Abstract
Folates, the generic term for the family of B vitamins, are derived entirely from dietary sources, and are key one-carbon donors required for de novo nucleotide and methionine synthesis. These highly hydrophilic molecules utilize genetically distinct and functionally diverse transport systems to enter cells: the reduced folate carrier (RFC), the proton-coupled folate transporter (PCFT), and the folate receptors. Each plays a unique role in mediating folate transport across epithelia and into systemic tissues. With the recent discovery of the mechanism of intestinal folate absorption, and the clarification of the genetic basis for the autosomal recessive disorder, hereditary folate malabsorption, involving loss-of-function mutations in PCFT protein, it is now possible to piece together how these folate transporters contribute, both individually and collectively, to folate homeostasis in humans. This review focuses on the physiological roles of these major folate transporters with a brief consideration of their impact on the pharmacological activities of antifolates.
1. Introduction
Folates are a family of B9 vitamins that play a critical role in key biosynthetic processes in mammalian cells. These one-carbon donors are required for purine nucleotide and thymidylate synthesis and, hence, are essential for the de novo production of RNA and DNA. Folates are also required for vitamin B12-dependent synthesis of methionine, from which S-adenosylmethionine is formed, required for methylation of DNA, histones, lipids and neurotransmitters (1). Mammalian cells, unlike bacteria, do not have the metabolic machinery to synthesize folates. Hence, folate requirements must be met entirely from dietary sources. Traditionally, folates were derived from foods such as liver and dark green leafy vegetables. The recent commercial fortification of cereals, grains, and bread with folic acid now represents an important source of folates. This has resulted in a rise in tissue and blood folate levels (2). Because of the hydrophilic nature of the charged folate molecule, there is minimal passive diffusion across cell membranes. Rather, highly specific transporters are required to mediate intestinal folate absorption and the transport of folates into systemic tissues. Yet, despite the importance of these vitamins and their membrane transport, aspects of folate physiology, particularly relating to their transport, were uncharacterized and/or misunderstood until recently.
There is considerable information, acquired over many decades, regarding the properties of two folate transporters. (i) The reduced folate carrier (RFC), a member of the superfamily of solute carriers (SLC19A1), is an anionic exchanger and a major route of delivery of folates to systemic tissues at physiological pH (3,4). (ii) Two folate receptors (FRs) (FRα and FRβ), embedded in the cell membrane by a glycosylphosphoinositol (GPI) anchor, transport folates via receptor-mediated endocytosis at neutral to mildly acidic pH (5,6). Recently, a third folate transporter was identified, the proton-coupled folate transporter (PCFT). PCFT also belongs to the superfamily of solute carriers (SLC46A1) and functions optimally at low pH (7,8). PCFT accounts for the low-pH folate transport activity in many normal and malignant tissues, most notably the small intestine. The critical role that PCFT plays in intestinal folate absorption was established by the demonstration of loss-of-function mutations in this protein in patients with hereditary folate malabsorption (HFM) (7,9,10).
Structural analogs of folate compounds have been used for treating cancer and autoimmune diseases (11,12). These include methotrexate (MTX) and, more recently, pemetrexed, (12,13). These agents utilize the same transporters as physiological folates to enter tumor cells. Transport is an important determinant of antifolate activities and impaired transport is an important mechanism of resistance (12).
This review considers how these three transport systems contribute both individually and collectively to folate homeostasis in man and to delivery of antifolates in the treatment of cancer.
2.0 The tetrahydrofolate cofactors: interconversions and utilization in folate-dependent biochemical reactions
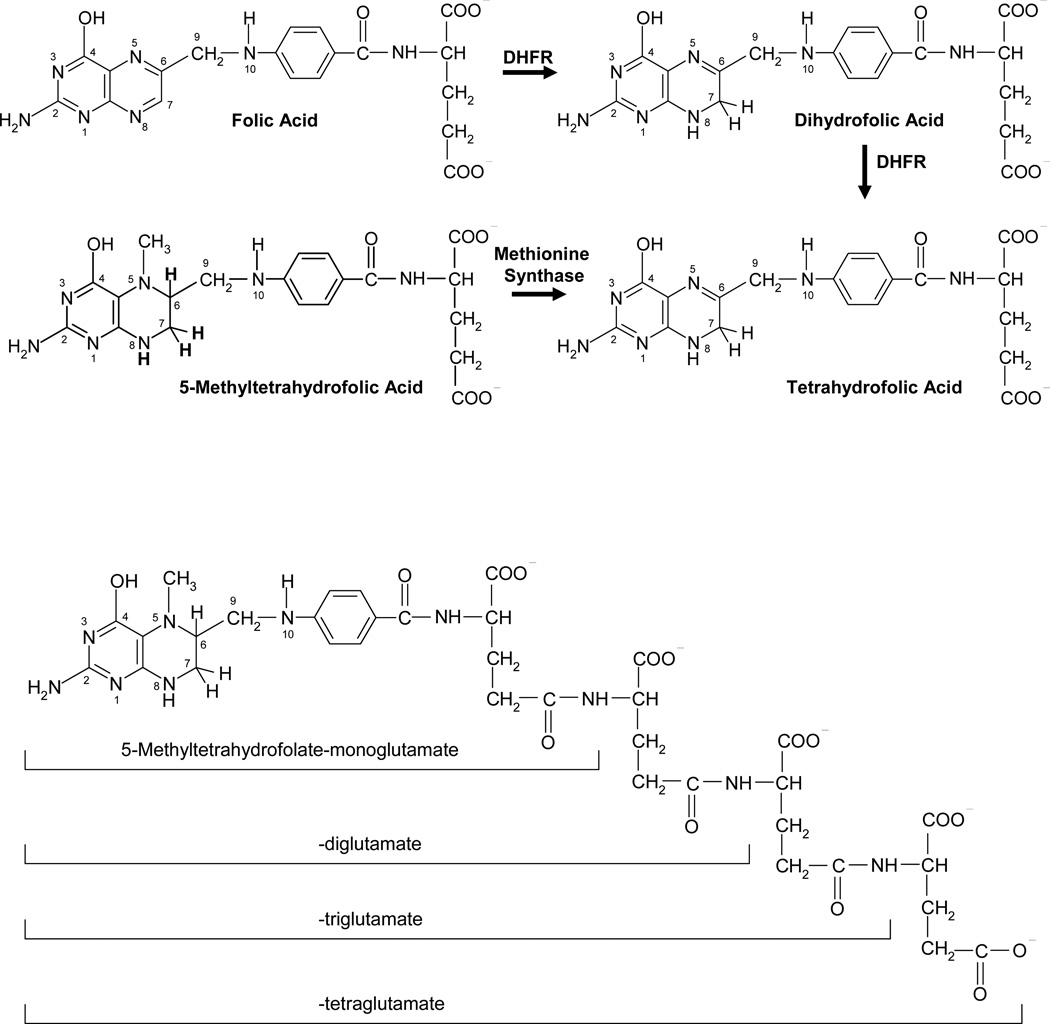
The folate molecule consists of a pteridine moiety with A and B rings linked at carbon 6 by a methylene bridge to p–aminobenzoylglutamic acid (Figure 1). “Folate” is a generic term for members of the B9 family of vitamins and will be used as such in this review. The parent structure is folic acid, a pharmacologic agent not found in nature. Because of its stability, folic acid has been used as a folate source in cell culture medium and in vitamins; it is reduced at the B ring first to dihydrofolate (DHF) and then to tetrahydrofolate (THF) within cells (Figure 1). Folic acid is also used to discriminate among folate transport routes for which it has markedly different affinities. THF is a good substrate for the enzyme, folylpolyglutamate synthetase (FPGS), which progressively adds glutamate molecules at the gamma carboxyl residue through an amide bond to form glutamate-peptide chains of varying length (Figure 1). THF polyglutamates associate with methyl, formyl, methylene, or methenyl one-carbon moieties at the N5 and/or N10 positions that serve as one-carbon donors in biosynthetic reactions (1). The folate polyglutamate derivatives are, with few exceptions, the preferred substrates for one-carbon transfer reactions over their non-polyglutamate (hereafter, designated “monoglutamate”) precursors (14).
Figure 1.
Upper Panel: Structures of representative folate compounds. Folic acid is reduced to dihydrofolic acid at the positions 7,8 of the B ring which, in turn, is reduced to tetrahydrofolic acid at the positions 5,6 of the B ring in reactions mediated by dihydrofolate reductase (DHFR). Endogenous folic acid is not found in cells. Rather, its presence is related to the consumption of folic acid added to cereals and grains and vitamins. The major oxidized folate in cells is dihydrofolic acid. 5-Methyltetrahydrofolic acid (5-methylTHF) is the major dietary form and the major folate found in blood. It is formed endogenously by the reduction of 5,10-methylene-tetrahydrofolic acid (5,10-methyleneTHF). With the utilization of 5-methylTHF in methionine synthesis, tetrahydrofolate (THF) is generated. These relationships are depicted in Figure 2. The two carboxyl groups of the glutamate moiety are fully ionized at physiological pH.
Lower Panel: Structure of 5-methylTHF tetraglutamate. This is one of the 5-methylTHF forms found in nature and in mammalian cells. This is a poly-anion and a very poor substrate for folate transporters and multidrug resistance associated proteins.
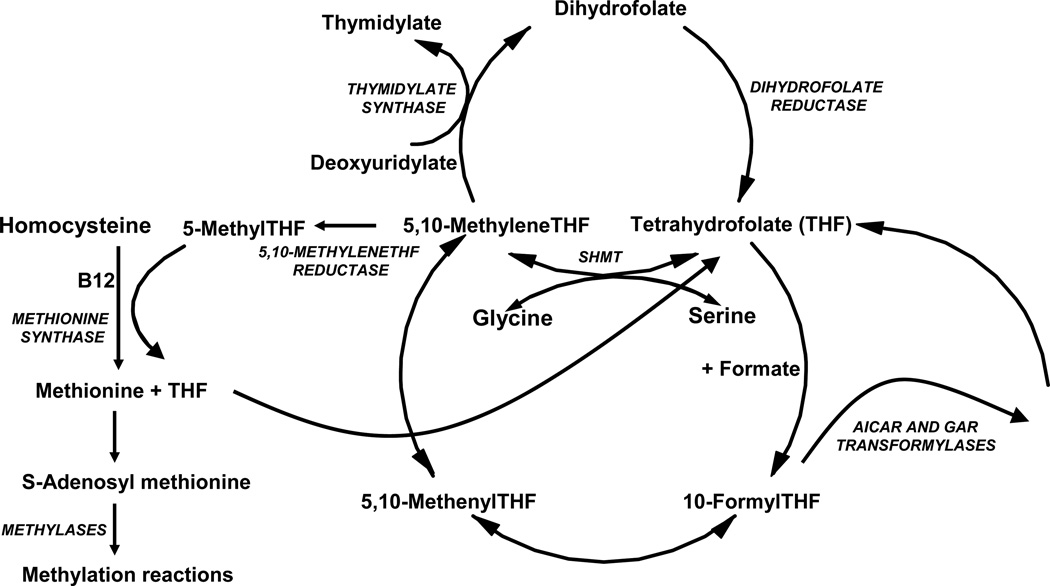
The predominant natural folate in the diet and blood of humans and rodents is 5-methylTHF (Figure 1). Utilization of this form in cells begins with the transfer of a methyl group to homocysteine, in a vitamin B12-dependent reaction mediated by methionine synthase, to form methionine (Figure 2). The unsubstituted THF released then reacts with formate (via 10-formylTHF synthetase) to produce 10-formylTHF which donates two carbons to the synthesis of the purine ring in reactions mediated first by β-glycinamideribonucleotide (GAR) transformylase and then by 5-amino-4-imidazolecarboxamide ribonucleotide (AICAR) transformylase. 10-FormylTHF also undergoes dehydration to 5,10-methenylTHF which in turn is reduced to 5,10-methyleneTHF. 5,10-MethyleneTHF is also formed from THF by serinehydroxymethyltransferase which interconverts serine to glycine. 5,10-MethyleneTHF is reduced in an irreversible reaction by 5,10-methyleneTHF reductase (MTHFR) to 5-methylTHF. 5,10-MethyleneTHF is required for de novo synthesis of thymidylate, catalyzed by thymidylate synthase, in which a one-carbon moiety is transferred to deoxyuridylate along with a reducing equivalent from the pteridine B ring. This results in oxidation of the THF moiety to DHF. Because of its rapidity and irreversibility, this reaction has the potential to deplete cellular THF cofactors which rapidly interconvert to 5,10-methyleneTHF by mass action as this folate is oxidized. However, THF cofactor levels are maintained because of the rapid reduction of DHF to THF, mediated by dihydrofolate reductase (DHFR) (Figure 2). When DHFR is inhibited by MTX, aminopterin, or the more recent PT523, there is rapid interconversion of THF cofactors to DHF, resulting in cessation of one-carbon-dependent processes (12,15,16).
Figure 2.
Folate metabolic pathways. 5-MethylTHF enters the metabolic cycle following transport into cells where, with homocysteine, it is utilized in the synthesis of methionine mediated by methionine synthase and vitamin B12. The THF product then acquires a carbon from formate or serine, at the N5, N10, or shared between the N5 and N10 positions that, through a series of interconversions, results in several other folate forms that provide carbons for purine, thymidylate, and methionine synthesis. Abbreviations: (AICAR, 5-amino-4-imidazolecarboxamide ribonucleotide; GAR, β-glycinamideribonucleotide; SHMT, serinehydroxymethyltransferase.
3.0 The folate transporters
3.1 The reduced folate carrier
(RFC,SLC19A1) was the first folate transporter described at the kinetic, thermodynamic and molecular levels (3,17–19) . The gene that encodes human RFC (hRFC) is located on chromosome 21q22.3. The Km for 5-methylTHF, 5-formylTHF, and MTX influx mediated by RFC is in the 3–7 µM range; the Km for PT523 is much lower. RFC has a very low affinity for folic acid (Ki ∼ 150–200 µM). The high affinity of RFC for PT523, its very low affinity for folic acid, and its neutral pH optimum clearly distinguish this carrier from PCFT. MTX is frequently used to characterize RFC-mediated transport because it is not metabolized over short intervals and because of the ease and accuracy of influx determinations and distinguishing between free and tightly bound drug within cells (17).
