Abstract
Context
Patients with multiple colorectal adenomas may carry germline mutations in the APC or MUTYH genes.
Objectives
To determine the prevalence of pathogenic APC and MUTYH mutations in patients who had undergone genetic testing and compare the prevalence and clinical characteristics of APC and MUTYH mutation carriers.
Design, Setting and Participants
This cross-sectional study consisted of 8676 unrelated individuals who had undergone full gene sequencing and large rearrangement analysis of the APC gene and targeted sequence analysis for the two most common MUTYH mutations (Y179C and G396D) between 2004 and 2011. Individuals with either mutation underwent full MUTYH gene sequencing. We evaluated APC and MUTYH mutation prevalence by polyp burden and the clinical characteristics associated with a pathogenic mutation using logistic regression analyses.
Main Outcome Measure
Deleterious mutations in APC and MUTYH genes.
Results
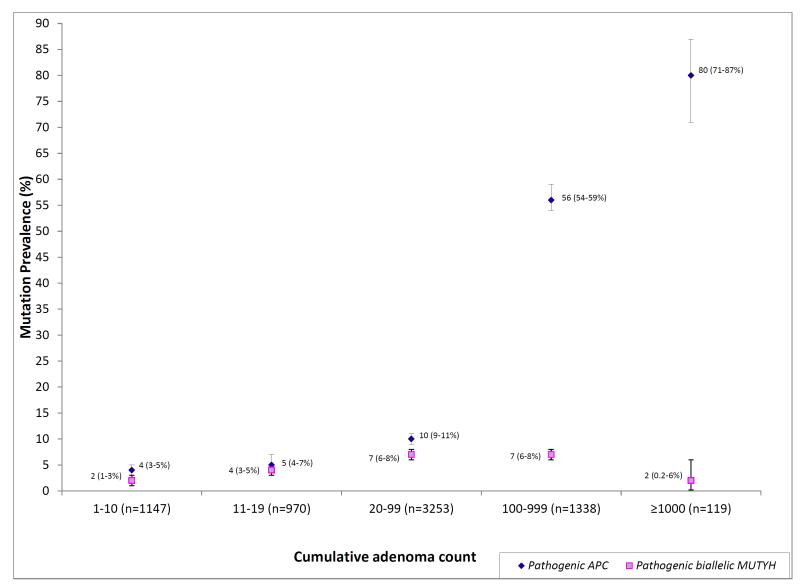
Colorectal adenomas were reported in 7225 individuals; 1457 with classic polyposis (≥ 100 adenomas) and 3253 with attenuated polyposis (20-99 adenomas). The prevalence of APC and biallelic MUTYH mutations was 95/119 (80%, 95%CI 71-87%) and 2/119 (2%, 95%CI 0.2-6%) among individuals with ≥ 1000 adenomas, 756/1338 (56%, 95%CI 54-59%) and 94/1338 (7%, 95%CI 6-8%) among individuals with 100-999 adenomas, 326/3253 (10%, 95%CI (9-11%) and 233/3253 (7%, 95%CI 6-8%) among individuals with 20-99 adenomas, and 50/970 (5%, 95%CI 4-7%) and 37/970 (4%, 95%CI 3-5%) among those with 10-19 adenomas.
Conclusions
Among patients with multiple colorectal adenomas, APC and MUTYH mutation prevalence varied considerably by adenoma count including within those with a classic polyposis phenotype. APC mutations predominate in patients with classic polyposis, whereas prevalence of APC and MYH mutations is similar in attenuated polyposis. These findings require external validation.
The presence of multiple colorectal adenomas may be attributable to the autosomal dominant polyposis syndrome familial adenomatous polyposis (FAP) due to germline mutations in the APC gene 1. Individuals with APC mutations may present with “classic polyposis” (≥100 adenomas) and develop thousands of adenomas in the second or third decade. Approximately 10% of individuals with APC mutations may have milder disease with 20-99 adenomas at an older age of onset 2. Multiple colorectal adenomas may also arise secondary to mutations in the MUTYH gene 3-4. Individuals with MUTYH-associated polyposis (MAP) are at an increased risk of CRC that may develop in the presence of few polyps 5.
Although it is established that the clinical presentation of FAP and MAP may overlap, two important issues warrant further study. First, the relative contribution of biallelic MUTYH mutations to APC mutations in individuals with multiple adenomas is unknown. Current estimates have been derived from highly selected clinic-based patients with multiple adenomas and no APC mutation 6-9. Studies evaluating the prevalence of both APC and MUTYH mutations in attenuated polyposis have been small, and their findings have not been validated 10-11. Second, guidelines for when genetic evaluation should be performed in individuals with multiple colorectal adenomas vary and data to support them are limited 12-15.
We evaluated the frequency of APC and MUTYH mutations by the number of colorectal adenomas among individuals who had undergone clinical genetic testing. We also studied the relationship between the number of adenomas and age at adenoma and CRC diagnosis and the prevalence of pathogenic APC or MUTYH mutations to inform future guidelines for genetic testing in individuals with multiple adenomas.
METHODS
Study Population
This cross-sectional study was performed on 8903 individuals, whose health care providers submitted blood samples for genetic testing for APC and MUTYH mutations to a commercial laboratory (Myriad Genetic Laboratories, Inc., Salt Lake City, UT) between 2004 and 2011 as part of their clinical care due to the patient’s personal and/or family history of CRC and/or colorectal polyps. Healthcare providers completed a prespecified test order form that included age at testing, ancestry [Western/Northern European, Central/East European, Ashkenazi, Latin American/Caribbean, African, Asian, Near East/Middle Eastern, Native American, other], cancer history (colorectal cancer, endometrial cancer, other), age at cancer diagnosis, age at colorectal adenoma diagnosis and adenoma count [1, 2-5, 6-9, 10-19, 20-99, 100-999 and ≥ 1000], and family history of cancer (relative, cancer site, age at diagnosis) and colorectal adenomas in first-, second- and third-degree relatives. We excluded 227 individuals for whom both personal and family histories were missing.
