Abstract
The renin–angio tensin system (RAS) is now regarded as an integral component in not only the development of hypertension, but also in physiologic and pathophysiologic mechanisms in multiple tissues and chronic disease states. While many of the endocrine (circulating), paracrine (cell-to-different cell) and autacrine (cell-to-same cell) effects of the RAS are believed to be mediated through the canonical extracellular RAS, a complete, independent and differentially regulated intracellular RAS (iRAS) has also been proposed. Angiotensinogen, the enzymes renin and angiotensin-converting enzyme (ACE) and the angiotensin peptides can all be synthesized and retained intracellularly. Angiotensin receptors (types I and 2) are also abundant intracellularly mainly at the nuclear and mitochondrial levels. The aim of this review is to focus on the most recent information concerning the subcellular localization, distribution and functions of the iRAS and to discuss the potential consequences of activation of the subcellular RAS on different organ systems.
Keywords: Renin-angiotensin system, Intracellular, Hypertension, Cardiovascular disease, Angiotensin peptides, Angiotensin receptors
1. Introduction: endocrine, paracrine, autacrine and intracellular renin–angiotensin systems
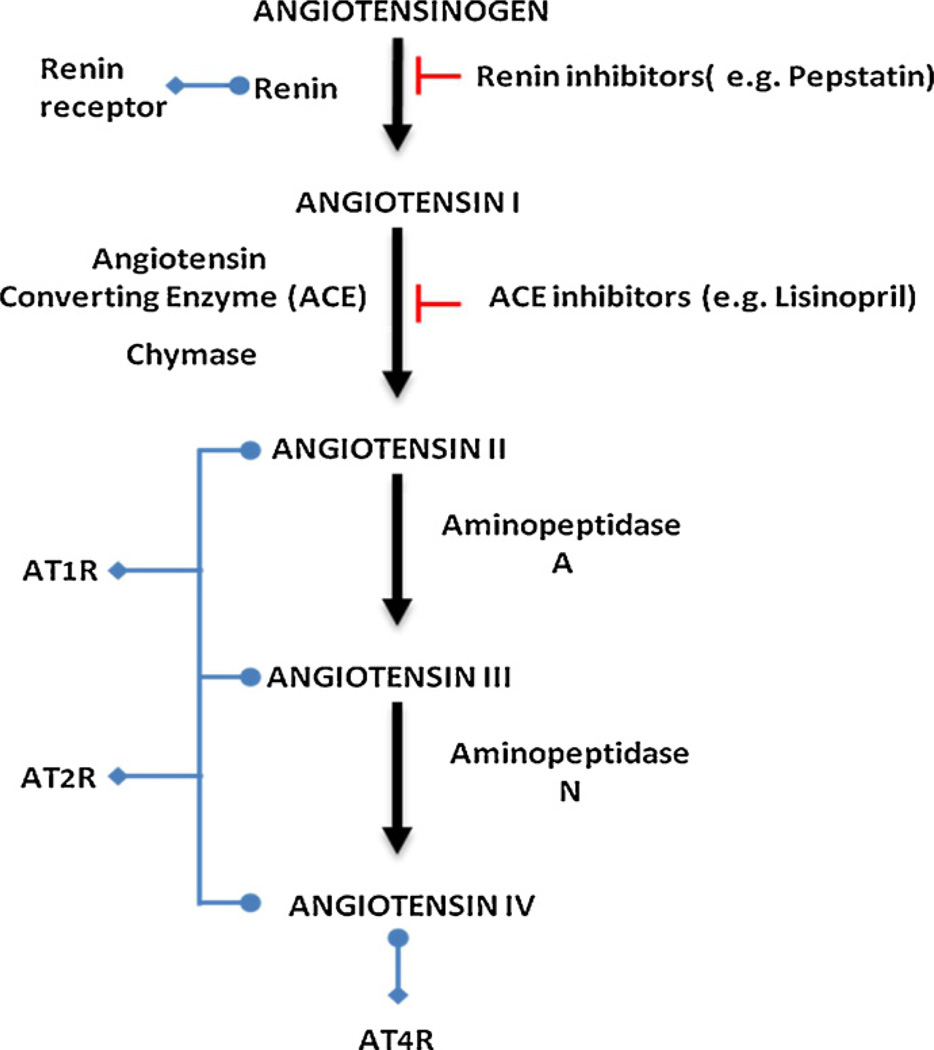
Over the past century, a substantial body of research has documented and characterized the renin–angiotensin system (RAS) that began with the discovery of renin by Tigerstedt and Bergman. Our understanding of the RAS has evolved from a simplified endocrine system that plays a role in blood pressure (BP) regulation to independently functioning local tissue hormonal systems (cardiac, vascular, renal, adrenal, pancreatic, pulmonary, integumentary, skeletal and CNS) [14,68]. To add to the complexity of the system a growing body of research is documenting the presence of subcellular functional units of RAS [30–33,38,52,60,61,69,72,81] in the nucleus and mitochondria [1,26]. Whether at the systemic circulating, local tissue or subcellular level, the system consists mainly of a two-step enzymatic cascade, catalyzed by renin, angiotensin-converting enzyme (ACE), and peptidases generating biologically active peptides, angiotensin (Ang) II, III and IV. Ang II, the major RAS effector peptide, acts predominantly through two Ang receptor subtypes, type-1 (AT1R) and type-2 (AT2R) (Fig. 1).
Fig. 1.
Diagrammatic representation of the renin-angiotensin system depicting the peptide cascade and interactions of enzymes, proteins and receptors.
Most of the early research focused on the endocrine RAS, wherein circulating angiotensin II produced and processed in liver (angiotensinogen), kidney (renin) and endothelial cells (ACE) acts on target tissues leading to regulation of BP through renal sodium and water reabsorption and systemic vasoconstriction. Although these actions were long thought to be caused primarily by the endocrine RAS, several organ systems were discovered to possess a local copy of the RAS with autacrine (affecting same cell) or paracrine (affecting neighboring cells) actions. The components of these local RASs were found in peripheral tissues such as kidneys, heart, vasculature, adrenal glands, liver, spleen, skeletal muscles and immune cells, all of which locally produce Ang II [35,54,57]. These local systems seem to be independently regulated and compartmentalized from the systemic circulation [79].
Most of the paracrine and autacrine effects of RAS are thought to derive from internalization of different components of the system into the cell where they exert specific effects on cell growth and differentiation [10,51,70]. Accumulating evidence from several laboratories is pointing to a version of the RAS that is not secreted [30–33,52,61,62,69,72,81]. Thus, the system peptide effectors are synthesized and retained entirely within the cells of origin: the intracrine RAS. Ang receptors AT1Rs and AT2Rs are also abundant intracellularly mainly at the nuclear and mitochondrial levels [1,26].
