Abstract
Background
Annual colorectal cancer screening with fecal occult blood test (FOBT) is a non-invasive alternative to screening colonoscopy once every ten years. If false-positive FOBT results are common, then many patients selecting an FOBT regimen will be exposed to the same invasive testing as those selecting a colonoscopy regimen. The objective of this study was to estimate the probability of experiencing a false-positive after receiving annual FOBT screening for 10 years.
Methods
Medical records for patients age 50–79 years receiving FOBT screening with Hemoccult Sensa between 1997 and 2009 at Group Health of Washington State captured the date and results of each FOBT, along with subsequent colorectal cancer diagnoses. We used logistic regression to analyze associations between patient characteristics and odds of a positive FOBT with no invasive cancer diagnosis within one year (FOBT+, CRC−). We estimated the probability of receiving at least one FOBT+, CRC− result after 10 years of screening.
Results
We observed 181,950 FOBTs from 94,637 individuals. Older patients, males, and non-white patients were significantly more likely to receive FOBT+, CRC− results (p < 0.001 for all risk factors). After 10 years of annual FOBT, 23.0% (95% confidence interval [18.2, 27.0]) will receive at least one FOBT+, CRC− result.
Conclusions
Most patients participating in annual FOBT screening over 10 years will not have a positive result, reinforcing the potential value of this regimen as a non-invasive alternative to colonoscopy.
Impact
Annual stool-based screening is a screening alternative resulting in substantially fewer colonoscopies than once per decade colonoscopy.
Keywords: Colorectal cancer, false-positive, fecal occult blood test, screening
Introduction
Colorectal cancer is one of the leading causes of cancer mortality in the US (1). Screening with either fecal occult blood test (FOBT) or colonoscopy has been found to reduce cancer mortality (2–6). While FOBT has the benefit of being non-invasive, its modest sensitivity, and focus on detection of preclinical cancer rather than precursor adenomas, necessitates frequent screening (7). Various organizations such as the American Cancer Society and U.S. Preventive Services Task Force recommend annual screening with FOBT as an effective means of reducing colorectal cancer mortality (8, 9). Given the imperfect specificity of FOBT, frequent screening over the course of decades may lead many individuals who are cancer-free to receive positive test results, defined as false-positive test results. Appropriate diagnostic evaluation of a positive FOBT includes colonoscopy. Therefore, if the risk of receiving false-positive test results after a decade of screening with FOBT is high, a more efficient use of resources might be to simply recommend that all individuals receive colonoscopy once every 10 years.
There is no empirical information, from patients seen in clinical practice, on the proportion of patients who will receive a false-positive test result over the course of an FOBT screening regimen. Previous studies of FOBT based on data from clinical practice have focused on test sensitivity and specificity at a single screening round (10, 11). However, current screening guidelines recommend annual FOBTs beginning at age 50 and continuing until age 75 for average risk individuals (9), resulting in 25 FOBTs over the course of a lifetime of screening. Studies based on simulation models have predicted the total number of colonoscopies resulting from this regimen of annual FOBT (12). However, these models rely on strong assumptions about the correlation of repeated tests. For example, Cancer Intervention and Surveillance Network models assume that given an individual's true disease state, repeated tests are independent. Studies of data from clinical practice making use of statistical methods that allow for correlation of test results within individuals are needed to provide information about the proportion of patients following a regimen of annual FOBT who receive a false-positive result and are referred for colonoscopy over the course of the screening regimen.
In this study we investigated the cumulative false-positive risk of FOBT after 10 years of annual or biennial screening. Using data from Group Health, an integrated health care system in Washington State, we described characteristics of individuals receiving false-positive test results and estimated the proportion that will receive a false-positive result after 10 years of screening.
Materials and Methods
Study population
We evaluated risk of a false-positive FOBT using data from Group Health, a large integrated health care system in Washington State serving approximately 600,000 members. Group Health’s colorectal cancer screening guidelines have called for annual or biennial FOBT since 1997. Beginning in 1997, Group Health recommended FOBT screening with unrehydrated Hemoccult SENSA (Beckman Coulter, Inc, Fullerton, CA). From 1997 until 2004, guidelines recommended screening with FOBT every 2 years for people of average risk. Guidelines were updated in 2004 to recommend FOBT every 1–2 years, and again in 2006 to recommend annual FOBT. Colonoscopy has been the recommended standard of care after a positive FOBT since colorectal cancer screening guidelines were first put forth in 1997.