The study was investigator initiated and approved by the Dana-Farber Cancer Institute institutional review board.
Laboratory Methods
Clinical genetic testing consisted of full gene sequencing and large rearrangement analysis of the APC gene. Full gene sequence determination was performed in the forward and reverse direction of approximately 8532 base pairs comprising 15 exons and 420 adjacent non-coding intronic base pairs. For large rearrangement analyses, all exons of APC were examined for evidence of deletions and duplications by standard Southern blot methods. All individuals also underwent DNA sequence analysis of specific portions of MUTYH exons 7 and 13 designed to detect the two most common MUTYH mutations (Y179C, G396D). Full MUTYH gene sequencing was performed if one of the two most common mutations was identified. Individuals with deleterious mutations or “suspected deleterious” mutations were defined as mutation-positive. “Suspected deleterious” mutations included genetic variants for which the available evidence indicated likelihood, but not proof, that the mutation is deleterious. Genetic testing techniques did not change during the study period (2004-2011).
Statistical Methods
The primary outcome was the presence of pathogenic APC or pathogenic biallelic MUTYH mutations. Covariates of interest included the number and age at diagnoses of adenomas, the presence of and age at CRC diagnosis, and the presence of CRC in a first-degree relative (FDR). In individuals diagnosed with the same cancer more than once, the age at diagnosis was defined as the youngest age at diagnosis. Age was categorized a priori into the following categories (< 30, 30-39, 40-49, and ≥ 50 years). For individuals with adenomas identified more than once, a cumulative adenoma count was computed. Adenoma count was analyzed as an ordinal variable (<10, 10-19, 20-99, 100-999, and ≥ 1000 adenomas).
Bivariable analyses were used to assess the association between mutation status and covariates of interest. Chi-square tests were performed for categorical variables and t-tests for continuous data. Results were reported as odds ratios with 95% confidence intervals. A two-sided p-value of < 0.05 was considered statistically significant.
Multiple imputation was used to obtain estimates for missing data [adenoma count (398/7225, 5%), age at adenoma diagnosis (1912/7225, 26%), and age at CRC diagnosis (67/2306, 3%)] 16. The coefficients of five rounds of imputation (AregImpute, R) were combined to obtain the final estimates for missing data. Multivariable logistic regression analysis was performed on the imputed dataset to assess the independent associations of the presence of a pathogenic mutation (APC or biallelic MUTYH) and covariates of interest. Multinomial logistic regression analyses were used to examine the differences in phenotypic characteristics between individuals with a pathogenic APC mutation and biallelic MUTYH mutations and to derive the probability of these mutations based on clinical characteristics. Statistical analyses were performed using SAS software (9.2, SAS Institute Inc, Cary, NC) and R (2.11.0, R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Of the 8676 individuals included in the study, 4324 (50%) were male and 6323 (73%) were of European ancestry (Table 1). One thousand five hundred and eight (17%) individuals had a pathogenic APC mutation, 422 (5%) had biallelic pathogenic MUTYH mutations, 168 (2%) had a monoallelic pathogenic MUTYH alteration, and 6578 (76%) had a non-pathogenic APC or MUTYH alteration or no alteration in either gene.
Table 1.
Patient Characteristics (n=8676±)
|
APC
(n=1508) |
Biallelic
MUTYH |
Total
(n=8676 ) |
|
| Characteristics | (n=422) | ||
| Male —n (%) | 765 (51) | 211 (50) | 4324 (50) |
| Ancestry*—n (%) | |||
| European | 1022 (68) | 307 (73) | 6323 (73) |
| Non-European | 525 (35) | 91 (22) | 2192 (25) |
| None specified | 210 (14) | 75 (18) | 1410 (16) |
| Personal history of colorectal adenoma —n (%) | 1380 (91) | 401 (95) | 7225 (83) |
| ≥ 1000 adenomas | 95 (7) | 2 (0.5) | 119 (2) |
| 100-999 adenomas | 756 (55) | 94 (23) | 1338 (19) |
| 20-99 adenomas | 326 (24) | 233 (58) | 3253 (45) |
| 10-19 adenomas | 50 (4) | 37 (9) | 970 (13) |
| <10 adenomas | 44 (3) | 19 (5) | 1147 (16) |
| Missing adenoma count | 109 (8) | 16 (4) | 398 (6) |
|
Median age first colorectal adenoma diagnosis —
yr (IQR) |
30 (20-41) | 47 (39-52) | 47 (34-55) |
| Personal history of CRC —n (%) | 328 (22) | 162 (38) | 2306 (27) |
| Colorectal cancer and adenoma | 286 (87) | 149 (92) | 1779 (77) |
| Colorectal cancer alone | 42 (13) | 13 (8) | 527 (23) |
| Median age at CRC diagnosis —yr (IQR) | 36 (27-45) | 46 (39-52) | 46 (36-56) |
| First-degree relative with CRC —n (%) | 600 (40) | 102 (24) | 2660 (31) |
1508 (17%) with pathogenic APC mutation, 422 (5%) with biallelic pathogenic MUTYH mutations, 168 (2%) with monoallelic pathogenic MUTYH alteration, 6578 (76%) with non-pathogenic APC or MUTYH alteration or no alteration in either gene
1097 individuals reported more than one ancestry, IQR: Interquartile range
Overall, 7225 (83%) individuals were reported to have a history of adenomas with a median age of 47 years at adenoma diagnosis and 517 (6%) individuals were reported to have extra-intestinal manifestations associated with a familial polyposis syndrome. Of the remaining 1451 (17%) individuals without a history of adenomas, 527 (36%) had a personal history of CRC and 184 (13%) had a history of either a cancer that was not CRC or an extra-intestinal manifestation associated with familial polyposis. A personal history of CRC was reported in 2306 (27%) individuals, 1779 (77%) of whom had a history of both CRC and adenomas. Approximately, one third of the study population reported a first-degree relative who had a history of CRC.
