Abstract
Background
The molecular mechanisms that underlie clonidine exacerbation of behavioral impairment caused by ethanol are not fully known. We tested the hypothesis that NOS-NO signaling in the locus coeruleus (LC) is implicated in this phenomenon.
Methods
Male, Sprague-Dawley rats, with intracisternal and jugular vein cannulae implanted 6 days earlier, were tested for drug-induced behavioral impairment. The latter was assessed as the duration of loss of righting reflex (LORR) and rotorod performance every 15 min until the rat recovered to the baseline walk criterion (180 s). In a separate cohort, we measured p-nNOS, p-eNOS, and p-ERK1/2 in the LC following drug treatment, vehicle, or NOS inhibitor.
Results
Rats that received clonidine (60 μg/kg, i.v.) followed by ethanol (1 or 1.5 g/kg, i.v.) exhibited synergistic impairment of rotorod performance. Intracisternal (i.c.) pretreatment with non-selective NOS inhibitor L-NAME (0.5 mg) or selective nNOS inhibitor N-propyl-L-arginine (NPLA, 1 μg) exacerbated the impairment of rotorod performance caused by clonidine-ethanol combination. Exacerbation of behavioral impairment was caused by L-NAME enhancement of the effect of ethanol, not clonidine. L-NAME did not influence blood ethanol levels; thus, the interaction was pharmacodynamic. LORR caused by clonidine (60 μg/kg, i.v.)-ethanol (1 g/kg, i.v.) combination was abolished by selective inhibition of central eNOS (L-NIO, 10 μg i.c.) but not by nNOS inhibition under the same conditions. Western blot analyses complemented the pharmacological evidence by demonstrating that clonidine-ethanol combination inhibits phosphorylation (activation) of nNOS (p-nNOS) and increases the level of phosphorylated eNOS (p-eNOS) in the LC; the change in p-nNOS was paralleled by similar change in LC p-ERK1/2. NOS inhibitors alone did not affect the level of nitrate/nitrite, p-nNOS, p-eNOS, or p-ERK1/2 in the LC.
Conclusions
Alterations in NOS-derived NO in the LC underlie clonidine-ethanol induced behavioral impairment. A decrease in nNOS activity, due at least partly to a reduction in nNOS phosphorylation, mediates rotorod impairment, while enhanced eNOS activity contributes to LORR, elicited by clonidine-ethanol combination.
Keywords: Ethanol, Clonidine, Neuronal NOS, Endothelial NOS, Locus coeruleus
Introduction
The interactions between alcohol and prescription drug medications, such as the antihypertensive drug clonidine, are cause for serious public health concern. The antagonistic hemodynamic interaction between ethanol and clonidine renders the antihypertensive effect of clonidine less effective (El-Mas and Abdel-Rahman, 1999; El-Mas and Abdel-Rahman, 2001; Mao and Abdel-Rahman, 1998), while an equally important behavioral interaction between these two drugs produces a clinically serious adverse effect. The synergistic behavioral impairment elicited by clonidine-ethanol interaction has been reported (Bender and Abdel-Rahman, 2009; Mao and Abdel-Rahman, 1996) and supported by findings from interactions with related drug classes (Czarnecka et al., 1986; Durcan et al., 1991; Idanpaan-Heikkila et al., 1995; Kushikata et al., 2002). Although the sedative effect of clonidine is utilized in some clinical applications, such as analgesia, anesthesia, or treatment of withdrawal symptoms (Fauler and Verner, 1993; Khan et al., 1999), the combined use of ethanol and clonidine and the resultant synergistic behavioral impairment is a greater concern for patients taking clonidine unsupervised as an antihypertensive agent.
The mechanisms underlying this synergistic interaction are still being elucidated. Our recent study (Bender and Abdel-Rahman, 2009) has identified an important role for the central α2A-adrenergic receptor in the behavioral interaction between clonidine and ethanol. Many studies including our own have implicated nitric oxide synthase (NOS)-derived nitric oxide (NO) in the neurobiological effects of clonidine and ethanol when administered alone (e.g. Adams et al., 1994; El-Mas et al., 2008; Nassar and Abdel-Rahman, 2008; Pokk et al., 2001; Soares de Moura et al., 2001; Vassiljev et al., 1998). NOS-NO signaling plays a role in a variety of critical processes, including neurotransmission (Clementi et al., 1995; Kiss and Vizi, 2001; Kosenko et al., 2003), vasorelaxation (Bredt et al., 1992), cytotoxicity (Clementi et al., 1995; Nelson et al., 2003), cell proliferation (Cha et al., 2001), gene transcription (Clementi et al., 1995), locomotor activity (Pechanova et al., 2006), and hypotension (Li and Abdel-Rahman, 2001; Moreira et al., 2004; Sy et al., 2001). However, the effect of ethanol or clonidine on NOS activity or NO levels and the relationship of NOS-NO signaling to behavioral impairment remain controversial. Ethanol-induced behavioral impairment has been attributed to augmentation (Adams et al., 1994; Pokk et al., 2001; Vassiljev et al., 1998) or attenuation (Ikeda et al., 1999; Phung and Black, 1999) of NOS-NO signaling, depending on the system studied. Reports are similarly contradictory for clonidine-induced behavioral impairment (Soares de Moura et al., 2001; Vulliemoz et al., 1996). Therefore, NOS-NO signaling has been implicated one way or the other in the behavioral effects of both ethanol and clonidine when administered alone. Further, NOS-derived NO inhibits firing of LC neurons (Xu et al., 1994), which are implicated in the production of ethanol and/or clonidine behavioral effects (De Sarro et al., 1987; Verbanck et al., 1991).
Although there are three isoforms of NOS, most studies investigating the effects of ethanol or clonidine on central NOS-NO signaling have focused on utilizing non-selective NOS or selective nNOS inhibitors. Nevertheless, all three isozymes are found in the brain (Syapin, 1998) and could potentially contribute to the mechanism of synergy between clonidine and ethanol (Bender and Abdel-Rahman, 2009). Further, neuronal NOS (nNOS) and endothelial NOS (eNOS) seem to have differential effects on behavioral responses (Jiang et al., 2002; Rickard and Gibbs, 2003). Importantly, since peak behavioral synergy occurs within the first 30 min of clonidine-ethanol interaction (Bender and Abdel-Rahman, 2009), the involvement of inducible NOS (iNOS), which requires 1-2 hr for induction (Syapin, 1998), seems unlikely. At the cellular level, nNOS and eNOS activity is regulated by phosphorylation at specific serine residues by kinases (Bredt et al., 1992). Extracellular signal-regulated protein kinase (ERK, also known as p42/44 MAPK) has been shown to increase NOS activity by enhancing NOS phosphorylation (Wyatt et al., 2002). Both clonidine (α2A-adrenergic receptor agonist) and ethanol have been linked to activation of ERK signaling (Alblas et al., 1993; Bachtell et al., 2002). Nevertheless, no attempt has been made to investigate the role of NOS-NO signaling in the LC in the behavioral interaction between clonidine and ethanol or to elucidate the relative contribution of nNOS vs. eNOS to the behavioral impairment caused by the drug combination.
The aim of these studies was to test the hypothesis that a synergistic enhancement of NOS-NO signaling in the LC underlies clonidine exacerbation of ethanol-evoked behavioral impairment. Therefore, the first objective of these experiments was to investigate the overall involvement of central NOS-NO signaling in the behavioral effects of clonidine-ethanol combination. This was achieved by evaluating behavioral responses (rotorod impairment and LORR) elicited by clonidine-ethanol combination in the absence or presence of the non-selective NOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME). The effect of L-NAME on the behavioral responses elicited by either drug (clonidine or ethanol) was investigated to elucidate the relative contribution of either drug to the NOS-NO signaling and whether the NOS inhibitor influenced blood ethanol levels. Second, we utilized the selective nNOS (N-propyl-L-arginine, NPLA) or eNOS (N(5)-(1-iminoethyl)-L-ornithine, L-NIO) inhibitor to elucidate the contribution of either isoform to the behavioral impairment caused by the drug combination. Finally, we complemented the pharmacological studies and directly tested our hypothesis by measuring nitrate/nitrite level in the LC and blood and conducting Western blot studies to investigate the effects of NOS inhibitor, clonidine, ethanol, and clonidine-ethanol combination on the level of p-nNOS and p-eNOS as well as pERK1/2 in the LC. The cellular and neurochemical studies were conducted on brains collected at a time that coincides with peak synergistic behavioral impairment caused by the drug combination.
Methods
Animals
Male, Sprague-Dawley rats were obtained from Taconic Farms (Germantown, New York) and housed individually in a controlled environment room with a constant temperature of 23 ± 1°C, humidity of 50 ± 10%, and a 12:12 hr light-dark cycle. Food (Prolab Rodent Chow, Prolab RMH 3000, Granville Milling, Creedmoor, NC) and water were available ad libitum. At least 2 days were allowed for acclimatization prior to surgical or experimental manipulation. Animals weighed approximately 325-375 g on the day of behavioral testing. All surgical, experimental, and animal care procedures were performed in accordance with National Institutes of Health and institutional animal care and use committee guidelines.