We included all individuals age 50–79 with no prior colorectal cancer residing in the 13 Washington-state counties covered by the Puget Sound Surveillance Epidemiology and End Results (SEER) program who received an FOBT between 1997 and 2009. Those with a prior history of inflammatory bowel disease were excluded from the study because they are not considered to be average-risk. We included individuals’ first and all subsequent FOBTs up until the first of: a colonoscopy, a colorectal cancer diagnosis, death, or disenrollment from Group Health. If multiple FOBTs were observed within a 90-day period, we assumed only the final FOBT in the sequence was conclusive and included only this final test. Group Health’s Institutional Review Board approved this study.
Measures and definitions
A positive FOBT (FOBT+) was defined as at least 1 of 3 slides positive for occult blood. Colorectal cancer diagnoses were obtained from the Puget Sound SEER program. The use of other colorectal cancer screening tests, including colonoscopy, was ascertained using automated electronic medical records. We classified colonoscopies as screening or diagnostic on the basis of ICD-9 and CPT codes for conditions and procedures that indicated signs or symptoms of colorectal cancer. Specifically, we used the algorithm of Fisher (13), which classified a colonoscopy as screening or diagnostic based on the presence of symptoms or diagnoses such as gastrointestinal bleeding, diarrhea, and benign neoplasms of the colon in the prior year. This approach has been demonstrated to identify screening colonoscopies with a sensitivity of 83% and a specificity of 76%. All colonoscopies performed within one year of a positive FOBT were defined to be diagnostic, regardless of the determination of the algorithm.
We investigated the cumulative risk of two outcomes: 1) a positive FOBT with no cancer detected within 12 months (FOBT+, CRC−) and 2) a positive FOBT followed by a diagnostic colonoscopy within 12 months with no cancer detected (FOBT+, Colonoscopy, CRC−). Some positive FOBTs may have been followed by a colonoscopy at which an adenoma was detected. However, adenoma detection was not captured in our data sources and is not the focus of this analysis.
Statistical analysis
We computed the proportion of observed FOBTs with FOBT+, CRC− results. In addition, we computed the proportion of FOBTs followed by a colorectal cancer diagnosis within 12 months and the proportion of positive FOBTs followed by a colorectal cancer diagnosis within 12 months (true-positive results). We stratified individuals by presence or absence of an FOBT+, CRC− test result at the first screening round. We report the distribution of age, sex, race, and year of first exam stratified by receipt of an FOBT+, CRC− result at the first screening round. We computed the elapsed time between successive FOBTs. We report the proportion of FOBT+, CRC− results stratified by elapsed time since the prior exam as well as by number of prior FOBTs. We also computed the proportion of exams resulting in an FOBT+, Colonoscopy, CRC− result using this stratification.
We analyzed the risk of FOBT+, CRC− results at the exam level (Analysis 1) and at the person level (Analysis 2). Analysis 1 investigated risk factors associated with increased odds of an FOBT+, CRC− result for individual FOBTs. We conducted this analysis using a discrete survival model in which the odds correspond to discrete hazards estimated via logistic regression adjusting for age, year of exam, sex, race, and number of prior negative FOBTs. The discrete survival approach accounts for within-person correlation by conditioning on the prior history of exam results (14). Number of prior tests was modeled as a categorical variable with separate categories for each of 0, 1, 2, 3, and 4 prior tests. All tests with more than 4 prior FOBTs were included in a single category. Calendar year was divided into 2-year intervals, with the exception of 2007 – 2009 which was treated as a single category. Age was treated as a categorical variable with 5-year increments. At the exam level we also modeled the association between patient characteristics and FOBT+, Colonoscopy, CRC− results using the above approach. Reported p-values are based on Wald tests for the statistical significance of covariates in Analysis 1 logistic regression models.
For Analysis 2, we estimated the person-level cumulative risk of FOBT+, CRC− results associated with annual or biennial screening regimens. Person-level results represent the probability that an individual will receive one or more FOBT+, CRC− result after 10 years of either annual or biennial screening. Analysis 2 used a censoring bias adjusted discrete survival model (15) to allow for the possibility of dependent censoring (see Supplementary Material 1). This approach first uses logistic regression to estimate covariate effects associated with risk of a first FOBT+, CRC− result at each screening round adjusting for screening round number and censoring time. The resulting logistic regression model is then used to estimate the probability of an FOBT+, CRC− result for each screening round, conditional on a specific censoring time and covariate profile. Finally, estimates from individual rounds are aggregated to the person level to obtain the probability of receiving at least one FOBT+, CRC− result over the course of 10 years of screening using the standard actuarial estimator, the discrete time analogue of the Kaplan-Meier estimator (16), and results are marginalized over the distribution of censoring times.