RFC generates only small transmembrane chemical gradients. However, folates are negatively charged due to the two glutamate carboxyl groups that are fully ionized at physiological pH (Figure 1). When this is considered within the context of the membrane potential, RFC actually produces a substantial electrochemical-potential difference for folates across cell membranes (17). The energy source for this uphill process is unique. RFC function is not directly linked to ATP hydrolysis, neither is it Na+ nor H+-dependent (20,21). Rather, RFC-mediated transport is highly sensitive to the transmembrane anion gradient, in particular, the organic phosphate gradient (20,21). Organic phosphates are highly concentrated in cells where they are synthesized in ATP-dependent reactions and are largely retained. Their resulting asymmetrical distribution across cell membranes provides the driving force for RFC-mediated uphill transport of folates into cells (21).
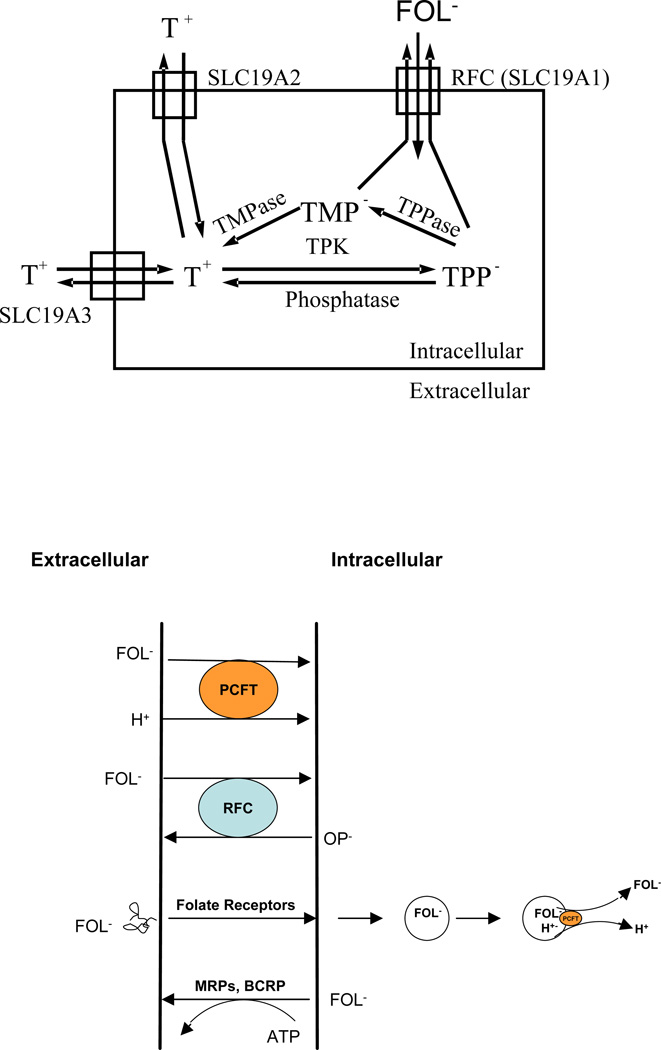
The most substantive support for this model comes from studies on thiamine transport and metabolism. There are two other members of the SLC19 family, SLC19A2 and SLC19A3, with ∼40% homology to RFC (22–26). However, both are thiamine, not folate, transporters. Neither does RFC transport thiamine. However, after thiamine is transported into cells, it is converted to thiamine pyrophosphate and thiamine monophosphate (27); both are transported by RFC (Kis of ∼ 25 µM and 32 µM, respectively) (28,29). When cells are exposed to thiamine, these phosphorylated derivatives accumulate intracellularly such that the higher the level of RFC expression, the lower the level of cellular accumulation of thiamine pyrophosphate due to enhanced export mediated by RFC. Likewise, when cells are loaded with MTX, accumulation of the phosphorylated derivatives of thiamine is enhanced due to MTX inhibition of their export by RFC (28). These observations can only be explained if MTX and phosphorylated derivatives of thiamine use the same carrier, RFC. These interactions among thiamine, its phosphorylated derivates, and folates are illustrated in Figure 3 (upper panel).
Figure 3.
Upper panel: Interactions among thiamine, its phosphorylated derivatives, folates, and the SLC19 family of transporters. Thiamine (T+), a cation, is transported into cells via SLC19A2 and SLC19A3. Within cells thiamine is metabolized to thiamine pyrophosphate (TPP−), an anion, in a reaction mediated by thiamine pyrophosphate kinase (TPK). TPP− can be hydrolyzed to thiamine monophosphate (TMP−), also an anion, mediated by thiamine pyrophosphatase (TPPase). Both are substrates for the reduced folate carrier (RFC-SLC19A1) and are exported by that mechanism. Folates enter cells via RFC and are not substrates for the thiamine transporters. Likewise, thiamine is not a substrate for RFC. Lower panel: A schema of the current known folate transporters. The proton-coupled folate transporter (PCFT) is a folate (FOL−)-H+ symporter that functions most efficiently in an acidic extracellular environment. The reduced folate carrier (RFC) is an anion exchanger that utilizes the transmembrane organic phosphate (OP−) gradient to achieve uphill transport into cells. Folate receptors (FRs) α and β are high- affinity folate binding proteins that transport folates into cells via an endocytic mechanism. Once in the cytoplasm, the vesicle acidifies, folate is release from receptor and is exported from the endosome via PCFT. Folate monoglutamates are exported from cells via the multidrug-associated resistance proteins (MRPs) and the breast cancer resistant protein (BCRP).
Additional evidence for utilization of RFC by structurally unrelated anions comes from the observation that folate influx is enhanced in RFC-containing membrane vesicles by phosphate or sulfate in the trans-compartment (30). Likewise, depletion of extracellular anions impairs efflux of folates from cells, an activity that is restored when extracellular anions such as thiamine pyrophosphate, AMP, or phosphate are added (20).
Thus, RFC is not influenced by the cellular energy charge, per se. Indeed, inhibition of anaerobic or aerobic metabolism resulting in ATP depletion enhances rather than diminishes the transmembrane folate gradient mediated by RFC (31–33). This is likely due to (i) inhibition of ATP-binding cassette proteins that export folates and oppose the concentrative impact of RFC (34), and (ii) maintenance of the net transmembrane adenine nucleotide gradient since ADP and AMP generated from ATP are more potent inhibitors of RFC-mediated folate transport than ATP (20,21).
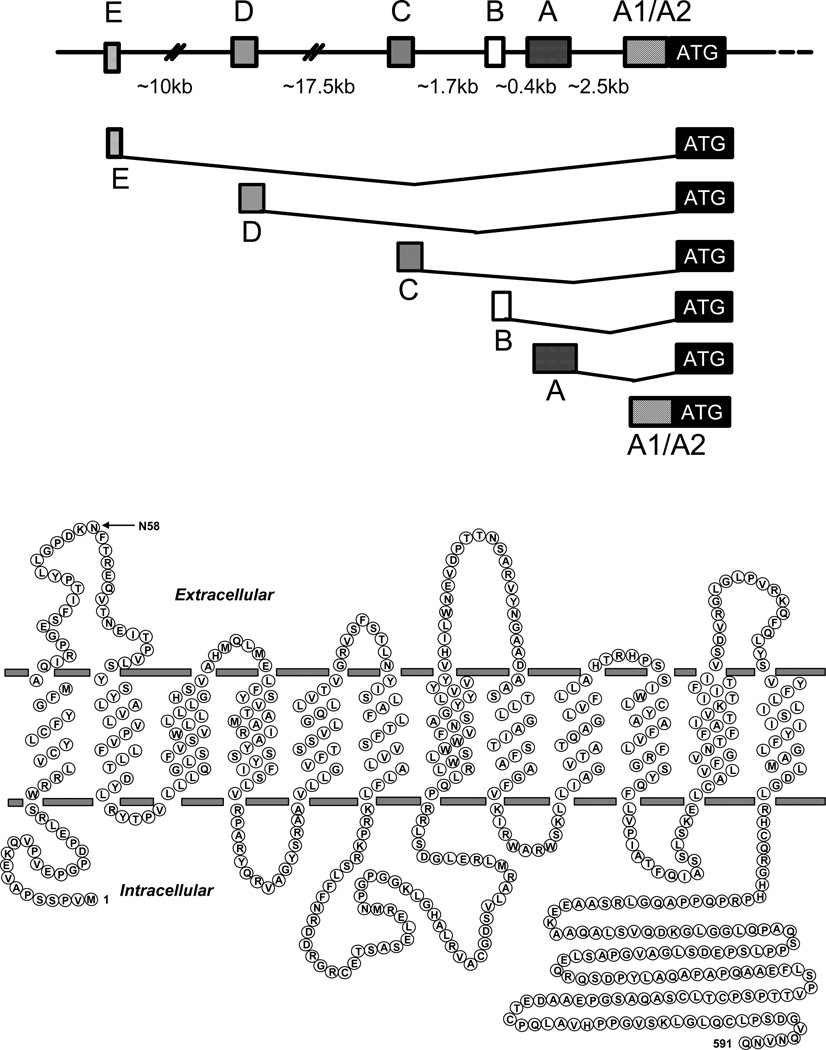
RFC is characterized by twelve membrane-spanning transmembrane domains (TMDs) and cytoplasmically oriented N- and C-termini (35–37) (Figure 4). hRFC is N-glycosylated at a N-glycosylation consensus site in the loop connecting TMDs 1 and 2 (at Asn58) (38). A large loop domain that connects TMDs 6 and 7 is poorly conserved between species and can be replaced altogether by a non-homologous segment from the SLC19A2 carrier (39). When separate TMD1–6 and TMD7–12 hRFC half-molecules are co-expressed in hRFC-null cells, they are targeted to the plasma membrane surface and restore transport activity (40). Hence, the role of the TMD6–7 loop domain is primarily to provide appropriate spacing between the TMD1–6 and TMD7–12 segments for optimal transport.
Figure 4.
Upstream RFC gene structure and membrane topology. Upper Panel: A schematic of the upstream region of the human RFC gene including up to 6 alternate non-coding regions, each preceded by a separate promoter, spanning ∼35 kb upstream of the major translational start site (shown). The alternate promoters transcribe unique RFC transcripts each with a distinct 5’ untranslated region (5’UTRs) fused to a common coding sequence. Alternate splicing for the A1/A2, A, B, and D 5’UTRs have been described. For the A1/A2 and A 5’UTRs, upstream AUGs occur in-frame within the RFC non-coding sequence and result in N-terminally modified hRFC protein isoforms, with 64 and 22 additional N-terminal amino acids, respectively. Lower Panel: A schematic is shown for the membrane topology of the human RFC including 12 transmembrane domains (TMDs), internally oriented N- and C-termini, the large cytosolic loop between TMDs 6 and 7, and the N-glycosylation site at Asparagine 58.
Studies with RFC mutants implicated conserved amino acids in TMDs 1, 2, 3, 4, 8, 10, and 11 as important to transport (4) and recent results with N-hydroxysuccinimide 3H-MTX radioaffinity labeling localized binding of the substrate gamma carboxyl group to Lys411 in TMD11 of hRFC (41). By exhaustive cysteine-scanning mutagenesis of a “cysteine-less” hRFC molecule, amino acids localized in TMDs 4, 5, 7, 8, 10, and 11 were implicated in forming the putative substrate binding pocket (42,43) consistent with crystal structures for the bacterial MFS proteins, LacY and GlpT (44,45).
RFC is ubiquitously expressed with patterns of localization (e.g., intestine, hepatocytes, renal epithelial cells, choroid plexus) that suggest its integral role in specialized tissue functions important for in vivo folate homeostasis (46,47). hRFC is intricately regulated by transcriptional controls involving as many as 6 alternate promoters and non-coding regions that generate up to 15 distinct 5’ untranslated regions (UTRs) fused to a common coding sequence (4) (Figure 4). The net level of hRFC transcripts among different tissues likely result from both ubiquitous (e.g., Sp, USF) and tissue-specific (Ap1, C/EBP) transcription factors that, when combined, transactivate or repress transcription in response to tissue-specific stimuli. Other factors likely important in the regulation of this gene include promoter methylation, general promoter architecture, and chromatin structure (4).
The diverse hRFC 5’UTRs are subject to posttranscriptional controls including effects on 5’CAP-dependent translation and transcript stabilities (48). For many 5’UTRs, the effects on steady-state hRFC transcripts and proteins are subtle and can be overshadowed by differences in promoter activities. However, for non-coding exons A1/A2, A, and D, there were profound decreases in steady-state hRFC compared to other 5’UTRs (48). For the A1/A2 and A 5’UTRs, upstream AUGs occur in-frame within hRFC coding sequence and result in N-terminally modified hRFC protein isoforms, with 64 and 22 additional N-terminal amino acids, respectively (48,49). The biological significance of these modified hRFC proteins is unclear.
High frequency polymorphisms have been described in hRFC involving both the coding region (G80A) and promoters A1/A2 and A (49–52). G80A hRFC was associated with an increased risk of fetal abnormalities (53,54) and MTX toxicity in patients with acute lymphoblastic leukemia (ALL) (55). However, the clinical significance of G80A remains controversial (4,56). The presence of a 61 bp repeat polymorphism in the hRFC-A promoter may protect against neural tube defects (57).
3.2 The proton-coupled folate transporter (PCFT)
is a recently discovered folate carrier (SLC46A1) (7,58,59). There are two other members of this family (SLC46A2 and SLC46A3) but their functions are not known. The gene that encodes human PCFT is located on chromosome 17q11.2. PCFT was initially reported to be a low-affinity, pH-independent, heme transporter (heme influx Km of 125 µM) that mediates heme-associated iron absorption in the small intestine (60); however, subsequent studies were not confirmatory (7,58). Rather, PCFT is a high-affinity folate transporter with a low pH optimum. Its identification now explains the molecular basis for the long-recognized low pH folate transport activities in normal and malignant mammalian cells (8,61–65).
Folate transport mediated by human, mouse and rat PCFTs is electrogenic, indicating that there is a net translocation of positive charges as each folate molecule is transported (7,59,66,67). Folate-induced currents in Xenopus oocytes microinjected with PCFT cRNA increase as the pH is decreased in parallel to increased transport of tritiated folates (7,66). This is opposite to what is observed for RFC where transport activity falls with decreasing pH from pH 7.4 and is negligible below a pH of 6.0–6.5 (68,69). If folates are bivalent anions, as has been assumed to be the case, more than two protons must be co-transported with each folate molecule to account for the positive charge of the PCFT-folate-proton complex. Hence, PCFT functions as a folate-proton symporter: the downhill flow of protons via PCFT is coupled to the uphill flow of folates into cells via PCFT (Figure 3). Early studies with rodent jejunal apical brush-border membrane vesicles provided insight into the energetics of what would later be recognized as PCFT-mediated transport. A transvesicular pH gradient resulted in increased unidirectional folate transport, and substantial transmembrane folate concentration gradients, from the low- to high- pH compartment, consistent with a proton-coupled process (70).