Prevalence of APC and MUTYH Mutations Among Individuals with Colorectal Adenomas
Of the 7225 (83%) individuals with a reported history of colorectal adenomas, 1457 (20%) individuals had a classic polyposis phenotype [≥ 100 adenomas (1338 with 100-999 adenomas and 119 with ≥1000 adenomas)] and 3253 (45%) had an attenuated phenotype (20-99 adenomas) (Table 2).
Table 2.
Prevalence of Mutations by Adenoma Count (n=7225)
|
Cumulative
Adenoma Count |
≥ 1000
Classic Polyposis (n=119) |
100-999
Classic Polyposis (n=1338) |
20-99
Attenuated Polyposis (n=3253) |
10-19
(n=970) |
< 10
(n=1147) |
Missing Adenoma
Count (n=398) |
Total
(n=7225) |
||||||
| Alteration |
n/
total |
% (95%CI) |
n/
total |
% (95%CI) |
n/
total |
% (95%CI) |
n/
total |
% (95%CI) |
n/
total |
% (95%CI) |
n/
total |
% (95%CI) | n (%) |
| Pathogenic APC mutation |
95 | 80 (71-87) |
756 | 56 (54-59) |
326 | 10 (9-11) |
50 | 5 (4-7) |
44 | 4 (3-5) |
109 | 27 (23-32) |
1380 (19) |
| CRC in FDR | 36/ 44 |
82 (68-87) |
295/ 457 |
65 (60-69) |
142/ 954 |
15 (13-17) |
18/ 287 |
6 (4-10) |
19/ 372 |
5 (3-8) |
39/ 121 |
32 (24-41) |
549 (40) |
| No CRC in FDR | 59/ 75 |
79 (71-87) |
461/ 881 |
52 (49-56) |
184/ 2299 |
8 (7-9) |
32/ 683 |
5 (3-6) |
25/ 775 |
5 (2-5) |
70/ 277 |
25 (20-31) |
831 (60) |
| Pathogenic biallelic MUTYH mutation |
2 | 2 (0.2-6) |
94 | 7 (6-8) |
233 | 7 (6-8) |
37 | 4 (3-5) |
19 | 2 (1-3) |
16 | 4 (2-6) |
401 (6) |
| CRC in FDR | 2/ 44 |
4 (0.60-15) |
25/ 457 |
5 (4-8) |
52/ 954 |
5 (4-7) |
5/ 287 |
2 (0.5-4) |
4/ 372 |
1 (0.29-3) |
6/ 121 |
5 (2-10) |
94 (23) |
| No CRC in FDR | 0/ 75 |
0 (0-5) |
69/ 881 |
8 (6-10) |
181/ 2299 |
8 (7-9) |
32/ 683 |
5 (3-6) |
15/ 775 |
2 (1-3) |
10/ 277 |
4 (2-6) |
307 (77) |
| Pathogenic monoallelic MUTYH mutation |
0 | 0 (0-3) |
15 | 1 (0.6-2) |
74 | 2 (2-3) |
12 | 1 (0.6-2) |
28 | 2 (2-3) |
6 | 2 (0.6-3) |
135 (2) |
| Non-pathogenic/ no alteration |
22 | 18 (12-26) |
473 | 35 (33-38) |
2620 | 81 (79-82) |
871 | 90 (88-92) |
1056 | 92 (90-94) |
267 | 67 (62-72) |
5309 (73) |
Of the 119 individuals with ≥ 1000 adenomas, 95 (80%, 95%CI 71-87) had a pathogenic APC mutation and 2 (2%, 95%CI 0.2-6) had biallelic MUTYH mutations. In contrast, among 1338 individuals with 100-999 adenomas, 756 (56%, 95%CI 54-59) had an APC mutation and 94 (7%, 95%CI 6-8) had biallelic MUTYH mutations. The presence of a first-degree relative (FDR) with CRC did not significantly influence APC or MUTYH mutation prevalence in individuals with ≥ 1000 adenomas.
Of the 3253 individuals with 20-99 polyps, 326 (10%, 95%CI 9-11) had a pathogenic APC mutation and 233 (7%, 95%CI 6-8) had biallelic MUTYH mutations. In these patients with an attenuated FAP phenotype, having a FDR with CRC was associated with a higher APC mutation prevalence than if no such history existed (15%, 95% CI 13-17 and 8%,95% CI 7-9).
Of the 970 individuals with 10-19 adenomas, APC and biallelic MUTYH mutations were present in 50 (5%, 95%CI 4-7) and 37 (4%, 95%CI 3-5) respectively. The majority of mutation carriers did not report a family history of CRC.
Overall, the prevalence of APC and MUTYH mutations varied with adenoma count, with APC mutation rate progressively increasing with increasing polyp burden, and MUTYH mutation rates remaining relatively constant across different categories (Figure 1).
Figure 1.
Prevalence of APC and MUTYH Alterations by Adenoma Count (n=7225)
Association between Phenotypic Characteristics and a Pathogenic Mutation in Either Gene
We performed bivariable and multivariable logistic regression analyses to evaluate the association of a pathogenic mutation in either gene with clinical characteristics (Table 3). In the multivariable logistic regression analysis, controlling for a family history of CRC in a FDR, individuals with 10-19 adenomas were significantly more likely to have pathogenic APC mutation or biallelic MUTYH mutations than those with <10 adenomas (OR 2.7; 95%CI 1.9-3.7). The odds of a mutation increased with adenoma count [20-99 (OR 6.4; 95% CI 4.9-8.4); 100-999 (OR 30.7; 95% CI 23.4-40.3), ≥ 1000 (OR 77.5; 95% CI 45.3-132.4)]. Colorectal adenomas prior to age 50 years were associated with an increased likelihood of pathogenic APC/biallelic MUTYH mutations, which increased progressively with earlier age at diagnosis [40-49 (OR 2.4; 95%CI 2.0-2.8); 30-39 (OR 4.2; 95%CI 3.5-5.2); < 30 (OR 8.7; 95%CI 7.1-10.6)].
