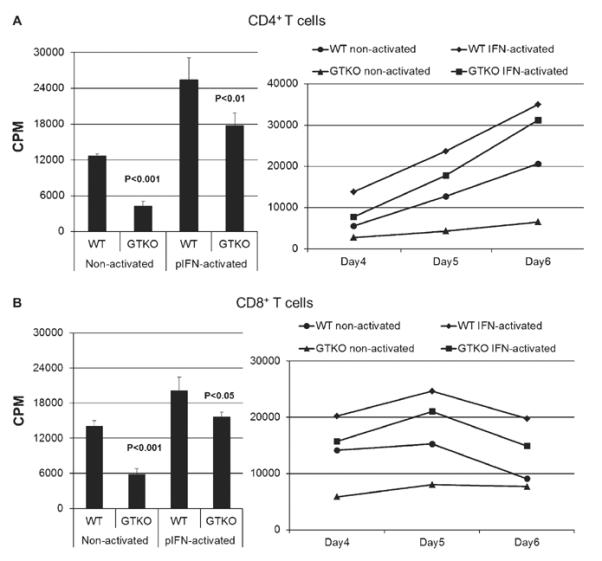
(A) The proliferative response of human CD4+ T cells (n = 3) to WT and GTKO porcine aortic endothelial cells (pAECs) before and after activation by pIFN-γ (left), where the response was significantly less to GTKO pAECs before (P < 0.001) and after (P < 0.01) activation. Additionally, MLRs were harvested on three consecutive days (4, 5, and 6) where CD4+ T-cell proliferation was consistently lower in response to GTKO pAECs. 3H incorporation values are presented as counts per minute (CPM). Data represent the mean (±SEM) and are representative of three different experiments. (B) The proliferative response of human CD8+ T cells (n = 3) to WT and GTKO pAECs before and after activation by pIFN-γ (left), where the response was significantly less to GTKO pAECs before (P < 0.001) and after (P < 0.05) activation. Additionally, MLRs were harvested on three consecutive days (4, 5, and 6) where CD8+ T-cell proliferation was consistently lower in response to GTKO pAECs. 3H incorporation values are presented as CPM. Data represent the mean (±SEM) and are representative of three different experiments.
Human PBMC proliferative response to non-activated and pIFN-γ-activated WT and GTKO pAECs
In a larger human sample size (n = 15), we further tested the cellular response to WT and GTKO pAECs, before and after pIFN-γ activation. Although there was some variability in the proliferative response of human PBMCs to pAECs in MLR, GTKO pAECs consistently induced significantly less human PBMC proliferation than WT pAECs before (P < 0.05) and after (P < 0.01) activation (Fig. 3A).
Fig. 3.
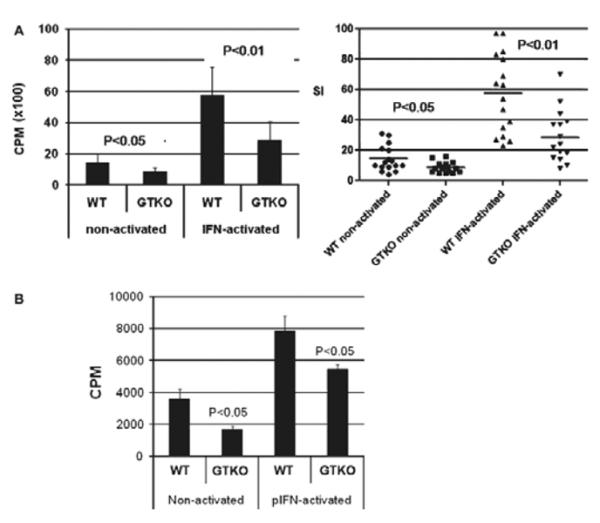
(A) The proliferative response of human peripheral blood mononuclear cells (PBMCs) (n = 15) to WT and GTKO porcine aortic endothelial cells (pAECs) before and after activation by pIFN-γ. This response was significantly less to GTKO pAECs before (P < 0.05) and after (P < 0.01) activation. PBMC proliferation is presented as counts per minute (CPM) for 3H incorporation (left) and in units of SI (right). Data represent the mean (±SEM) and are shown. (B) The proliferative response of baboon PBMCs (n = 4) to WT and GTKO pAECs before and after activation by pIFN-γ. The response to GTKO pAECs was significantly less before (P < 0.05) and after (P < 0.05) activation. 3H incorporation values are presented as CPM. Data represent the mean (±SEM) and are representative of three different experiments.