Surgical Procedures
All surgical procedures were conducted under sterile conditions as reported in our recent study (Bender and Abdel-Rahman, 2009). Animals were anesthetized with sodium pentobarbital (60 mg/kg, i.p.). A stainless steel guide cannula (23 G) was inserted approximately 6 mm into the skull between the occipital bone and the cerebellum so that its tip protruded into the cisterna magna. The cannula was secured in place with small metal screws and dental acrylic cement (Durelon, Thompson Dental Supply, Raleigh, NC) as described previously (El-Mas and Abdel-Rahman, 2001). Stainless steel wire (0.012” diameter) inside the guide cannula served as a block until the day of the experiment. Intracisternal cannulation was not performed in animals used for neurochemical studies. All animals were vascularly catheterized for intravenous (i.v.) administration of drugs. For behavioral studies, the jugular vein was isolated, and a polyethylene-50, gas sterilized catheter filled with heparinized saline (100 U/mL) was inserted approximately 2 cm into the vessel. In some animals, the carotid artery was similarly catheterized for collection of blood samples during behavioral testing. For neurochemical studies, the femoral vein was isolated, and two smaller catheters (polyethylene-10 tubing connected to polyethylene-50 tubing, gas sterilized) filled with heparinized saline were inserted into the abdominal vena cava. All catheters were: (i) secured by thread to the blood vessel and muscle tissue, (ii) tunneled subcutaneously (s.c.) and exteriorized at the back of the neck between the shoulder blades, (iii) flushed with heparinized saline, and (iv) closed with sterilized metal pins. For carotid artery catheterizations, muscle around the trachea was sutured prior to closing the wound. The neck or leg wound was swabbed with povidone-iodine solution and closed by wound clips. All rats received postoperative care that comprised buprenorphine hydrochloride (0.03 mg/kg, s.c.) and penicillin G benzathine/penicillin G procaine (100,000 units/kg, s.c). Following recovery from anesthesia, the rats were provided with wet food (rat chow softened in water) to facilitate food intake and gain in body weight post-surgery. Rats were allowed to recover for approximately 6 days before the experiment following intracisternal cannulation or 2 days following femoral catheterization only as in our reported studies (El-Mas and Abdel-Rahman, 2001; Mao and Abdel-Rahman, 1996).
Behavioral Testing Protocol
Behavioral impairment was assessed by performance on a rotorod (Treadmill for Rats 7700, Ugo Basile Biological Research Apparatus, Italy) operated at constant speed (∼11 RPM). Prior to surgery, rats were trained to walk on the rotorod for 180 seconds consecutively without falling off as in reported studies (Bender and Abdel-Rahman, 2009; Dar, 1998). All animals were successfully trained to this baseline criterion. On the day of the experiment, all animals were retested to ensure that they could still meet the baseline criterion post-surgery. Animals received an intracisternal (i.c.) injection (pretreatment) of pharmacological inhibitor or vehicle (artificial cerebrospinal fluid, aCSF) followed by two i.v. injections of drug (60 μg/kg clonidine, 1 or 1.5 g/kg ethanol, or their combination) and/or vehicle (saline) depending on the experiment. The duration of pretreatment varied depending on the pharmacological inhibitor used. Briefly, after removing the block, a 30 G stainless steel injector was inserted into the guide cannula to deliver the pharmacological inhibitor or aCSF to the cisterna magna by a microsyringe connected to the injector with polyethylene tubing. A volume of 3-4 μL was delivered by hand over 30-60 sec. After 5-min, the injector was removed and the block was replaced for the duration of testing. The drug given i.v. was routinely followed by saline flush to ensure complete drug delivery. A 10-min injection interval between clonidine and ethanol was employed throughout these studies based on our earlier findings (Bender and Abdel-Rahman, 2009; Mao and Abdel-Rahman, 1996). Loss of righting reflex (LORR) was assessed by placing the animal on its back and recording the duration (in minutes) before it righted itself (all four paws touching the floor). Rotorod performance was assessed every 15 min after the two i.v. injections until recovery to the baseline criterion was achieved or until the end of the experiment (3 hr max). Each assessment consisted of a maximum of 3 walk trials, with a 180 sec cutoff time as reported (Bender and Abdel-Rahman, 2009; Dar, 1998). The highest walk time (180 sec max.) was recorded for each time point. Steps were taken to ensure that animals were not injured by falling. No cardiovascular parameters (e.g. blood pressure) were measured in these studies. Animals were euthanized by pentobarbital overdose at the end of the experiment. Correct placement of the intracisternal cannula was determined by i.c. injection of fast green dye.
Blood Alcohol Concentration (BAC)
In some animals, arterial blood samples (0.1 ml/sample) were collected every 15 min for the first 60 min following ethanol administration. Arterial catheters used to withdraw blood during testing were flushed with heparinized saline after each sample. All blood samples were collected on ice in heparinized microcentrifuge tubes and centrifuged at 4°C for 10 min at 5000 RPM. Supernatants were transferred to fresh tubes and stored at −80°C. Blood alcohol concentration (BAC) was determined by a standard enzymatic assay (Bernt and Gutmann, 1974) with UV-vis spectrophotometric detection (Beckman DU 640 or Nanodrop ND-1000 Spectrophotometer).
Western Blot Analysis
Brains were collected for neurochemical studies 15 min after the drug and/or saline injections – the time corresponding to peak drug-induced behavioral impairment (Bender and Abdel-Rahman, 2009). Animals received a lethal dose of sodium pentobarbital (i.p.), and following decapitation, brains were removed, flash frozen in 2-methylbutane (cooled on dry ice for at least 30 min), and stored at −80°C until use. Brains were equilibrated to −20°C and sectioned with a cryostat (HM 505E, Microm International GmbH, Waldorf, Germany) rostrally to the locus coeruleus (LC) according to atlas coordinates (Paxinos and Watson, 1998). Tissue from the LC was collected bilaterally using a 0.75-mm punch instrument as described in other studies (e.g. Mouledous et al., 2007) from approximately −10.04 mm to −9.30 mm from bregma (Paxinos and Watson, 1998). Tissue was homogenized on ice by sonication in cell lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM activated sodium orthovanadate) containing protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN). After centrifugation at 4°C at 10,000 RPM for 20 min, protein in the supernatant was quantified using a standard Bio-Rad protein assay system (Bio-Rad Laboratories, Hercules, CA). Protein extracts (20 μg per lane) were denatured at 97°C for 5-10 min, separated on NuPAGE Novex Bis-Tris 4-12% SDS-PAGE gels (Invitrogen, Carlsbad, CA) using MOPS NuPAGE running buffer, and electroblotted to nitrocellulose membranes (cold transfer buffer: 230 mM glycine, 25 mM Tris, 0.7 mM SDS, 20% methanol). Positive controls for actin (Sigma, St. Louis, MO), phosphorylated neuronal or endothelial NOS (rat cerebrum or human endothelial cell lysate, respectively, BD Transduction/BD Biosciences, San Jose, CA), and p42/44 MAPK (rat cerebrum) as well as a marker (SeeBlue® Plus2 Pre-Stained Standard, Invitrogen, CA) were loaded on each gel as appropriate. Non-specific binding sites on the membranes were blocked at room temperature in wash buffer (10 mM Tris, 150 mM NaCl, 0.2% 0.5 M EDTA pH 8.0, 0.01% Triton X-100) containing 5% non-fat milk for 1-2 hr. The membranes were then incubated overnight at 4°C with rabbit polyclonal antibodies to phospho-nNOS (pSer1416) (1:400, Affinity BioReagents, Golden, CO), phospho-eNOS (pSer1179) (1:100, Zymed/Invitrogen, Carlsbad, CA), or p42/44 MAPK (also known as pERK1/2, 1:100, Promega, Madison, WI) in block solution. The blots were washed 4× then incubated for 60 min at room temperature with anti-rabbit IgG horseradish peroxidase-linked secondary antibody (1:2,000, GE Healthcare, Piscataway, NJ). After 4 more washes, protein was detected on the blots by enhanced chemiluminescence and exposure to x-ray film. Equivalent sample loading was confirmed by stripping the membranes with Blot Fresh Stripping Reagent (SignaGen, Gaithersburg, MD) and reprobing with rabbit anti-actin antibody (1:2,000, Sigma); all data were expressed as values normalized to actin. Although multiple gels were run due to the number of samples, control and treated samples were loaded on each gel.
Nitrate/Nitrite (NO×) Assay
Portions of the protein homogenate supernatants collected for Western blot analyses were ultrafiltered through Microcon, Ultracel YM-10 filters (Millipore/Fisher, Bedford, MA) by centrifuging at 25°C for 20 min at 10,000 RPM. Total nitrate/nitrite (NO×) concentrations of LC homogenates and plasma from blood samples were determined by standard colorimetric assay (kit from Cayman Chemical, Ann Arbor, MI) using Griess reagents and a 3 hr incubation period. Values for LC homogenates were normalized to protein as in our recent studies (El-Mas and Abdel-Rahman, 2009; El-Mas et al., 2008).
Protocols and Experimental Groups
Experiment 1. Behavioral effect of clonidine, ethanol or their combination
Four groups of rats were used to investigate the effect of a single drug (clonidine or ethanol) or their combination on animal behavior assessed as rotorod performance and LORR. The data generated under this experiment also served as controls for the data generated following pretreatment with selective or nonselective NOS inhibitors under experiment 2. Therefore, all rats that comprised the 4 groups received i.c. aCSF (vehicle for NOS inhibitors) pretreatment 90 min prior to the following i.v. injections: (i) saline + saline, (ii) clonidine (60 μg/kg) + saline, (iii) saline + ethanol (1.5 g/kg), or (iv) clonidine (60 μg/kg) + ethanol (1.5 g/kg).