Table 3.
Association between Phenotypic Characteristics and APC and Biallelic MUTYH Mutation Status
| Mutation | APC or Biallelic MUTYH (n=1930) | APC (n=1508) | Biallelic MUTYH (n=422) | |||
|
Covariate of Interest
(n) |
Bivariable Odds
Ratio (95%CI) |
Multivariable Odds Ratio (95%CI)*∞» |
Bivariable Odds
Ratio (95%CI) |
Multinomial Odds
Ratio (95%CI) ‡ |
Bivariable Odds
Ratio (95%CI) |
Multinomial Odds Ratio (95%CI)*∞ |
| Adenoma count | ||||||
| < 10(1218) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 10-19 (1020) | 1.6(1.2-2.2) | 2.7 (1.9-3.7) | 1.2(0.84-1.9) | 2.4(1.6-3.6) | 2.3 (1.3-4.0) | 2.9(1.7-5.1) |
| 20-99 (3420) | 3.5 (2.7-4.5) | 6.4 (4.9-8.4) | 2.7 (2.0-3.6) | 6.0 (4.3-8.2) | 4.6 (2.9-7.3) | 6.6(4.1-10.6) |
| 100-999 (1437 ) | 28.9 (22.2-37.7) | 30.7 (23.4-40.3) | 31.0 (23.0-42.0) | 40.1 (29.2-55.1) | 4.3 (2.6-7.1) | 12.5 (7.6-20.6) |
| ≥ 1000 (130) | 76.3 (45.8-127.2) | 77.5 (45.3-132.4) | 98.1 (58.3-165.0) | 124.0 (69.7-220.7) | 0.94(0.22-4.0) | 5.3 (1.2-24.2) |
|
Age at adenoma
diagnosis —yr |
||||||
| < 30 (1236) | 11.6(9.9-13.7) | 8.7(7.1-10.6) | 22.3 (18.3-27.2) | 15.4(12.2-19.5) | 0.36 (0.23-0.57) | 0.93 (0.57-1.5) |
| 30-39 (1092) | 5.0 (4.2-5.9) | 4.2 (3.5-5.2) | 7.5 (6.1-9.2) | 6.1 (4.8-7.8) | 1.5 (1.1-2.0) | 2.2(1.6-3.0) |
| 40-49 (1837) | 2.7 (2.3-3.2) | 2.4 (2.0-2.8) | 3.1 (2.5-3.8) | 2.7 (2.2-3.4) | 1.9(1.5-2.4) | 2.0 (1.6-2.6) |
| ≥ 50 (3060) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| History of CRC (2306) | 0.92 (0.82-1.0) | 1.7(1.3-2.2) | 0.73 (0.64-0.83) | 1.2(0.83-1.6) | 1.8(1.4-2.2) | 2.8 (2.0-3.8) |
| No CRC (6370 ) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
|
Age at CRC diagnosis
—yr |
||||||
| < 30 (270 ) | 4.3 (3.1-5.9) | 0.83 (0.52-1.3) | 8.4 (5.8-12.3) | 1.2(0.70-2.1) | 0.40(0.18-0.90) | 0.60 (0.20-1.8) |
| 30-39 (479) | 2.7 (2.0-3.5) | 1.2 (0.84-1.8) | 3.9 (2.7-5.6) | 1.5 (0.94-2.4) | 1.2(0.75-1.8) | 1.3 (0.77-2.2) |
| 40-49 (634) | 2.3 (1.7-3.0) | 1.8(1.3-2.6) | 2.5 (1.8-3.6) | 1.9(1.2-2.9) | 1.7 (1.2-2.5) | 1.8(1.2-2.8) |
| ≥ 50 (923 ) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
Phenotypic Differences Between Individuals with APC and Biallelic MUTYH Mutations
To examine the differences between the phenotypic characteristics of individuals with a pathogenic APC mutation and biallelic MUTYH mutations, we performed multinomial logistic regression analysis [logistic regression for a categorical dependant variable with ≥2 categories (APC, biallelic MUTYH, non-pathogenic APC or MUTYH alteration/no APC or MUTYH alteration/monoallelic MUTYH)] (Table 3). The odds of carrying a pathogenic APC mutation were significantly increased with greater than 10 adenomas [10-19 (OR 2.4; 95%CI 1.6-3.6); 20-99 (OR 6.0; 95%CI 4.3-8.2); 100-999 (OR 40.1; 95% CI 29.2-55.1); ≥1000 (OR 124.0; 95% CI 69.7-220.7)]. Age at adenoma diagnosis was also associated with an APC mutation [< 30 (OR 15.4; 95% CI 12.2-19.5); 30-39 (OR 6.1; 95% CI 4.8-7.8 ); 40-49 (OR 2.7; 95% CI 2.2-3.4)]. Individuals with 10-19 adenomas were significantly more likely to have biallelic MUTYH mutations than no mutation or a monoallelic MUTYH mutation. The odds of biallelic MUTYH mutations increased with increasing number of adenomas [10-19 (OR 2.9; 95%CI 1.7-5.1); 20-99 (OR 6.6; 95%CI 4.1-10.6); 100-999 (OR 12.5; 95%CI 7.6-20.6).