Baboon PBMC proliferative response to non-activated and pIFN-γ-activated WT and GTKO pAECs
As pig-to-baboon xenotransplantation is a common experimental model, we studied the proliferative response in MLR of naïve, healthy baboon PBMCs (n = 4) to WT and GTKO pAECs. Similar to that of human PBMCs, the proliferation of baboon PBMCs was significantly greater to WT than GTKO pAECs both before (P < 0.05) and after (P < 0.05) activation (Fig. 3B).
Human cytokine/chemokine production in response to WT and GTKO pAECs
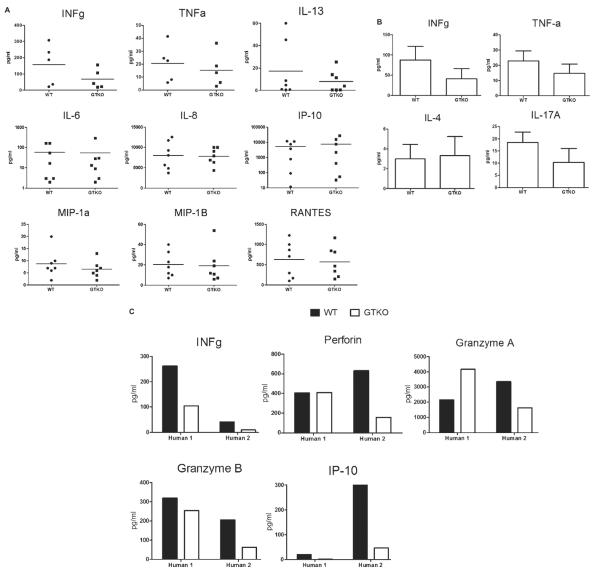
Supernatants from MLRs using human PBMCs, CD4+ T cells, or CD8+ T cells as responders were analyzed for cytokine/chemokine production. Very little or no cytokines (<1 pg/ml) were detected in supernatants obtained from pAECs cultured alone, with or without LPS activation, suggesting no cross-reactivity with pig cytokines (not shown). There was a tendency towards a higher production of IFN-γ, TNF-α and IL-13 by human PBMCs in response to WT than to GTKO pAECs. In contrast, human PBMCs produced nearly equivalent concentrations of IL-6, IL-8, IP-10, MIP-1α, MIP-1β, and RANTES in response to WT and GTKO pAECs (Fig. 4A). CD4+ T cells produced less IFN-γ, TNF-α, and IL-17A in response to GTKO pAECs (Fig. 4B) and nearly equivalent concentrations of IL-4. CD8+ T cells appeared to produce less IFN-γ, granzyme B, and IP-10 to GTKO pAECs (Fig. 4C), while granzyme A and perforin production revealed no clear trend.
Fig. 4.
Cytokine production by human peripheral blood mononuclear cells (n = 7) (A), human CD4+ T cells (n = 3) (B), and human CD8+ T cells (n = 2) (C) in response to either WT or GTKO porcine aortic endothelial cells (pAECs) in MLR. Supernatants were collected on day 3. Each value represents the average of duplicate. Cytokine concentrations are presented as pg/ml. It is noted that a log scale has been implemented in some graphs for clarity. Very little or no cytokines (<1 pg/ml) were detected in supernatants obtained from pAECs cultured alone, with or without LPS activation, suggesting that there was no Luminex bead cross-reactivity with pig cytokines (not shown).