In Analysis 2, models for the cumulative probability of FOBT+, CRC− results included characteristics of both the screening regimen and patient characteristics. The logistic regression models used to estimate the probability of an FOBT+, CRC− result conditioned on age at first exam, race, sex, year of the exam, screening round number, total number of screening rounds before censoring, and the elapsed time between FOBTs (for tests after the first). Age and calendar year were categorized as in Analysis 1. Screening round number and censoring time were treated as categorical with a separate category for each round. Elapsed time between tests was categorized as 90 days – 180 days, 180 days – 1.5 years, 1.5 – 2.5 years, 2.5 – 3.5 years, or 3.5 years. This model treats elapsed time since the prior FOBT as a time-varying covariate to allow screening intervals to vary within individual. For instance an individual with three observed FOBTs, at ages 51.2, 53.4, and 54.5 years would contribute three observations to the analysis. The first observation would be classified as a first screening test, the second would be classified as an interval of 1.5 – 2.5 years, and the third would be classified as an interval of 180 days – 1.5 years. Each observed test contributes to estimation of model parameters related to the associated interval (first exam, 180 days – 1.5 years, or 1.5 – 2.5 years).
We adapted a previously proposed discrete survival approach used to estimate cumulative false-positive probabilities to account for unique features of colorectal cancer screening (14). Specifically, we accounted for the fact that individuals receiving a diagnostic colonoscopy during the course of a decade of annual FOBT screening will no longer be eligible for future FOBT screening and hence are no longer at risk of FOBT+, CRC− results by treating diagnostic colonoscopy as a competing event. In survival analysis, both individuals who experience a censoring event and those experiencing a competing event are removed from the risk set. Individuals who experience a censoring event are assumed to be at the same risk of the event as those who are not censored, though the event can no longer be observed. In contrast, individuals who experience a competing event are assumed to no longer be at risk for the outcome of interest. In this analysis, death, diagnostic colonoscopy, and diagnosis with colorectal cancer were treated as competing events. We used methods for estimating cumulative incidence curves in the presence of competing risks to account for these outcomes (15, 17). We censored individuals at the earliest of disenrollment from Group Health, screening colonoscopy, or the end of the study period. Unlike diagnostic colonoscopy, screening colonoscopy was not treated as a competing event because these individuals would have been eligible for additional FOBTs, had they chosen to continue screening with FOBT rather than switching to colonoscopy.
Standard errors were estimated based on 1,000 bootstrap replicates (see Supplementary Material 1). We report cumulative probabilities for annual and biennial screening regimens for FOBT+, CRC− results and FOBT+, Colonoscopy, CRC− results. Ten years of annual screening was defined as 10 screening exams with an interval of 180 days – 1.5 years between exams. Ten years of biennial screening was defined to be 5 exams separated by an interval of 1.5 – 2.5 years. Age-, race-, and sex-specific estimates were combined using indirect standardization to provide overall estimates of risk. All cumulative risk estimates were based on risk levels for calendar years 2007 – 2009. For reference purposes, we also report the cumulative true-positive probability after 10 years of annual or biennial FOBT screening using the same methodology as described above.
We defined statistical significance using a two-sided alpha-level of 0.05. Analyses were performed in R 2.15.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
We observed 181,950 FOBTs from 94,637 individuals. Overall, the rate of FOBT+, CRC− results was 4.4%, the colorectal cancer incidence rate was 3.5 per 1,000 tests, and the true-positive rate for FOBT was 1.9 per 1,000 tests. In our cohort, 39% of individuals received flexible sigmoidoscopy at some point during study follow-up, 8% received barium enema, and 0.03% received CT colonography. The majority of individuals contributed only a single examination to our analysis (53.4%), while more than five exams were observed for 2.7%. At their first FOBT, the majority of individuals were age 50–59 years (54.9%). Individuals observed to receive an FOBT+, CRC− result at their first test were more likely to be older, male, and non-white than were those with no FOBT+, CRC− result at their first screening test (Table 1).
Table 1.
Characteristics of study population stratified by FOBT result (positive (FOBT+) or negative (FOBT−)) at first test.