Predicted Probability of APC and Biallelic MUTYH Mutations
The multinomial logistic regression model (eTable 1) was also used to derive the predicted probability of pathogenic APC and MUTYH mutations based on phenotypic characteristics and family history of CRC. The c-statistic for APC and MUTYH was 0.81 (95% CI 0.73-0.89) and 0.59 (95% CI 0.49-0.68) when the model included the number of adenomas alone, 0.88 (95%CI 0.82-0.95) and 0.59 (95%CI 0.49-0.69) when the model included the number and age at adenoma diagnoses, 0.89 (95%CI 0.82-0.95) and 0.65 (95%CI 0.55-0.74) when the presence of CRC and age at CRC diagnosis were added to the model and finally 0.89 (95%CI 0.82-0.95) and 0.66 (95%CI 0.56-0.75) respectively when the presence of a FDR with CRC was also included in the model. To illustrate how the prediction probabilities derived from these models may be used in a clinical setting and the differences in APC and MUTYH mutation probability based on clinical characteristics, twenty clinical scenarios with their respective predicted mutation probabilities are presented in Table 4. For example, for an individual with multiple adenomas diagnosed at age 20 and no history of CRC in a FDR, the probability of APC and biallelic MUTYH mutations range from 97% (95%CI 93.4-100.0) and 0.5% (95%CI 0.0-1.9) with ≥ 1000 adenomas to 89% (95%CI 83.0-95.2) and 3% (95%CI 0.0-6.9) with 100-999 adenomas, to 59% (95%CI 49.3-68.6) and 8% (95%CI 2.7-13.4) with 20-99 adenomas, and 38% (95%CI 28.6-47.7) and 6% (95%CI 1.4-10.7) with 10-19 adenomas.
Table 4.
Predicted Probability of Pathogenic APC or Biallelic MUTYH Mutations Based on Clinical Phenotype
|
Clinical
Scenario |
Number
of Adenomas (n) |
Age at
First Adenoma Diagnosis (yr) |
CRC
Diagnosis (yes/no) |
Age at
CRC Diagnosis (yr) |
FDR with CRC
(yes/no) |
APC Mutation
Probability (95% CI) |
MUTYH Mutation
Probability (95% CI) |
| 1 | 10-19 | 20 | No | - | No | 38 (28.6-47.7) | 6 (1.4-10.7) |
| 2 | 10-19 | 50 | No | - | No | 7 (1.8-11.7) | 6 (1.6-11.2) |
| 3 | 10-19 | 50 | Yes | 50 | No | 2 (0.0-4.7) | 6 (1.4-10.8) |
| 4 | 10-19 | 50 | No | - | Yes | 11 (5.1-17.5) | 5 (0.8-9.4) |
| 5 | 10-19 | 50 | Yes | 50 | Yes | 3 (0.0 -7.0) | 5 (0.7-9.3) |
|
| |||||||
| 6 | 20-99 | 20 | No | - | No | 59 (49.3-68.6) | 8 (2.7-13.4) |
| 7 | 20-99 | 50 | No | - | No | 15 (7.9-21.8) | 12 (5.7-18.5) |
| 8 | 20-99 | 50 | Yes | 50 | No | 5 (0.5-8.8) | 12 (6.0-18.9) |
| 9 | 20-99 | 50 | No | - | Yes | 24 (15.3-32.0) | 9 (3.5-14.8) |
| 10 | 20-99 | 50 | Yes | 50 | Yes | 8 (2.7-13.3) | 10 (4.2-16.0) |
|
| |||||||
| 11 | 100-999 | 20 | No | - | No | 89 (83.0-95.2) | 3 (0.0-6.9) |
| 12 | 100-999 | 50 | No | - | No | 51 (41.0-60.6) | 11 (5.2-17.7) |
| 13 | 100-999 | 50 | Yes | 50 | No | 23 (14.5-30.9) | 17 (9.4-24.0) |
| 14 | 100-999 | 50 | No | - | Yes | 65 (55.8-74.5) | 7 (2.0 -11.9) |
| 15 | 100-999 | 50 | Yes | 50 | Yes | 35 (25.2-43.9) | 12 (5.7-18.4) |
| 16 | ≥1000 | 20 | No | - | No | 97 (93.4-100.0) | 0.5 (0.0-1.9) |
| 17 | ≥1000 | 50 | No | - | No | 78 (70.5-86.6) | 2 (0.0-5.4) |
| 18 | ≥1000 | 50 | Yes | 50 | No | 52 (41.9-61.5) | 3 (0.0-6.4) |
| 19 | ≥1000 | 50 | No | - | Yes | 87 (79.9-93.3) | 1 (0.0-3.4) |
| 20 | ≥1000 | 50 | Yes | 50 | Yes | 64 (55.0-73.8) | 3 (0.0-6.4) |
COMMENTS
We evaluated the relative frequencies of mutations in the APC and MUTYH genes in a large number of individuals who had undergone genetic testing. Our results help further inform the evolution in the understanding of the genetic epidemiology of the classic hereditary colorectal cancer syndrome, FAP, and shed some light on the important differences in disease patterns between carriers of APC mutations versus those with biallelic MUTYH mutations.
The clinical syndrome of FAP was first reported in 1847. In 1975, Bussey described the clinical characteristics of patients with hundreds to thousands of colorectal polyps 17. In 1991, the adenomatous polyposis coli (APC) gene was cloned and found to be mutated in FAP patients 18-20. MAP was described in 2002 when Al-Tassan et al. noted biallelic germline mutations in the base excision repair gene MUTYH in a family with recessive inheritance of multiple colorectal adenomas and CRC 3.
Previous studies (predating the discovery of MAP) have reported widely varying prevalence of pathogenic APC mutations among individuals with a classic polyposis phenotype (52% to 82%) likely due to varying mutation analysis techniques and patient selection 21-26. However, these studies primarily involved small cohorts that were geographically and ethnically homogeneous. After the discovery of MUTYH, APC mutation-negative probands with classic FAP were screened for MUTYH mutations. These relatively small studies reported MUTYH mutation prevalence rates ranging from 7.5% to 20% in classic polyposis 6, 8.