While the substrate specificity for PCFT shares some similarity with RFC, there are important differences including a marked difference in pH optima that impacts affinities for transport substrates. The RFC influx Kms are comparable at pHs 7.4 and 5.5. The fall in transport with decreasing pH is due instead to a marked decrease in influx Vmax (69). In contrast, for PCFT the influx Km for folates is increased and Vmax is decreased with an increase in pH from 5.5 to 7.4. However, the pattern and magnitude of these changes varies among different transport substrates (7). Most notable is the relatively small change in influx Km and Vmax for pemetrexed, compared to other folates, with increasing pH. Because of this, PCFT mediates pemetrexed pharmacological effects at physiological pH (7,67). Hence, like the divalent metal-ion transporter, DMT1, PCFT mediates transport of folates in the absence of a proton gradient (71). RFC and PCFT are both stereospecific for 5-formylTHF (7,72).
The PCFT expression pattern in human tissues provides clues as to its functional roles. In murine and human tissues, there are high levels of PCFT transcripts in small intestine, kidney, liver, placenta, retina, and brain. Within the intestine, the highest PCFT levels are found in the proximal jejunum and duodenum (7,66,73).
Information on PCFT secondary structure is emerging. Hydropathy analyses predict a protein with twelve TMDs and amino- and carboxy- termini oriented to the cytoplasm (Figure 5). Th e latter was confirmed by immunofluorescence studies of hemagglutin-tagged PCFT (66,74). The loop domain between the first and second TMDs must be extracellular since the two putative N-glycosylation consensus sites in this region are glycosylated. N-glycosylation does not appear to be required for PCFT intracellular trafficking nor function (74).
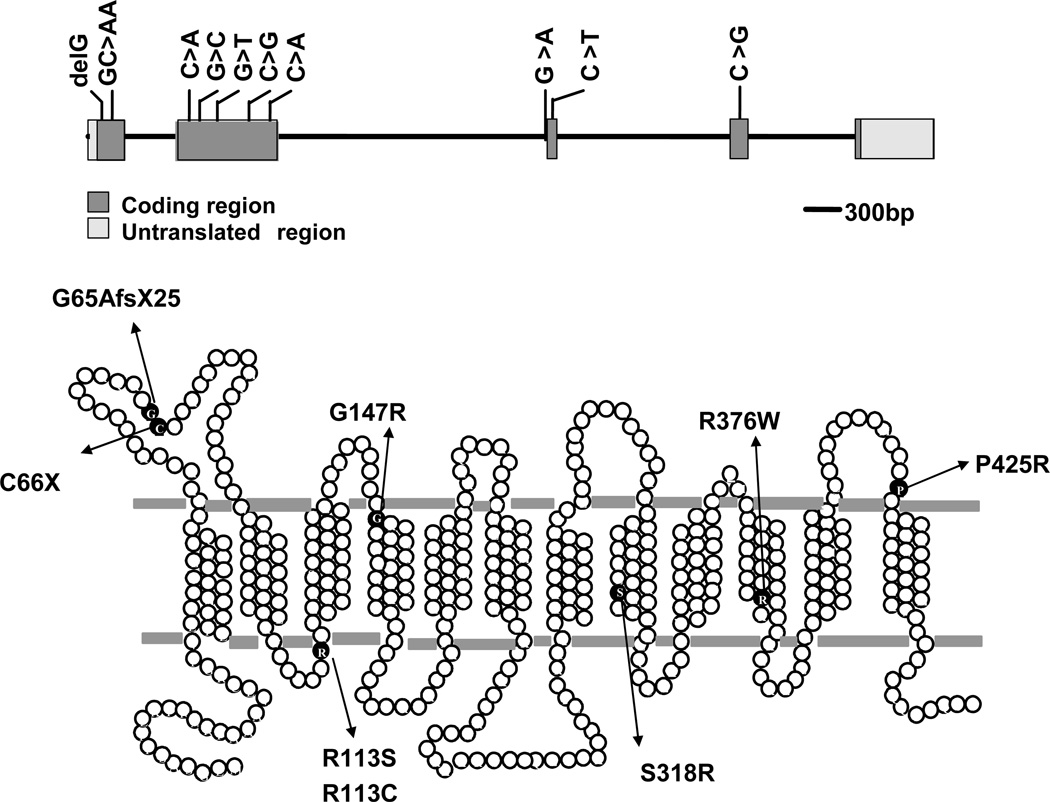
Figure 5.
The genomic organization and predicted secondary structure of PCFT and the spectrum of reported mutations in the protein derived from patients with the autosomal recessive disorder, hereditary folate malabsorption (HFM). Upper Panel: The genomic organization of PCFT; the positions of the various base mutations in the first through fourth exons are indicated. Lower Panel: A schematic of a predicted human PCFT topology showing confirmed N-glycosylation sites between the first and second TMDs with the N- and C- termini oriented to the cytoplasm. The location and identity of the mutated amino acid residues involved with HFM are indicated.
Both RFC (47) and PCFT (66) expressions are markedly increased in small intestine when mice are fed a folate-deficient diet. However, the underlying regulatory mechanisms for these changes await definition.
3.2.1 The impact of pH on the relative contributions of RFC and PCFT to the transport of folates; focus on intestinal folate absorption
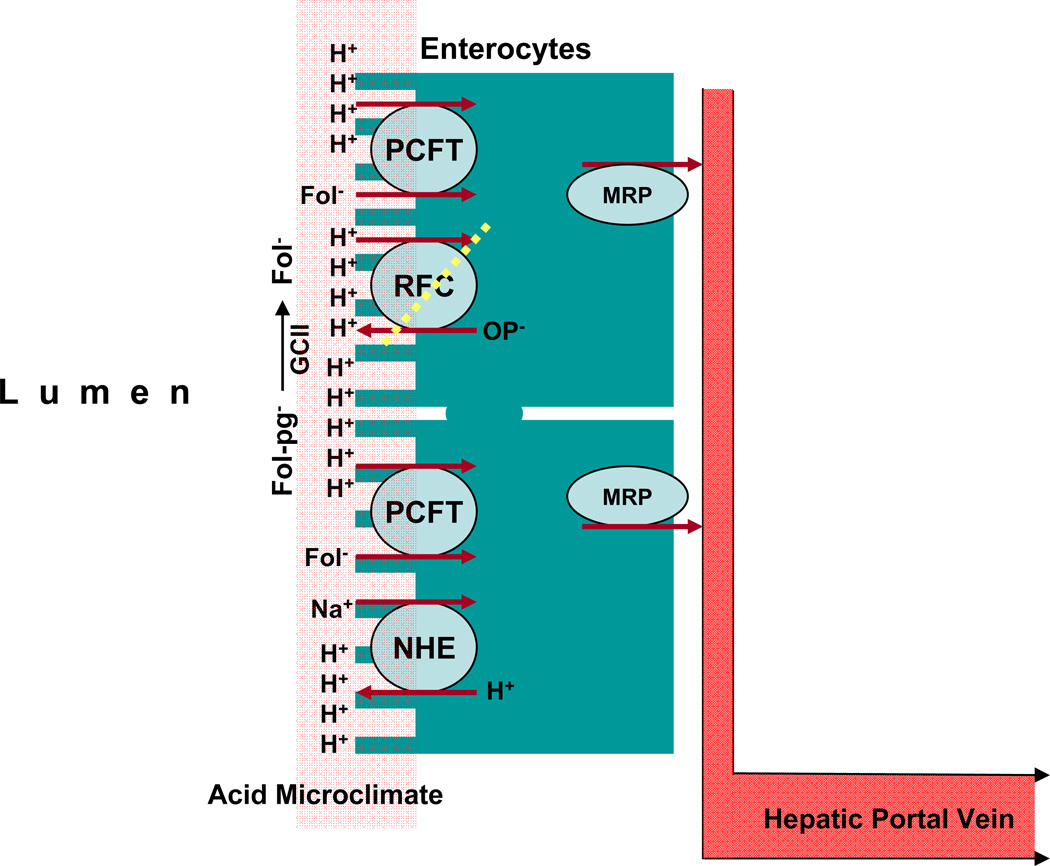
The pH at the transport interface is an important determinant of the relative contributions of RFC and PCFT to overall folate uptake when these transporters are expressed at the same site in the same tissue, as is frequently the case. When the pH is 7.4, PCFT transport function is low to negligible (depending on the folate substrate), and RFC function is maximal. As the pH drops, RFC function decreases and PCFT activity increases. At some point, RFC transport disappears altogether and PCFT becomes the sole transport route (7,66,69). From the physiological perspective, the critical transport interfaces where there is an acid pH include: (i) the microclimate of the duodenum and proximal jejunum apical brush-border membranes where several Na+/H+ exchangers generate pHs of 5.8–6.0, and where folates are absorbed from the diet (75–78); and (ii) within acidified endosomes containing FRs (pH ∼6.5) from which, following internalization from the cell surface, folates are exported into the cytoplasm (79) (see Section 3.3). It is possible that there are other, as yet unidentified, transport interfaces where ambient pH is low due to localized activities of Na+/H+ exchangers.
RFC and PCFT are expressed at the apical brush-border membrane of the proximal jejunum (58,66,80,81); however, the acidic pH clearly favors PCFT over RFC (Figure 6). Indeed, there is a large literature that demonstrates a low-pH optimum at this site for folate transport and a Km for folic acid at or below 1µM, properties clearly different from RFC. These encompass a broad spectrum of systems: intact intestine in man and rodents; isolated intestinal loops, rings and everted sacs in vitro; and isolated intestinal cells or cell lines of intestinal origin (82–84).
Figure 6.
Schematic of the sequence of events that occur in the proximal jejunum during the process of hydrolysis and absorption of dietary folate polyglutamates. Dietary 5-methylTHF polyglutamates (Fol-pg−) are hydrolyzed to monoglutamate by glutamate carboxypeptidase II (GCII) and then transported into cells via PCFT; both reactions have a low pH optimum and are favored by the low pH within the microenvironment at the jejunal villi (indicated in pink) that is generated by NHE Na+/H+ exchangers. Cellular folates exit the basolateral membrane by an, as yet, unconfirmed mechanism likely related to a member(s) of the multidrug-associated protein (MRP) family of ABC cassette exporters.
Yet, despite this, because RFC was identified at the intestinal apical brush-border membrane and there was no other known transporter at this location, absorption was attributed to RFC. Indeed, the functional discrepancies between intestinal transport and RFC-mediated transport were attributed to post-translational modifications of RFC that altered its functional properties, although such modifications were never identified (85,86). Further evidence that the low-pH folate transport activity is due to a carrier genetically distinct from RFC came from studies in which RFC was deleted from the genome, mutated with loss-of- function, or was silenced. In these studies, with a variety of cell lines including those of intestinal origin, transport at pH 7.4 was lost or markedly diminished but the low pH transport activity was preserved (62,87–89). However, it remains unexplained how in one report siRNA to RFC suppressed folate transport activity in rat-derived intestinal epithelia cells at low, but not physiological pH (90).
Ultimately, the critical role that PCFT plays in intestinal folate absorption was unequivocally established with the demonstration that subjects with HFM invariably have loss-of-function mutations in the PCFT gene (see Section 4.0).
3.3 The folate receptors
FRs are very high affinity folate binding proteins, encoded by three distinct genes designated α, β, and γ, located on chromosome 11 (6,91). FRs are homologous proteins (68–79% identical amino acid sequences), characterized by disparate patterns of tissue expression (see below). FRα and FRβ are both GPI-anchored proteins that mediate folate transport (5,6,91,92).
FRs have high affinity for folic acid (Kd 1–10 nM). FRα and FRβ from both human and murine sources exhibit different specificities for (6S) and (6R) diastereomers of reduced folates such as 5-methylTHF and 5-formylTHF (93,94). The 50-fold lower binding affinity of (6S) 5-methylTHF for FRβ compared to FRα was attributed to Leu49, Phe104, and Gly164 in FRβ since replacement of these residues with the corresponding residues from FRα (Ala, Val, and Glu, respectively) reconstituted the FRα phenotype (95).
Mechanistically, folate internalization by membrane-associated FRs involves a receptor-mediated endocytosis (5,6,91,96,97). The process is initiated when a folate molecule binds to a FR on the cell surface. This is followed by invagination of the plasma membrane at that site and the formation of a vesicle (endosome), which migrates into the cytoplasm where it is acidified to a pH of ∼ 6.5, resulting in dissociation of the folate from the FR complex (79). Folate ligand is then exported into the cytoplasm (96,97). This trans-endosomal exporter was thought to be an anion exchanger and require a trans-endosomal pH gradient (96,98,99). Most recently, PCFT was proposed to account for folate export from endosomes (7,59,100) (Figure 3, see below).
The physiologic roles of FRs are unclear in all but a few cases. While FRs α and β can transport folate into cells, this is inefficient compared to transporters such as RFC (101,102). FRα is expressed in epithelial cells of the kidney, choroid plexus, retina, uterus, and placenta (6,103). In proximal renal tubule cells, FRα is expressed at the apical (luminal) surface. In retinal pigment epithelium, FRα is expressed on the basolateral membrane (104,105). FRα is also expressed in tumors, particularly non-mucinous adenocarcinomas of the ovary, uterus and cervix (103). For ovarian carcinomas, FRα expression correlates with histologic grade and stage (106). FRα is negatively regulated by the estrogen receptor (107). Dexamethasone, a glucocorticoid receptor agonist, is a positive regulator of FRα (108).
FRβ is expressed during normal myelopoiesis and is present in placenta, spleen, thymus, and in CD34+ monocytes (109–112). A non-functional form of FRβ was reported in CD34+ human hematopoietic cells (111). FRβ is expressed in leukemic blasts in chronic myelogenous leukemia and in acute myelogenous leukemia (AML) (110,112,113). In AML, FRβ expression is induced by treatment with retinoid receptor agonists including all-trans-retinoic acid (110). This is independent of cell differentiation or proliferation status and was not observed in non-myeloid cells.
3.4 Folate transport mediated by ATP-binding cassette transport proteins and members of the SLC21 and SLC22 families of solute carriers
Beyond the transporters that are highly specific for folates, there are other potential folate transport routes. Relevant ATP-binding cassette exporters include the multidrug resistance-associated proteins, MRP1–5 (ABCC1-ABCC5) and the breast cancer resistance protein, BCRP, (ABCG2) (34,114,115). These are low affinity, high capacity transporters (Kms ∼0.2 – 2 mM for folates/antifolates). Members of this family are widely expressed in mammalian cells and suppress the level of free folates or antifolates that accumulate in most cells grown in vitro (see Section 3.1) (34). Some of the shorter chain-length polyglutamate folates may be weak substrates for MRPs. However, in general, folate polyglutamates are retained within cells (12,34,116).