| FOBT- (N = 90,271) |
FOBT+ (N = 4,366) |
|||
|---|---|---|---|---|
| N | % | N | % | |
| Age at first exam | ||||
| 50–54 | 29541 | 32.7 | 1251 | 28.7 |
| 55–59 | 20058 | 22.2 | 884 | 20.2 |
| 60–64 | 14025 | 15.5 | 681 | 15.6 |
| 65–69 | 10868 | 12 | 576 | 13.2 |
| 69–74 | 8827 | 9.8 | 536 | 12.3 |
| 75–79 | 6952 | 7.7 | 438 | 10 |
| Sex* | ||||
| Male | 40394 | 44.7 | 2292 | 52.5 |
| Female | 49874 | 55.3 | 2074 | 47.5 |
| Race | ||||
| White | 66609 | 73.8 | 2895 | 66.3 |
| Asian/Pacific Islander | 6262 | 6.9 | 469 | 10.7 |
| Black | 2492 | 2.8 | 168 | 3.8 |
| American Indian/Alaskan Native | 399 | 0.4 | 17 | 0.4 |
| Unknown/other | 14509 | 16.1 | 817 | 18.7 |
| Year of first exam | ||||
| 1997–1998 | 24132 | 26.7 | 1144 | 26.2 |
| 1999–2000 | 16447 | 18.2 | 873 | 20 |
| 2001–2002 | 13742 | 15.2 | 646 | 14.8 |
| 2003–2004 | 11181 | 12.4 | 537 | 12.3 |
| 2005–2006 | 9481 | 10.5 | 401 | 9.2 |
| 2007–2009 | 15288 | 16.9 | 765 | 17.5 |
Sex was missing for 3 individuals with FOBT-results at their first test.
At their first FOBT, 4.6% of individuals received an FOBT+, CRC− result and 74.9% of these received a subsequent colonoscopy within 12 months (Table 2). The probability of FOBT+, CRC− results decreased in subsequent screening rounds while the probability that an FOBT+, CRC− result would be followed-up with a colonoscopy increased. An FOBT+, CRC− result was also less likely to be followed up with colonoscopy when only 90 – 180 days had elapsed since the prior FOBT compared to longer intervals between FOBTs.
Table 2.
Number and percentage of FOBT+, CRC− results and number and percentage of positive FOBTs followed by colonoscopy stratified by number of prior screening tests and elapsed time since prior test.
| FOBT+, CRC− N |
FOBT+, CRC− % |
Colonoscopy following FOBT+, N |
Colonoscopy following FOBT+, % |
|
|---|---|---|---|---|
| Screening round | ||||
| 1 | 4366 | 4.6 | 3269 | 74.9 |
| 2 | 1755 | 4.0 | 1448 | 82.5 |
| 3 | 737 | 3.4 | 627 | 85.1 |
| 4 | 346 | 3.1 | 302 | 87.3 |
| 5+ | 273 | 2.7 | 239 | 87.5 |
| Elapsed time since prior FOBT* | ||||
| 90 – 180 days | 59 | 7.2 | 45 | 76.3 |
| 180 days – 1.5 years | 862 | 3.5 | 723 | 83.9 |
| 1.5 – 2.5 years | 1006 | 3.3 | 860 | 85.5 |
| 2.5 – 3.5 years | 519 | 3.5 | 428 | 82.5 |
| >3.5 years | 665 | 3.9 | 560 | 84.2 |
First FOBTs are excluded from analyses stratified by elapsed time since prior test.
Older age was significantly associated with increased odds of an FOBT+, CRC− result (odds ratio (OR) = 1.80, 95% confidence interval (CI) [1.64, 1.98] for age 75 – 79 years compared to age 50 – 54 years) (Table 3). Individuals of non-white race were also significantly more likely to receive FOBT+, CRC− results. Asian/Pacific Islander individuals were at highest risk with an OR of 1.88 compared to white individuals (95% CI [1.74, 2.03], p < 0.001). Women were significantly less likely to receive an FOBT+, CRC− result (OR = 0.75, 95% CI [0.71, 0.79], p < 0.001). Having received a prior negative FOBT was associated with significantly lower risk of an FOBT+, CRC− result (p < 0.001), while later years of examination were associated with increased risk of an FOBT+, CRC− result compared to 1997–1998 (p ranging from <0.001 to 0.65).
Table 3.
Association between odds of FOBT+, CRC− results at an individual screening round and patient characteristics and between colonoscopy risk among those with FOBT+ results and patient characteristics based on logistic regression.