1.1. The intracellular RAS
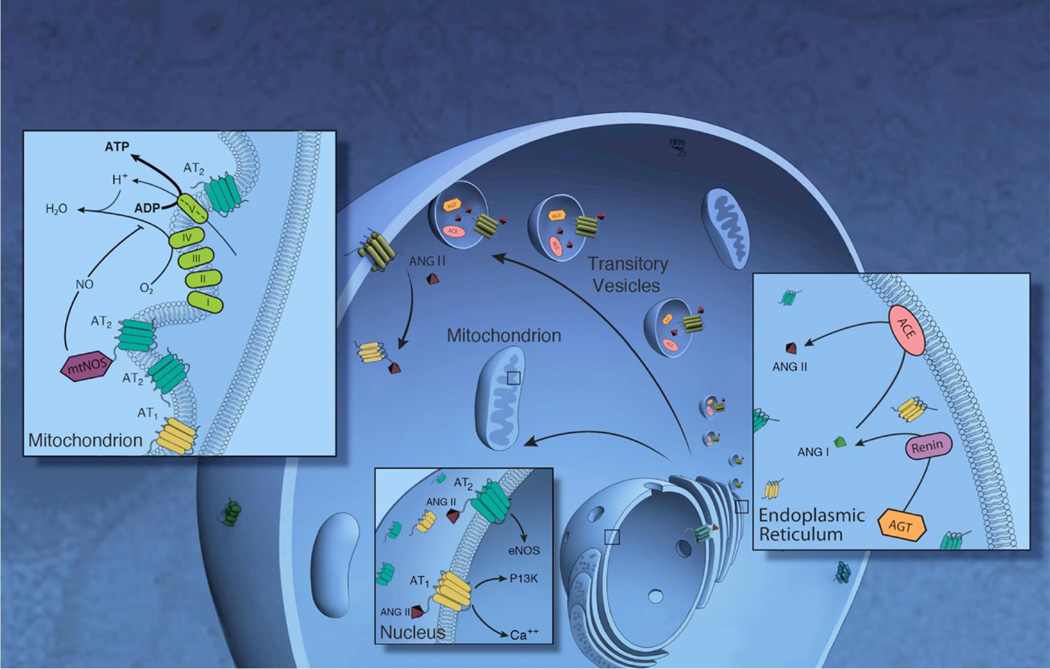
Hints of the presence of an intracellular RAS can be dated back to the early 1970s [68]. Using radio-labeled Ang II, prominent subcellular binding sites for Ang II were evident [64]. Although the source (internalization, chaperoning, trafficking), exact location and actions of such a system were and remain today not fully understood, a growing body of evidence suggests that such intracellular (intracrine) RASs may be present through two different theoretical pathways (Fig. 2) [39]. The first such pathway regards the intracellular RAS as a transient system the components of which are transported intracellularly via secretory vesicles on the cell surface. These secretory vesicles contain renin, angiotensinogen and ACE providing for intravesicular generation of Ang II. Intracellularly formed Ang II would consequently be utilized intracellularly and/or secreted [30–33,52]. The second pathway involves a non-secreted constitutive system composed of a differently spliced non-secreted renin isoform, a membrane bound ACE [2,9,16] and Ang receptors mainly localized in the nucleus and mitochondria [12,44,77]. A third theoretical possibility is a hybrid system via interactions between the constitutive and secreted intracellular RASs. Current evidence suggests that intracellular RASs play distinct roles, are subcellularly compartmentalized and are regulated separately from the canonical autacrine/paracrine RAS, both of which require a secreted peptide (Tables 1–3; Fig. 3) [13,14,20,23,37,53,74].
Fig. 2.
Scheme foriRAS. In endoplasmic reticulum(ER), renin cleaves angiotensinogen to ANG I, which is subsequently processed to ANG II by angiotensin-converting enzyme (ACE). The different components including the processing enzymes, ang peptides and receptors can be transported intracellularly via secretory vesicles to the cell surface. These different components can be transported directly either to the mitochondria or the nucleus. In the mitochondria, Ang II binds to mtAT2 Rsand stimulates NO formation through mtNOS, suppressing mitochondrial oxygen consumption. Nuclear Ang II can stimulate NO formation (via AT2Rs) or Ca2+ and phosphoinositol 3 kinase (PI3K) (via AT1Rs).
Table 1.
Structure, subcellular localization, distribution, function and pathophysiology of intracellular RAS receptors.
| Receptor | AT1R | AT2R | (Pro)Renin | AT4R | Ang (1–7)R |
|---|---|---|---|---|---|
| Structure | GPCR 395aa |
GPCR 364aa |
Single transmembrane 350aa |
Insulin-regulated aminopeptidase 916aa |
GPCR Mas |
| Subcellular localization | Nucleus Mitochondria |
Nucleus Mitochondria |
Endoplasmic reticulum | Unknown | Perinuclear Nucleus |
| Cell type | Neurons Hepatocytes Cardiac myocytes and fibroblasts Renal proximal tubule cells Skeletal myocytes Monocytes |
Neurons Hepatocytes Cardiac myocytes and fibroblasts Renal proximal tubule cells Skeletal myocytes |
Glomerular mesangial Distal nephrons Collecting ducts Neurons Endothelium (coronary and kidney) Smooth muscle cells |
Unclear but detected in brain, aorta, heart, kidney, liver, lung and uterus. |
Glomerular mesangial Cerebral cortex Endothelial cells, Cardiomyocytes, proximal tubules |
| Function | Nuclear and mitochondrial ROS stimulation Mitochondrial oxygen consumption |
NO formation in nucleus and mitochondria Mitochondrial oxygen consumption |
Cell hypertrophy, intracellular pH homeostasis and G protein-coupled endocytosis and recycling |
Unclear | Activation of nuclear phosphatase activity |
| Pathophysiology | Nuclear and mitochondrial oxidative stress and damage |
Nuclear and mitochondrial oxidative stress and damage |
Nephroangiosclerosis Liver fibrosis Cardiac and aortic hypertrophy |
Unclear | Nuclear oxidative stress DNA damage |
Table 3.
Structure, subcellular localization and distribution of intracellular RAS processing enzymes.
| Enzyme | Renin | ACE | Aminopetidase A | Aminopetidase N |
|---|---|---|---|---|
| Structure | 340 amino acid peptide | Zinc- and chloride-dependent metallopeptidase(1277aa) |
Zinc-dependent membrane-bound aminopeptidase |
Zinc-dependent membrane-bound aminopeptidase |
| Subcellular localization | Cytoplasm Nucleus Mitochondria |
Cytoplasm Nucleus Mitochondria Endoplasmic reticulum |
Cytoplasm | Cytoplasm |
| Cell type | Adrenal glands, brain and heart |
Mesangial, smooth muscle and endothelial cells |
Brain Kidney |
Brain Kidney |
| Mesangial, smooth muscle and endothelial cells |
||||
| Function | Mitochondrial bioenergetics |
Peptide modification | Unknown | Unknown |
| Junctional conductance | ||||
| Apoptosis calcium influx | ||||
| Pathology | Fluid homeostasis and BP regulation |
Genetic hypertension | Unknown | Unknown |
BP, blood pressure.