From a physiological perspective, MRP2 plays a critical role in the export of folates across the bile canalicular membrane, as demonstrated by impaired biliary secretion of MTX in MRP2 (−/−) mice (117). The role that MRP family members play in the vectorial transport of folates in other tissues is less clear. Of particular interest is their potential role in the export of folates from jejunal enterocytes. MRP2 is expressed at the apical membrane which would oppose absorption mediated by PCFT (118). Expression of MRP3 at the basolateral membrane of rat jejunum (119) suggests its role in the transport of folates across the jejunal enterocyte by mediating export across the basolateral membrane. MRPs1–4 and BCRP are expressed in human jejunum although relative levels have not been firmly established (120,121). While MRP1 and MRP 4 were localized to the basolateral membrane of some tissues, their localization in human jejunum is not known (34).
The SLC21 family of facilitative carriers transport organic anions and the SLC22 family transport both organic anions and cations (122–124). Both are expressed in tissues that mediate vectorial transport, i.e, intestine, kidney, and liver. Some of these carriers transport folates and antifolates. Of the SLC21 members, OAT-K1 and OAT-K2 are kidney-specific and expressed at the apical brush-border membrane. These transporters have Kms for MTX and 5-methylTHF of ∼2 µM and could play a role in reabsorption of folates at the proximal tubule (125). Of the SLC22 members, OAT1, OAT2, and OAT3 are expressed at the basolateral membrane of renal tubules (126). These transporters generally have low affinities for MTX (10–700 µM), depending on the expression system (127–131). Since RFC is also present at the basolateral membrane, it is unlikely that the SLC22 transporters play a major role in renal reabsorption of folates.
4.0 Genetic deletion of folate transporter genes: the PCFT-null phenotype, hereditary folate malabsorption (HFM), and what can be learned from this disorder about the role of PCFT (and RFC) in folate biology
RFC and the FRs have been genetically deleted in the mouse. RFC heterozygous (+/−) mice have no phenotype. Loss of both RFC alleles (−/−) is embryonic lethal; however, mice can be brought to term and live for 1–2 weeks when pregnant females receive parenteral folic acid. At death these animals have evidence of failure of erythropoiesis in bone marrow, liver, and spleen (132). More recent studies on RFC (−/−) mice from less folate-supplemented pregnant females reveal a variety of congenital malformations (133). Mice heterozygous (+/−) for FRα have no phenotype. Loss of both alleles (−/−) is embryonic lethal; the embryos are arrested at the time of neural tube closure (134). If, however, pregnant females receive parental 5-formylTHF throughout gestation, the pups are normal and can be maintained without any apparent abnormalities through adulthood on a standard chow (R. Finnell, personal communication).
The human PCFT-null genotype/phenotype is known within the context of the rare autosomal recessive disorder, HFM (7,9,10,135). The major defects in HFM are: (i) impaired intestinal folate absorption, resulting in severe systemic folate deficiency; and (ii) impaired transport of folates into the central nervous system associated with very low to undetectable folate in the cerebrospinal fluid (CSF).
Individuals who are PCFT (+/−) are completely normal. Infants with HFM are normal at birth. Neural tube defects are not seen, indicating that there is adequate placental delivery of folates from mother to fetus. Signs of HFM begin several months after birth, presumably when folate stores, provided by the mother during gestation, are depleted. The infants develop anemia, sometimes pancytopenia, reflecting folate deficiency in rapidly proliferating hematopoietic cells. Gastrointestinal involvement manifests as severe diarrhea. Immune deficiency due to hypoimmunoglobulinemia is frequent, resulting in infections usually associated with immune-compromised states (i.e. Pneumocystis carinii and cytomegalovirus pneumonia). Patients frequently have neurological signs including seizures, neuro-developmental defects, and/or mental retardation. Systemic signs of the disease can be completely reversed by low-dose parenteral folate or higher-dose oral folate which normalizes blood folate levels. However, very high blood folate levels are required to achieve CSF folate levels in the normal range (135).
Eight families with HFM have been reported to date with loss-of-function mutations in PCFT (Four additional families not as yet reported; Goldman, unpublished). The mutations are indicated in Figure 5 (7,9,10,136). No mutations were detected in RFC or FRα in patients with HFM. These observations unequivocally establish PCFT as the mechanism by which folates are absorbed across the apical brush-border membrane of the proximal jejunum under normal conditions. This is consistent with the low pH (5.8–6.0) at the jejunal brush-border membrane and observations that: (i) when jejunal pH is decreased, as occurs with pancreatic insufficiency, folate absorption is increased (137); and (ii) when the pH is increased, as occurs with ingestion of antacids or after gastrectomy, intestinal folate absorption is decreased (138).
The clinical observation that high-doses of oral folates can restore systemic folate levels in individuals with HFM raises the question of how intestinal folate is absorbed under these conditions (135). Defects due to PCFT mutations that decrease affinities for folates could be overcome by high doses of oral folates. However, in several cases, mutations resulted in a stop codon, a frameshift in exon 1, or a splicing defect, resulting in the absence of PCFT protein. In other cases, point mutations resulted in defects in trafficking/stability and/or a complete loss of intrinsic function (7,9,10,136). Since passive diffusion of folates is assumed to be negligible even in the presence of pharmacological doses of folates, it is likely that absorption is mediated by RFC, albeit inefficiently, under these conditions.
5.0 The folate-one-carbon journey from food to macromolecules
This section traces the pathway of folates ingested and the various folate transporters that mediate folate transport across epithelia and into systemic tissues.
5.1 Hydrolysis of folate polyglutamates
The major natural folate forms in the diet are polyglutamate congeners of 5-methylTHF that are not substrates for the absorptive process (139,140). This is consistent with the recent demonstration that affinities of PCFT for the di- to penta- glutamates of MTX are very low (66). Rather, folate polyglutamate derivatives must be hydrolyzed to their monoglutamate forms by glutamate carboxypeptidase II in human intestine and γ-glutamyl hydrolase in rat intestine (141,142). The γ-glutamate hydrolytic reaction has a low pH optimum like the absorptive process, consistent with the low pH at the surface of mucosal cells in the proximal jejunum.
5.2 Intestinal Absorption
Once folate monoglutamate is generated, it is transported across the apical brush border membrane of the proximal jejunum mediated by PCFT. Because PCFT transport is concentrative, driven by the transmembrane proton gradient, the high folate levels in enterocytes should facilitate folate efflux across the basolateral membrane into the periserosal space and, from there, enter the vascular system. The mechanism of export from enterocytes is not clear; neither RFC nor PCFT is expressed at the basolateral membrane. However, MRPs are expressed at this site, particularly MRP3 (34), and may represent the route of export of folates (Section 3.4).
5.3 Transport to, and disposition in, the liver
After intestinal absorption, folates enter the hepatic portal system and are delivered to the hepatic sinusoids in apposition to the hepatocyte basolateral (sinusoidal) membrane. PCFT is highly expressed in the liver, and localizes to the sinusoidal membrane (Wang and Goldman, unpublished). This is consistent with a dominant low pH folate transport activity in hepatocytes and in sinusoidal membranes derived from hepatocytes (143,144). In the latter, concentrative folate transport is produced with a transvesicular pH gradient. There is minimal transport activity at pH 7.4, consistent with a low level of RFC activity (145,146), although RFC is detected at the hepatocyte membrane (80). It is unclear as to the pH within hepatic sinusoids where hepatic portal vein blood derived from the intestine, pancreas, and spleen mixes with hepatic artery blood. It has been proposed that a Na+/H+ antiporter at the sinusoidal membrane may generate a local extracellular region of high acidity (147), as is the case in the proximal jejunum.
Folates that enter the liver have three potential destinations. (i) Folates can be converted to polyglutamate storage forms or (ii) they can be secreted into the bile at the hepatic canalicular membrane, mediated by MRP2 (117), whereby they return to the duodenum and jejunum and are subsequently reabsorbed, thus completing the cycle of enterohepatic circulation. (iii) Folate monoglutamates, formed by hydrolysis of polyglutamate forms stored in hepatocytes, or delivered directly from the hepatic portal vein, enter the hepatic vein, ultimately reaching the systemic circulation whereby they accumulate in, and meet the one-carbon requirements of, peripheral tissues. Folates in blood also encounter epithelial barriers separating compartments containing highly folate-dependent tissues. Notable are neural tissues isolated by the vascular blood-brain-barrier and blood-choroid plexus-CSF-barrier.
5.4 Transport into systemic tissues
Membrane transport of folates into systemic tissues via the arterial system where the pH is 7.4 is mediated by the RFC. While PCFT is often co-expressed with RFC, PCFT function would be minimal and not likely contribute to the delivery of folates systemically unless, as suggested above, there are tissues where Na+/H+ exchangers produce acid microenvironments at transport interfaces. PCFT may also play an important role in FRα–mediated transport (see Sections 3.3 and 5.6).
5.5 Filtration and reabsorption in the kidney
Blood folates, not bound to serum proteins, are filtered at the glomerulus but, due to a highly efficient reabsorptive mechanism, little or none is lost in the urine at physiological levels of folate intake (148). The initial step in reabsorption involves tight binding to FRα highly expressed at the luminal brush-border membrane of proximal tubules. The affinities of folates for this site are consistent with their affinities for FRα (149,150) and the urinary clearance of folates is inversely proportional to their affinities for FRα (151). In the FRα (−/−) mouse, (maintained on standard chow – see above, Section 4) there is increased folic acid clearance, and decreased 5-methylTHF uptake (152). Folates accumulate to high levels in the kidney, reflecting the large component bound to FRα. This is followed by slow internalization of receptors, release of folate into the cytoplasm, and export at the basolateral membrane by RFC where the pH is optimal for carrier function (80,148,149). Several SLC21 solute carriers are expressed at the apical brush-border membrane of proximal renal tubule cells (see Section 3.4) and probably contribute to renal folate absorption, particularly at high folate loads, when FRα-mediated absorption is saturated (152). The role of PCFT in renal folate reabsorption is not clear. PCFT mRNA is highly expressed in the kidney but its exact location has not been defined (7,66). In renal brush-border membrane vesicles, the folate flux and net transport is optimal at low pH when the pH of the trans-compartment is 7.4, consistent with a PCFT-mediated process (153,154). The pH in the proximal tubule starts at 7.4 and falls to ∼6.8 as filtrate passes down the tubule, bicarbonate is reabsorbed, and chloride is secreted (155). Under these conditions, should PCFT be present, only modest PCFT-mediated transport at the brush-border membrane would be expected.
While a rigorous analysis of renal folate clearance in subjects with HFM has not been reported, some information can be gleaned from published studies. In one patient with HFM, the clearance of folate from blood, after an intravenous bolus, was not different from that of a normal subject over the first 2−3 hours (156). In another case, clearance in a patient with HFM over this interval was similar to what was observed in historical controls (148,157). These observations suggest that PCFT is not required for reabsorption of folates in the proximal tubule. However, since the initial phase of folate clearance from blood is influenced by uptake and retention in systemic tissues and tight binding of filtered folate to FRα, more than a few hours may be necessary to fully decipher the role of PCFT in the reabsorptive process. It is also possible that at least a component of the FR-mediated folate reabsorption in the proximal tubule represents a transcytosis in which vesicles formed at the brush-border membrane retain folate as they transit intact to the basolateral membrane where their contents are discharged to the peritubular space. However, uptake of tritiated folate alone, or folate coupled to colloidal gold particles, into renal tubular epithelial cells is consistent with translocation of vesicles across the brush-border membrane, followed by recycling back to that membrane via dense apical tubules (158,159).
5.6 Transport of folates across the blood-brain-barrier and the choroid plexus-CSF-barrier
There are two potential routes of delivery of substrates to the brain, the blood-brain-barrier at the cerebral vascular endothelium, and the blood-CSF-barrier at the choroid plexus. RFC (80) and PCFT (Zhao et al, unpublished) proteins are localized to the vascular blood-brain-barrier. FRα message is neither expressed in brain nor is protein detected at the blood-brain-barrier (160,161). The high rate of delivery of 5-methylTHF to brain parenchyma is consistent with folate extraction from blood mediated predominantly at the vascular blood-brain-barrier (162). Transport of 5-methylTHF into human brain capillaries is saturable and can be inhibited by low levels of 5-methylTHF or folic acid (162). While this has been interpreted as implicating FRα in this process, despite the absence of the receptor in brain, this is also consistent with a PCFT-mediated process for which folic acid and 5-methylTHF have comparable affinities. However, there is no evidence that the pH at this transport interface is acidic.
The choroid plexus represents the pathway for the active transport of substrates and metabolites into and out of the CSF (163). The concentration of most small molecules in CSF is less than in blood (163). The opposite is the case for folates. The usual CSF:blood folate ratio is 2–3:1 (135), consistent with concentrative folate transport across the choroid plexus. It is clear that PCFT is required to achieve usual blood:CSF folate gradients, since this ratio is reversed in subjects with HFM (135). Evidence that FRα plays a role in this process comes from the observations that: (i) this protein is highly expressed in choroid plexus; and (ii) blocking autoantibodies to FR are found in the blood of patients with cerebral folate deficiency. In this disorder, there are low levels of CSF folate even though folate intestinal absorption and folate blood levels are normal (164–168). These subjects do not have mutations in FRα, RFC, or PCFT (164).
Studies on transport of folates into the choroid plexus in vivo and in vitro further substantiate a role for this organ in the transport of folates (161,162,169,170). PCFT protein is expressed at the basolateral membrane of ependymal cells at the capillary interface (R. Zhao et al., unpublished); RFC is expressed at the apical membrane at the CSF interface (80). FRα mRNA is expressed in the choroid plexus, and is visualized at the basolateral and, to a greater extent, apical basolateral membranes (161,171–174). While both interfaces are likely at neutral pH, Na+/H+ antiporters expressed at the basolateral membrane (175) could produce an acid microclimate that facilitates PCFT function, as occurs in the proximal small intestine. One possible model that includes FRα and PCFT envisions FRα-mediated endocytosis across the basolateral membrane, generating a folate gradient across ependymal cells, followed by the downhill flow of folate across the apical membrane into the CSF mediated by RFC. While RFC produces small folate gradients directed into cells, it mediates very rapid bidirectional fluxes of folates (176). According to this paradigm, PCFT facilitates FRα-mediated endocytosis by serving as the route for folate export from acidified endosomes into the cytoplasm (Figure 3). As observed for transport across the vascular blood-brain-barrier, 5-methylTHF appears to be the preferred substrate for transport across the choroid plexus (162,170,177).