| FOBT+, CRC− | Colonoscopy following FOBT+ | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | ||||||
| 50–54 | 1 | ref | ref | 1 | ref | ref |
| 55–59 | 1.08 | (0.99, 1.17) | 0.091 | 0.91 | (0.73, 1.12) | 0.371 |
| 60–64 | 1.25 | (1.14, 1.36) | <0.001 | 0.93 | (0.74, 1.16) | 0.520 |
| 65–69 | 1.42 | (1.30, 1.56) | <0.001 | 0.90 | (0.72, 1.13) | 0.358 |
| 69–74 | 1.61 | (1.47, 1.76) | <0.001 | 0.85 | (0.67, 1.06) | 0.151 |
| 75–79 | 1.80 | (1.64, 1.98) | <0.001 | 0.58 | (0.46, 0.72) | <0.001 |
| Year of exam | ||||||
| 1997–1998 | 1 | ref | ref | 1 | ref | ref |
| 1999–2000 | 1.19 | (1.09, 1.30) | <0.001 | 1.74 | (1.43, 2.12) | <0.001 |
| 2001–2002 | 1.11 | (1.01, 1.22) | 0.023 | 2.00 | (1.61, 2.47) | <0.001 |
| 2003–2004 | 1.19 | (1.08, 1.32) | <0.001 | 2.63 | (2.07, 3.34) | <0.001 |
| 2005–2006 | 1.02 | (0.92, 1.14) | 0.650 | 1.95 | (1.52, 2.49) | <0.001 |
| 2007–2009 | 1.14 | (1.04, 1.24) | 0.005 | 2.48 | (2.00, 3.07) | <0.001 |
| Sex | ||||||
| Male | 1 | ref | ref | 1 | ref | ref |
| Female | 0.75 | (0.71, 0.79) | <0.001 | 0.91 | (0.8, 1.03) | 0.136 |
| Race | ||||||
| White | 1 | ref | ref | 1 | ref | ref |
| Asian/Pacific Islander | 1.88 | (1.74, 2.03) | <0.001 | 0.76 | (0.63, 0.92) | 0.005 |
| Black | 1.47 | (1.29, 1.68) | <0.001 | 1.13 | (0.81, 1.57) | 0.483 |
| American Indian/Alaskan Native | 1.42 | (1.03, 1.96) | 0.035 | 1.20 | (0.50, 2.90) | 0.688 |
| Number of prior FOBTs | ||||||
| 0 | 1 | ref | ref | 1 | ref | ref |
| 1 | 0.77 | (0.73, 0.83) | <0.001 | 1.48 | (1.25, 1.76) | <0.001 |
| 2 | 0.64 | (0.58, 0.70) | <0.001 | 1.66 | (1.29, 2.13) | <0.001 |
| 3 | 0.57 | (0.50, 0.64) | <0.001 | 1.90 | (1.32, 2.75) | 0.001 |
| 4+ | 0.48 | (0.42, 0.55) | <0.001 | 1.94 | (1.29, 2.91) | 0.002 |
Among those with positive results, odds of receiving a colonoscopy in the following year were significantly lower for Asian/Pacific Islander individuals compared to white individuals (OR = 0.76, 95% CI [0.63,0.92]). Those with positive results who were screened in more recent years were highly statistically significantly more likely to receive a follow-up colonoscopy (OR = 2.48 in 2007–2009 compared to 1997–1998, 95% CI [2.00, 3.07]), as were those with a greater number of prior FOBT examinations (OR = 1.94, 95% CI [1.29, 2.91] for individuals with 4 or more prior FOBTs compared to those with no prior tests). The oldest individuals included in our study (age 75 – 79 years) were significantly less likely to receive a colonoscopy following a positive FOBT compared to the youngest individuals (age 50 – 54 years) (OR = 0.58, 95% CI [0.46, 0.72]).
Overall, we estimated the percentage of individuals receiving an FOBT+, CRC− test result after 10 years of annual screening with FOBT to be 23.0% (95% CI: (18.2, 27.0)) (Table 4). Under biennial screening this risk was reduced to 10.4% (95% CI: (9.2, 10.6)). Risks were lower for individuals who began screening at age 50 and higher for individuals who began screening at age 65. After 10 years of screening, the cumulative probability of a true-positive result was 0.6% under annual screening and 0.4% under biennial screening. Thus, an increase in true-positive probability of 0.2% associated with adopting annual compared to biennial screening came at the cost of an increase in the cumulative probability of FOBT+, CRC− results of 12.6%, an incremental benefit of 0.016 cancers detected per FOBT+, CRC− result.
Table 4.
Estimated percentage of individuals receiving an FOBT+, CRC− result or FOBT+, Colonoscopy, CRC− result after 10 years (95% CI) under annual or biennial screening. Overall estimates were adjusted via indirect standardization to the age and race distribution of the population. Starting age-specific estimates were adjusted via indirect standardization to the race distribution for that age group. All estimates are based on risk levels for 2007 – 2009.