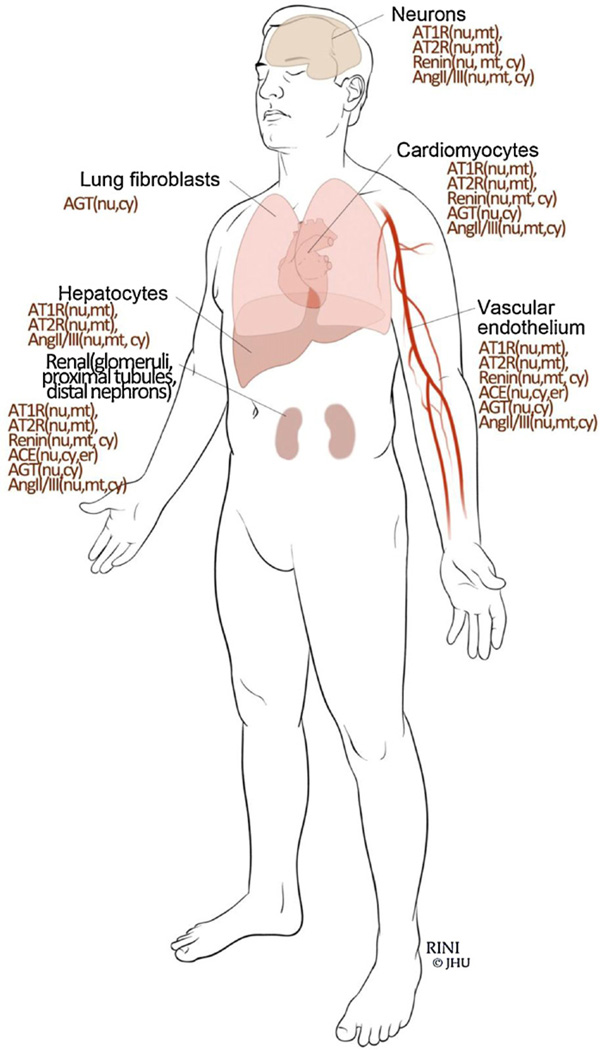
Fig. 3.
Cellular distribution of different iRAS components.
In order for such an intracellular RAS to be functional and independently operated, all of the components of RAS must be confined within the same cell. Here we will dissect the intracellular RAS in terms of biochemical structure, subcellular localization and function beginning with the parent peptide substrate angiotensinogen through the renin and angiotensin converting enzymes through the generation of biologically active peptides, peptide interactions with angiotensin receptors and their distal signaling pathways.
2. Processing enzymes
2.1. Intracellular renin (iRenin)
Renin is the first and rate-limiting enzyme in the RAS. Renin processes angiotensinogen into Ang I. Two forms of renin are expressed: secreted prorenin (sRenin) and a non-secreted intracellular form of active renin (iRenin) [12,44,77]. The non-secreted intracellular renin (iRenin) is constitutively active and is synthesized exclusively for use within the cell as it lacks the signal peptide that allows it to be secreted. Evidence for iRenin was discovered in brain, adrenal glands and heart [12,44]. Intracellularly, iRenin has been detected in the cytosol, nucleus and mitochondria [40,41,82]. A link between nuclear AT1R and intracellular renin regulation has been described, providing evidence that the iRAS is subcellularly compartmentalized [22]. Subcellularly, iRenin is transported into mitochondria via a mechanism dependent on ATP synthesis and the potential of the mitochondrial membrane, pointing to a potential role in mitochondrial bioenergetics [12].
The regulation of the iRenin seems to be independent of that of sRenin; adrenal renin is upregulated in response to an increase in serum potassium (hyperkalemia) while circulating renin is decreased. Also post-nephrectomy, almost all detectable circulating renin is eliminated, while adrenal renin is increased [19,55]. iRenin is also upregulated in the heart after myocardial infarction and in kidney cells with high glucose conditions [11,40,41]. Whether this form of iRenin has specific intracellular actions remains unknown. Over-expression of mitochondrial renin resulted in enhanced apoptosis [82]. Intracellular renin administration was reported to increase inward calcium current in cardiomyocytes [18]. iRenin is also involved in cell-to-cell communication in heart muscle through control of junctional conductance [17].
Physiologically, iRenin has similar functions to those observed with over production of sRenin. iRenin when over-expressed in mouse brain also resulted in changes in fluid homeostasis and BP regulation [80].
2.2. Intracellular angiotensin converting enzyme (iACE)
ACE is a peptidyl-dipeptidase that is found in a soluble secreted form and an ectoenzyme intracellular membrane-bound form that is involved in the intracellular generation of Ang II [2,9,16]. Similar to renin, intracellular iACE has been also localized to cell cytoplasm and nuclei [4,80]. Abundant iACE has also been localized to the endoplasmic reticulum [71].
Current evidence demonstrates differential regulation of extracellular and iACE. While high glucose content had no effect on extracellular ACE, it increased iACE by 10-fold [16]. It is also interesting to note that despite that the commonly prescribed ACE inhibitors that are effective in blocking the extracellular ACE, ACE inhibitors have failed to block intracellular ACE [16], which may be explained by lack of intracellular uptake of these agents.
At the subcellular level, iACE has been shown to be involved in modification of intracellular peptides at the level of the endoplasmic reticulum [71]. The gene encoding nuclear ACE has been suggested as a possible genetic marker for hypertension [4].
Over-expression of endoplasmic reticulum ACE in monocytes is associated with changes in the processing and presentation of peptides including antigens associated with MHC class I molecules and resulted in an enhanced inflammatory response [71].
2.3. Intracellular aminopeptidases A and N
Aminopeptidases A (APA) and N (APN) are zinc metallopeptidase enzymes that respectively degrade Ang II to form Ang III and Ang III to form Ang IV. Both enzymes are abundant in brain and the apical plasma membranes of renal proximal tubule cells [63,65,66]. The respective roles of APA and APN at the cellular level are currently unknown.
3. Angiotensin peptides
3.1. Intracellular angiotensinogen (iAGT)
The requisite parent peptide of the RAS is angiotensinogen, a 452 amino acid α-2-globulin. In contrast to the previous concept that angiotensinogen is exclusively produced in the liver, current evidence strongly supports the generation of angiotensinogen locally in central and peripheral tissues. The messenger RNA encoding for angiotensinogen has been found in the adrenal gland, pituitary, ovary, uterus, heart, kidney, pancreas, adipocytes, endothelial cells and brain [5–8,21,43,53].
As with iRenin and iACE, iAGT has been reported to be produced in a non-glycosylated form by post-translational modification and is therefore not secreted [72]. iAGT has been localized to cytoplasm and nuclei. The mechanism regulating the biosynthesis and accumulation of angiotensinogen intracellularly is also similar to that of iRenin and iACE. High glucose conditions both in animal experiments and tissue culture lead to accumulation of iAGT, in marked contrast to extracellular angiotensinogen which remained relatively unchanged [16,42,75,76,80].