5.7 Transport of folates across the placenta
FRα, RFC, and PCFT are all highly expressed in the placenta (7,46,109,172,178), although the location of, and relationship among, these transporters has not been established. However, the observation that low levels of folic acid, but not MTX, inhibit maternal to fetal [3H]folic acid transport across guinea pig placenta suggests that FRα plays an important role in the initial phase of folate transport from maternal blood at the apical membrane of the syncytiotrophoblast (179,180). Further evidence of a role for FRα in placental transport involves the inhibitory effects of bafilomycin A, which blocks the V-type proton pump, or FCCP, which abolishes the transmembrane proton gradient, on folic acid transport into tumor cells of trophoblastic origin (JAR). This is presumably due to their inhibitory effects on folate export from endosomes in the endocytic pathway (99). Transport of folic acid in the BeWo trophoblastic cell line is maximal at pH 5.0, reflecting PCFT-mediated transport, and decreases as pH is increased, although a second peak of activity at pH 7.4 is also detected, likely reflecting RFC-mediated transport (181). Vectorial transport of folic acid across BeWo cells, from the apical compartment (maternal side) at pH 5.5, separated by a semi-permeable membrane from the basal compartment (fetal side) at pH 7.4, was far greater than transport from the basal to apical compartments when the pH gradient was reversed (182). This suggests a higher level of PCFT activity at the apical than basal interface.
Because the placenta is fetal in origin, and because PCFT (−/−) subjects with HFM do not have developmental defects associated with folate deficiency, PCFT does not appear to be necessary for placental transport of folate from mother to embryo or fetus despite its high level of placental expression and its potential role in FRα-mediated endocytosis. The histories of families with HFM reveal that PCFT (+/−) mothers can support a normal pregnancy. Unanswered as yet is whether the loss of two PCFT alleles will affect maternal-to-fetus folate delivery. The oldest reported female with HFM is now age 28 and has had normal menarche but has not, as yet, become pregnant (10,183,184).
6.0 Clinical Implications/Applications
Folate transport is a critical determinant of folate sufficiency and the integrity of one-carbon metabolism in man. Hence, expression of folate transporters, as determined by genetic, epigenetic and other regulatory factors, is of considerable importance. The requirements for RFC and FRα in embryonic development, and PCFT in infancy, are clear. Folate deficiency during adult life may be associated with transport variations that contribute to other pathophysiologic states including cancer, cardiovascular disease, and neurological disorders (185–187). Folate deficiency in pregnant women is a major factor in fetal abnormalities (188). Mild transport variations could be compounded by alterations in activities of folate-dependent interconverting and biosynthetic enzymes for which there are known allelic variations (e.g., C677T in MTHFR) that impact on cellular distributions of individual THF cofactor forms (189). The cumulative effects could be impaired nucleotide biosynthesis, impaired repair of DNA damage, DNA hypomethylation resulting in aberrant oncogene expression, or DNA hypermethylation resulting in silencing of protective genes such as tumor suppressors (185).
An understanding of the molecular basis for HFM now makes possible a definitive diagnosis of this disorder in families. This allows genetic counseling along with prenatal and in vitro testing. Individualization of treatment with specific folates may be possible, based upon the functional characteristics and specificity of individual mutant PCFTs in patients with HFM.
RFC-mediated transport is also key to antitumor activities of antifolate chemotherapeutic drugs such as MTX (4,12). Early on, it was recognized that intrinsic and acquired resistance to MTX is frequently due to impaired transport in cell lines (190–192) and is a factor in resistance to MTX in ALL and osteogenic sarcoma (193–195). Enhanced accumulations of MTX and its polyglutamates accompany increased levels of hRFC transcripts and copies of chromosome 21 in hyperdiploid B-precursor ALL (196).
Recently, pemetrexed was approved for the treatment of cancer in the United States, more than fifty-years following the introduction of MTX (13). Pemetrexed has an affinity for RFC comparable to that of MTX. However, pemetrexed differs from MTX in that its affinity for PCFT is sufficiently high, even at physiological pH, that its activity is preserved in tumor cells that are MTX resistant due to loss of RFC function (13,87,197).
7.0 Research in progress and outstanding research questions
The transcriptional and post-transcriptional controls that contribute to tissue-specific expression of RFC have been largely defined (4). While there have been extensive studies of high frequency sequence polymorphisms in the hRFC coding and noncoding regions in relation to a variety of pathological states (4,56), these associations remain controversial. The recent production of a “humanized” RFC mouse in which the mouse RFC gene is functionally replaced by the hRFC gene locus (198) holds promise for extending in vitro findings of hRFC regulation and function including polymorphic hRFC gene variants to a clinically relevant in vivo model. This will allow correlations with dietary folate status and biological manifestations of folate deficiency.
Structure-function studies of hRFC have provided substantive insights into its membrane topology, N-glycosylation, important domains and amino acids, and three dimensional helix packing associations (4). Recent studies indicate that there are higher order oligomers (e.g., dimers) of hRFC (Z. Hou and L.H. Matherly, unpublished), as has been observed for other transporters (199). Clarification of RFC oligomeric character is especially important to understanding carrier structure and function, including the mechanism of concentrative folate transport. The existence of homo-oligomeric hRFC may also play an important role in antifolate resistance through potential “dominant-negative” interactions between mutant and wild-type hRFCs that result in alterations in function and/or trafficking defects.
Since PCFT was only recently discovered, there is only scant information on the structural properties of this transporter. A predicted secondary structure was described and localization of the N- and C-termini and the first extracellular loop was verified (66,74). Studies are ongoing to identify key amino acid residues that are determinants of substrate binding, proton-coupling, and carrier mobility. Future studies will emphasize helix packing and tertiary structure using approaches such as cysteine-scanning mutagenesis and accessibility methods (35,200). Of course, the finding of higher order hRFC oligomers raises the intriguing possibility that oligomeric PCFT may be important, as well. All these studies of PCFT structure and function would be enhanced considerably by the availability of a three dimensional structural model of this transporter. The extrapolation of a prokaryotic model based upon the GlpT crystal structure to PCFT is appealing (136) since GlpT is also proton-coupled although, unlike PCFT, this is a proton antiporter (201). Extrapolation of this and other models to PCFT or other transporters will require rigorous experimental verification, as noted above for RFC.
Since folate deficiency is associated with increased risk of colorectal cancer, deciphering the role of RFC and PCFT in the delivery of folates to the large intestine and their regulation at this site, within the context of dietary and environmental factors, will be of considerable importance. These studies need to take into consideration the recent paradigm that folate deficiency results in an increased frequency of oncogenesis, but once cancers are formed, excess folates may accelerate tumor growth (202,203). For RFC, causal associations with loss of carrier function and colorectal cancer were described in mouse models (204,205).
The hypothesis that PCFT is required for FR function, by serving as a route of export from acidified endosomes, broadens the potential biological importance of this carrier. Clarification of this issue could establish a critical role for PCFT in the transport of folates across the blood-choroid plexus-barrier and clarify why transport into this compartment is impaired in HFM. Additional information is also required to clarify the functional role of PCFT in other tissues in which it is highly expressed, particularly those expected to harbor a neutral pH milieu. This relates, in particular, to the extent to which PCFT mediates folate transport from the hepatic portal vein across the sinusoidal membrane to hepatic cells, reabsorption in the proximal renal tubule, transport across the placenta to the fetus, and transport across the choroid plexus and into brain parenchyma at the blood-brain-barrier. These questions will be best addressed by studies on a PCFT-null mouse.
The role that PCFT plays in antifolate delivery to tumor cells requires further exploration. PCFT has a high affinity for pemetrexed and, when transfected into tumor cells, selectivity augments the drug’s growth inhibitory activity without a salutary effect on other antifolates such as MTX, PT523 or raltitrexed which have a much lower affinity for PCFT at physiological pH (67,197). It will be important to determine whether PCFT might contribute more to the activity of pemetrexed, or at all to the activities of other antifolates, when transport occurs within solid tumors in vivo where cells are hypoxic, the microenvironment is somewhat acidic, and PCFT would be expected to operate more efficiently (206–208). Likewise, because of the high Km s for antifolate transport at physiological pH, it will be important to determine the role PCFT plays in the delivery of antifolates at the high blood levels achieved in clinical regimens under conditions in which RFC is saturated.
Finally, there continues to be an ongoing interest in the development of new-generation antifolates. Antifolate thymidylate synthase inhibitors were designed with very low affinity for RFC but very high affinity for FRα, so as to minimize toxicity due to uptake via RFC which is ubiquitously expressed, and to maximize uptake via FRs that are more selectively expressed in certain tumors (209–211). An analogous strategy was very recently described for a series of highly potent FR-targeted antifolate GARFT inhibitors (212). It will be of particular interest to determine whether the activities of these novel FR-targeted agents will be limited by their efficacies as substrates for PCFT which may be required for their optimal export from endosomes during the endocytic cycle.
Acknowledgements
The authors wish to thank the reviewers of this manuscript for their very helpful suggestions. This work was supported by grants CA53535 (LHM) and CA82621 (IDG) from the National Institutes of Health and from the Mesothelioma Applied Research Foundation (IDG).
Reference List
- 1.Stokstad ELR. Historical perspective on key advances in the biochemistry and physiology of folates. In: Picciano MF, E. Stokstad LR, editors. Folic Acid Metabolism in Health and Disease. New York: Wiley-Liss; 1990. pp. 1–21. [Google Scholar]
- 2.Jacques PF, et al. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–1454. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 3.Matherly LH, Goldman DI. Membrane transport of folates. Vitam Horm. 2003;66:403–456. doi: 10.1016/s0083-6729(03)01012-4. [DOI] [PubMed] [Google Scholar]
- 4.Matherly LH, Hou Z, Deng Y. Human reduced folate carrier: translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev. 2007;26:111–128. doi: 10.1007/s10555-007-9046-2. [DOI] [PubMed] [Google Scholar]
- 5.Kamen BA, Smith AK. A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev. 2004;56:1085–1097. doi: 10.1016/j.addr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007;26:141–152. doi: 10.1007/s10555-007-9048-0. [DOI] [PubMed] [Google Scholar]
- 7.Qiu A, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Zhao R, Goldman ID. The molecular identity and characterization of a Proton-coupled Folate Transporter--PCFT; biological ramifications and impact on the activity of pemetrexed. Cancer Metastasis Rev. 2007;26:129–139. doi: 10.1007/s10555-007-9047-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhao R, et al. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood. 2007;110:1147–1152. doi: 10.1182/blood-2007-02-077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min SH, et al. The clinical course and genetic defect in the PCFT in a 27-year-old woman with Hereditary folate malabsorption. J Pediatr. 2008;153:435–437. doi: 10.1016/j.jpeds.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2008;47:249–255. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- 12.Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;22:7431–7457. doi: 10.1038/sj.onc.1206946. [DOI] [PubMed] [Google Scholar]
- 13.Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6:404–417. doi: 10.1158/1535-7163.MCT-06-0343. [DOI] [PubMed] [Google Scholar]
- 14.Shane B. Folylpolyglutamate synthesis and role in the regulation of one-carbon metabolism. Vitam Horm. 1989;45:263–335. doi: 10.1016/s0083-6729(08)60397-0. [DOI] [PubMed] [Google Scholar]
- 15.Matherly LH, et al. The effects of 4-aminoantifolates on 5-formyltetrahydrofolate metabolism in L1210 cells. J Biol Chem. 1987;262:710–717. [PubMed] [Google Scholar]
- 16.Seither RL, et al. Folate-pool interconversions and inhibition of biosynthetic processes after exposure of L1210 leukemia cells to antifolates. J Biol Chem. 1989;264:17016–17023. [PubMed] [Google Scholar]
- 17.Goldman ID, Lichtenstein NS, Oliverio VT. Carrier-mediated transport of the folic acid analogue methotrexate, in the L1210 leukemia cell. J Biol Chem. 1968;243:5007–5017. [PubMed] [Google Scholar]
- 18.Goldman ID, Matherly LH. The cellular pharmacology of methotrexate. Pharmacol & Ther. 1985;28:77–102. doi: 10.1016/0163-7258(85)90083-x. [DOI] [PubMed] [Google Scholar]