| FOBT+, CRC− % (95% CI) |
FOBT+, Colonoscopy, CRC− % (95% CI) |
|
|---|---|---|
| Overall | ||
| Annual | 23.0 (18.2, 27.0) | 20.5 (16.1, 24.7) |
| Biennial | 10.4 (9.2, 10.6) | 8.8 (7.6, 8.9) |
| Starting age 50 | ||
| Annual | 20.1 (15.7, 23.7) | 18.4 (14.2, 22.3) |
| Biennial | 9.1 (7.9, 9.3) | 7.8 (6.7, 8.0) |
| Starting age 65 | ||
| Annual | 27.7 (21.5, 32.9) | 24.8 (19.6, 29.9) |
| Biennial | 12.5 (10.8, 12.9) | 10.6 (8.9, 11.0) |
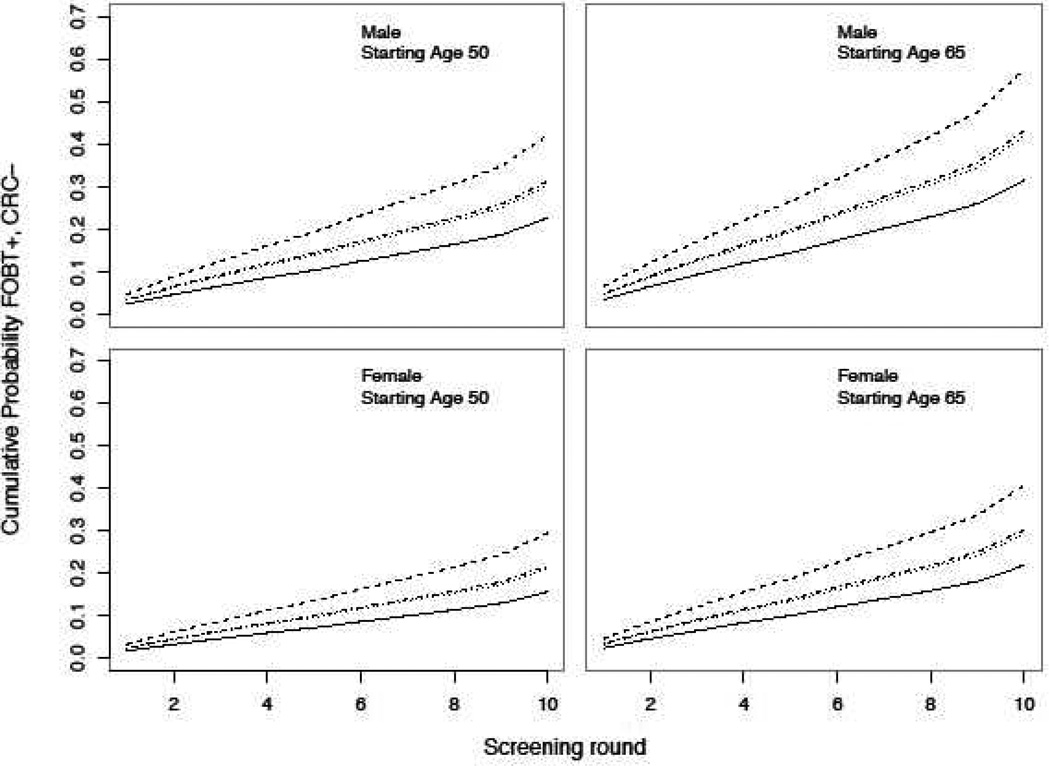
Differences in the probability of an FOBT+, CRC− result between individuals of differing race were small after a single screening FOBT but were substantially magnified after 10 years of annual screening (Figure 1). For instance, among women who began screening at age 50 the probability of an FOBT+, CRC− result on the first FOBT was 1.8% for white women and 3.4% for Asian women. However, the probability of having received at least one FOBT+, CRC− result after 10 screening examinations was 15.7% for white women and 29.5% for Asian women. Similar trends were observed for men and individuals who began screening at age 65.
Figure 1.
Cumulative probability of receiving at least one FOBT+, CRC− result after 1 – 10 years of annual FOBT screening stratified by starting age, race, and sex. All estimates are based on risk levels for 2007 – 2009. Solid = white, dashed = Asian, dotted = black, dashed/dotted = American Indian/Alaskan Native.
Discussion
In a large study of repeat screening with FOBT, we found that 23% of individuals screened annually and 10% of individuals screened biennially with no subsequent colorectal cancer diagnosis within one year will receive at least one positive test result after 10 years of screening. The majority of individuals with a positive FOBT result will also receive a follow-up diagnostic colonoscopy. It is reassuring that the imperfect specificity of FOBT does not mitigate its value as a non-invasive alternative to colonoscopy. After a decade of annual screening with FOBT rather than screening with once per decade colonoscopy, over 75% of individuals will have avoided exposure to colonoscopy and the concomitant risk of adverse events.