3.2. Intracellular Ang I
The decapeptide Ang I is formed by the action of renin on angiotensinogen. Similar to the non-secreted iRenin, a non-secreted Angiotensin I have been reported in several tissues including pancreatic cells and cardiac myofibroblasts and is involved in organogenesis and tissue repair [25,36,50,60]. Rising levels of iRenin were associated with increased intracellular Ang levels leading to activation of the intracardiac RAS associated with cardiac damage, providing evidence for the presence of a complete and independently regulated intracellular RAS.
3.3. Intracellular Ang II
The octapeptide Ang II is the major effector of the renin/ACE/Ang II/AT receptor axis. Ang II provided the earliest hints of presence of intracellular angiotensin system. The demonstration of release of Ang II by cultured cardiac myocyte undergoing mechanical stretch was among the first observations pointing to the ability of the cells to generate their own Ang II. This secreted form of Ang II was hypothesized to be generated in intracellular secretory vesicles (Fig. 2). Within these secretory vesicles, renin, AGT and ACE co-localized and resulted in Ang II production [30–33,52].
Ang II can also be produced and retained intracellularly from iAGT [14]. iAng II has been detected in several cell types including renal juxtaglomerular cells, renal proximal tubule cells, cardiac myocytes, hepatoma cells, vascular smooth muscle cells and several types of brain cells [13]. Following the tracks of iAGT, iAngII has been detected in cytoplasm, nuclei and mitochondria [1,13,30,74–76].
iANG II production is elevated in cardiac cells under high glucose conditions [37]. iAng II alters cellular proliferation and signal transduction and elevates blood pressure [23]. iAng II functions through cytoplasmic protein interactions and through nuclear translocation, receptor binding, and transcriptional regulation of gene expression at the level of the nucleus and mitochondria [14].
4. RAS receptors
4.1. Intracellular RAS receptors
Most of the known actions of Ang II are mediated by AT1 and AT2Rs, which are seven transmembrane glycoproteins with 30% sequence homology. The relative expression of angiotensin receptors in the mitochondria, nucleus or plasma membranes determines in many cases the physiological consequences of intracellular RAS activation. In addition to the classical AT1 and AT2Rs, newer additions to the RAS receptor family include the renin receptor, the AT4 receptor and Ang (1–7) receptor.
4.2. Intracellular renin receptor
The intracellular renin receptor consists of 350 amino acids with a single transmembrane domain and specifically binds renin. Binding of renin to its receptor increases the catalytic activity of renin approximately 4–5-fold, increasing the rate of Ang I generation [56].
The intracellular localization of the renin receptor has been demonstrated primarily in the perinuclear zone, but also in the endoplasmic reticulum. Intracellular renin receptor activation (possibly by the non-secreted form of renin) caused a significant increase in cell number and a concomitant decrease in apoptosis of cardiomyoblasts [56].
4.3. Intracellular AT1R
Despite the absence of a subcellular targeting sequence (mitochondrial or nuclear) in AT1Rs, these receptors have been localized both in the mitochondria and the nucleus. Nuclear AT1 Rs have been reported in renal cortex and medulla, spleen and cardiac cells. Compared to the plasma membrane, nuclear AT1Rs are more abundant in renal cortex cells (2-fold) and liver cells (20-fold) [47,49,58,78]. Mitochondrial AT1Rs have been reported in renal cortex and cardiac cells. Although the levels of mitochondrial AT1Rs are low in young animals, we have demonstrated a significant increase in the expression of AT1Rs with aging [1]. A similar increase in the nuclear AT1Rs was observed with aging [28].
Functionally, specific nuclear AT1Rs activation engenders a dose-dependent increase in nuclear calcium concentration, increased monocyte chemo-attractant protein, transforming growth factor, sodium hydrogen exchanger-3 (NHE3) and reactive oxygen species [59].
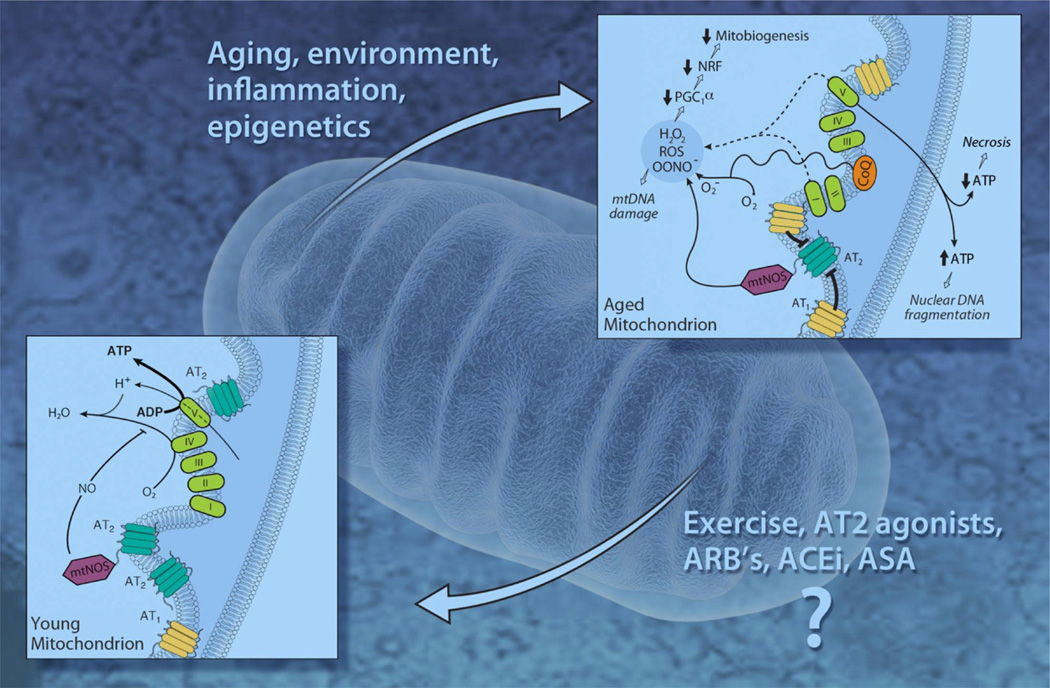
The role of AT1Rs in the mitochondria remains obscure; treating aging animals with AT1R blocker losartan for 20 weeks did not affect the expression of mitochondrial AT1Rs, but led to a significant increase in the number of mitochondrial AT2Rs and improved bioenergetics of the aging mitochondria (Fig. 4) [1].
Fig. 4.
A hypothetical model for changes in mitochondrial angiotensin receptors with aging. Note that with aging the balance is tipped toward more expression of mtAT1Rs and less mtAT2Rs. The imbalance between mtAT1Rs and mtAT2Rs with aging may accelerate the development of age related oxidative stress, mtDNA mutations, apoptosis and senescence. ACEi, ACE inhibitor; ARBs, Ang II receptor blockers.