- 19.Sirotnak FM, Tolner B. Carrier-mediated membrane transport of folates in mammalian cells. Annu Rev Nutr. 1999;19:91–122. doi: 10.1146/annurev.nutr.19.1.91. [DOI] [PubMed] [Google Scholar]
- 20.Henderson GB, Zevely EM. Structural requirements for anion substrates of the methotrexate transport system of L1210 cells. Arch Biochem Biophys. 1983;221:438–446. doi: 10.1016/0003-9861(83)90162-5. [DOI] [PubMed] [Google Scholar]
- 21.Goldman ID. The characteristics of the membrane transport of amethopterin and the naturally occurring folates. Ann N Y Acad Sci. 1971;186:400–422. doi: 10.1111/j.1749-6632.1971.tb46996.x. [DOI] [PubMed] [Google Scholar]
- 22.Labay V, et al. Mutations in SLC19A2 cause thiamine-responsive megaloblastic anaemia associated with diabetes mellitus and deafness. Nat Genet. 1999;22:300–304. doi: 10.1038/10372. [DOI] [PubMed] [Google Scholar]
- 23.Fleming JC, et al. The gene mutated in thiamine-responsive anaemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat Genet. 1999;22:305–308. doi: 10.1038/10379. [DOI] [PubMed] [Google Scholar]
- 24.Diaz GA, et al. Mutations in a new gene encoding a thiamine transporter cause thiamine- responsive megaloblastic anaemia syndrome. Nat Genet. 1999;22:309–312. doi: 10.1038/10385. [DOI] [PubMed] [Google Scholar]
- 25.Dutta B, et al. Cloning of the human thiamine transporter, a member of the folate transporter family. J Biol Chem. 1999;274:31925–31929. doi: 10.1074/jbc.274.45.31925. [DOI] [PubMed] [Google Scholar]
- 26.Rajgopal A, et al. SLC19A3 encodes a second thiamine transporter, ThTr2. Biochim Biophys Acta. 2001;1537:175–178. doi: 10.1016/s0925-4439(01)00073-4. [DOI] [PubMed] [Google Scholar]
- 27.Rindi G, Laforenza U. Thiamine intestinal transport and related issues: recent aspects. Proc Soc Exp Biol Med. 2000;224:246–255. doi: 10.1046/j.1525-1373.2000.22428.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhao R, et al. Impact of the reduced folate carrier on the accumulation of active thiamin metabolites in murine leukemia cells. J Biol Chem. 2000;276:1114–1118. doi: 10.1074/jbc.M007919200. [DOI] [PubMed] [Google Scholar]
- 29.Zhao R, Gao F, Goldman ID. Reduced folate carrier transports thiamine monophosphate: an alternative route for thiamine delivery into mammalian cells. Am J Physiol Cell Physiol. 2002;282:C1512–C1517. doi: 10.1152/ajpcell.00547.2001. [DOI] [PubMed] [Google Scholar]
- 30.Yang C-H, Sirotnak FM, Dembo M. Interaction between anions and the reduced folate/methotrexate transport system in L1210 cell plasma membrane vesicles: directional symmetry and anion specificity for differential mobility of loaded and unloaded carrier. J Membr Biol. 1984;79:285–292. doi: 10.1007/BF01871067. [DOI] [PubMed] [Google Scholar]
- 31.Goldman ID. Transport energetics of the folic acid analogue, methotrexate, in L1210 cells: Enhanced accumulation by metabolic inhibitors. J Biol Chem. 1969;244:3779–3785. [PubMed] [Google Scholar]
- 32.Fry DW, White JC, Goldman ID. Effects of 2,4-dinitrophenol and other metabolic inhibitors on the bidirectional carrier fluxes, net transport, and intracellular binding of methotrexate in Ehrlich ascites tumor cells. Cancer Res. 1980;40:3669–3673. [PubMed] [Google Scholar]
- 33.Dembo M, Sirotnak FM, Moccio DM. Effects of metabolic deprivation on methotrexate transport in L1210 leukemia cells: further evidence for separate influx and efflux systems with different energetic requirements. J Membr Biol. 1984;78:9–17. doi: 10.1007/BF01872527. [DOI] [PubMed] [Google Scholar]
- 34.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 35.Cao W, Matherly LH. Analysis of the membrane topology for transmembrane domains 7–12 of the human reduced folate carrier by scanning cysteine accessibility methods. Biochem J. 2004;378:201–206. doi: 10.1042/BJ20031288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson PL, Flintoff WF. Topological and functional analysis of the human reduced folate carrier by hemagglutinin epitope insertion. J Biol Chem. 1999;274:16269–16278. doi: 10.1074/jbc.274.23.16269. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Matherly L. Analysis of membrane topology of the human reduced folate carrier protein by hemagglutinin epitope insertion and scanning glycosylation insertion mutagenesis. Biochim Biophys Acta. 2002;1564:333–342. doi: 10.1016/s0005-2736(02)00467-4. [DOI] [PubMed] [Google Scholar]
- 38.Wong SC, et al. Effects of the loss of capacity for N-glycosylation on the transport activity and cellular localization of the human reduced folate carrier. Biochim Biophys Acta. 1998;1375:6–12. doi: 10.1016/s0005-2736(98)00118-7. [DOI] [PubMed] [Google Scholar]
- 39.Liu XY, Witt TL, Matherly LH. (3 A.D.) Restoration of high level transport activity by human reduced folate carrier/ThT1 chimeric transporters: Role of the transmembrane domain 6/7 linker region in reduced folate carrier function. Biochem J. 369:31–37. doi: 10.1042/BJ20020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witt TL, Stapels SE, Matherly LH. Restoration of transport activity by co-expression of human reduced folate carrier half-molecules in transport-impaired K562 cells: localization of a substrate binding domain to transmembrane domains 7–12. J Biol Chem. 2004;279:46755–46763. doi: 10.1074/jbc.M408696200. [DOI] [PubMed] [Google Scholar]
- 41.Deng Y, et al. Role of lysine 411 in substrate carboxyl group binding to the human reduced folate carrier, as determined by site-directed mutagenesis and affinity inhibition. Mol Pharmacol. 2008;73:1274–1281. doi: 10.1124/mol.107.043190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou Z, et al. Transmembrane domains 4, 5, 7, 8, and 10 of the human reduced folate carrier are important structural or functional components of the transmembrane channel for folate substrates. J Biol Chem. 2006;281:33588–33596. doi: 10.1074/jbc.M607049200. [DOI] [PubMed] [Google Scholar]
- 43.Hou Z, et al. Localization of a substrate binding domain of the human reduced folate carrier to transmembrane domain 11 by radioaffinity labeling and cysteine-substituted accessibility methods. J Biol Chem. 2005;280:36206–36213. doi: 10.1074/jbc.M507295200. [DOI] [PubMed] [Google Scholar]
- 44.Abramson J, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 45.Huang Y, et al. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 46.Whetstine JR, Flatley RM, Matherly LH. The human reduced folate carrier gene is ubiquitously and differentially expressed in normal human tissues: identification of seven non-coding exons and characterization of a novel promoter. Biochem J. 2002;367:629–640. doi: 10.1042/BJ20020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu M, et al. Structure and regulation of the murine reduced folate carrier gene: identification of four noncoding exons and promoters and regulation by dietary folates. J Biol Chem. 2005;280:5588–5597. doi: 10.1074/jbc.M412662200. [DOI] [PubMed] [Google Scholar]
- 48.Payton SG, et al. Effects of 5’ untranslated region diversity on the posttranscriptional regulation of the human reduced folate carrier. Biochim Biophys Acta. 2007;1769:131–138. doi: 10.1016/j.bbaexp.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flatley RM, et al. Primary acute lymphoblastic leukemia cells use a novel promoter and 5’noncoding exon for the human reduced folate carrier that encodes a modified carrier translated from an upstream translational start. Clin Cancer Res. 2004;10:5111–5122. doi: 10.1158/1078-0432.CCR-04-0116. [DOI] [PubMed] [Google Scholar]
- 50.Chango A, et al. A polymorphism (80G->A) in the reduced folate carrier gene and its associations with folate status and homocysteinemia. Mol Genet Metab. 2000;70:310–315. doi: 10.1006/mgme.2000.3034. [DOI] [PubMed] [Google Scholar]
- 51.Whetstine JR, et al. Single nucleotide polymorphisms in the human reduced folate carrier: characterization of a high-frequency G/A variant at position 80 and transport properties of the His(27) and Arg(27) carriers. Clin Cancer Res. 2001;7:3416–3422. [PubMed] [Google Scholar]
- 52.Whetstine JR, Witt TL, Matherly LH. The human reduced folate carrier gene is regulated by the AP2 and Sp1 transcription factor families and a functional 61 base pair polymorphism. J Biol Chem. 2002;277:43873–43880. doi: 10.1074/jbc.M208296200. [DOI] [PubMed] [Google Scholar]
- 53.De Marco P, et al. Reduced folate carrier polymorphism (80A-->G) and neural tube defects. Eur J Hum Genet. 2003;11:245–252. doi: 10.1038/sj.ejhg.5200946. [DOI] [PubMed] [Google Scholar]
- 54.Morin I, et al. Evaluation of genetic variants in the reduced folate carrier and in glutamate carboxypeptidase II for spina bifida risk. Mol Genet Metab. 2003;79:197–200. doi: 10.1016/s1096-7192(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 55.Laverdiere C, et al. Polymorphism G80A in the reduced folate carrier gene and its relationship to methotrexate plasma levels and outcome of childhood acute lymphoblastic leukemia. Blood. 2002;100:3832–3834. doi: 10.1182/blood.V100.10.3832. [DOI] [PubMed] [Google Scholar]
- 56.Human Reduced Folate Carrier Gene and Transcript Variants: Functional, Physiologic, and Pharmacologic Consequences. Current Pharmacogenetics. 2004;2:287–298. [Google Scholar]
- 57.O’Leary VB, et al. Reduced folate carrier polymorphisms and neural tube defect risk. Mol Genet Metab. 2006;87:364–369. doi: 10.1016/j.ymgme.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 58.Nakai Y, et al. Functional characterization of human PCFT/HCP1 heterologously expressed in mammalian cells as a folate transporter. J Pharmacol Exp Ther. 2007;322:469–476. doi: 10.1124/jpet.107.122606. [DOI] [PubMed] [Google Scholar]
- 59.Umapathy NS, et al. Cloning and functional characterization of the proton-coupled electrogenic folate transporter and analysis of its expression in retinal cell types. Invest Ophthalmol Vis Sci. 2007;48:5299–5305. doi: 10.1167/iovs.07-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shayeghi M, et al. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 61.Henderson GB, Strauss BP. Characteristics of a novel transport system for folate compounds in wild-type and methotrexate-resistant L1210 cells. Cancer Res. 1990;50:1709–1714. [PubMed] [Google Scholar]
- 62.Zhao R, et al. A prominent low-pH methotrexate transport activity in human solid tumor cells: Contribution to the preservation of methotrexate pharmacological activity in HeLa cells lacking the reduced folate carrier. Clin Cancer Res. 2004;10:718–727. doi: 10.1158/1078-0432.ccr-1066-03. [DOI] [PubMed] [Google Scholar]
- 63.Kuhnel JM, Chiao JH, Sirotnak FM. Contrasting effects of oncogene expression on two carrier-mediated systems internalizing folate compounds in Fisher rat 3T3 cells. J Cell Physiol. 2000;184:364–372. doi: 10.1002/1097-4652(200009)184:3<364::AID-JCP11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 64.Sierra EE, Goldman ID. Characterization of folate transport mediated by a low pH route in mouse L1210 leukemia cells with defective reduced folate carrier function. Biochem Pharmacol. 1998;55:1505–1512. doi: 10.1016/s0006-2952(97)00673-4. [DOI] [PubMed] [Google Scholar]
- 65.Assaraf YG, Babani S, Goldman ID. Increased activity of a novel low pH folate transporter associated with lipoplilic antifolate resistance in Chinese hamster ovary cells. J Biol Chem. 1998;273:8106–8111. doi: 10.1074/jbc.273.14.8106. [DOI] [PubMed] [Google Scholar]
- 66.Qiu A, et al. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am J Physiol Cell Physiol. 2007;293:C1669–C1678. doi: 10.1152/ajpcell.00202.2007. [DOI] [PubMed] [Google Scholar]
- 67.Zhao R, et al. The proton-coupled folate transporter (PCFT): impact on pemetrexed transport and on antifolate activities as compared to the reduced folate carrier. Mol Pharmacol. 2008;74:854–862. doi: 10.1124/mol.108.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sierra EE, et al. pH dependence of methotrexate transport by the reduced folate carrier and the folate receptor in L1210 leukemia cells -Further evidence for a third route mediated at low pH. Biochem Pharmacol. 1997;53:223–231. doi: 10.1016/s0006-2952(96)00730-7. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Zhao R, Goldman ID. Characterization of a folate transporter in HeLa cells with a low pH optimum and high affinity for pemetrexed distinct from the reduced folate carrier. Clin Cancer Res. 2004;10:6256–6264. doi: 10.1158/1078-0432.CCR-04-0645. [DOI] [PubMed] [Google Scholar]
- 70.Schron CM, Washington C, Jr, Blitzer BL. The transmembrane pH gradient drives uphill folate transport in rabbit jejunum. Direct evidence for folate/hydroxyl exchange in brush border membrane vesicles. J Clin Invest. 1985;76:2030–2033. doi: 10.1172/JCI112205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mackenzie B, et al. Divalent metal-ion transporter DMT1 mediates both H+ -coupled Fe2+ transport and uncoupled fluxes. Pflugers Arch. 2006;451:544–558. doi: 10.1007/s00424-005-1494-3. [DOI] [PubMed] [Google Scholar]
- 72.Sirotnak FM, et al. Stereospecificity at carbon 6 of formyltetrahydrofolate as a competitive inhibitor of transport and cytotoxicity of methotrexate in vitro. Biochem Pharmacol. 1979;28:2993–2997. doi: 10.1016/0006-2952(79)90599-9. [DOI] [PubMed] [Google Scholar]
- 73.Inoue K, et al. Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model. Am J Physiol Gastrointest Liver Physiol. 2008;294:G660–G668. doi: 10.1152/ajpgi.00309.2007. [DOI] [PubMed] [Google Scholar]
- 74.Unal ES, et al. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT) Biochim Biophys Acta. 2008;1178:1407–1414. doi: 10.1016/j.bbamem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yun CH, et al. Structure/function studies of mammalian Na-H exchangers--an update. J Physiol. 1995;482:1S–6S. doi: 10.1113/jphysiol.1995.sp020558. 1S-6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McEwan GT, et al. A combined TDDA-PVC pH and reference electrode for use in the upper small intestine. J Med Eng Technol. 1990;14:16–20. doi: 10.3109/03091909009028758. [DOI] [PubMed] [Google Scholar]
- 77.Ikuma M, et al. Effects of aging on the microclimate pH of the rat jejunum. Biochim Biophys Acta. 1996;1280:19–26. doi: 10.1016/0005-2736(95)00261-8. [DOI] [PubMed] [Google Scholar]