Experiment 2. Effect of non-selective or selective NOS inhibition on behavioral impairment caused by clonidine-ethanol combination
A total of 9 groups of rats (in addition to the control groups from experiment 1) was used in this experiment to investigate the effect of nonselective or selective NOS inhibition on behavioral effects elicited by clonidine (60 μg/kg) given alone or in combination with one of two doses (1 or 1.5 g/kg) of ethanol. The doses of clonidine and ethanol are based on preliminary and reported findings (Bender and Abdel-Rahman, 2006; Bender and Abdel-Rahman, 2009; Mao and Abdel-Rahman, 1996). Three groups of rats received pretreatment with the nonselective NOS inhibitor L-NAME (0.5 mg, i.c.) 90 min prior to i.v. injections of the following: (i) clonidine (60 μg/kg) + saline, (ii) saline + ethanol (1.5 g/kg), or (iii) clonidine (60 μg/kg) + ethanol (1.5 g/kg). The effects of a larger dose (1 mg, i.c.) of L-NAME or the selective nNOS inhibitor NPLA (1 μg, i.c.) on the synergistic behavioral impairment elicited by clonidine (60 μg/kg) + ethanol (1.5 g/kg) were investigated in two additional groups. L-NAME doses and pretreatment time were chosen based on preliminary experiments and reported findings (Ayers et al., 1997; Noda et al., 1995). Blood samples from these 5 groups, in addition to the ethanol and clonidine + ethanol control groups from experiment 1, were collected for blood alcohol analysis as described above. Given the relatively high blood ethanol concentration achieved following the administration of the 1.5 g/kg dose of ethanol, subsequent behavioral and neurochemical studies were conducted with 1 g/kg dose of ethanol; BAC achieved following this dose are comparable to levels achieved following social drinking (Abdel-Rahman, 1989; El-Mas and Abdel-Rahman, 1998). Two control groups, added for the lower ethanol dose, received aCSF (i.c.) pretreatment 90 min prior to i.v. injections of: (i) saline + ethanol (1 g/kg) or (ii) clonidine (60 μg/kg) + ethanol (1 g/kg). To investigate the effect of selective NOS inhibition on clonidine-ethanol interaction, the last two groups of rats received NPLA (1 μg, i.c.) or L-NIO (10 μg, i.c.) pretreatment 15 min prior to i.v. injections of clonidine (60 μg/kg) + ethanol (1 g/kg). NPLA and L-NIO doses were selected based on reported findings (El-Haddad et al., 2002; Ferreira et al., 1999). The shorter pretreatment time (as opposed to 90 min) was based on preliminary experiments as well as several studies employing times no more than 30 min (Jiang et al., 2002; Klamer et al., 2004; Li et al., 2003).
Experiment 3. Effect of clonidine, ethanol, and their combination on ERK1/2, eNOS, and nNOS phosphorylation in the locus coeruleus
For neurochemical studies, four groups of rats, which received no pretreatment, were given two i.v. injections as follows: (i) saline + saline, (ii) clonidine (60 μg/kg) + saline, (iii) saline + ethanol (1 g/kg), or (iv) clonidine (60 μg/kg) + ethanol (1 g/kg). Brain tissue from the locus coeruleus (LC) was collected, as described above, 15 min after i.v. injections (time of peak clonidine-ethanol behavioral synergy) and processed for measurements of protein levels of p42/44 MAPK (p-ERK1/2) and phosphorylated neuronal (p-nNOS) and endothelial (p-eNOS) NOS by Western blot as described in our previous study (Bender and Abdel-Rahman, 2009). Total nitrate/nitrite (NO×) levels in the LC tissue homogenates were also determined as in our previous studies (El-Mas and Abdel-Rahman, 2009; El-Mas et al., 2008). Behavioral testing was conducted in two additional groups of rats, which were pretreated with the p-ERK1/2 inhibitor PD98059 (6 μg, i.c.) 10 min prior to i.v. injections of (i) saline + saline or (ii) clonidine (60 μg/kg) + ethanol (1 g/kg). The dose and pretreatment time for PD98059 were selected based on reported findings (Lin et al., 2004).
Experiment 4. Effect of central NOS inhibition on ERK1/2, eNOS and nNOS phosphorylation in the locus coeruleus
In this experiment we investigated whether pretreatment with the NOS inhibitors used prior to clonidine-ethanol combination (Experiment 2) reduced NOS phosphorylation and nitrate/nitrite level or influenced pERK1/2 in the LC. Furthermore, the possibility that the centrally injected NOS inhibitors leaked into peripheral circulation and inhibited peripheral NOS was investigated in this experiment by measuring plasma nitrate/nitrite level in treatment and control groups. Four groups of rats received two i.v. injections of saline (control vehicle for clonidine and ethanol) following i.c. pretreatment with: (i) aCSF, (ii) L-NAME (0.5 mg), (iii) NPLA (1 μg), or (iv) L-NIO (10 μg). As described in Experiment 3, brain tissue from the LC was collected for Western blot analysis of p42/44 MAPK (p-ERK1/2), p-nNOS, and p-eNOS as well as total nitrate/nitrite (NO×) levels. Blood samples (femoral artery catheter) taken before and after NOS inhibitor pretreatment were also assayed for total NO× level.
Drug Preparation
All drugs and chemicals were obtained commercially. Ethanol (Pharmco, Brookfield, CT) was given as a 100% solution as in earlier studies from our lab (e.g. Bender and Abdel-Rahman, 2009; Mao and Abdel-Rahman, 1996). Clonidine hydrochloride (Sigma, St. Louis, MO) was prepared in sterile saline (0.9% sodium chloride, Abbott Labs, North Chicago, IL). Saline injections were given at 0.1 mL/100 g body weight. Artificial CSF was prepared as: 123 mM NaCl, 0.86 mM CaCl2, 3 mM KCl, 0.89 mM MgCl2 • 6 H2O, 0.5 mM NaH2PO4, 0.25 mM Na2HPO4, and 25 mM NaHCO3. L-NAME (Sigma), NPLA and L-NIO dihydrochloride (Tocris Bioscience), and fast green dye (Sigma) were prepared in aCSF. PD98059 (Sigma) was dissolved in dimethyl sulfoxide (DMSO, Sigma) and delivered i.c. over 5 min.
Data Analysis and Statistical Procedures
Data were input into Excel and Prism for graphical analysis and calculation of averages ± S.E.M. and area under the curve (AUC). Statistical analyses were performed using SPSS or Prism software. Rotorod data were analyzed by repeated measures ANOVA to determine differences in time course trends. Differences at each time point were determined by multiple one-way ANOVA's. Rotorod AUC and LORR treatment averages were compared by one-way ANOVA or unpaired t-test (two-tailed) as specified in Results. Behavioral synergy was evaluated with rotorod AUC data, where a linear combination of means was used within the ANOVA setting (to take advantage of the pooling of error terms) to compare the sum of the single drug treatment groups (clonidine or ethanol) to combination treatment groups. The linear combination of means was achieved through the use of a contrast coefficient, where the sum of the two single drug groups (each denoted “1”) could be compared by ANOVA with the combination group (denoted “-1”). For post hoc comparisons, Tukey's test was used when data had equal variance, and the Games-Howell test was used when data had unequal variance. Differences in blood alcohol concentration (BAC) were determined by two-way ANOVA (time × treatment) followed by Bonferroni post hoc tests. Western blot bands were scanned and quantified by measuring the integrated density (mean density × area) using NIH Image software (version 1.37). Data were normalized in relation to actin, tested for normality (Shapiro-Wilk test) to identify and exclude outlier values, and analyzed by univariate ANOVA which included a blocking factor to remove variability associated with running samples on multiple gels. Treatment differences were determined by least significant difference (LSD) post-hoc tests. For graphical presentation, group means are shown as % saline or aCSF control. Treatment means for nitrate/nitrite concentration (NO×) data were compared by one-way ANOVA. In all analyses, p<0.05 was considered statistically significant.
Results
Effect of clonidine, ethanol, or their combination on behavioral performance
Animals treated with clonidine or ethanol alone exhibited impaired rotorod performance that peaked at 15-20 min before returning to baseline rotorod performance within 90 min (Fig. 1A). A similar pattern was evident in the rats that received the drug combination except that the magnitude of impairment was significantly greater [AUC, one-way ANOVA between aCSF pretreated groups, F(3,30) = 30.883, p<0.05] than that caused by either drug and lasted much longer (Fig. 1A, C). As shown in Fig. 1C, clonidine synergistically enhanced ethanol-evoked impairment of rotorod performance [ANOVA with contrast, 1 = sum of clonidine and ethanol AUC, −1 = clonidine-ethanol AUC, F(2,23) = 23.643, p<0.05]. Similarly, pretreatment with clonidine enhanced ethanol-evoked LORR (Fig. 1D). Vehicle (aCSF or saline) treated animals exhibited no behavioral deficits.
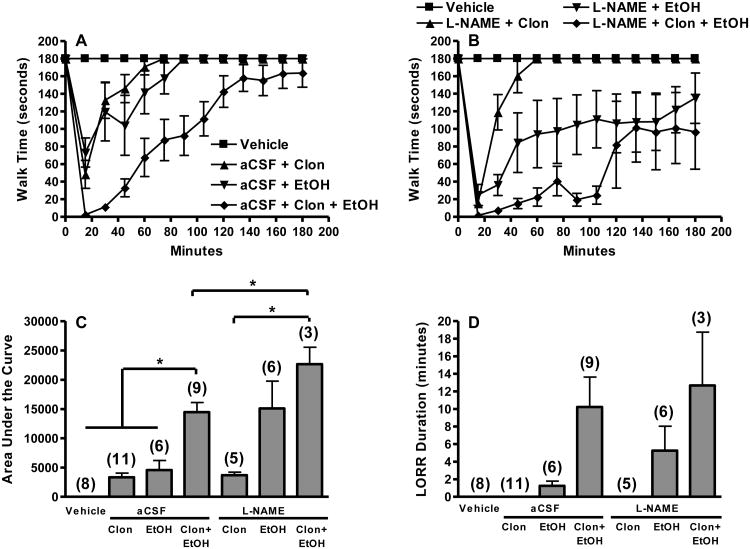
Figure 1.