4.4. Intracellular AT2Rs
AT2Rs are G-protein-coupled receptors also lacking a mitochondrial or nuclear targeting sequence. However, AT2Rs have been reported both in the nucleus and mitochondria of several cell types. Nuclear AT2Rs have been reported in the renal cortex [29] and in cardiomyocytes [78]. We reported the co-localization of AT2Rs and endogenous Ang II and Ang III on mitochondrial inner membrane in several human, mouse and rat cell types including human skeletal muscle cells and monocytes, rat cardiac cells and mouse cardiomyocytes, renal tubular cells, neuronal cells, vascular endothelial cells, and hepatocytes [1]. The relative expression of the mitochondrial AT2Rs (per each mitochondrion) was much higher (∼40-fold) as compared to the total number of cell surface AT2Rs on human monocytes. It is also interesting that relative expression of mitochondrial AT2Rs was tissue-dependent. Hepatocytes had the highest expression of mitochondrial AT2Rs per mitochondrion followed by cardiac cells and even lower in kidney cells. The importance of the difference in mitochondrial AT2R numbers among different tissues in terms of function or changes in bioenergetics is yet to be determined.
In contrast to observations with AT1Rs, the aging process is associated with a stepwise reduction in the expression of both nuclear and mitochondrial AT2Rs. Gwathmey et al. [28] have demonstrated that while nuclear AT2Rs formed the majority (80%) of Ang receptors in young adult kidney cells, the balance changed with advancing age toward a vast majority of AT1Rs (85%). Similarly, we have demonstrated that aging is associated with a reduction in the total number of mitochondrial AT2Rs, and that chronic administration of Ang receptor blockers in vivo restored the balance and increased the expression of mitochondrial AT2Rs.
Functionally, both in the mitochondria [1] and nucleus [27], activation of AT2Rs was coupled to nitric oxide (NO) formation.
Stimulation of AT2Rs in isolated mitochondria by the selective agonist CGP-42112A resulted in a dose-dependent increase in NO production and suppressed respiratory oxygen consumption presumably due to its competition with O2 for binding to cytochrome C oxidase. This suppression of respiration was reversed by the coadministration of AT2R specific antagonist PD-123319 or by NO synthase (NOS) inhibitor L-NAME.
4.5. Intracellular Ang (1–7) receptor (Mas oncogene receptor)
The Ang (1–7) receptor is a G-protein-coupled receptor with vasodilator properties and also linked to NO production. Ang (1–7) receptors were detected on nuclei of renal cells and have been linked to nuclear NO production [29]. Aging has a similar effect on Ang (1–7) receptors as AT2Rs with a shift in the Ang receptor profile from the AT2 and Ang (1–7) receptor subtypes to the AT1R isoform with advancing age. Functionally, in contrast to the effects of the AT2R antagonist in nuclei that increase ROS production, the Ang (1–7) receptor antagonist had no effect [28].
5. Functional iRAS Units (Fig. 2)
By definition for an iRAS unit to be deemed functional and independent, the angiotensin receptors and angiotensin II or III have to co-localize within a confined space, a signal has to be propagated and an effect has to be observed. A functional iRAS unit has been described both in the mitochondria and nucleus.
5.1. Functional nuclear unit
This unit consists of Ang II coupled to the three main angiotensin receptors AT1 R, AT2R and the Ang (1–7) receptor within the nuclear compartment [26–29,59].
Functionally, the nuclear AT1R is coupled to phosphoinositol-3 kinase and protein kinase C activation and ultimately to the production of reactive oxygen species. In contrast, both the nuclear AT2R and Ang (1–7) receptor have been linked to NO formation, possibly through nuclear NOS activation. Aging and steroid administration altered the balance between different nuclear angiotensin receptors (presumably attenuated nuclear AT2Rs and Ang (1–7) receptors and exaggerated AT1R) leading to enhanced nuclear ROS production, decreased NO production and the development of fetal-programmed hypertension [26–29,59].
5.2. Functional mitochondrial unit
We recently reported the identification of a functional mitochondrial angiotensin system consisting of Ang II/III coupled to predominantly mitochondrial AT2Rs and to a lesser extent to mitochondrial AT1Rs. Activation of the mitochondrial RAS was coupled to NO production and mitochondrial respiration. Based on analogous effects of surface membrane Ang receptors, other potential mechanisms for the observed effect of mitochondrial AT2Rs on mitochondrial respiration could include activation of phospholipase C, protein tyrosine phosphatases, or protein kinase phosphatases. Alternatively, downstream effects of NO, such as activation of cyclic GMP (cGMP) production and post-translational modification of mitochondrial targets could supersede the direct inhibition of mitochondrial respiration to account for the observed increase in oxygen consumption. Further investigation will be necessary to determine if the AT2R effect on respiration is NO- and/or cGMP-dependent.
As mentioned above, aging was associated with an imbalance between mitochondrial Ang receptors, with an increase in mitochondrial AT1Rs and a decrease in mitochondrial AT2Rs. Of particular interest is the possibility that the AT2Rs may counterbalance the detrimental effects of AT1Rs (Fig. 4). In this light, previous studies have shown that Ang II is released by cultured cardiac cells in response to stretch to promote hypertrophic growth [69] and/or apoptosis [45,46]. The latter effect on cell death was mediated by AT1Rs and involved p53 activation and a decrease in the ratio of Bcl-2 to Bax, regulators of mitochondrially-triggered apoptosis. Since protection of mitochondrial function and cell death via ischemic preconditioning is thought to involve the NO/cGMP axis [3,15,34], it will be interesting to find out in future studies if the mitochondrial RAS participates in this broad intrinsic protective pathway and what other components of the system are present within mitochondria.
The role of mitochondrial angiotensin system in ROS production is yet to be investigated. Increased mitochondrial production of ROS has an important role in mitochondrial protein oxidation and increased mitochondrial DNA mutations. Mitochondrial ROS has been also linked to senescence and to apoptosis in endothelial cells. The imbalance between mtAT1Rs and mtAT2Rs with aging may suggest a role for impaired MAS in the development of age related oxidative stress, mtDNA mutations, apoptosis and senescence (Fig. 4).
5.3. Pathophysiologic effects of intracellular RAS activation
5.3.1. Effects on the heart
The strongest evidence of pathological changes in intracardiac intracellular RAS comes from diabetic heart studies. High glucose conditions have been associated with activation of intracellular RAS in different cell types including heart (cardiomyocytes [75] and fibroblasts [74]), vascular smooth muscle cells [42], and renal mesangial cells [73]. Intracellular Ang II levels in cardiac myocytes are 3.4-fold higher in diabetic than non-diabetic patients and 5-fold higher in diabetic hypertensive patients than diabetic patients and were associated with enhanced oxidative damage, cardiac cell apoptosis and necrosis [24,76]. Interestingly the activation of intracellular RAS in diabetic hearts was not reversed by AT1Rs blocker administration that had no effects on intracellular Ang II generation [76].
5.3.2. Kidneys
The role of the intracellular RAS in development of kidney disease has been partially elucidated through the use of transgenic mice over-expressing a non-secreted form of intracellular AngII. This animal model is characterized phenotypically by elevated systolic and diastolic blood pressures and significant kidney pathology consistent with microthrombosis within glomerular capillaries and small vessels [67]. When the over-expression of intracellular Ang II was restricted to renal proximal tubules, elevation of blood pressure was again demonstrated, accompanied by 24-h decreases in urinary sodium excretion [48].