- 78.Said HM, Smith R, Redha R. Studies on the intestinal surface acid microclimate: developmental aspects. Pediatr Res. 1987;22:497–499. doi: 10.1203/00006450-198711000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Yang J, et al. Characterization of the pH of folate receptor-containing endosomes and the rate of hydrolysis of internalized acid-labile folate-drug conjugates. J Pharmacol Exp Ther. 2007;321:462–468. doi: 10.1124/jpet.106.117648. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, et al. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochim Biophys Acta. 2001;1513:49–54. doi: 10.1016/s0005-2736(01)00340-6. [DOI] [PubMed] [Google Scholar]
- 81.Subramanian VS, Marchant JS, Said HM. Apical membrane targeting and trafficking of the human proton-coupled transporter in polarized epithelia. Am J Physiol Cell Physiol. 2008;294:C233–C240. doi: 10.1152/ajpcell.00468.2007. [DOI] [PubMed] [Google Scholar]
- 82.Mason JB, Rosenberg IH. Intestinal absorption of folate. In: Johnson LR, editor. Physiology of the gastrointestinal tract, Third ed. New York: Raven Press; 1994. pp. 1979–1995. [Google Scholar]
- 83.Selhub J, Rosenberg IH. Folate transport in isolated brush border membrane vesicles from rat intestine. J Biol Chem. 1981;256:4489–4493. [PubMed] [Google Scholar]
- 84.Mason JB, et al. Carrier affinity as a mechanism for the pH-dependence of folate transport in the small intestine. Biochim Biophys Acta Bio-Membr. 1990;1024:331–335. doi: 10.1016/0005-2736(90)90362-r. [DOI] [PubMed] [Google Scholar]
- 85.Kumar CK, et al. Comparison of intestinal folate carrier clone expressed in IEC-6 cells and in Xenopus oocytes. Am J Physiol. 1998;274:C289–C294. doi: 10.1152/ajpcell.1998.274.1.C289. [DOI] [PubMed] [Google Scholar]
- 86.Chiao JH, et al. RFC-1 gene expression regulates folate absorption in mouse small intestine. J Biol Chem. 1997;272:11165–11170. doi: 10.1074/jbc.272.17.11165. [DOI] [PubMed] [Google Scholar]
- 87.Chattopadhyay S, et al. The inverse relationship between reduced folate carrier function and pemetrexed activity in a human colon cancer cell line. Mol Cancer Ther. 2006;5:438–449. doi: 10.1158/1535-7163.MCT-05-0243. [DOI] [PubMed] [Google Scholar]
- 88.Zhao R, Hanscom M, Goldman ID. The relationship between folate transport activity at low pH and reduced folate carrier function in human Huh7 hepatoma cells. Biochim Biophys Acta. 2005;1715:57–64. doi: 10.1016/j.bbamem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, et al. Preservation of folate transport activity with a low-pH optimum in rat IEC-6 intestinal epithelial cell lines that lack reduced folate carrier function. Am J Physiol Cell Physiol. 2005;288:C65–C71. doi: 10.1152/ajpcell.00307.2004. [DOI] [PubMed] [Google Scholar]
- 90.Balamurugan K, Said HM. Role of reduced folate carrier in intestinal folate uptake. Am J Physiol Cell Physiol. 2006;291:C189–C193. doi: 10.1152/ajpcell.00594.2005. [DOI] [PubMed] [Google Scholar]
- 91.Lu Y, Low P. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliv Rev. 2002;54:675–693. doi: 10.1016/s0169-409x(02)00042-x. [DOI] [PubMed] [Google Scholar]
- 92.Jansen G. Ann L. Jackman ed. Totowa, New Jersey: Humana Press; 1999. Receptor- and Carrier- Mediated Transport Systems for Folates and Antifolates; pp. 293–321. [Google Scholar]
- 93.Brigle KE, et al. Increased expression and characterization of two distinct folate-binding proteins in murine erythroleukemia cells. Biochem Pharmacol. 1994;47:337–345. doi: 10.1016/0006-2952(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 94.Wang X, et al. Differential stereospecificities and affinities of folate receptor isoforms for folate compounds and antifolates. Biochem Pharmacol. 1992;44:1898–1901. doi: 10.1016/0006-2952(92)90089-2. [DOI] [PubMed] [Google Scholar]
- 95.Maziarz KM, et al. Complete mapping of divergent amino acids responsible for differential ligand binding of folate receptors alpha and beta. J Biol Chem. 1999;274:11086–11091. doi: 10.1074/jbc.274.16.11086. [DOI] [PubMed] [Google Scholar]
- 96.Kamen BA, et al. Delivery of folates to the cytoplasm of MA104 cells is mediated by a surface membrane receptor that recycles. J Biol Chem. 1988;263:13602–13609. [PubMed] [Google Scholar]
- 97.Rothberg KG, et al. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990;110:637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kamen BA, Smith AK, Anderson RGW. The folate receptor works in tandem with a probenecid-sensitive carrier in MA104 cells in vitro. J Clin Invest. 1991;87:1442–1449. doi: 10.1172/JCI115150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prasad PD, et al. Functional coupling between a bafilomycin A1-sensitive proton pump and a probenecid-sensitive folate transporter in human placental choriocarcinoma cells. Biochim Biophys Acta Mol Cell Res. 1994;1222:309–314. doi: 10.1016/0167-4889(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 100.Andrews NC. When is a heme transporter not a heme transporter? When it’s a folate transporter. Cell Metab. 2007;5:5–6. doi: 10.1016/j.cmet.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 101.Sierra EE, et al. Comparison of transport properties of the reduced folate carrier and folate receptor in murine L1210 leukemia cells. Biochem Pharmacol. 1995;50:1287–1294. doi: 10.1016/0006-2952(95)94097-y. [DOI] [PubMed] [Google Scholar]
- 102.Spinella MJ, et al. Distinguishing between folate receptor-α-mediated transport and reduced folate carrier-mediated transport in L1210 leukemia cells. J Biol Chem. 1995;270:7842–7849. doi: 10.1074/jbc.270.14.7842. [DOI] [PubMed] [Google Scholar]
- 103.Parker N, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 104.Smith SB, et al. Expression of folate receptor alpha in the mammalian retinol pigmented epithelium and retina. Invest Ophthalmol Vis Sci. 1999;40:840–848. [PubMed] [Google Scholar]
- 105.Chancy CD, et al. Expression and differential polarization of the reduced-folate transporter-1 and the folate receptor alpha in mammalian retinal pigment epithelium. J Biol Chem. 2000;275:20676–20684. doi: 10.1074/jbc.M002328200. [DOI] [PubMed] [Google Scholar]
- 106.Toffoli G, et al. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997;74:193–198. doi: 10.1002/(sici)1097-0215(19970422)74:2<193::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 107.Kelley KM, Rowan BG, Ratnam M. Modulation of the folate receptor alpha gene by the estrogen receptor: mechanism and implications in tumor targeting. Cancer Res. 2003;63:2820–2828. [PubMed] [Google Scholar]
- 108.Tran T, et al. Enhancement of folate receptor alpha expression in tumor cells through the glucocorticoid receptor: a promising means to improved tumor detection and targeting. Cancer Res. 2005;65:4431–4441. doi: 10.1158/0008-5472.CAN-04-2890. [DOI] [PubMed] [Google Scholar]
- 109.Ratnam M, et al. Homologous membrane folate binding proteins in human placenta: Cloning and sequence of cDNA. Biochemistry. 1989;28:8249–8254. doi: 10.1021/bi00446a042. [DOI] [PubMed] [Google Scholar]
- 110.Wang H, et al. Differentiation-independent retinoid induction of folate receptor type beta, a potential tumor target in myeloid leukemia. Blood. 2000;96:3529–3536. [PubMed] [Google Scholar]
- 111.Reddy JA, et al. Expression and functional characterization of the beta-isoform of the folate receptor on CD34(+) cells. Blood. 1999;93:3940–3948. [PubMed] [Google Scholar]
- 112.Ross JF, et al. Folate receptor type beta is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer. 1999;85:348–357. doi: 10.1002/(sici)1097-0142(19990115)85:2<348::aid-cncr12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 113.Elnakat H. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56:1067–1084. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 114.Assaraf YG. The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resist Updat. 2006;9:227–246. doi: 10.1016/j.drup.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 115.Wielinga P, et al. The human multidrug resistance protein MRP5 transports folates and can mediate cellular resistance against antifolates. Cancer Res. 2005;65:4425–4430. doi: 10.1158/0008-5472.CAN-04-2810. [DOI] [PubMed] [Google Scholar]
- 116.Fry DW, Yalowich JC, Goldman ID. Rapid formation of poly-gamma-glutamyl derivatives of methotrexate and their association with dihydrofolate reductase as assessed by high pressure liquid chromatography in the Ehrlich ascited tumor cell in vitro. J Biol Chem. 1982;257:1890–1896. [PubMed] [Google Scholar]
- 117.Masuda M, et al. Methotrexate is excreted into the bile by canalicular multispecific organic anion transporter in rats. Cancer Res. 1997;57:3506–3510. [PubMed] [Google Scholar]
- 118.Mottino AD, et al. Expression and localization of multidrug resistant protein mrp2 in rat small intestine. J Pharmacol Exp Ther. 2000;293:717–723. [PubMed] [Google Scholar]
- 119.Rost D, et al. Expression and localization of the multidrug resistance-associated protein 3 in rat small and large intestine. Am J Physiol Gastrointest Liver Physiol. 2002;282:G720–G726. doi: 10.1152/ajpgi.00318.2001. [DOI] [PubMed] [Google Scholar]
- 120.Taipalensuu J, et al. Correlation of gene expression of ten drug efflux proteins of the ATP-binding cassette transporter family in normal human jejunum and in human intestinal epithelial Caco-2 cell monolayers. J Pharmacol Exp Ther. 2001;299:164–170. [PubMed] [Google Scholar]
- 121.Seithel A, et al. Variability in mRNA expression of ABC- and SLC-transporters in human intestinal cells: comparison between human segments and Caco-2 cells. Eur J Pharm Sci. 2006;28:291–299. doi: 10.1016/j.ejps.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 122.Koepsell H, Endou H. The SLC22 drug transporter family. Pflugers Arch. 2004;447:666–676. doi: 10.1007/s00424-003-1089-9. [DOI] [PubMed] [Google Scholar]
- 123.Sekine T, Miyazaki H, Endou H. Molecular physiology of renal organic anion transporters. Am J Physiol Renal Physiol. 2006;290:F251–F261. doi: 10.1152/ajprenal.00439.2004. [DOI] [PubMed] [Google Scholar]
- 124.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 125.Masuda S. Functional characteristics and pharmacokinetic significance of kidney-specific organic anion transporters, OAT-K1 and OAT-K2, in the urinary excretion of anionic drugs. Drug Metab Pharmacokinet. 2003;18:91–103. doi: 10.2133/dmpk.18.91. [DOI] [PubMed] [Google Scholar]
- 126.Rizwan AN, Burckhardt G. Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm Res. 2007;24:450–470. doi: 10.1007/s11095-006-9181-4. [DOI] [PubMed] [Google Scholar]
- 127.Uwai Y, et al. Methotrexate-loxoprofen interaction: involvement of human organic anion transporters hOAT1 and hOAT3. Drug Metab Pharmacokinet. 2004;19:369–374. doi: 10.2133/dmpk.19.369. [DOI] [PubMed] [Google Scholar]
- 128.Takeda M, et al. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J Pharmacol Exp Ther. 2002;302:666–671. doi: 10.1124/jpet.102.034330. [DOI] [PubMed] [Google Scholar]
- 129.Nozaki Y, et al. Quantitative evaluation of the drug-drug interactions between methotrexate and nonsteroidal anti-inflammatory drugs in the renal uptake process based on the contribution of organic anion transporters and reduced folate carrier. J Pharmacol Exp Ther. 2004;309:226–234. doi: 10.1124/jpet.103.061812. [DOI] [PubMed] [Google Scholar]
- 130.Cha SH, et al. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59:1277–1286. doi: 10.1124/mol.59.5.1277. [DOI] [PubMed] [Google Scholar]
- 131.VanWert AL, Sweet DH. Impaired clearance of methotrexate in organic anion transporter 3 (Slc22a8) knockout mice: a gender specific impact of reduced folates. Pharm Res. 2008;25:453–462. doi: 10.1007/s11095-007-9407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao R, et al. Rescue of embryonic lethality in reduced folate carrier-deficient mice by maternal folic acid supplementation reveals early neonatal failure of hematopoietic organs. J Biol Chem. 2001;276:10224–10228. doi: 10.1074/jbc.c000905200. [DOI] [PubMed] [Google Scholar]
- 133.Gelineau-van Waes J, et al. Embryonic development in the reduced folate carrier knockout mouse is modulated by maternal folate supplementation. Birth Defects Res A Clin Mol Teratol. 2008;82:494–507. doi: 10.1002/bdra.20453. [DOI] [PubMed] [Google Scholar]
- 134.Piedrahita JA, et al. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat Genet. 1999;23:228–232. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- 135.Geller J, et al. Hereditary folate malabsorption: family report and review of the literature. Medicine (Baltimore) 2002;81:51–68. doi: 10.1097/00005792-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 136.Lasry I, et al. A novel loss of function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function. Blood. 2008 doi: 10.1182/blood-2008-04-150276. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 137.Russell RM, et al. Influence of intraluminal pH on folate absorption: studies in control subjects and in patients with pancreatic insufficiency. J Lab Clin Med. 1979;93:428–436. [PubMed] [Google Scholar]
- 138.Russell RM, et al. Effect of antacid and H2 receptor antagonists on the intestinal absorption of folic acid. J Lab Clin Med. 1988;112:458–463. [PubMed] [Google Scholar]
- 139.Rosenberg IH, et al. Absorption of polyglutamic folate: participation of deconjugating enzymes of the intestinal mucosa. N Engl J Med. 1969;280:985–988. doi: 10.1056/NEJM196905012801804. [DOI] [PubMed] [Google Scholar]
- 140.Butterworth CE, Jr, Baugh CM, Krumdieck C. A study of folate absorption and metabolism in man utilizing carbon-14--labeled polyglutamates synthesized by the solid phase method. J Clin Invest. 1969;48:1131–1142. doi: 10.1172/JCI106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Halsted CH. The intestinal absorption of dietary folates in health and disease. J Am Coll Nutr. 1989;8:650–658. doi: 10.1080/07315724.1989.10720340. [DOI] [PubMed] [Google Scholar]
- 142.Shafizadeh TB, Halsted CH. gamma-Glutamyl hydrolase, not glutamate carboxypeptidase II, hydrolyzes dietary folate in rat small intestine. J Nutr. 2007;137:1149–1153. doi: 10.1093/jn/137.5.1149. [DOI] [PubMed] [Google Scholar]
- 143.Horne DW, Reed KA. Transport of methotrexate in basolateral membrane vesicles from rat liver. Arch Biochem Biophys. 1992;298:121–128. doi: 10.1016/0003-9861(92)90102-3. [DOI] [PubMed] [Google Scholar]
- 144.Horne DW. Na+ and pH dependence of 5-methyltetrahydrofolic acid and methotrexate transport in freshly isolated hepatocytes. Biochim Biophys Acta Bio-Membr. 1990;1023:47–55. doi: 10.1016/0005-2736(90)90008-c. [DOI] [PubMed] [Google Scholar]