Exacerbation of clonidine-ethanol evoked behavioral impairment following NOS inhibition is predominantly mediated by enhancement of ethanol action. Rotorod time courses (A) are shown for control groups pretreated i.c. with aCSF 90 min prior to i.v. injections of: (i) saline + saline (Vehicle), (ii) 60 μg/kg clonidine + saline (aCSF + Clon), (iii) saline + 1.5 g/kg ethanol (aCSF + EtOH), or (iv) 60 μg/kg clonidine + 1.5 g/kg ethanol (aCSF + Clon + EtOH). Rotorod time courses (B) are shown for groups pretreated i.c. with 0.5 mg L-NAME 90 min prior to i.v. injections of: (i) 60 μg/kg clonidine + saline (L-NAME + Clon), (ii) saline + 1.5 g/kg ethanol (L-NAME + EtOH), or (iii) 60 μg/kg clonidine + 1.5 g/kg ethanol (L-NAME + Clon + EtOH). The Vehicle group is replotted in panel B for comparison. Corresponding area under the curve (AUC, C) and LORR durations (D) are shown together for both aCSF and L-NAME pretreated groups. The number of animals per group is shown in parentheses (*, p<0.05). Rotorod time course trends differed between: (i) Clon + EtOH and all other aCSF groups (panel A), and (ii) L-NAME + Clon and L-NAME + Clon + EtOH (panel B) (repeated measures ANOVA). Clonidine-ethanol treated groups (panel A and B) were significantly different from vehicle and single drug treated groups until the 105 min time point as determined by multiple one-way ANOVA's (p<0.05). Clonidine synergistically enhanced ethanol-evoked behavioral impairment. The AUC for aCSF + Clon + EtOH was significantly greater than the sum of the AUC for aCSF + Clon and aCSF + EtOH. Due to unequal variance (Games-Howell post hoc test), LORR data did not reach significance.
Effect of non-selective or selective NOS inhibition or pERK1/2 inhibition on behavioral impairment caused by clonidine-ethanol combination
The effect of the non-selective NOS inhibitor L-NAME (0.5 or 1 mg, i.c.) or the selective nNOS inhibitor NPLA (1 μg, i.c.) on rotorod impairment and LORR elicited by 60 μg/kg clonidine + 1.5 g/kg ethanol combination are shown in Fig. 1 and 2. Interestingly, while L-NAME (0.5 mg) did not alter rotorod impairment elicited by clonidine (Fig. 1C), it enhanced ethanol-evoked rotorod impairment to a level similar to that produced by clonidine-ethanol combination (Fig. 1C). L-NAME (0.5 mg) pretreated rats that received clonidine-ethanol combination treatment exhibited significantly more impairment on the rotorod than those that received clonidine alone [AUC, one-way ANOVA between L-NAME (0.5 mg) pretreated groups, F(2,11) = 5.706, p<0.05, Fig. 1C]. Compared to aCSF, L-NAME (0.5 or 1 mg, i.c.) pretreatment enhanced the behavioral impairment (rotorod performance and LORR) caused by clonidine-ethanol combination (Fig. 1, 2). This enhancement was significant for clonidine-ethanol induced rotorod impairment following L-NAME (0.5 mg) pretreatment compared to aCSF pretreatment [AUC, unpaired t-test, t(10) = -2.512, p<0.05, Fig. 1C]. As shown in Fig. 2, NPLA (1 μg, i.c.) pretreatment significantly enhanced impairment of rotorod performance caused by 60 μg/kg clonidine + 1.5 g/kg ethanol combination [AUC, one-way ANOVA between clonidine-ethanol treated groups, F(2,19) = 4.678, p<0.05, Fig. 2B]. Although the difference did not achieve statistical significance, LORR responses caused by the drug combination in animals pretreated with L-NAME or NPLA paralleled rotorod responses [LORR, one-way ANOVA between all groups in figure, F(6,41) = 5.224, p>0.05, Fig. 1D; LORR, one-way ANOVA between clonidine-ethanol treated groups, F(2,19) = 2.385, p>0.05, Fig. 2C].
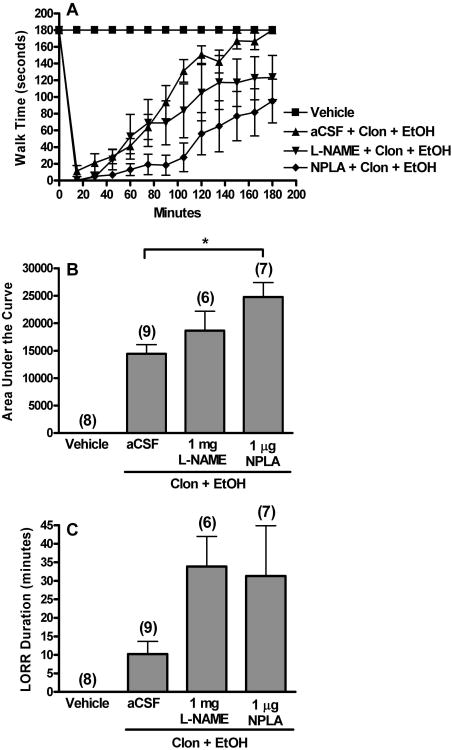
Figure 2.
Non-selective NOS inhibition or selective nNOS inhibition exacerbates clonidine-ethanol evoked behavioral impairment. Rotorod time courses (A), corresponding area under the curve (AUC, B), and LORR durations (C) are shown for groups pretreated i.c. with aCSF, 1 mg L-NAME, or 1 μg NPLA 90 min prior to i.v. injections of clonidine (60 μg/kg, Clon) + ethanol (1.5 g/kg, EtOH). The aCSF + saline + saline (Vehicle) and aCSF + Clon + EtOH groups are replotted from Fig. 1 for comparison. The number of animals per group is shown in parentheses (*, p<0.05). The aCSF and NPLA pretreated groups differed in rotorod time course trends (repeated measures ANOVA) as well as time points from 90-165 min (multiple one-way ANOVA's).
Because the 1.5 g dose of ethanol resulted in relatively high BAC, which was further increased by NPLA pretreatment (see below), we reduced the dose of ethanol to 1 g/kg in another set of experiments in which the effect of nNOS-selective inhibitor (NPLA) or eNOS-selective inhibitor (L-NIO) on behavioral impairment induced by clonidine (60 μg/kg) + ethanol (1 g/kg) combination was investigated. The lower dose (1 g/kg) of ethanol was also used in the neurochemical studies discussed below. In rats pretreated with i.c. aCSF (vehicle for NOS inhibitors), clonidine (60 μg/kg) + ethanol (1 g/kg) combination elicited synergistic behavioral impairment [ANOVA with contrast, 1 = sum of clonidine (60 μg/kg, Fig. 1) and ethanol (1 g/kg, Fig. 3) AUC, -1 = clonidine-ethanol (Fig. 3) AUC, F(2,29) = 25.066, p<0.05]. As shown in Fig. 3, NPLA (1 μg, i.c.) or L-NIO (10 μg, i.c.) pretreatment exacerbated rotorod impairment caused by clonidine-ethanol combination compared to aCSF pretreatment [AUC, one-way ANOVA between ethanol and three combination treated groups, F(3,28) = 25.302, p<0.05, Fig. 3B]. LORR elicited by clonidine-ethanol combination was not altered by NPLA pretreatment (Fig. 3C). By contrast, L-NIO abolished the LORR caused by clonidine-ethanol combination [LORR, one-way ANOVA between ethanol and three combination treated groups, F(3,31) = 3.388, p<0.05, Fig. 3C]. Finally, pharmacological inhibition of ERK1/2 phosphorylation with PD98059 did not produce any behavioral deficits per se (Table 1, PD98059 + Saline + Saline). However, at the selected dose (6 μg, i.c.), while PD98059 only slightly increased rotorod impairment induced by clonidine-ethanol combination [AUC, unpaired t-test, t(15) = -1.210, p>0.05], it prolonged LORR duration [LORR, unpaired t-test, t(18) = -1.970, p=0.064, Table 1].
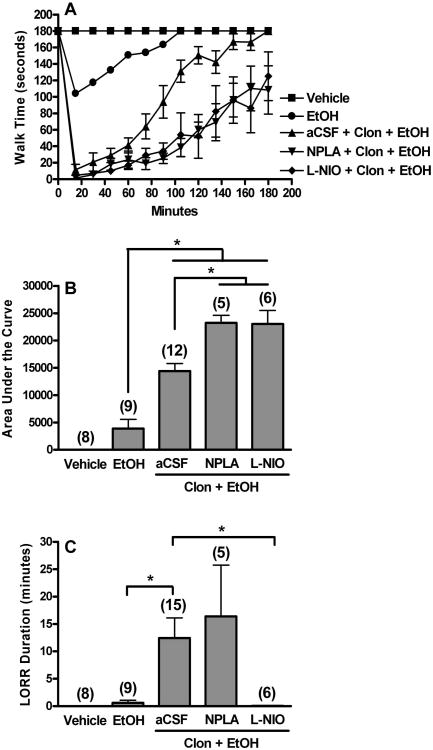
Figure 3.
Selective nNOS (see also Fig. 2) or eNOS inhibition exacerbates clonidine-ethanol evoked rotorod impairment while selective eNOS, but not nNOS, inhibition abolishes clonidine-ethanol induced LORR. Rotorod time courses (A), corresponding area under the curve (AUC, B), and LORR durations (C) are shown for groups pretreated i.c. with aCSF, NPLA (1 μg), or L-NIO (10 μg) 15 min prior to i.v. injections of clonidine (60 μg/kg) + ethanol (1 g/kg). The aCSF + saline + saline (Vehicle) treated group is replotted from Fig. 1 for comparison. The aCSF pretreated control group for the lower dose (1 g/kg) of ethanol (EtOH) is also shown. Clonidine synergistically enhanced ethanol (1 g/kg)-evoked behavioral impairment. The AUC for aCSF + Clon + EtOH was significantly greater than the sum of the AUC for aCSF + Clon (plotted in Figure 1) and aCSF + EtOH. The number of animals per group is shown in parentheses (*, p<0.05). (Note: A few additional animals were tested only for LORR in the aCSF + Clon + EtOH group.) Rotorod time course trends differed between: (i) EtOH and all other groups, and (ii) aCSF + Clon + EtOH and NPLA + Clon + EtOH (repeated measures ANOVA). Oneway ANOVA's at each time point indicated that EtOH differed from combination treated groups (15-150 min), and aCSF + Clon + EtOH differed from both the NPLA pretreated group (90-120 min) and the L-NIO pretreated group (120 min).