The results of our study, in which all individuals were tested for both APC and MUTYH mutations, indicate that there is significant heterogeneity in mutation prevalence even among individuals with a classic polyposis phenotype. Among individuals with ≥1000 adenomas, 80% (95%CI 71-87) had a pathogenic APC mutation, and MUTYH played a minor role (2%, 95%CI 0.2-6). The distribution and prevalence of mutations was markedly different, however, in individuals with 100-999 adenomas (still considered classic polyposis) - only 56% (95%CI 54-59) were APC carriers, and a higher proportion (7%, 95%CI 6-8) had biallelic MUTYH mutations. No pathogenic APC or MUTYH mutations were detected in 18% (95%CI 12-26) of individuals with ≥1000 adenomas and 35% (95% CI 33-38) with 100-999 adenomas, potentially attributable in part to genes that have not been identified.
In contrast, in the 3253 individuals with attenuated polyposis, prevalence rates of pathogenic APC and MUTYH mutations were similar (10%, 95%CI 9-11 and 7%, 95%CI 6-8 respectively). This MUTYH prevalence rate is lower than prior reports from smaller cohorts of attenuated polyposis patients, where estimates have ranged from 22% to 29% 6-9, 27-29, 11, 30.
We did not evaluate the genotype-phenotype correlation among individuals with APC mutations as has been previously reported, as this study aimed to highlight the clinical characteristics associated with a pathogenic mutation in either of the two familial polyposis genes (APC or MUTYH) and the differences in these characteristics between mutation carriers. Ten or more adenomas and young onset adenomas (< 50 years) were associated with a mutation in either gene (APC or MUTYH). There was an incremental increase in the odds of a mutation with an increasing number of adenomas and earlier age at adenoma diagnosis. Individuals with ≥ 10 adenomas and young onset adenomas (prior to 50 years) were significantly more likely to have an APC mutation. The presence of ≥ 10 adenomas was associated with a pathogenic MUTYH mutation but in contrast to individuals with an APC mutation, the odds of a mutation did not incrementally increase with earlier age at diagnosis and were highest between 30-49 years.
The study population is both a weakness and strength. This was not a population-based study, and subjects had undergone testing based on a personal or family history suggestive of a polyposis syndrome by health care providers who may have had variable expertise in genetic evaluation; therefore prevalence estimates, particularly in the groups with fewer numbers of individuals must be interpreted with caution due to potential ascertainment and referral bias 31. Nonetheless, this cohort is representative of individuals for whom genetic testing for APC and MUTYH genes should be considered and reflects the characteristics of the population at risk. We did not verify the pathology of polyps or the clinical data provided on the test order form. Although data were provided by health care providers whose specific specialty or training was not reported on the form, other studies using similar methods of data collection for cohorts tested for familial CRC syndromes have been externally validated, suggesting that the data are likely to be accurate, and are likely not to vary between the groups being compared 32-33. We also used multiple imputation techniques for missing data so as to minimize selection bias which has been demonstrated to be particularly important in genetic association studies, where missing data may be distributed differentially and may generate spurious associations 34. However, results obtained from using both complete case data and imputed data were similar.
The test order form did not elicit a history of hyperplastic polyps which have been reported in small cohorts with MAP 35. However, only a small percentage of patients with MAP present with hyperplastic polyposis and adenomatous polyps and CRC remain the most common clinical presentation. Targeted sequence analysis was performed to detect the two most common MUTYH mutations Y179C and G396D and full MUTYH gene sequencing was performed in a small percentage of individuals. It is however known that Y179C and G396D mutations account for the vast majority of mutant alleles in individuals of Northern American and European ancestry that comprised the majority of our study subjects 8, 36-38. The use of MUTYH gene rearrangement analysis and allele-specific APC analysis which have recently been reported, but are not widely available commercially, may result in a small improvement in the yield of testing 39.
Through evaluation of the phenotypic differences between mutation carriers in this large study, a pattern has emerged. Overall, in individuals with multiple adenomas, the APC mutation rate progressively increases with increasing polyp burden whereas the MUTYH mutation rate remains relatively constant across different categories. Furthermore, the prevalence of APC mutations varies significantly among individuals with classic polyposis (≥ 1000 adenomas: 80%, 95%CI 71-87; 100-999 adenomas: 56%, 95%CI 54-59). In contrast, biallelic MUTYH mutations are rare in individuals with ≥ 1000 adenomas and their prevalence is relatively constant among individuals with < 1000 adenomas. Our evaluation of individuals who underwent genetic testing due to a personal or family history suggestive of a familial polyposis syndrome suggests that genetic evaluation for APC and MUTYH mutations may be considered in individuals with 10 or more adenomas. However, our results are derived from a selected cohort of high-risk individuals, and need to be validated in larger populations of unselected patients. The mutation probabilities presented may assist providers in their decision to recommend genetic evaluation and counsel patients undergoing genetic testing. However, it remains important to also consider the limitations of genetic testing at the present time- a third of patients with a classic FAP phenotype are found to not carry a mutation in either the APC or MUTYH gene. Such individuals should undergo periodic re-evaluation as other susceptibility genes are identified.
Supplementary Material
eTable 1. Equation for Prediction of Pathogenic APC and Biallelic MUTYH Mutations
ACKNOWLEDGEMENT
Funding/Support: National Cancer Institute (R25 CA 092203 (SG), K07 CA151769 (FK)) and National Institute of Health (K24113433 (SS)). No grant support was obtained from Myriad Genetics Laboratories Inc. to support the study.