- 145.Horne DW, Reed KA, Said HM. Transport of 5-methyltetrahydrofolate in basolateral membrane vesicles of rat liver. Am J Physiol Gastrointest Liver Physiol. 1992;262:G150–G158. doi: 10.1152/ajpgi.1992.262.1.G150. [DOI] [PubMed] [Google Scholar]
- 146.Horne DW, et al. 5-Methyltetrahydrofolate transport in basolateral membrane vesicles from human liver. Am J Clin Nutr. 1993;58:80–84. doi: 10.1093/ajcn/58.1.80. [DOI] [PubMed] [Google Scholar]
- 147.Arias IM, Forgac M. The sinusoidal domain of the plasma membrane of rat hepatocytes contains an amiloride-sensitive Na+/H+ antiport. J Biol Chem. 1984;259:5406–5408. [PubMed] [Google Scholar]
- 148.Goresky CA, Watanabe H, Johns DG. The renal excretion of folic acid. J Clin Invest. 1963;42:1841–1849. doi: 10.1172/JCI104868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Selhub J, Nakamura S, Carone FA. Renal folate absorption and the kidney folate binding protein. II. Microinfusion studies. Am J Physiol. 1987;252:F757–F760. doi: 10.1152/ajprenal.1987.252.4.F757. [DOI] [PubMed] [Google Scholar]
- 150.Selhub J, Rosenberg IH. Demonstration of high-affinity folate binding activity associated with the brush border membranes of rat kidney. Proc Natl Acad Sci U S A. 1978;75:3090–3093. doi: 10.1073/pnas.75.7.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Selhub J, et al. Renal folate absorption and the kidney folate binding protein. I. Urinary clearance studies. Am J Physiol. 1987;252:F750–F756. doi: 10.1152/ajprenal.1987.252.4.F750. [DOI] [PubMed] [Google Scholar]
- 152.Birn H, et al. Renal tubular reabsorption of folate mediated by folate binding protein 1. J Am Soc Nephrol. 2005;16:608–615. doi: 10.1681/ASN.2004080711. [DOI] [PubMed] [Google Scholar]
- 153.Bhandari SD, Joshi SK, McMartin KE. Folate binding and transport by rat kidney brush-border membrane vesicles. Biochim Biophys Acta. 1988;937:211–218. doi: 10.1016/0005-2736(88)90243-x. [DOI] [PubMed] [Google Scholar]
- 154.Bhandari SD, Fortney T, McMartin KE. Analysis of the pH dependence of folate binding and transport by rat kidney brush border membrane vesicles. Proc Soc Exp Biol Med. 1991;196:451–456. doi: 10.3181/00379727-196-43215. [DOI] [PubMed] [Google Scholar]
- 155.Jaramillo-Juarez F, Aires MM, Malnic G. Urinary and proximal tubule acidification during reduction of renal blood flow in the rat. J Physiol. 1990;421:475–483. doi: 10.1113/jphysiol.1990.sp017956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Santiago-Borrero PJ, et al. Congenital isolated defect of folic acid absorption. J Pediatr. 1973;82:450–455. doi: 10.1016/s0022-3476(73)80119-2. [DOI] [PubMed] [Google Scholar]
- 157.Lanzkowsky P, Erlandson ME, Bezan AI. Isolated defect of folic acid absorption associated with mental retardation and cerebral calcification. Blood. 1969;34:452–465. [PubMed] [Google Scholar]
- 158.Birn H, Nielsen S, Christensen EI. Internalization and apical-to-basolateral transport of folate in rat kidney proximal tubule. Am J Physiol. 1997;272:F70–F78. doi: 10.1152/ajprenal.1997.272.1.F70. [DOI] [PubMed] [Google Scholar]
- 159.Birn H, Selhub J, Christensen EI. Internalization and intracellular transport of folate-binding protein in rat kidney proximal tubule. Am J Physiol Cell Physiol. 1993;264:C302–C310. doi: 10.1152/ajpcell.1993.264.2.C302. [DOI] [PubMed] [Google Scholar]
- 160.Weitman SD, Frazier KM, Kamen BA. The folate receptor in central nervous system malignancies of childhood. J Neurooncol. 1994;21:107–112. doi: 10.1007/BF01052894. [DOI] [PubMed] [Google Scholar]
- 161.Kennedy MD, et al. Evaluation of folate conjugate uptake and transport by the choroid plexus of mice. Pharm Res. 2003;20:714–719. doi: 10.1023/a:1023421232689. [DOI] [PubMed] [Google Scholar]
- 162.Wu D, Pardridge WM. Blood-brain barrier transport of reduced folic acid. Pharm Res. 1999;16:415–419. doi: 10.1023/a:1018829920158. [DOI] [PubMed] [Google Scholar]
- 163.Segal MB. Transport of nutrients across the choroid plexus. Microsc Res Tech. 2001;52:38–48. doi: 10.1002/1097-0029(20010101)52:1<38::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 164.Moretti P, et al. Brief report: autistic symptoms, developmental regression, mental retardation, epilepsy, and dyskinesias in CNS folate deficiency. J Autism Dev Disord. 2008;38:1170–1177. doi: 10.1007/s10803-007-0492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moretti P, et al. Cerebral folate deficiency with developmental delay, autism, and response to folinic acid. Neurology. 2005;64:1088–1090. doi: 10.1212/01.WNL.0000154641.08211.B7. [DOI] [PubMed] [Google Scholar]
- 166.Ramaekers VT, et al. Autoantibodies to folate receptors in the cerebral folate deficiency syndrome. N Engl J Med. 2005;352:1985–1991. doi: 10.1056/NEJMoa043160. [DOI] [PubMed] [Google Scholar]
- 167.Schwartz RS. Autoimmune folate deficiency and the rise and fall of “horror autotoxicus”. N Engl J Med. 2005;352:1948–1950. doi: 10.1056/NEJMp058034. [DOI] [PubMed] [Google Scholar]
- 168.Ramaekers VT, et al. Folate receptor autoimmunity and cerebral folate deficiency in low-functioning autism with neurological deficits. Neuropediatrics. 2007;38:276–281. doi: 10.1055/s-2008-1065354. [DOI] [PubMed] [Google Scholar]
- 169.Spector R, Lorenzo AV. Folate transport by the choroid plexus in vitro. Science. 1975;187:540–542. doi: 10.1126/science.1167256. [DOI] [PubMed] [Google Scholar]
- 170.Spector R, Lorenzo AV. Folate transport in the central nervous system. Am J Physiol. 1975;229:777–782. doi: 10.1152/ajplegacy.1975.229.3.777. [DOI] [PubMed] [Google Scholar]
- 171.Weitman SD, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–3401. [PubMed] [Google Scholar]
- 172.Weitman SD, et al. Cellular localization of the folate receptor: potential role in drug toxicity and folate homeostasis. Cancer Res. 1992;52:6708–6711. [PubMed] [Google Scholar]
- 173.Selhub J, Franklin WA. The folate-binding protein of rat kidney. Purification, properties, and cellular distribution. J Biol Chem. 1984;259:6601–6606. [PubMed] [Google Scholar]
- 174.Patrick TA, et al. Folate receptors as potential therapeutic targets in choroid plexus tumors of SV40 transgenic mice. J Neurooncol. 1997;32:111–123. doi: 10.1023/a:1005713115147. [DOI] [PubMed] [Google Scholar]
- 175.Segal MB. The choroid plexuses and the barriers between the blood and the cerebrospinal fluid. Cell Mol Neurobiol. 2000;20:183–196. doi: 10.1023/A:1007045605751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhao R, et al. Impact of overexpression of the reduced folate carrier (RFC1), an anion exchanger, on concentrative transport in murine L1210 leukemia cells. J Biol Chem. 1997;272:21207–21212. doi: 10.1074/jbc.272.34.21207. [DOI] [PubMed] [Google Scholar]
- 177.Levitt M, et al. Transport characteristics of folates in cerebrospinal fluid; a study utilizing doubly labeled 5-methyltetrahydrofolate and 5-formyltetrahydrofolate. J Clin Invest. 1971;50:1301–1308. doi: 10.1172/JCI106609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Maddox DM, et al. Reduced-folate carrier (RFC) is expressed in placenta and yolk sac, as well as in cells of the developing forebrain, hindbrain, neural tube, craniofacial region, eye, limb buds and heart. BMC Dev Biol. 2003;3:6. doi: 10.1186/1471-213X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sweiry JH, Yudilevich DL. Transport of folates at maternal and fetal sides of the placenta: lack of inhibition by methotrexate. Biochim Biophys Acta. 1985;821:497–501. doi: 10.1016/0005-2736(85)90055-0. [DOI] [PubMed] [Google Scholar]
- 180.Sweiry JH, Yudilevich DL. Characterization of folate uptake in guinea pig placenta. Am J Physiol. 1988;254:C735–C743. doi: 10.1152/ajpcell.1988.254.6.C735. [DOI] [PubMed] [Google Scholar]
- 181.Keating E, et al. Comparison of folic acid uptake characteristics by human placental choriocarcinoma cells at acidic and physiological pH. Can J Physiol Pharmacol. 2006;84:247–255. doi: 10.1139/y05-129. [DOI] [PubMed] [Google Scholar]
- 182.Takahashi T, et al. Carrier-mediated transport of folic acid in BeWo cell monolayers as a model of the human trophoblast. Placenta. 2001;22:863–869. doi: 10.1053/plac.2001.0742. [DOI] [PubMed] [Google Scholar]
- 183.Poncz M, et al. Therapy of congenital folate malabsorption. J Pediatr. 1981;98:76–79. doi: 10.1016/s0022-3476(81)80541-0. [DOI] [PubMed] [Google Scholar]
- 184.Poncz M, Cohen A. Long-term treatment of congenital folate malabsorption. J Pediatr. 1996;129:948. doi: 10.1016/s0022-3476(96)70057-4. [DOI] [PubMed] [Google Scholar]
- 185.Kim YI. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev. 2004;13:511–519. [PubMed] [Google Scholar]
- 186.Davey SG, Ebrahim S. Folate supplementation and cardiovascular disease. Lancet. 2005;366:1679–1681. doi: 10.1016/S0140-6736(05)67676-3. [DOI] [PubMed] [Google Scholar]
- 187.Mischoulon D, Raab MF. The role of folate in depression and dementia. J Clin Psychiatry. 2007;68(Suppl 10):28–33. 28–33. [PubMed] [Google Scholar]
- 188.Eichholzer M, Tonz O, Zimmermann R. Folic acid: a public-health challenge. Lancet. 2006;367:1352–1361. doi: 10.1016/S0140-6736(06)68582-6. [DOI] [PubMed] [Google Scholar]
- 189.Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71:121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- 190.Kessel D, Hall TC, Roberts D. Uptake as a determinant of methotrexate response in mouse leukemias. Science. 1965;150:752–754. doi: 10.1126/science.150.3697.752. [DOI] [PubMed] [Google Scholar]
- 191.Hakala MT. On the role of drug penetration in amethopterin resistance of Sarcoma-180 cells in vitro. Biochim Biophys Acta. 1965;102:198–209. doi: 10.1016/0926-6585(65)90213-x. [DOI] [PubMed] [Google Scholar]
- 192.Fischer GA. Detective transport of amethopterin (methotrexate) as a mechanism of resistance to the antimetabolite in L5178Y leukemic cells. Biochem Pharmacol. 1962;11:1233–4. 1233–1234. doi: 10.1016/0006-2952(62)90200-9. [DOI] [PubMed] [Google Scholar]
- 193.Ge Y, et al. Prognostic role of the reduced folate carrier, the major membrane transporter for methotrexate, in childhood acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Clin Cancer Res. 2007;13:451–457. doi: 10.1158/1078-0432.CCR-06-2145. [DOI] [PubMed] [Google Scholar]
- 194.Guo W, et al. Mechanisms of methotrexate resistance in osteosarcoma. Clin Cancer Res. 1999;5:621–627. [PubMed] [Google Scholar]
- 195.Levy AS, et al. Reduced folate carrier and dihydrofolate reductase expression in acute lymphocytic leukemia may predict outcome: a Children’s Cancer Group Study. J Pediatr Hematol Oncol. 2003;25:688–695. doi: 10.1097/00043426-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 196.Zhang L, et al. Reduced folate carrier gene expression in childhood acute lymphoblastic leukemia: relationship to immunophenotype and ploidy. Clin Cancer Res. 1998;4:2169–2177. [PubMed] [Google Scholar]
- 197.Zhao R, et al. Selective preservation of pemetrexed pharmacological activity in HeLa cells lacking the reduced folate carrier; association with the presence of a secondary transport pathway. Cancer Res. 2004;64:3313–3319. doi: 10.1158/0008-5472.can-03-3953. [DOI] [PubMed] [Google Scholar]
- 198.Patterson D, et al. A humanized mouse model for the reduced folate carrier. Mol Genet Metab. 2008;93:95–103. doi: 10.1016/j.ymgme.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Veenhoff LM, Heuberger EH, Poolman B. Quaternary structure and function of transport proteins. Trends Biochem Sci. 2002;27:242–249. doi: 10.1016/s0968-0004(02)02077-7. [DOI] [PubMed] [Google Scholar]
- 200.Karlin A, Akabas MH. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–45. 123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- 201.Lemieux MJ. Eukaryotic major facilitator superfamily transporter modeling based on the prokaryotic GlpT crystal structure. Mol Membr Biol. 2007;24:333–341. doi: 10.1080/09687680701496507. [DOI] [PubMed] [Google Scholar]
- 202.Song J, et al. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 2000;60:5434–5440. [PubMed] [Google Scholar]
- 203.Song J, et al. Chemopreventive effects of dietary folate on intestinal polyps in Apc+/−Msh2−/− mice. Cancer Res. 2000;60:3191–3199. [PubMed] [Google Scholar]
- 204.Ma DW, et al. Folate transport gene inactivation in mice increases sensitivity to colon carcinogenesis. Cancer Res. 2005;65:887–897. [PMC free article] [PubMed] [Google Scholar]
- 205.Lawrance AK, et al. Genetic and nutritional deficiencies in folate metabolism influence tumorigenicity in Apcmin/+ mice. J Nutr Biochem. 2007;18:305–312. doi: 10.1016/j.jnutbio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 206.Helmlinger G, et al. Interstitial pH and pO2 gradients in solid tumors in vivo: high- resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 207.Tredan O, et al. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 208.Raghunand N, et al. Plasmalemmal pH-gradients in drug-sensitive and drug-resistant MCF-7 human breast carcinoma xenografts measured by 31P magnetic resonance spectroscopy. Biochem Pharmacol. 1999;57:309–312. doi: 10.1016/s0006-2952(98)00306-2. [DOI] [PubMed] [Google Scholar]
- 209.Theti DS, et al. Selective delivery of CB300638, a cyclopenta[g]quinazoline-based thymidylate synthase inhibitor into human tumor cell lines overexpressing the alpha-isoform of the folate receptor. Cancer Res. 2003;63:3612–3618. [PubMed] [Google Scholar]
- 210.Henderson EA, et al. Targeting the alpha-folate receptor with cyclopenta[g]quinazoline-based inhibitors of thymidylate synthase. Bioorg Med Chem. 2006;14:5020–5042. doi: 10.1016/j.bmc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 211.Gibbs DD, et al. BGC 945, a novel tumor-selective thymidylate synthase inhibitor targeted to alpha-folate receptor-overexpressing tumors. Cancer Res. 2005;65:11721–11728. doi: 10.1158/0008-5472.CAN-05-2034. [DOI] [PubMed] [Google Scholar]
- 212.Deng Y, et al. Synthesis and Discovery of High Affinity Folate Receptor-Specific Glycinamide Ribonucleotide Formyltransferase Inhibitors with Antitumor Activity. J Med Chem. 2008 doi: 10.1021/jm8003366. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
9.0 Website
- 213.For an in-depth up-to-date review of hereditary folate Malabsorption from the clinical and genetic perspectives. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=folate-mal.