Table 1. Effect of p-ERK1/2 inhibition (PD98059, 6 μg, i.c.) on rotorod impairment (expressed as area under the curve, AUC) and LORR induced 10 min later by i.v. injections of clonidine (60 μg/kg) + ethanol (1 g/kg).
Values expressed as mean ± S.E.M.
| Treatment | N | AUC | N | LORR Duration (min) |
|---|---|---|---|---|
| aCSF + Saline + Saline1 | 8 | 0 ± 0 | 8 | 0.00 ± 0.00 |
| PD98059 + Saline + Saline | 3 | 0 ± 0 | 3 | 0.00 ± 0.00 |
| aCSF + Clonidine + Ethanol1,2 | 12 | 14,421 ± 1374 | 15 | 12.43 ± 3.69 |
| PD98059 + Clonidine + Ethanol2 | 5 | 17,544 ± 2274 | 5 | 29.20 ± 10.07 |
Data from aCSF pretreated rats (Fig. 3) are presented for comparison.
Comparison of clonidine-ethanol treated groups by unpaired t-test (p>0.05).
Blood alcohol concentrations (BAC) achieved following 1.5 g/kg ethanol administration alone (after saline, vehicle) or after clonidine in the absence (aCSF pretreatment, vehicle) or presence of NOS inhibitor pretreatment (behavioral data shown in Fig. 1 and 2) are shown in Table 2. In general, all pretreatments (except for 0.5 mg L-NAME + clonidine) resulted in greater blood ethanol concentration (Table 2) compared to ethanol alone. However, the difference was only significant (based on two-way ANOVA followed by Bonferroni post hoc tests) in the case of NPLA + clonidine pretreatment (Table 2). Blood ethanol level declined as expected over the time course.
Table 2. Effects of pretreatment with clonidine (60 μg/kg, i.v.) alone or in combination with L-NAME (0.5 mg or 1 mg, i.c.) or NPLA (1 μg, i.c.) on blood alcohol concentration (BAC, mg/dL) achieved following ethanol (1.5 g/kg, i.v.) administration.
| Treatment | 15 min | 30 min | 45 min | 60 min |
|---|---|---|---|---|
| Ethanol | 115.95 ± 8.43 (6)* | 109.49 ± 10.69 (6)* | 98.36 ± 13.54 (3)* | 77.95 ± 9.12 (3)* |
| Clonidine + Ethanol | 146.45 ± 11.60 (6) | 125.99 ± 9.51 (6) | 114.39 ± 9.16 (5) | 99.37 ± 11.30 (5) |
| L-NAME (0.5 mg) + Ethanol | 142.98 ± 12.62 (6) | 122.68 ± 15.03 (6)* | 118.82 ± 11.19 (5) | 107.35 ± 7.77 (4) |
| L-NAME (0.5 mg) + Clonidine + Ethanol | 110.66 ± 16.67 (2)* | 109.13 ± 17.57 (2) | 100.74 ± 15.92 (2) | 89.10 ± 17.44 (2) |
| L-NAME (1 mg) + Clonidine + Ethanol | 156.07 ± 6.22 (3) | 134.37 ± 14.13 (3) | 117.81 ± 12.52 (3) | 100.55 ± 10.25 (3) |
| NPLA + Clonidine + Ethanol | 188.15 ± 19.06 (7) | 171.64 ± 18.54 (7) | 157.12 ± 16.02 (7) | 141.51 ± 15.54 (7) |
Values are mean ± S.E.M. N values are indicated in parentheses.
p<0.05 compared with NPLA + Clonidine + Ethanol treatment by two-way ANOVA (Bonferroni post hoc tests indicate significant effects of time and treatment but not their interaction).
Effect of clonidine, ethanol, and their combination on p-ERK1/2, phosphorylated NOS, and nitrate/nitrite levels in the locus coeruleus (LC)
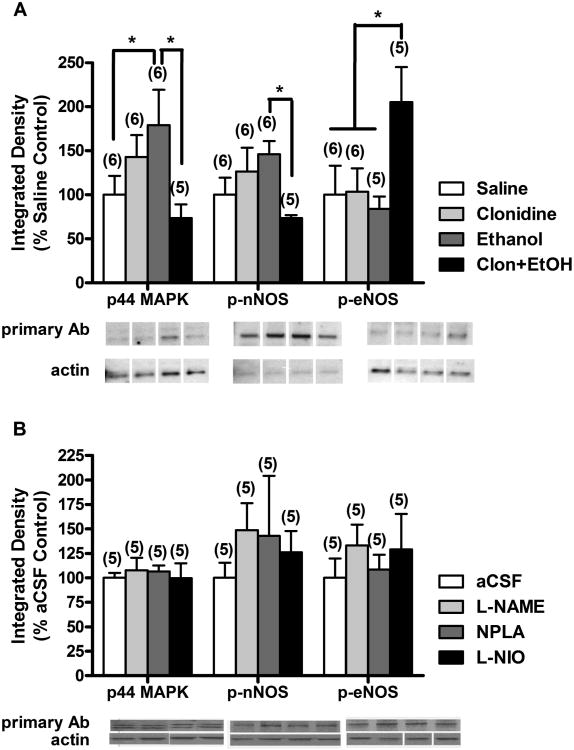
Levels of p-ERK1/2 in LC of animals treated with clonidine (60 μg/kg, i.v.) or ethanol (1 g/kg, i.v.) were increased compared to saline treated animals (Fig. 4A), though the difference was only significant for ethanol treatment. Interestingly, clonidine-ethanol combination treatment resulted in a decrease in p-ERK1/2 (Fig. 4A), which was significantly lower than levels found in ethanol-treated animals [p44 MAPK univariate ANOVA, F(3,18) = 23.855, p<0.05, LSD post hoc test]. The same expression pattern was observed for levels of phosphorylated (Ser1416) nNOS in the LC, where clonidine or ethanol treatment slightly increased the phosphorylated (active) nNOS (p-nNOS) level compared to saline treated animals and combination treatment decreased the level of p-nNOS to a significantly lower level than in ethanol treated animals [p-nNOS univariate ANOVA, F(3,18) = 28.671, p<0.05, LSD post hoc test, Fig. 4A]. LC level of phosphorylated (Ser1179) eNOS (p-eNOS) was not different for clonidine- or ethanol-treated, compared to saline-treated, animals; however, combination treatment increased p-eNOS level over control (saline treatment) and single-drug (clonidine or ethanol) treatment level [p-eNOS univariate ANOVA, F(3,17) = 17.706, p<0.05, LSD post hoc test, Fig. 4A]. Central inhibition of NOS with L-NAME (0.5 mg), NPLA (1 μg), or L-NIO (10 μg) did not significantly alter the level of p42/44 MAPK (p-ERK1/2), p-nNOS, or p-eNOS in LC compared to aCSF control (Fig. 4B).
Figure 4.
Inhibition of nNOS activity in the locus coeruleus (LC) is partly mediated by clonidine-ethanol induced attenuation of p-ERK1/2 (p42/44 MAPK) levels. LC homogenates were processed for measurements of levels of pERK1/2 (44 kDa band; p44 MAPK), Ser1416-phosphorylated nNOS (p-nNOS), and Ser1179-phosphorylated eNOS (p-eNOS) by Western blot in animals treated with: (A) i.v. injections of (i) saline + saline (Saline), (ii) 60 μg/kg clonidine + saline (Clonidine), (iii) saline + 1 g/kg ethanol (Ethanol), or (iv) 60 μg/kg clonidine + 1 g/kg ethanol (Clon+EtOH) or (B) i.v. injections of saline + saline following i.c. injections of (i) aCSF, (ii) 0.5 mg L-NAME, (iii) 1 μg NPLA, or (iv) 10 μg L-NIO. The number of animals per group is shown in parentheses (*, p<0.05). Data was normalized to actin and expressed as % saline control (A) or % aCSF control (B). Representative Western blot bands are presented below the graph for each primary antibody (Ab) and actin. Note: Actin representative bands may appear different because they are from different gels, as indicated by the white space between lanes; however, these bands correspond to the samples represented above them. The MAPK (pERK1/2) blot shows both the p42 and p44 kDa bands; however, there was no significant difference between treatments in the level of the 42 kDa band (data not shown).
Total nitrate/nitrite (NO×) level measured in the same LC homogenates used for Western blot analyses showed no significant difference between treatments (Table 3), nor did the NO× level in blood change significantly following treatment with NOS inhibitor (unpaired t-tests between blood samples taken before and after NOS inhibitor administration, data not shown).
Table 3. Total NO× (nitrate/nitrite) concentration in locus coeruleus tissue homogenates from animals treated with clonidine (60 μg/kg, i.v.), ethanol (1 g/kg, i.v.), NOS inhibitor, or vehicle.
Values are expressed as μmol/g protein (mean ± S.E.M.).
| Treatment | Total NO× Concentration in LC Homogenates |
|---|---|
| Saline (i.v.) | 1.96 ± 0.08 (6) |
| Clonidine | 2.28 ± 0.26 (6) |
| Ethanol | 2.42 ± 0.57 (6) |
| Clonidine + Ethanol | 2.67 ± 0.33 (6) |
| aCSF (i.c.) + Saline (i.v.) | 2.57 ± 0.32 (5) |
| L-NAME (0.5 mg, i.c.) + Saline (i.v.) | 3.24 ± 0.50 (5) |
| NPLA (1 μg, i.c.) + Saline (i.v.) | 2.48 ± 0.44 (5) |
| L-NIO (10 μg, i.c.) + Saline (i.v.) | 2.89 ± 0.31 (5) |
N values are indicated in parentheses. There were no significant treatment differences in NO× level of LC homogenates (one-way ANOVA).