Overall, we found the one-time risk of a positive FOBT without subsequent colorectal cancer diagnosis within one year to be 4–5%. This is lower than estimates of the false-positive risk of FOBT of around 10% from previous studies of FOBT in average-risk screening populations (10, 11). This may be attributable to differences in the demographic characteristics of our study population. Notably, these prior studies included a larger Asian population, and we found risk of a false-positive result to be significantly higher in this group compared to white individuals.
Risk of positive FOBT results without subsequent colorectal cancer diagnosis varied substantially by sex and race. The risk in Asian individuals was twice that in white individuals. The differences in risk associated with sex and race could be attributable to greater use of FOBT as a diagnostic test in these populations. That is, if FOBT is used in symptomatic patients, who have an underlying condition that results in bleeding in the gastrointestinal tract, this will tend to increase the sensitivity and decrease the specificity of FOBT. Several recent studies have found that non-white individuals are less likely to be screened with colonoscopy compared to white individuals (18, 19). This may suggest a cultural bias against colonoscopy, which could increase rates of use of FOBT as a diagnostic test in response to individual preferences, increasing the positivity rate. Another possible explanation is greater deviation from dietary recommendations in these populations. Differences in false-positive risk may reflect ethnic differences in dietary practices. Alternatively, individuals with language barriers may not receive native language dietary recommendations and therefore may be less able to comply with guidelines. The observed very high risk of positive FOBT results among Asian individuals with no subsequent cancer diagnosis is of particular concern because rates of colorectal cancer are generally lower in this population (20), calling into question the ratio of harms and benefits.
Increases in the risk of positive FOBT without subsequent colorectal cancer diagnosis for older individuals may be due to increased bleeding. This could be attributable to greater use of aspirin or oral anticoagulants. While some studies have shown the positive predictive value of FOBT to be reduced in individuals taking aspirin and anti-coagulants (21), other studies have found no difference (22).
We observed substantial changes in the odds that positive results would be followed by colonoscopy over the course of the study period. Trends in the diagnostic evaluation of a positive FOBT are consistent with changes in clinical practice at Group Health. In November 1998, Group Health began an electronic registry to track patients with a positive FOBT who had not received a complete diagnostic evaluation. Subsequently, in 2003 an audit system was instituted to track and document follow-up care for these individuals. A previous study found that these efforts coincided with significant improvements in rates of colonoscopy following positive FOBT (23). Our study confirms these findings and indicates ongoing improvements through 2009. It is likely that colonoscopy is contraindicated for some individuals who did not receive diagnostic colonoscopy.
The effectiveness of screening with FOBT is limited by the extent to which individuals are willing to utilize this test and adhere to guidelines for repeat screening. A previous study found that 23% of eligible individuals in the Group Health population screened with FOBT within a two year period, and 44% of screened individuals subsequently screened again within two years of the initial test (24). The prevalence of FOBT screening in our population is slightly higher than the national rate of 17% based on the National Health Interview Study (25) likely because our population was fully insured. However, given the modest rates of compliance with annual FOBT screening both in Group Health population and nationally, FOBT screening is unlikely to be reaching its full potential benefit.
Colorectal cancer screening with guaiac-based FOBT is being replaced by fecal immunochemical testing (FIT). Several studies have shown improved specificity for FIT compared to FOBT in average risk populations (10, 11). The lower false-positive risk of FIT at individual screening rounds compared to FOBT implies that the long-term cumulative probability of receiving a false-positive test result would also be lower. Since the transition to FIT began in 2008, estimates of the long term burden of screening with FIT will not be available for a minimum of 5 years. In the interim, our estimates for FOBT provide an upper bound for the risk associated with annual screening with FIT.
Strengths of this study include its large sample size, long follow-up period allowing us to observe some individuals over many years of repeat FOBT screening, and evaluation of an average risk population receiving screening as part of standard clinical care. Our study has several limitations. Our estimates are based on a model that incorporates between-test correlation and accounts for the timing of multiple tests. Still, none of the individuals in our sample underwent annual (or biennial) testing over a ten year period, and so our estimates are based on some extrapolation. Not all individuals with a positive FOBT received a diagnostic colonoscopy within a year and others may have had a diagnostic colonoscopy that missed the cancer, so that some cancers may have remained clinically occult during this period resulting in misclassification of some true-positive FOBT results. We expect that such misclassification constituted only a small fraction of FOBT+, CRC− results in our study because the majority of individuals with positive FOBTs did receive diagnostic colonoscopy and colorectal cancer diagnosis is rare, even among those with positive FOBTs. Our estimates thus constitute an upper bound on false-positive risk. Additionally, our data did not include information about adenomas detected at colonoscopy subsequent to a positive FOBT. Colorectal cancer was surely prevented in a small fraction of these individuals through removal of a lesion destined to transition to cancer. However, because our objective was to determine the proportion of individuals following a regimen of FOBT that would experience a colonoscopy and thus receive the same harms and benefits as a regimen of screening colonoscopy, we believe including FOBTs followed by adenoma detection with other positive FOBTs where no cancer was subsequently detected is appropriate. Even allowing for these considerations, which would tend to inflate our cumulative risk estimates, we found that the risk after 10 years was relatively low.