Discussion
It has been reported previously that WT pAECs treated with α-galactosidase induce less human PBMC proliferation than untreated cells in vitro [20,21]. Prior observations by our group suggest that the human cellular proliferative response is reduced against GTKO as compared with WT cells [16,17]. Sæthre et al. [19] have demonstrated that the immune responses of human whole blood to WT and GTKO pAECs result in different cytokine production. In the present study, we aimed to explore the correlation between Gal expression and the primate cellular response.
First, to determine whether Gal expression correlates with the magnitude of human cellular proliferation to pig cells, we treated WT pAECs with α-galactosidase. The human PBMC response to treated cells was reduced in correlation with Gal expression in a dose-dependent manner. Also, both human CD4+ and CD8+ T cells proliferated less against GTKO than WT pAECs. The cellular responses of 15 different humans against GTKO and WT pAECs confirm this reduced proliferation. In addition, baboon PBMCs proliferate less to GTKO pAECs than WT, and we observed a similar trend against GTKO PBMCs (not shown). Unlike humans, both baboons and pigs express N-glycolylneuraminic acid (the Hanganutziu-Deicher antigen), suggesting that the increased human cellular response to Gal-expressing cells is not related to the expression of N-glycolylneuraminic acid on pig cells. Collectively, these data suggest that recognition of the Gal oligosaccharide on pig cells might induce an amplification of the primate direct T-cell response to pig antigens. We believe that in MLR, the direct pathway of antigen recognition is the major and initial response, although the indirect pathway might be playing a role in the later phase of the T-cell response. Whether the reduced cellular response in the absence of Gal expression is associated with a Gal-specific memory T-cell response remains unknown.
The first cells of a vascularized xenograft encountered by recipient T cells are those of the vascular endothelium. In MLR, pAECs obtained from WT and GTKO pigs were used as stimulators. Although the WT and GTKO pigs were not MHC identical, they were from the same genetic background. On pAECs, it is known that there is cross-species recognition of SLA class II by human CD4+ T cells [22] and SLA class I by human CD8+ T cells [23], as well as porcine CD86 by human T cells [24]. We assessed SLA class I and II expression on both WT and GTKO pAECs before and after IFN-γ activation prior to their use as stimulators in MLR. While SLA class I was constitutively expressed, SLA class II was minimally expressed, each in a comparable amount on both WT and GTKO pAECs before activation. After activation, SLA class I was slightly upregulated, while SLA class II was strongly upregulated, also comparably on both WT and GTKO pAECs. This suggests that the reduced response of human T cells to GTKO pAECs in comparison with WT pAECs was not related to different levels of SLA class I and II expression.
Together with T-cell receptors, cytokines influence T-cell responses, T-cell survival, and proliferation [25]. In correlation with human T-cell proliferation, we assessed cytokine production by PBMCs and T cells in response to WT and GTKO pAECs. Human PBMCs produced nearly equivalent levels of cytokines/chemokines in response to WT and GTKO pAECs. Although T cells from only two humans were tested, there was a tendency towards discrepant production of selected cytokines by T cells. CD4+ T cells tended to produce less IFN-γ, TNF-α, and IL-17A in response to GTKO pAECs, and CD8+ T cells tended to produce less IFN-γ, granzyme B, and IP-10 to GTKO pAECs. The response of human PBMCs is more likely to reflect a combined innate and adaptive cellular response.
In rodents, it has been shown that IP-10, MIP-1α, and MIP-1β were upregulated in rejected islet xenografts [26]. In light of this, our results suggest the production of such chemoattractants by innate immune cells might not be affected by the lack of Gal expression on pig cells. In contrast to data by Sæthre et al., we detected no significant difference in human chemokine or IL-6 production by PBMCs against WT and GTKO cells. Their data showed reduced human IL-6 and chemokine production (e.g., IL-8, MIP-1α, MIP-1β, and RANTES) from human whole blood against GTKO pAECs as opposed to WT pAECs [19]. It is important to note, however, that our results were obtained from incubations of pAECs with human PBMCs rather than whole blood and therefore were obtained in the absence of antibody binding and complement activation.