5.3.3. Central nervous system
Evidence from transgenic mice supports a role for brain intracellular RAS activation on fluid homeostasis and blood pressure regulation. Double-transgenic mice expressing human intracellular renin and human angiotensinogen driven by glial fibrillary acidic protein exhibited an increase in drinking volume and higher mean arterial pressures compared with control littermates [40,41].
6. Future perspectives
There is currently considerable evidence that different components of RAS exist intracellularly, not in a random un-consorted fashion but rather forming fully integrated potentially functional units intracellularly at the level of mitochondria and nucleus. This has now been demonstrated in several species and tissue types. It is also apparent that these intracellular RAS units exert interesting biological effects that can be similar to or distinct from the classical functions observed with stimulation of the autacrine/paracrine and endocrine systems. Given the critical role of mitochondria in metabolism and apoptosis in all tissues, and especially in aging organisms, and the vital role of the nucleus in controlling essentially all biological functions of the cell, studies focusing on the pharmacological and molecular manipulation of the intracellular RAS on a variety of chronic diseases may be useful in determining the utility of improving mitochondrial and nuclear function in aging humans.
Table 2.
Structure, subcellular localization and distribution of intracellular RAS peptides.
| Peptide | Agt | Ang I | Ang II | Ang III | Ang IV |
|---|---|---|---|---|---|
| Structure | 452 amino acids peptide |
Ten-aminoacid peptide |
Eight-amino acid peptide |
Seven-amino acid peptide |
Six-aminoacid peptide |
| Subcellular localization | Cytoplasm Nucleus |
Cytoplasm | Cytoplasm Nucleus Mitochondria perinuclear region |
Unclear | Unclear |
| Cell type | Cardiac myocytes Mesangial, smooth muscle and endothelial cells |
Neurons Hepatocytes Cardiac myocytes and fibroblasts Renal proximal tubule cells Skeletal myocytesVSMC |
Unclear | Unclear |
VSMC, vascular smooth muscle cells.
Acknowledgement
National Institute on Aging Grant K23 AG035005 (PMA).
References
- 1.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA. 2011;108:14849–14854. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade MC, Quinto BM, Carmona AK, Ribas OS, Boim MA, Schor N, et al. Purification and characterization of angiotensin I-converting enzymes from mesangial cells in culture. J Hypertens. 1998;16:2063–2074. doi: 10.1097/00004872-199816121-00031. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 4.Camargo de Andrade MC, DiMarco GS, de Paulo Castro Teixeira V, Mortara RA, Sabatini RA, Pasquero JB, et al. Expression and localization of N-domain ANG I-converting enzymes in mesangial cells in culture from spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2006;290:F364–F375. doi: 10.1152/ajprenal.00110.2005. [DOI] [PubMed] [Google Scholar]
- 5.Campbell DJ. Circulating and tissue angiotensin systems. J Clin Invest. 1987;79:1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell DJ, Habener JF. Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J Clin Invest. 1986;78:31–39. doi: 10.1172/JCI112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell DJ, Habener JF. Cellular localization of angiotensinogen gene expression in brown adipose tissue and mesentery: Quantification of messenger ribonucleic acid abundance using hybridization in situ. Endocrinology. 1987;121:1616–1626. doi: 10.1210/endo-121-5-1616. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DJ, Habener JF. Hybridization in situ studies of angiotensinogen gene expression in rat adrenal and lung. Endocrinology. 1989;124:218–222. doi: 10.1210/endo-124-1-218. [DOI] [PubMed] [Google Scholar]
- 9.Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Mukhin YV, Garnovskaya MN, Thielen TE, Iijima Y, Huang C, et al. A functional angiotensin II receptor-GFP fusion protein: evidence for agonistdependent nucleartranslocation. Am J Physiol Renal Physiol. 2000;279:F440–F448. doi: 10.1152/ajprenal.2000.279.3.F440. [DOI] [PubMed] [Google Scholar]
- 11.Clausmeyer S, Reinecke A, Farrenkopf R, Unger T, Peters J. Tissue-specific expression of a rat renin transcript lacking the coding sequence for the prefragment and its stimulation by myocardial infarction. Endocrinology. 2000;141:2963–2970. doi: 10.1210/endo.141.8.7623. [DOI] [PubMed] [Google Scholar]
- 12.Clausmeyer S, Sturzebecher R, Peters J. An alternative transcript of the rat renin gene can result in a truncated prorenin that is transported into adrenal mitochondria. Circ Res. 1999;84:337–344. doi: 10.1161/01.res.84.3.337. [DOI] [PubMed] [Google Scholar]
- 13.Cook JL, Re RN. Lessons from in vitro studies and a related intracellular angiotensin II transgenic mouse model. Am J Physiol Regul Integr Comp Physiol. 2012;302:R482–R493. doi: 10.1152/ajpregu.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook JL, Zhang Z, Re RN. In vitro evidence for an intracellular site of angiotensin action. Circ Res. 2001;89:1138–1146. doi: 10.1161/hh2401.101270. [DOI] [PubMed] [Google Scholar]
- 15.Costa AD, Pierre SV, Cohen MV, Downey JM, Garlid KD. cGMP signalling in pre- and post-conditioning: the role of mitochondria. Cardiovasc Res. 2008;77:344–352. doi: 10.1093/cvr/cvm050. [DOI] [PubMed] [Google Scholar]
- 16.Cristovam PC, Gloria MA, Melo GB, Gomes JA. ACE-dependent and chymasedependent angiotensin II generation in normal and glucose-stimulated human mesangial cells. Exp Biol Med (Maywood) 2008;233:103–143. doi: 10.3181/0708-RM-229. [DOI] [PubMed] [Google Scholar]
- 17.De Mello WC. Influence of intracellular renin on heart cell communication. Hypertension. 1995;25:1172–1177. doi: 10.1161/01.hyp.25.6.1172. [DOI] [PubMed] [Google Scholar]
- 18.De Mello WC. Renin increments the inward calcium current in the failing heart. J Hypertens. 2006;24:1181–1186. doi: 10.1097/01.hjh.0000226209.88312.db. [DOI] [PubMed] [Google Scholar]
- 19.Doi Y, Atarashi K, Franco-Saenz R, Mulrow PJ. Effect of changes in sodium or potassium balance, and nephrectomy, on adrenal renin and aldosterone concentrations. Hypertension. 1984;6:I124–I129. doi: 10.1161/01.hyp.6.2_pt_2.i124. [DOI] [PubMed] [Google Scholar]
- 20.Dzau VJ. Circulating versus local renin-angiotensin system in cardiovascular homeostasis. Circulation. 1988;77:I4–I13. [PubMed] [Google Scholar]