Recommendations for colorectal cancer currently include screening regimens consisting of colonoscopy, flexible sigmoidoscopy, or FOBT. The decision of which regimen to adopt should be based on individual preferences, informed by the benefits and harms of the test. In the case of FOBT, individuals may prefer this test because it is non-invasive, does not require bowel preparation, and does not carry risks of adverse events. As the false-positive risk of FOBT increases, more individuals screened with FOBT will be evaluated with diagnostic colonoscopy mitigating the benefits of choosing this regimen. Our results support the idea that annual stool-based screening is a way to screen for colorectal cancer with substantially fewer colonoscopies compared to screening with colonoscopy once every ten years.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the National Cancer Institute of the National Institutes of Health (grant numbers R03CA15007, U01CA152959).
Footnotes
Conflicts of interest: None
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2008 Incidence and Mortality Web-based Report. [Accessed June 13, 2012];2012 Available at: http://www.cdc.gov/uscs.
- 2.Hewitson P, Glasziou P, Irwig L, Towler B, Watson E. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev. 2007:CD001216. doi: 10.1002/14651858.CD001216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 4.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 5.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 6.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 7.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 8.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 9.US Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 10.Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. New England Journal of Medicine. 1996;334:155–159. doi: 10.1056/NEJM199601183340304. [DOI] [PubMed] [Google Scholar]
- 11.Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. Journal of the National Cancer Institute. 2007;99:1462–1470. doi: 10.1093/jnci/djm150. [DOI] [PubMed] [Google Scholar]
- 12.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:659–669. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher DA, Grubber JM, Castor JM, Coffman CJ. Ascertainment of colonoscopy indication using administrative data. Dig Dis Sci. 2011;55:1721–1725. doi: 10.1007/s10620-010-1200-y. [DOI] [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society Series B-Statistical. 1972;34:187–220. [Google Scholar]
- 15.Hubbard R, Miglioretti D. A semiparametric censoring bias model for estimating the cumulative risk of a false-positive screening test under dependent censoring. Biometrics. 2013;69:245–253. doi: 10.1111/j.1541-0420.2012.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelfand AE, Smith AF. Sampling-based approaches to calculating marginal densities. Journal of the American Statistical Association. 1990;85:398–409. [Google Scholar]
- 17.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Doubeni CA, Laiyemo AO, Reed G, Field TS, Fletchers RH. Socioeconomic and Racial Patterns of Colorectal Cancer Screening among Medicare Enrollees in 2000 to 2005. Cancer Epidemiology Biomarkers & Prevention. 2009;18:2170–2175. doi: 10.1158/1055-9965.EPI-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in Colorectal Cancer Test Use among Vulnerable Populations in the United States. Cancer Epidemiology Biomarkers & Prevention. 2011;20:1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SEER. SEER Stat Fact Sheets: Colon and Rectum. 2013 May 23; 2013 Available from: http://seer.cancer.gov/statfacts/html/colorect.html.
- 21.Sawhney MS, McDougall H, Nelson DB, Bond JH. Fecal Occult Blood Test in Patients on Low-Dose Aspirin, Warfarin, Clopidogrel, or Non-steroidal Anti-inflammatory Drugs. Digestive Diseases and Sciences. 2010;55:1637–1642. doi: 10.1007/s10620-010-1150-4. [DOI] [PubMed] [Google Scholar]
- 22.Bini EJ, Rajapaksa RC, Weinshel EH. Positive predictive value of fecal occult blood testing in persons taking warfarin. American Journal of Gastroenterology. 2005;100:1586–1592. doi: 10.1111/j.1572-0241.2005.41979.x. [DOI] [PubMed] [Google Scholar]
- 23.Miglioretti DL, Rutter CM, Bradford SC, Zauber AG, Kessler LG, Feuer EJ, et al. Improvement in the diagnostic evaluation of a positive fecal occult blood test in an integrated health care organization. Medical care. 2008;46:S91–S96. doi: 10.1097/MLR.0b013e31817946c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenton JJ, Elmore JG, Buist DS, Reid RJ, Tancredi DJ, Baldwin LM. Longitudinal adherence with fecal occult blood test screening in community practice. Annals of family medicine. 2010;8:397–401. doi: 10.1370/afm.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100:2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.