Even if primate cellular migration to WT and GTKO xenografts is comparable, our data suggest reduced T-cell proliferation and cytokine production to the GTKO because of lack of Gal. It is already known that Gal expression plays a role in the interaction between other cell types (e.g., monocytes and pAECs). Galectin-3 confers an affinity for Gal to monocytes, but it is only minimally expressed on lymphocytes [27]. In addition, it is possible the interaction between human T cells and porcine oligosaccharides may not be restricted to Gal. Unidentified non-Gal antigens also almost certainly play roles in xenograft rejection [28–32]. However, it is unknown whether they, like Gal, amplify the primate T-cell response.
In summary, our data indicate that the absence of, or reduced, Gal expression on pig cells is associated with a significantly reduced primate cellular response in vitro. This effect is evidenced by observed differences in T-cell proliferation (and perhaps also by reduced production of selected cytokines). Prior studies achieving prolonged survival of organs from GTKO pigs in non-human primates have attributed that success primarily to reduced antibody binding is because of a lack of Gal expression [7,8]. However, in light of our results, it seems that a reduced human T-cell response against GTKO xenografts may also be contributing to their survival. The specific mechanisms by which the absence of Gal downregulates the T-cell response remain uncertain.
Acknowledgments
Work in our laboratory is supported in part by NIH grant 1U19AI090959-01 and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc, Blacksburg, VA. The baboons used in the study were from the Oklahoma University Health Sciences Center Division of Animal Resources, which is supported by NIH P40 sponsored grant RR012317-09.
Abbreviations
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- pAECs
porcine aortic endothelial cells
- PBMCs
peripheral blood mononuclear cells
- SLA
swine leukocyte antigen
- WT
wild-type
References
- 1.Good AH, Cooper DK, Malcolm AJ, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in man. Transplant Proc. 1992;24:559–562. [PubMed] [Google Scholar]
- 2.Cooper DK. Depletion of natural antibodies in non-human primates—a step towards successful discordant xenografting in man. Clin Transplantation. 1992;6:178–183. [PubMed] [Google Scholar]
- 3.Oriol R, Ye Y, Koren E, Cooper DK. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993;56:1433–1442. doi: 10.1097/00007890-199312000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Galili U. Interaction of the natural anti-Gal antibody with [alpha]galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993;14:480–482. doi: 10.1016/0167-5699(93)90261-i. [DOI] [PubMed] [Google Scholar]
- 5.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolber-Simonds D, Lai L, Watt SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 8.Tseng YL, Kuwaki K, Dor FJ, et al. [alpha]1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80:1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 9.Pierson RN, III, Dorling A, Ayares D, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16:263–280. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada K, Sachs DH, Dersimonian H. Human antiporcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155:5249–5256. [PubMed] [Google Scholar]
- 11.Batten P, Mccormack AM, Page CS, Yacoub MH, Rose ML. Human T cell responses to human and porcine endothelial cells are highly sensitive to cyclosporin A and FK506 in vitro. Transplantation. 1999;68:1552–1560. doi: 10.1097/00007890-199911270-00020. [DOI] [PubMed] [Google Scholar]