Discussion
In the present studies, we utilized pharmacological interventions (non-selective and selective NOS inhibitors and pERK1/2 inhibitor) and conducted biochemical and molecular studies to elucidate the role of NOS-NO signaling in the LC in synergistic behavioral impairment elicited by clonidine-ethanol combination. The most important findings of these studies are: (i) non-selective NOS inhibition (L-NAME) enhances clonidine-ethanol induced rotorod impairment and LORR duration (Fig. 1, 2), (ii) L-NAME enhancement of clonidine-ethanol induced behavioral impairment is due primarily to enhancement of ethanol effects (Fig. 1C, 1D), (iii) selective nNOS inhibition enhances clonidine-ethanol induced behavioral impairment (both rotorod and LORR tests, Fig. 3), (iv) selective eNOS inhibition enhances clonidine-ethanol induced rotorod impairment but abolishes clonidine-ethanol evoked LORR (Fig. 3), and (v) clonidine-ethanol treatment tends to decrease p-ERK1/2 and p-nNOS levels, while increasing p-eNOS level in the LC (Fig. 4A). Together, the present findings yield insight into the mechanistic roles of the two constitutive NOS isoforms in the LC in the synergistic behavioral impairment caused by clonidine-ethanol combination.
The synergistic behavioral interaction between clonidine and ethanol that we originally reported based on LORR testing (Mao and Abdel-Rahman, 1996) has been replicated and extended to include rotorod performance in the present and in our recent (Bender and Abdel-Rahman, 2009) studies. Differences in neurobiological mechanisms that mediate LORR and impairment of rotorod performance allowed us to elucidate the role of the constitutive NOS isoforms (nNOS and eNOS) in the synergistic impairment of the two behaviors elicited by clonidine-ethanol combination.
As a first step, we opted to evaluate the overall contribution of NOS-NO signaling to the synergistic behavioral impairment elicited by clonidine-ethanol combination. Rotorod impairment evoked by clonidine (60 μg/kg)-ethanol (1.5 g/kg) combination was slightly enhanced by central L-NAME pretreatment (0.5 or 1 mg). LORR duration was also increased by L-NAME at the higher (1 mg) dose. These findings, which contradict our hypothesis, seem to suggest that the mechanism of synergy underlying the behavioral interaction between ethanol and clonidine involves a decrease in NOS-NO signaling. This notion agrees with several studies that demonstrated the ability of L-NAME itself to evoke behavioral impairment (Adams et al., 1994; Del Bel et al., 2005; Pokk et al., 2001; Vassiljev et al., 1998). These reported findings raise the possibility that L-NAME exacerbation of the behavioral impairment caused by clonidine-ethanol combination was merely the result of adding a third drug that caused behavioral impairment. It is imperative to note that the doses of L-NAME used in our study had no impact on rotorod performance or righting reflex when administered alone. More importantly, L-NAME does not generally exacerbate behavioral impairment caused by another drug in our model system as discussed below.
We demonstrate the interesting finding that L-NAME exacerbation of synergistic behavioral impairment caused by clonidine-ethanol combination was a result of its interaction with ethanol. Whereas L-NAME had no effect on clonidine-evoked behavioral impairment, it substantially enhanced ethanol-induced rotorod impairment (Fig. 1B, C). Importantly, in agreement with reported findings (Adams and Cicero, 1998), blood ethanol levels were not affected by L-NAME pretreatment. Together, these findings highlight the important role for central NOS-NO signaling in clonidine-ethanol evoked behavioral impairment and rule out potential contributions of nonspecific behavioral effects or changes in blood ethanol concentration caused by L-NAME.
The hypothesis that differential modulation of nNOS and eNOS activities may underlie the behavioral impairment caused by clonidine-ethanol combination was first tested by investigating the effect of selective nNOS inhibitor (NPLA) on the rotorod impairment and LORR caused by clonidine (60 μg/kg)-ethanol (1.5 g/kg) combination. The findings that NPLA pretreatment significantly enhanced the synergistic behavioral impairment caused by the drug combination seem to suggest that an overall reduction in NO, particularly nNOS-derived NO underlies the synergistic behavioral responses. Notably, NPLA-pretreated animals that subsequently received clonidine-ethanol combination exhibited significantly higher BAC compared to animals given only ethanol (Table 2). Nevertheless, the possibility that the higher BAC played a major role in NPLA exacerbation of the behavioral impairment caused by clonidine-ethanol combination is ruled out because a similar exacerbation occurred following L-NAME (0.5 mg) in the absence of any change in BAC (Table 2). Moreover, while there are no reports regarding the pharmacokinetic interaction (including effects on ethanol metabolizing enzymes) between ethanol and NPLA, one report showed that ethanol metabolism was slowed (i.e. BAC remained elevated) following a different nNOS selective inhibitor (7-nitroindazole, 7-NI) (Vassiljev et al., 1998). It appears therefore that pharmacokinetic interactions depend upon the specific inhibitor being used and further studies are needed in order determine specific pharmacokinetic interactions between ethanol and NPLA and other NOS inhibitors used in our model system. It is notable, however, that BAC obtained with the 1.5 g/kg ethanol was much higher than the levels achieved following social drinking. For this reason, we conducted additional studies in which a smaller dose (1 g/kg) of ethanol was combined with the same dose (60 μg/kg) of clonidine. This drug combination (Fig. 3) caused synergistic behavioral impairment, as previously reported (Bender and Abdel-Rahman, 2009), in the presence of BAC comparable to that achieved following social drinking (El-Mas and Abdel-Rahman, 1998). This dose regimen was adopted in subsequent studies with NPLA and L-NIO as well as in the neurochemical studies. NPLA pretreatment similarly exacerbated the impairment of rotorod performance caused by the lower dose (Fig. 3) as it did with the higher dose (Fig. 2) of ethanol when combined with clonidine. On the other hand, NPLA exacerbation of LORR occurred only with the combination that contained the higher dose of ethanol (Fig. 2).
We report the intriguing finding that while NPLA did not alter LORR induced by clonidine-ethanol (1 g/kg) combination, L-NIO abolished LORR under the same conditions. These findings support the hypothesis that eNOS-derived NO is involved in production of LORR, while nNOS-derived NO counterbalances or plays a minimal role in LORR caused by combining clonidine with a moderate (1 g/kg) dose of ethanol. Together, these findings suggest that clonidine-ethanol combination activates eNOS to produce LORR, but inhibits nNOS to impair rotorod performance; the latter seems to be affected by ethanol dose because it is more evident in the presence of the higher dose (1.5 g/kg) dose of ethanol. Alternately, nNOS-derived NO may counterbalance signaling implicated in rotorod impairment caused by clonidine-ethanol combination; therefore, inhibition of nNOS would remove this opposition, allowing clonidine-ethanol signaling to proceed unchecked. This suggestion could be supported by demonstrating the ability of clonidine-ethanol treatment to cause simultaneous activation of eNOS and inhibition of nNOS signaling in a neuroanatomical structure that is implicated in rotorod performance and LORR and serves as a target for both drugs. As discussed earlier, the LC is considered a prime candidate for testing this hypothesis. Although alternate explanations and the involvement of other brain areas in the interaction may be equally valid, one interesting possibility involves differential regulation of nNOS and eNOS activation via phosphorylation. Therefore, we investigated the effects of clonidine, ethanol, or their combination on the levels of phosphorylated (active) nNOS (p-nNOS) and eNOS (p-eNOS) in the LC.
The neurochemical (Western blot) studies were conducted at a time (15 min) that coincided with peak behavioral interaction. We show that p-nNOS and p-eNOS levels tended to be lower and higher, respectively, in the LC of clonidine-ethanol treated animals compared to saline treated controls (Fig. 4A). Interestingly, while p-eNOS level was not affected by either drug alone, p-nNOS level was increased over control (saline) by clonidine or ethanol treatment. However, there are no reports on the effect of clonidine or ethanol on phosphorylated forms of NOS, and the molecular mechanisms underlying the responses to single drug versus combination treatment, obtained in the present study, remain to be elucidated. Nonetheless, our pharmacological and neurochemical studies have identified p-nNOS and p-eNOS in the LC as molecular targets that underlie, at least partly, the synergistic behavioral impairment elicited by clonidine-ethanol combination. Moreover, this differential alteration in the level of the active forms of nNOS and eNOS might explain the contradictory reports on clonidine or ethanol effects on NOS-NO signaling (Davis and Syapin, 2005; Soares de Moura et al., 2001; Vulliemoz et al., 1996).
Although selective and nonselective NOS inhibitors are routinely used to investigate the role of NOS isoform(s) in a particular phenomenon, the possibility must be considered that significant reduction by these agents of NOS may influence the response to subsequent drug treatment(s) and confound data interpretation. Another potential confounding factor in our study is the possibility that the centrally administered NOS inhibitor might have leaked into general circulation and inhibited peripheral NOS activity. This is particularly important because ethanol and clonidine were administered systemically in the present investigation, and we showed in a recent study (El-Mas et al., 2008) that a similar dose of ethanol increased blood NO×. Our findings suggest that at the dose used, none of the three NOS inhibitors influenced the level of p-nNOS or p-eNOS or their upstream regulator pERK1/2 in the LC. These findings were confirmed by a lack of a change in NO× in the same area. Finally, lack of change in plasma NO× level following intracisternal administration of any of the three NOS inhibitors argues against peripheral interaction between NOS inhibitors and their peripheral targets. Furthermore, the new findings that centrally administered NOS inhibitors differently influenced the behavioral responses elicited by clonidine-ethanol combination without affecting peripherally mediated NO× generation might argue against significant involvement of blood NO×, generated by clonidine-ethanol combination, in the behavioral responses reported in the present study. Future studies will directly address this issue by measuring blood NO× in clonidine-ethanol treated animals in the absence or presence of centrally administered NOS inhibitor.