- 21.Dzau VJ, Ellison KE, Brody T, Ingelfinger J, Pratt RE. A comparative study of the distributions of renin and angiotensinogen messenger ribonucleic acids in rat and mouse tissues. Endocrinology. 1987;120:2334–2338. doi: 10.1210/endo-120-6-2334. [DOI] [PubMed] [Google Scholar]
- 22.Eggena P, Zhu JH, Clegg K, Barrett JD. Nuclear angiotensin receptors induce transcription of renin and angiotensinogen mRNA. Hypertension. 1993;22:496–501. doi: 10.1161/01.hyp.22.4.496. [DOI] [PubMed] [Google Scholar]
- 23.Ellis B, Li XC, Miguel-Qin E, Gu V, Zhuo JL. Evidence for a functional intracellular angiotensin system in the proximal tubule of the kidney. Am J Physiol Regul IntegrComp Physiol. 2012;302:R494–R509. doi: 10.1152/ajpregu.00487.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, et al. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez A, Lopez B, Diez J. Fibrosis in hypertensive heart disease: Role of the renin-angiotensin-aldosterone system. Med Clin North Am. 2004;88:83–97. doi: 10.1016/s0025-7125(03)00125-1. [DOI] [PubMed] [Google Scholar]
- 26.Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul IntegrComp Physiol. 2012;302:R518–R530. doi: 10.1152/ajpregu.00525.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, et al. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol. 2009;296:F1484–F1493. doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz D, Chappell MC. Angiotensin-(1–7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension. 2010;55:166–171. doi: 10.1161/HYPERTENSIONAHA.109.141622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz D, et al. Nuclear angiotensin-(1–7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol. 2010;299:F983–F990. doi: 10.1152/ajprenal.00371.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt MK, Ramos SP, Geary KM, Norling LL, Peach MJ, Gomez RA, et al. Colocalizationand release of angiotensin and renin in renal cortical cells. Am J Physiol. 1992;263:F363–F373. doi: 10.1152/ajprenal.1992.263.3.F363. [DOI] [PubMed] [Google Scholar]
- 31.Inagami T, Nakamuru M, Pandey KN, Naruse M, Naruse K, Misono K, et al. Intracellular action of renin, angiotensin production and release. J Hypertens Suppl. 1986;4:S11–S16. [PubMed] [Google Scholar]
- 32.Inagami T, Mizuno K, Nakamuru M, Pandey KN, Naruse M, Naruse K, et al. The renin-angiotensin system: an overview of its intracellular function. Cardiovasc Drugs Ther. 1988;2:453–458. doi: 10.1007/BF00051182. [DOI] [PubMed] [Google Scholar]
- 33.Inagami T, Mizuno K, Naruse K, Okamura T, Kawamura M. Intracellular formation and release of angiotensins from juxtaglomerular cells. Kidney Int Suppl. 1990;30:S33–S37. [PubMed] [Google Scholar]
- 34.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takakura A, Carpenter CB, et al. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18:1093–1102. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- 36.Katwa LC, Campbell SE, Tyagi SC, Lee SJ, Cicila GT, Weber KT. Cultured myofibroblasts generate angiotensin peptides de novo. J Mol Cell Cardiol. 1997;29:137–186. doi: 10.1006/jmcc.1997.0376. [DOI] [PubMed] [Google Scholar]
- 37.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: a new paradigm. Trends Endocrinol Metab. 2007;18:208–214. doi: 10.1016/j.tem.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: implications in cardiovascular remodeling. Curr Opin Nephrol Hypertens. 2008;17:168–173. doi: 10.1097/MNH.0b013e3282f521a8. [DOI] [PubMed] [Google Scholar]
- 39.Kumar R, Thomas CM, Yong QC, Chen W, Baker KM. The intracrine renin-angiotensin system. Clin Sci (Lond) 2012;123:273–284. doi: 10.1042/CS20120089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Localization of renin expressing cells in the brain, by use of a REN-eGFP transgenic model. Physiol Genomics. 2004;16:240–246. doi: 10.1152/physiolgenomics.00131.2003. [DOI] [PubMed] [Google Scholar]
- 41.Lavoie JL, Liu X, Bianco RA, Beltz TG, Johnson AK, Sigmund CD. Evidence supporting a functional role for intracellular renin in the brain. Hypertension. 2006;47:461–466. doi: 10.1161/01.HYP.0000203308.52919.dc. [DOI] [PubMed] [Google Scholar]
- 42.Lavrentyev EN, Estes AM, Malik KU. Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ Res. 2007;101:455–464. doi: 10.1161/CIRCRESAHA.107.151852. [DOI] [PubMed] [Google Scholar]
- 43.Lee HU, Campbell DJ, Habener JF. Developmental expression of the angiotensinogen gene in rat embryos. Endocrinology. 1987;121:1335–1342. doi: 10.1210/endo-121-4-1335. [DOI] [PubMed] [Google Scholar]
- 44.Lee-Kirsch MA, Gaudet F, Cardoso MC, Lindpaintner K. Distinct renin isoforms generated by tissue-specific transcription initiation and alternative splicing. Circ Res. 1999;84:240–246. doi: 10.1161/01.res.84.2.240. [DOI] [PubMed] [Google Scholar]
- 45.Leri A, Claudio PP, Li Q, Wang X, Reiss K, Wang S, et al. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the bcl-2-to-bax protein ratio in the cell. J Clin Invest. 1998;101:1326–1342. doi: 10.1172/JCI316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leri A, Liu Y, Li B, Fiordaliso F, Malhotra A, Latini R, et al. Up-regulation of AT(1) and AT(2) receptors in postinfarcted hypertrophied myocytes and stretch-mediated apoptotic cell death. Am J Pathol. 2000;156:1663–1672. doi: 10.1016/S0002-9440(10)65037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li XC, Zhuo JL. Intracellular ANG II directly induces in vitro transcription of TGF-beta1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Cell Physiol. 2008;294:C1034–C1045. doi: 10.1152/ajpcell.00432.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li XC, et al. Intrarenal transfer of an intracellular fluorescent fusion of angiotensin II selectively in proximal tubules increases blood pressure in rats and mice. Am J Physiol Renal Physiol. 2011;300:F1076–F1088. doi: 10.1152/ajprenal.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Licea H, Walters MR, Navar LG. Renal nuclear angiotensin II receptors in normal and hypertensive rats. Acta Physiol Hung. 2002;89:427–438. doi: 10.1556/APhysiol.89.2002.4.3. [DOI] [PubMed] [Google Scholar]
- 50.Lijnen PJ, Petrov VV. Role of intracardiac renin-angiotensi-aldosterone system in extracellular matrix remodeling. Methods Find Exp Clin Pharmacol. 2003;25:541–564. doi: 10.1358/mf.2003.25.7.778094. [DOI] [PubMed] [Google Scholar]
- 51.Lu D, Yang H, Shaw G, Raizada MK. Angiotensin II-induced nuclear targeting of the angiotensin type 1 (AT1) receptor in brain neurons. Endocrinology. 1998;139:365–375. doi: 10.1210/endo.139.1.5679. [DOI] [PubMed] [Google Scholar]