Research Support: This work was supported by National Cancer Institute (R25 CA 092203 (SG), K07 CA151769 (FK)), and National Institute of Health (K24113433 (SS))
Footnotes
Author Contributions: Dr. Grover, Dr. Kastrinos and Dr. Syngal had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Grover and Kastrinos contributed equally to the manuscript
Study concept and design: Grover, Syngal
Acquisition of data: Burbidge, Wenstrup
Analysis and interpretation of data: Grover, Kastrinos, Steyerberg, Cook, Dewanwala, Syngal
Drafting of the manuscript: Grover, Syngal
Critical revision of the manuscript for important intellectual content: Grover, Kastrinos, Steyerberg, Cook, Dewanwala, Burbidge, Wenstrup, Syngal
Statistical analysis: Grover, Kastrinos, Steyerberg, Cook, Syngal
Obtained funding: Grover, Syngal
Administrative, technical, or material support: Burbidge, Wenstrup, Dewanwala
Study supervision: Grover, Kastrinos, Syngal
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Richard Wenstrup and Lynn Anne Burbidge are employees of Myriad Genetic Laboratories Inc. and receive stock options. Dr. Syngal has been a consultant for Archimedes, Inc., Quest Diagnostics, Inc., Interquest, Inc., Cequent, Inc., and has received stock options from Marinabio, Inc. and travel /accommodations/meeting expenses unrelated to activities listed from Myriad Genetic Laboratories Inc. Dr. Kastrinos is a consultant for Marinabio, Inc. Dr. Grover is an employee of UpToDate, Inc.
Role of the Sponsor: The funding organizations had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Independent Statistical Analysis: Data sets were forwarded by investigators at Myriad Genetics Laboratories, Inc. to independent investigators Shilpa Grover, M.D., M.P.H. at the Dana-Farber Cancer Institute, Boston, MA and Fay Kastrinos, M.D., M.P.H at the Herbert Irving Comprehensive Cancer Center, New York, NY, and Sapna Syngal, MD. M.P.H.. Drs. Grover, Kastrinos, and Steyerberg performed independent statistical analyses. None of the coauthors who are not employed by Myriad Genetics, Inc. received any funding for the study from Myriad Genetics Laboratories, Inc.
REFERENCES
- 1.Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001 Apr;10(7):721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 2.Van Duijvendijk P, Slors JF, Taat CW, et al. Quality of life after total colectomy with ileorectal anastomosis or proctocolectomy and ileal pouch-anal anastomosis for familial adenomatous polyposis. Br J Surg. 2000;87(5):590–596. doi: 10.1046/j.1365-2168.2000.01442.x. [DOI] [PubMed] [Google Scholar]
- 3.Al-Tassan N, Chmiel NH, Maynard J, et al. Inherited variants of MYH associated with somatic G:C-->T:A mutations in colorectal tumors. Nat Genet. 2002 Feb;30(2):227–232. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]
- 4.Jones S, Emmerson P, Maynard J, et al. Biallelic germline mutations in MYH predispose to multiple colorectal adenoma and somatic G:C-->T:A mutations. Hum Mol Genet. 2002 Nov 1;11(23):2961–2967. doi: 10.1093/hmg/11.23.2961. [DOI] [PubMed] [Google Scholar]
- 5.Cleary SP, Cotterchio M, Jenkins MA, et al. Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology. 2009 Apr;136(4):1251–1260. doi: 10.1053/j.gastro.2008.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sieber OM, Lipton L, Crabtree M, et al. Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in MYH. N Engl J Med. 2003 Feb 27;348(9):791–799. doi: 10.1056/NEJMoa025283. [DOI] [PubMed] [Google Scholar]
- 7.Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003 Jul 5;362(9377):39–41. doi: 10.1016/S0140-6736(03)13805-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Baudhuin LM, Boardman LA, et al. MYH mutations in patients with attenuated and classic polyposis and with young-onset colorectal cancer without polyps. Gastroenterology. 2004 Jul;127(1):9–16. doi: 10.1053/j.gastro.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 9.Croitoru ME, Cleary SP, Berk T, et al. Germline MYH mutations in a clinic-based series of Canadian multiple colorectal adenoma patients. J Surg Oncol. 2007 May 1;95(6):499–506. doi: 10.1002/jso.20724. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen M, Hes FJ, Nagengast FM, et al. Germline mutations in APC and MUTYH are responsible for the majority of families with attenuated familial adenomatous polyposis. Clin Genet. 2007 May;71(5):427–433. doi: 10.1111/j.1399-0004.2007.00766.x. [DOI] [PubMed] [Google Scholar]
- 11.Venesio T, Molatore S, Cattaneo F, Arrigoni A, Risio M, Ranzani GN. High frequency of MYH gene mutations in a subset of patients with familial adenomatous polyposis. Gastroenterology. 2004 Jun;126(7):1681–1685. doi: 10.1053/j.gastro.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Giardiello FM, Brensinger JD, Petersen GM. AGA technical review on hereditary colorectal cancer and genetic testing. Gastroenterology. 2001;121(1):198–213. doi: 10.1053/gast.2001.25581. [DOI] [PubMed] [Google Scholar]
- 13.Vasen HF, Moslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP) Gut. 2008 May;57(5):704–713. doi: 10.1136/gut.2007.136127. [DOI] [PubMed] [Google Scholar]
- 14.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009 Mar;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 15.Burt RW, Barthel JS, Dunn KB, et al. NCCN clinical practice guidelines in oncology. Colorectal cancer screening. J Natl Compr Canc Netw. 2010 Jan;8(1):8–61. doi: 10.6004/jnccn.2010.0003. [DOI] [PubMed] [Google Scholar]
- 16.Raedle J, Trojan J, Brieger A, et al. Bethesda guidelines: relation to microsatellite instability and MLH1 promoter methylation in patients with colorectal cancer. Ann Intern Med. 2001;135(8 Pt 1):566–576. doi: 10.7326/0003-4819-135-8_part_1-200110160-00007. [DOI] [PubMed] [Google Scholar]