An established signaling pathway implicated in activation (phosphorylation) of nNOS and eNOS is the p-ERK1/2–NOS pathway (Schonhoff et al., 2001; Wyatt et al., 2002). Therefore, we considered the possibility that the alterations in LC p-ERK1/2 level might underlie the observed changes in nNOS and/or eNOS in the LC in the present study. This possibility is supported by the findings that clonidine or ethanol can interact with and/or activate ERK1/2 signaling either directly or indirectly (Alblas et al., 1993; Bachtell et al., 2002). In agreement with these findings, the present study showed that either clonidine or ethanol treatment increased LC p-ERK1/2 level. However, we report for the first time, that clonidine-ethanol combination decreased p-ERK1/2 level in the LC. This pattern mimics the reduction in p-nNOS level, suggesting that a reduction in p-nNOS might be a consequence of reduced p-ERK1/2 in the LC of clonidine-ethanol treated rats. Also, NOS inhibition alone did not influence p-ERK1/2 level in the LC, suggesting that any reduction in p-ERK1/2 that contributed to enhanced behavioral impairment was due entirely to the action of clonidine-ethanol treatment. These neurochemical findings are consistent with the pharmacological finding in which the p-ERK1/2 inhibitor, PD98059, only slightly enhanced clonidine-ethanol induced rotorod impairment; this finding is expected if p-ERK1/2 is already suppressed by clonidine-ethanol treatment. The dose (6 μg) of PD98059 used in the present study was based on reported findings (Lin et al., 2004). On the other hand, the findings with LC p-ERK1/2 do not explain the increase in p-eNOS in clonidine-ethanol treated rats, which implicates p-ERK1/2 independent mechanisms in eNOS phosphorylation; this notion is supported by reported studies (Dudzinski and Michel, 2007). Together, the present studies support the hypothesis that reduction of nNOS phosphorylation in the LC, caused by clonidine-ethanol combination, occurs as a consequence of p-ERK1/2 inhibition, while enhancement of eNOS phosphorylation utilizes p-ERK-independent pathways.
We report that overall nitrate/nitrite level in the LC did not change following administration of clonidine, ethanol, or their combination (Table 3). It must be remembered that Western blot and selective NOS inhibition data revealed contrasting effects of clonidine-ethanol combination on nNOS (inhibition) and eNOS (enhancement) in the LC. These findings may explain, at least partly, the lack of significant change in nitrate/nitrite level in the LC of rats receiving the drug combination, although the contribution of iNOS, which was not investigated in the present study, needs to be considered.
Overall, the present studies suggest that NOS-NO signaling in the LC is implicated in the synergistic behavioral interaction between clonidine and ethanol. While the effects of clonidine, ethanol, and their combination are expected to involve different brain structures and signaling mechanisms, we focused on the LC because it mediates some of the behavioral effects of each drug when administered alone. Clonidine-ethanol combination elicits differential alteration in the level of active (phosphorylated) nNOS (inhibition) and eNOS (enhancement) in the LC. These neurochemical findings complement, and might explain, the pharmacological findings with non-selective (L-NAME) and selective (NPLA and L-NIO) NOS inhibitors that demonstrated the importance of both NOS isoforms in mediating impairment of rotorod performance and the greater role of eNOS in mediating LORR caused by clonidine-ethanol combination. A reduction in LC p-ERK1/2 might explain the reduced phosphorylation of nNOS in clonidine-ethanol treated rats while the p-ERK1/2-independent cellular mechanism implicated in the enhanced phosphorylation of eNOS remains to be elucidated. Together, the present findings yield insight into NOS-NO signaling in the LC as a cellular mechanism that seems to underlie the synergistic behavioral impairment caused by clonidine-ethanol combination.
Acknowledgments
The authors would like to thank Dr. Kevin Obrien for his valuable assistance with the statistical analyses used in these experiments.
Supported by NIH grants 5F31AA015472 (TSB) and 2 R01 AA07839 (ARA)
References
- Abdel-Rahman AA. Reversal by ethanol of the hypotensive effect of clonidine in conscious spontaneously hypertensive rats. Hypertension. 1989;14:531–541. doi: 10.1161/01.hyp.14.5.531. [DOI] [PubMed] [Google Scholar]
- Adams ML, Cicero TJ. Alcohol intoxication and withdrawal: the role of nitric oxide. Alcohol. 1998;16:153–158. doi: 10.1016/s0741-8329(97)00185-7. [DOI] [PubMed] [Google Scholar]
- Adams ML, Meyer ER, Sewing BN, Cicero TJ. Effects of nitric oxide-related agents on alcohol narcosis. Alcohol Clin Exp Res. 1994;18:969–975. doi: 10.1111/j.1530-0277.1994.tb00068.x. [DOI] [PubMed] [Google Scholar]
- Alblas J, van Corven EJ, Hordijk PL, Milligan G, Moolenaar WH. Gi-mediated activation of the p21ras-mitogen-activated protein kinase pathway by alpha 2-adrenergic receptors expressed in fibroblasts. J Biol Chem. 1993;268:22235–22238. [PubMed] [Google Scholar]
- Ayers NA, Kapas L, Krueger JM. The inhibitory effects of N omega-nitro-L-arginine methyl ester on nitric oxide synthase activity vary among brain regions in vivo but not in vitro. Neurochem Res. 1997;22:81–86. doi: 10.1023/a:1027385522859. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002;302:516–524. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- Bender TS, Abdel-Rahman AA. Differential contribution of eNOS- and nNOS-derived NO to clonidine-ethanol induced behavioral impairment. Society for Neuroscience Annual Meeting. 2006;749.13 [Google Scholar]
- Bender TS, Abdel-Rahman AA. Alpha 2A-adrenergic receptor signaling underlies synergistic enhancement of ethanol-induced behavioral impairment by clonidine. Alcohol Clin Exp Res. 2009;33:408–418. doi: 10.1111/j.1530-0277.2008.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt E, Gutmann I. Ethanol determination with alcohol dehydrogenase and NAD. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Academic Press; New York: 1974. pp. 1499–1502. [Google Scholar]
- Bredt DS, Ferris CD, Snyder SH. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992;267:10976–10981. [PubMed] [Google Scholar]
- Cha MS, Lee MJ, Je GH, Kwak JY. Endogenous production of nitric oxide by vascular endothelial growth factor down-regulates proliferation of choriocarcinoma cells. Biochem Biophys Res Commun. 2001;282:1061–1066. doi: 10.1006/bbrc.2001.4682. [DOI] [PubMed] [Google Scholar]
- Clementi E, Vecchio I, Corasaniti MT, Nistico G. Nitric oxide modulates agonist-evoked Ca2+ release and influx responses in PC12-64 cells. Eur J Pharmacol. 1995;289:113–123. doi: 10.1016/0922-4106(95)90176-0. [DOI] [PubMed] [Google Scholar]
- Czarnecka E, Pietkiewicz B, Skretkowicz J. Investigations of central interaction of ethanol and clonidine. Pol J Pharmacol Pharm. 1986;38:157–166. [PubMed] [Google Scholar]
- Dar MS. Involvement of kappa-opioids in the mouse cerebellar adenosinergic modulation of ethanol-induced motor incoordination. Alcohol Clin Exp Res. 1998;22:444–454. [PubMed] [Google Scholar]
- Davis RL, Syapin PJ. Interactions of alcohol and nitric-oxide synthase in the brain. Brain Res Brain Res Rev. 2005;49:494–504. doi: 10.1016/j.brainresrev.2005.01.008. [DOI] [PubMed] [Google Scholar]
- De Sarro GB, Ascioti C, Froio F, Libri V, Nistico G. Evidence that locus coeruleus is the site where clonidine and drugs acting at alpha 1- and alpha 2-adrenoceptors affect sleep and arousal mechanisms. Br J Pharmacol. 1987;90:675–685. doi: 10.1111/j.1476-5381.1987.tb11220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bel EA, Guimaraes FS, Bermudez-Echeverry M, Gomes MZ, Schiaveto-de-souza A, Padovan-Neto FE, Tumas V, Barion-Cavalcanti AP, Lazzarini M, Nucci-da-Silva LP, de Paula-Souza D. Role of nitric oxide on motor behavior. Cell Mol Neurobiol. 2005;25:371–392. doi: 10.1007/s10571-005-3065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan MJ, Lister RG, Linnoila M. Evidence for central alpha-2 adrenoceptors, not imidazoline binding sites, mediating the ethanol-attenuating properties of alpha-2 adrenoceptor antagonists. J Pharmacol Exp Ther. 1991;258:576–582. [PubMed] [Google Scholar]