- 52.Mercure C, Ramla D, Garcia R, Thibault G, Deschepper CF, Reudelhuber TL. Evidence for intracellular generation of angiotensin II in rat juxtaglomerular cells. FEBS Lett. 1998;422:395–399. doi: 10.1016/s0014-5793(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 53.Naftilan AJ, Zuo WM, Ingelfinger J, Ryan TJ, Jr, Pratt RE, Dzau VJ. Localization and differential regulation of angiotensinogen mRNA expression in the vessel wall. J Clin Invest. 1991;87:1300–1311. doi: 10.1172/JCI115133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nahmod KA, Vermeulen ME, Raiden S, Salomone G, Gamberale R, Fernandez-Culotti P, et al. Control of dendritic cell differentiation by angiotensin II. FASEB J. 2003;17:491–493. doi: 10.1096/fj.02-0755fje. [DOI] [PubMed] [Google Scholar]
- 55.Nakamaru M, Misono KS, Naruse M, Workman RJ, Inagami T. A role for the adrenal renin-angiotensin system in the regulation of potassium-stimulated aldosterone production. Endocrinology. 1985;117:1772–1778. doi: 10.1210/endo-117-5-1772. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen G, Dalarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977;57:313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- 58.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. lewis rat. Am J Physiol Renal Physiol. 2006;290:F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 59.Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun. 2009;384:149–154. doi: 10.1016/j.bbrc.2009.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perez-Diaz I, Guzman C, Olivares-Reyes JA, Ramirez T, Gutierrez-Reyes G, Hiriart M, et al. Evidence of an intracellular angiotensin-generating system and non-AT1, non-AT2 binding site in a human pancreatic cell line. Pancreas. 2011;40:701–707. doi: 10.1097/MPA.0b013e318215a891. [DOI] [PubMed] [Google Scholar]
- 61.Peters J. Secretory and cytosolic(pro)renin in kidney, heart, and adrenal gland. J Mol Med (Berl) 2008;86:711–714. doi: 10.1007/s00109-008-0328-0. [DOI] [PubMed] [Google Scholar]
- 62.Peters J. Local renin-angiotensin systems in the adrenal gland. Peptides. 2012;34:427–432. doi: 10.1016/j.peptides.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 63.Pullman TN, Oparil S, Carone FA. Fate of labeled angiotensin II microinfused into individual nephrons in the rat. Am J Physiol. 1975;228:747–751. doi: 10.1152/ajplegacy.1975.228.3.747. [DOI] [PubMed] [Google Scholar]
- 64.Re RN, Cook JL. Noncanonical intracrine action. J Am Soc Hypertens. 2011;5:435–448. doi: 10.1016/j.jash.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Reaux A, deMota N, Zini S, Cadel S, Fournie-Zaluski MC, Roques BP, et al. PC18, a specific aminopeptidase N inhibitor, induces vasopressin release by increasing the half-life of brain angiotensin III. Neuroendocrinology. 1999;69:370–376. doi: 10.1159/000054439. [DOI] [PubMed] [Google Scholar]
- 66.Reaux A, Iturrioz X, Vazeux G, Fournie-Zaluski MC, David C, Roques BP, et al. Aminopeptidase A, which generates one of the main effector peptides of the brain renin-angiotensin system, angiotensin III, has a key role in central control of arterial blood pressure. Biochem Soc Trans. 2000;28:435–440. [PubMed] [Google Scholar]
- 67.Redding KM, Chen BL, Singh A, Re RN, Navar LG, Seth DM, et al. Transgenic mice expressing an intracellular fluorescent fusion of angiotensin II demonstrate renal thrombotic microangiopathy and elevated blood pressure. Am J Physiol Heart Circ Physiol. 2010;298:H1807–H218. doi: 10.1152/ajpheart.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robertson AL, Jr, Khairallah PA, Angiotensin II. Rapid localization in nuclei of smooth and cardiac muscle. Science. 1971;172:1138–1139. doi: 10.1126/science.172.3988.1138. [DOI] [PubMed] [Google Scholar]
- 69.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 70.Sasamura H, Hein L, Saruta T, Pratt RE. Evidence for internalization of both type 1 angiotensin receptor subtypes (AT1a, AT1b) by a protein kinase C independent mechanism. Hypertens Res. 1997;20:295–300. doi: 10.1291/hypres.20.295. [DOI] [PubMed] [Google Scholar]
- 71.Shen XZ, Lukacher AE, Billet S, Williams IR, Bernstein KE. Expression of angiotensin-converting enzyme changes major histocompatibility complex class I peptide presentation by modifying C termini of peptide precursors. J Biol Chem. 2008;283:9957–9965. doi: 10.1074/jbc.M709574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sherrod M, Liu X, Zhang X, Sigmund CD. Nuclear localization of angiotensinogen in astrocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R539–R546. doi: 10.1152/ajpregu.00594.2004. [DOI] [PubMed] [Google Scholar]
- 73.Singh R, Leehey DJ. Effect of ACE inhibitors on angiotensin II in rat mesangial cells cultured in high glucose. Biochem Biophys Res Commun. 2007;357:1040–1045. doi: 10.1016/j.bbrc.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 74.Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol. 2008;294:H1675–H1684. doi: 10.1152/ajpheart.91493.2007. [DOI] [PubMed] [Google Scholar]
- 75.Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes. AmJ Physiol Heart Circ Physiol. 2007;293:H939–H948. doi: 10.1152/ajpheart.00391.2007. [DOI] [PubMed] [Google Scholar]
- 76.Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008;57:3297–3306. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sinn PL, Sigmund CD. Identification of three human renin mRNA isoforms from alternative tissue-specific transcriptional initiation. Physiol Genomics. 2000;3:25–31. doi: 10.1152/physiolgenomics.2000.3.1.25. [DOI] [PubMed] [Google Scholar]
- 78.Tadevosyan A, Maguy A, Villeneuve LR, Babin Y, Bonnefoy A, Allen BG, et al. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J Biol Chem. 2010;285:22338–22349. doi: 10.1074/jbc.M110.121749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Velez JCQ. The importance of the intrarenal renin-angiotensin system. Nat Clin Pract Nephrol. 2009;5:89–100. doi: 10.1038/ncpneph1015. [DOI] [PubMed] [Google Scholar]
- 80.Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol. 2004;286:F1039–F1045. doi: 10.1152/ajprenal.00371.2003. [DOI] [PubMed] [Google Scholar]
- 81.Vila-Porcile E, Corvol P. Angiotensinogen, prorenin, and renin are co-localized in the secretory granules of all glandular cells of the rat anterior pituitary: an immunoultrastructural study. J Histochem Cytochem. 1998;46:301–311. doi: 10.1177/002215549804600303. [DOI] [PubMed] [Google Scholar]
- 82.Wanka H, Kessler N, Ellmer J, Endlich N, Peters BS, Clausmeyer S, et al. Cytosolic renin is targeted to mitochondria and induces apoptosis in H9c2 rat cardiomyoblasts. J Cell Mol Med. 2009;13:2926–2937. doi: 10.1111/j.1582-4934.2008.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]