- 17.Bussey H. Familial polyposis coli. Family studies, histopathology, differential diagnosis, and results of treatment. Johns Hopkins University Press; Baltimore, Maryland: 1975. [Google Scholar]
- 18.Nishisho I, Nakamura Y, Miyoshi Y, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991 Aug 9;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 19.Kinzler KW, Nilbert MC, Su LK, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991 Aug 9;253(5020):661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 20.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991 Aug 9;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 21.Powell SM, Petersen GM, Krush AJ, et al. Molecular diagnosis of familial adenomatous polyposis. N Engl J Med. 1993 Dec 30;329(27):1982–1987. doi: 10.1056/NEJM199312303292702. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi Y, Ando H, Nagase H, et al. Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4452–4456. doi: 10.1073/pnas.89.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong JG, Davies DR, Guy SP, Frayling IM, Evans DG. APC mutations in familial adenomatous polyposis families in the Northwest of England. Hum Mutat. 1997;10(5):376–380. doi: 10.1002/(SICI)1098-1004(1997)10:5<376::AID-HUMU7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 24.Giarola M, Stagi L, Presciuttini S, et al. Screening for mutations of the APC gene in 66 Italian familial adenomatous polyposis patients: evidence for phenotypic differences in cases with and without identified mutation. Hum Mutat. 1999;13(2):116–123. doi: 10.1002/(SICI)1098-1004(1999)13:2<116::AID-HUMU3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Nagase H, Miyoshi Y, Horii A, et al. Screening for germ-line mutations in familial adenomatous polyposis patients: 61 new patients and a summary of 150 unrelated patients. Hum Mutat. 1992;1(6):467–473. doi: 10.1002/humu.1380010603. [DOI] [PubMed] [Google Scholar]
- 26.van der Luijt RB, Khan PM, Vasen HF, et al. Molecular analysis of the APC gene in 105 Dutch kindreds with familial adenomatous polyposis: 67 germline mutations identified by DGGE, PTT, and southern analysis. Hum Mutat. 1997;9(1):7–16. doi: 10.1002/(SICI)1098-1004(1997)9:1<7::AID-HUMU2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Gismondi V, Meta M, Bonelli L, et al. Prevalence of the Y165C, G382D and 1395delGGA germline mutations of the MYH gene in Italian patients with adenomatous polyposis coli and colorectal adenomas. Int J Cancer. 2004 May 1;109(5):680–684. doi: 10.1002/ijc.20054. [DOI] [PubMed] [Google Scholar]
- 28.Pinol V, Castells A, Andreu M, et al. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. Jama. 2005 Apr 27;293(16):1986–1994. doi: 10.1001/jama.293.16.1986. [DOI] [PubMed] [Google Scholar]
- 29.Russell AM, Zhang J, Luz J, et al. Prevalence of MYH germline mutations in Swiss APC mutation-negative polyposis patients. Int J Cancer. 2006 Apr 15;118(8):1937–1940. doi: 10.1002/ijc.21470. [DOI] [PubMed] [Google Scholar]
- 30.Filipe B, Baltazar C, Albuquerque C, et al. APC or MUTYH mutations account for the majority of clinically well-characterized families with FAP and AFAP phenotype and patients with more than 30 adenomas. Clin Genet. 2009 Sep;76(3):242–255. doi: 10.1111/j.1399-0004.2009.01241.x. [DOI] [PubMed] [Google Scholar]
- 31.Giardiello FM, Brensinger JD, Petersen GM, et al. The use and interpretation of commercial APC gene testing for familial adenomatous polyposis. N Engl J Med. 1997 Mar 20;336(12):823–827. doi: 10.1056/NEJM199703203361202. [DOI] [PubMed] [Google Scholar]
- 32.Balmana J, Stockwell DH, Steyerberg EW, et al. Prediction of MLH1 and MSH2 mutations in Lynch syndrome. Jama. 2006 Sep 27;296(12):1469–1478. doi: 10.1001/jama.296.12.1469. [DOI] [PubMed] [Google Scholar]
- 33.Balaguer F, Balmana J, Castellvi-Bel S, et al. Validation and extension of the PREMM1,2 model in a population-based cohort of colorectal cancer patients. Gastroenterology. 2008 Jan;134(1):39–46. doi: 10.1053/j.gastro.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clayton DG, Walker NM, Smyth DJ, Pask R. Population structure, differential bias and genomic control in a large-scale,case-control assocition study. Nat Genet. 2005;37(11):1243–1246. doi: 10.1038/ng1653. [DOI] [PubMed] [Google Scholar]
- 35.Boparai KS, Dekker E, Van Eeden S, et al. Hyperplastic polyps and sessile serrated adenomas as a phenotypic expression of MYH-associated polyposis. Gastroenterology. 2008 Dec;135(6):2014–2018. doi: 10.1053/j.gastro.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Croitoru ME, Cleary SP, Di Nicola N, et al. Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J Natl Cancer Inst. 2004 Nov 3;96(21):1631–1634. doi: 10.1093/jnci/djh288. [DOI] [PubMed] [Google Scholar]
- 37.Fleischmann C, Peto J, Cheadle J, Shah B, Sampson J, Houlston RS. Comprehensive analysis of the contribution of germline MYH variation to early-onset colorectal cancer. Int J Cancer. 2004 Apr 20;109(4):554–558. doi: 10.1002/ijc.20020. [DOI] [PubMed] [Google Scholar]
- 38.Farrington SM, Tenesa A, Barnetson R, et al. Germline susceptibility to colorectal cancer due to base-excision repair gene defects. Am J Hum Genet. 2005 Jul;77(1):112–119. doi: 10.1086/431213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castellsague E, Gonzalez S, Guino E, et al. Allele specific expression of APC in adenomatous polyposis families. Gastroenterology. 2010;139(2):439–447. doi: 10.1053/j.gastro.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Equation for Prediction of Pathogenic APC and Biallelic MUTYH Mutations