- 12.Oostingh G, Davies H, Bradley JA, Taylor C. Comparison of allogeneic and xenogeneic in vitro T-cell proliferative responses in sensitized patients awaiting kidney transplantation. Xenotransplantation. 2003;10:545–551. doi: 10.1034/j.1399-3089.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 13.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 14.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 15.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezzelarab M, Welchons D, Torres C, et al. Atorvastatin down-regulates the primate cellular response to porcine aortic endothelial cells in vitro. Transplantation. 2008;86:733–737. doi: 10.1097/TP.0b013e3181821cad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin YJ, Hara H, Tai HC, et al. Suppressive efficacy and proliferative capacity of human regulatory T cells in allogeneic and xenogeneic responses. Transplantation. 2008;86:1452–1462. doi: 10.1097/TP.0b013e318188acb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzelarab M, Ezzelarab C, Wilhite T, et al. Genetically-modified pig mesenchymal stromal cells: xenoantigenicity and effect on human T-cell xenoresponses. Xenotransplantation. 2011;18:183–195. doi: 10.1111/j.1399-3089.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 19.Sæthre M, Schneider M, Lambris J, et al. Cytokine secretion depends on Galα(1,3)Gal expression in a pig-to-human whole blood model. J Immunol. 2008;180:6346–6353. doi: 10.4049/jimmunol.180.9.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Li T, Ma Y. Reactivity of human peripheral blood lymphocyte to alpha-Gal on porcine aortic endothelial cell. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1998;12:359–362. [PubMed] [Google Scholar]
- 21.Li Y, Li T, Bu H, et al. Xenoantigenacity of Chinese Neijiang pig-related to xenotransplantation. Transplant Proc. 2000;32:875–876. doi: 10.1016/s0041-1345(00)01020-4. [DOI] [PubMed] [Google Scholar]
- 22.Bravery CA, Batten P, Yacoub MH, Rose ML. Direct recognition of SLA- and HLA-like class II antigens on porcine endothelium by human T cells results in T cell activation and release of interleukin-2. Transplantation. 1995;60:1024–1033. [PubMed] [Google Scholar]
- 23.Shishido S, Naziruddin B, Howard T, Mohanakumar T. Recognition of porcine major histocompatibility complex class I antigens by human CD8+ cytolytic T cell clones. Transplantation. 1997;64:340–346. doi: 10.1097/00007890-199707270-00028. [DOI] [PubMed] [Google Scholar]
- 24.Maher SE, Karmann K, Min W, et al. Porcine endothelial CD86 is a major costimulator of xenogeneic human T cells. Cloning, sequencing and functional expression in human endothelial cells. J Immunol. 1996;157:3838–3844. [PubMed] [Google Scholar]
- 25.Hirahara K, Vahedi G, Ghoreschi K, et al. Helper T-cell differentiation and plasticity: insights from epigenetics. Immunology. 2011;134:235–245. doi: 10.1111/j.1365-2567.2011.03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon MF, Kuziel WA, Mann DA, Simeonovic CJ. The role of chemokines and their receptors in the rejection of pig islet tissue xenografts. Xenotransplantation. 2003;10:164–177. doi: 10.1034/j.1399-3089.2003.01146.x. [DOI] [PubMed] [Google Scholar]
- 27.Jin R, Greenwald A, Peterson MD, Waddell TK. Human monocytes recognize porcine endothelium via the interaction of galectin 3 and alpha-GAL. J Immunol. 2006;177:1289–1295. doi: 10.4049/jimmunol.177.2.1289. [DOI] [PubMed] [Google Scholar]
- 28.Baumann B, Stussi G, Huggel K, Rieben R, Seebach J. Reactivity of human natural antibodies to endothelial cells from Gal[alpha](1,3)Gal-deficient pigs. Transplantation. 2007;83:193–201. doi: 10.1097/01.tp.0000250478.00567.e5. [DOI] [PubMed] [Google Scholar]
- 29.Hara H, Ezzelarab M, Rood PP, et al. Allosensitized humans are at no greater risk of humoral rejection of GTKO pig organs than other humans. Xenotransplantation. 2006;13:357–365. doi: 10.1111/j.1399-3089.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 30.Ezzelarab M, Hara H, Busch J, et al. Antibodies directed to pig non-Gal antigens in naïve and sensitized baboons. Xenotransplantation. 2006;13:400–407. doi: 10.1111/j.1399-3089.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 31.Rood PP, Hara H, Busch JL, et al. Incidence and cytotoxicity of antibodies in cynomolgus monkeys directed to nonGal antigens, and their relevance for experimental models. Transpl Int. 2006;19:158–165. doi: 10.1111/j.1432-2277.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 32.Yeh P, Ezzelarab M, Bovin N, et al. Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation. 2010;17:197–206. doi: 10.1111/j.1399-3089.2010.00579.x. [DOI] [PubMed] [Google Scholar]