- El-Haddad MA, Chao CR, Ma SX, Ross MG. Neuronal NO modulates spontaneous and ANG II-stimulated fetal swallowing behavior in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1521–1527. doi: 10.1152/ajpregu.00229.2001. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Ethanol selectively counteracts hypotension evoked by central I(1)-imidazoline but not alpha2-adrenergic receptor activation in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1998;32:382–389. doi: 10.1097/00005344-199809000-00008. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Ethanol counteraction of I1-imidazoline but not alpha-2 adrenergic receptor-mediated reduction in vascular resistance in conscious spontaneously hypertensive rats. J Pharmacol Exp Ther. 1999;288:455–462. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Evidence for the involvement of central I1 imidazoline receptor in ethanol counteraction of clonidine hypotension in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2001;38:417–426. doi: 10.1097/00005344-200109000-00010. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Chronic ethanol attenuates centrally-mediated hypotension elicited via alpha(2)-adrenergic, but not I(1)-imidazoline, receptor activation in female rats. Life Sci. 2009;84:111–118. doi: 10.1016/j.lfs.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Endotoxemia-mediated induction of cardiac inducible nitric-oxide synthase expression accounts for the hypotensive effect of ethanol in female rats. J Pharmacol Exp Ther. 2008;324:368–375. doi: 10.1124/jpet.107.127498. [DOI] [PubMed] [Google Scholar]
- Fauler J, Verner L. The pharmacokinetics of clonidine in high dosage. Eur J Clin Pharmacol. 1993;45:165–167. doi: 10.1007/BF00315500. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Santos AR, Calixto JB. The role of systemic, spinal and supraspinal L-arginine-nitric oxide-cGMP pathway in thermal hyperalgesia caused by intrathecal injection of glutamate in mice. Neuropharmacology. 1999;38:835–842. doi: 10.1016/s0028-3908(99)00006-4. [DOI] [PubMed] [Google Scholar]
- Idanpaan-Heikkila JJ, Bjorn M, Seppala T. The effects of ethanol in combination with the alpha 2-adrenoceptor agonist dexmedetomidine and the alpha 2-adrenoceptor antagonist atipamezole on brain monoamine metabolites and motor performance of mice. Eur J Pharmacol. 1995;292:191–199. doi: 10.1016/0926-6917(95)90012-8. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Komiyama T, Sato I, Himi T, Murota S. Neuronal nitric oxide synthase is resistant to ethanol. Life Sci. 1999;64:1623–1630. doi: 10.1016/s0024-3205(99)00099-5. [DOI] [PubMed] [Google Scholar]
- Jiang MH, Kaku T, Hada J, Hayashi Y. Different effects of eNOS and nNOS inhibition on transient forebrain ischemia. Brain Res. 2002;946:139–147. doi: 10.1016/s0006-8993(02)02870-6. [DOI] [PubMed] [Google Scholar]
- Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- Kiss JP, Vizi ES. Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 2001;24:211–215. doi: 10.1016/s0166-2236(00)01745-8. [DOI] [PubMed] [Google Scholar]
- Klamer D, Engel JA, Svensson L. The neuronal selective nitric oxide synthase inhibitor, Nomega-propyl-L-arginine, blocks the effects of phencyclidine on prepulse inhibition and locomotor activity in mice. Eur J Pharmacol. 2004;503:103–107. doi: 10.1016/j.ejphar.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Kosenko E, Llansola M, Montoliu C, Monfort P, Rodrigo R, Hernandez-Viadel M, Erceg S, Sanchez-Perez AM, Felipo V. Glutamine synthetase activity and glutamine content in brain: modulation by NMDA receptors and nitric oxide. Neurochem Int. 2003;43:493–499. doi: 10.1016/s0197-0186(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Kushikata T, Hirota K, Yoshida H, Kubota T, Ishihara H, Matsuki A. Alpha-2 adrenoceptor activity affects propofol-induced sleep time. Anesth Analg. 2002;94:1201–1206. doi: 10.1097/00000539-200205000-00028. table of contents. [DOI] [PubMed] [Google Scholar]
- Li GC, Abdel-Rahman AA. Direct evidence for NO mediation of the cardiovascular responses elicited by the NMDA receptor in the nucleus tractus solitarius of conscious rats. Faseb Journal. 2001;15:A212–A212. [Google Scholar]
- Li S, Ohgami Y, Dai Y, Quock RM. Antagonism of nitrous oxide-induced anxiolytic-like behavior in the mouse light/dark exploration procedure by pharmacologic disruption of endogenous nitric oxide function. Psychopharmacology (Berl) 2003;166:366–372. doi: 10.1007/s00213-002-1363-0. [DOI] [PubMed] [Google Scholar]
- Lin Y, Matsumura K, Tsuchihashi T, Fukuhara M, Fujii K, Iida M. Role of ERK and Rho kinase pathways in central pressor action of urotensin II. J Hypertens. 2004;22:983–988. doi: 10.1097/00004872-200405000-00021. [DOI] [PubMed] [Google Scholar]
- Mao L, Abdel-Rahman AA. Synergistic behavioral interaction between ethanol and clonidine in rats: role of alpha-2 adrenoceptors. J Pharmacol Exp Ther. 1996;279:443–449. [PubMed] [Google Scholar]
- Mao L, Abdel-Rahman AA. Ethanol counteraction of clonidine-evoked inhibition of norepinephrine release in rostral ventrolateral medulla of rats. Alcohol Clin Exp Res. 1998;22:1285–1291. [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Menani JV, Sato MA, Colombari E. Central blockade of nitric oxide synthesis reduces moxonidine-induced hypotension. Br J Pharmacol. 2004;142:765–771. doi: 10.1038/sj.bjp.0705853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouledous L, Diaz MF, Gutstein HB. Extracellular signal-regulated kinase (ERK) inhibition does not prevent the development or expression of tolerance to and dependence on morphine in the mouse. Pharmacol Biochem Behav. 2007;88:39–46. doi: 10.1016/j.pbb.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar N, Abdel-Rahman AA. Brainstem phosphorylated extracellular signal-regulated kinase 1/2-nitric-oxide synthase signaling mediates the adenosine A2A-dependent hypotensive action of clonidine in conscious aortic barodenervated rats. J Pharmacol Exp Ther. 2008;324:79–85. doi: 10.1124/jpet.107.129692. [DOI] [PubMed] [Google Scholar]
- Nelson EJ, Connolly J, McArthur P. Nitric oxide and S-nitrosylation: excitotoxic and cell signaling mechanism. Biol Cell. 2003;95:3–8. doi: 10.1016/s0248-4900(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Noda Y, Yamada K, Furukawa H, Nabeshima T. Involvement of nitric oxide in phencyclidine-induced hyperlocomotion in mice. Eur J Pharmacol. 1995;286:291–297. doi: 10.1016/0014-2999(95)00464-x. [DOI] [PubMed] [Google Scholar]
- Paxinos, Watson . The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Pechanova O, Jendekova L, Kojsova S, Jagla F. Possible role of nitric oxide in the locomotor activity of hypertensive rats. Behav Brain Res. 2006;174:160–166. doi: 10.1016/j.bbr.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Phung YT, Black SM. The synergistic action of ethanol and nerve growth factor in the induction of neuronal nitric oxide synthase. Alcohol Alcohol. 1999;34:506–510. doi: 10.1093/alcalc/34.4.506. [DOI] [PubMed] [Google Scholar]
- Pokk P, Sepp E, Vassiljev V, Vali M. The effects of the nitric oxide synthase inhibitor 7-nitroindazole on the behaviour of mice after chronic ethanol administration. Alcohol Alcohol. 2001;36:193–198. doi: 10.1093/alcalc/36.3.193. [DOI] [PubMed] [Google Scholar]
- Rickard NS, Gibbs ME. Hemispheric dissociation of the involvement of NOS isoforms in memory for discriminated avoidance in the chick. Learn Mem. 2003;10:314–318. doi: 10.1101/lm.59503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhoff CM, Bulseco DA, Brancho DM, Parada LF, Ross AH. The Ras-ERK pathway is required for the induction of neuronal nitric oxide synthase in differentiating PC12 cells. J Neurochem. 2001;78:631–639. doi: 10.1046/j.1471-4159.2001.00432.x. [DOI] [PubMed] [Google Scholar]
- Soares de Moura R, Rios AA, de Oliveira LF, Resende AC, de Lemos Neto M, Santos EJ, Correia ML, Tano T. The effects of nitric oxide synthase inhibitors on the sedative effect of clonidine. Anesth Analg. 2001;93:1217–1221. doi: 10.1097/00000539-200111000-00035. [DOI] [PubMed] [Google Scholar]
- Sy GY, Bruban V, Bousquet P, Feldman J. Nitric oxide and central antihypertensive drugs: one more difference between catecholamines and imidazolines. Hypertension. 2001;37:246–249. doi: 10.1161/01.hyp.37.2.246. [DOI] [PubMed] [Google Scholar]
- Syapin PJ. Alcohol and nitric oxide production by cells of the brain. Alcohol. 1998;16:159–165. doi: 10.1016/s0741-8329(97)00186-9. [DOI] [PubMed] [Google Scholar]
- Vassiljev V, Kalda A, Pokk P, Vali M, Zharkovsky A. The effects of the nitric oxide synthase inhibitor 7-nitroindazole on ethanol pharmacokinetics in rats after acute and chronic ethanol administration. Alcohol Alcohol. 1998;33:609–615. doi: 10.1093/alcalc/33.6.609. [DOI] [PubMed] [Google Scholar]
- Verbanck P, Seutin V, Massotte L, Dresse A. Yohimbine can induce ethanol tolerance in an in vitro preparation of rat locus coeruleus. Alcohol Clin Exp Res. 1991;15:1036–1039. doi: 10.1111/j.1530-0277.1991.tb05207.x. [DOI] [PubMed] [Google Scholar]
- Vulliemoz Y, Shen H, Virag L. Alpha-2 adrenoceptor agonists decrease cyclic guanosine 3′,5′-monophosphate in the mouse brain. Anesthesiology. 1996;85:544–550. doi: 10.1097/00000542-199609000-00013. [DOI] [PubMed] [Google Scholar]
- Wyatt AW, Steinert JR, Wheeler-Jones CP, Morgan AJ, Sugden D, Pearson JD, Sobrevia L, Mann GE. Early activation of the p42/p44MAPK pathway mediates adenosine-induced nitric oxide production in human endothelial cells: a novel calcium-insensitive mechanism. Faseb J. 2002;16:1584–1594. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Pieribone VA, Zhang X, Grillner S, Hokfelt T. A functional role for nitric oxide in locus coeruleus: immunohistochemical and electrophysiological studies. Exp Brain Res. 1994;98:75–83. doi: 10.1007/BF00229111. [DOI] [PubMed] [Google Scholar]