Abstract
Glomerulosclerosis is characterized by excessive deposition of extracellular matrix within the glomeruli of the kidney, glomerular cell death, and subsequent loss of functional glomeruli. While in physiological situations the levels of extracellular matrix components are kept constant by a tight balance between formation and degradation, in the case of injury that results in fibrosis there is increased matrix deposition relative to its breakdown. Multiple factors control matrix synthesis and degradation, thus contributing to the development of glomerulosclerosis. This review focuses primarily on the role of cell-matrix interactions, which play a critical role in governing glomerular cell cues in both healthy and diseased kidneys. Cell-extracellular matrix interactions are made possible by various cellular receptors including integrins, discoidin domain receptors, and dystroglycan. Upon binding to a selective extracellular matrix protein, these receptors activate intracellular signaling pathways that can either downregulate or upregulate matrix synthesis and deposition. This, together with the observation that changes in the expression levels of matrix receptors have been documented in glomerular disease, clearly emphasizes the contribution of cell-matrix interactions in glomerular injury. Understanding the molecular mechanisms whereby extracellular matrix receptors regulate matrix homeostasis in the course of glomerular injury is therefore critical for devising more effective therapies to treat and ideally prevent glomerulosclerosis.
Keywords: integrins, collagen, laminin, glomerulus, discoidin domain receptor, fibrosis, growth factors, dystroglycan
Introduction
Glomerulosclerosis, the process by which glomerular tissue is replaced by extracellular matrix (ECM), is the final common pathway for loss of functioning glomeruli. Glomerulosclerosis occurs when the normal response to renal injury, characterized by the synthesis, degradation, and remodeling of ECM components, is dysregulated such that matrix deposition prevails on its breakdown.
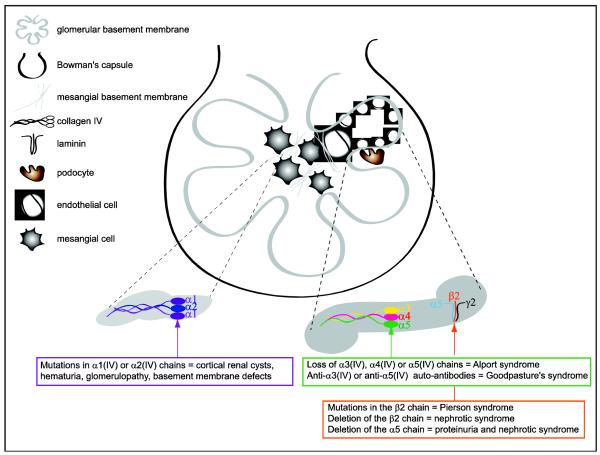
The glomerulus, the filtering unit of the kidney, has a complex structure which includes: 1) a capillary bed composed of specialized fenestrated endothelial cells; 2) mesangial cells, the principal mesenchymal cell type, which maintain the three-dimensional structure of the capillary bed; 3) terminally differentiated visceral epithelial cells called podocytes; and 4) the glomerular basement membrane (GBM) that separates the podocytes from the endothelial cells (Figure 1). The endothelial cells, GBM, and podocytes form the glomerular filtration barrier. Dysfunction of any of the four major components of the glomerulus due to genetic disorders, immune complex mediated injury, hemodynamic injury, or direct cytotoxic injury to specific glomerular cell components can result in glomerulosclerosis. Thus, it is imperative that we understand the molecular and cellular mechanisms that contribute to the homeostasis of the glomerular filtration barrier in order to devise new and more efficient tools to halt the progression of glomerulosclerosis and ideally prevent glomerular disease.
Figure 1.
Schematic representation of a glomerulus highlighting the three major cell components and the two major basement membranes. Diseases or glomerular phenotypes associated to loss of collagen IV chains (in both mesangial and GBMs), or laminin-521 (in the GBM) are highlighted. See text for details.
Although multiple factors contribute to the initiation and progression to glomerulosclerosis [1], in this review we will focus on the interactions between glomerular cells with the surrounding ECM, as these interactions play a critical role in regulating the response of the glomerulus to injury and progression to glomerulosclerosis. We will briefly describe the major matrix components, namely collagens and laminins, found the in adult glomerulus and how changes in their expression contribute to glomerular injury. We will then describe the role of three major matrix receptors, namely integrins, discoidin domain receptors (DDR), and dystroglycan in the control of glomerular homeostasis in healthy and diseased glomeruli. Finally, we will discuss the hope and tribulations of targeting these receptors for the treatment of glomerulosclerosis.
Glomerular extracellular matrix
In the glomerulus, ECM components provide structural stability to the glomerulus and interact with the three major glomerular cell components described above through integrin and non-integrin receptors, influencing cellular survival, proliferation, adhesion and matrix homeostasis [2]. Endothelial cells and podocytes lie on basement membranes, specialized ECM structures composed primarily by collagen IV and laminins (Figure 1). In addition to these two major components, matrices such as collagen XV, nidogens, and proteoglycans (i.e. perlecan, collagen XVIII, and agrin) can also be found in the GBM [1]. Collagen IV is the major matrix found in the mesangium and separates mesangial cells from each other’s, as well as mesangial cells from endothelial cells (Figure 1). In this review we describe briefly the contribution of collagen IV and laminins to glomerular homeostasis, as they are the two major components upregulated in the course of glomeruli injury and the ligands for both integrin and non-integrin receptors within the glomerulus.
Collagen IV is comprised of 6 chains, called α1-α6, that assemble in a selective manner giving rise to trimer molecules [3]. Several networks of collagen IV are present in the adult glomerulus. The α1α2α1(IV) network is found primarily in the mesangium, the α3α4α5(IV) network is the main component of the GBM, and the α1α2α1-α5α6α5(IV) network is present in the Bowman’s capsule [4]. The glomerular α1α2α1(IV) network provides structural stability and interacts with cellular receptors like integrins and DDRs [5, 6]. The absence of this network results in embryonic lethality [7], while mutations in the α1(IV) chain lead to cortical renal cysts, hematuria, basement membrane defects, glomerulopathy, and decreased glomerular filtration rate [8-10] (Figure 1). Unlike the α1α2α1(IV), the α3α4α5(IV) network is dispensable for kidney development, but is required for proper glomerular filtration, since patients lacking either the α3(IV), α4(IV) or the α5(IV) chain develop Alport Syndrome, a genetic disorder characterized by glomerulonephritis that progresses to end stage kidney failure [11] (Figure 1). In addition, production of auto-antibodies against the non collagenous domain of the α3(IV) or α5(IV) chain leads to Goodpasture’s disease, an immunological disease characterized by rapidly progressive glomerulonephritis [12] (Figure 1).
Laminins are glycoproteins composed by the assembly of an α, β and γ subunit [13] and are highly expressed in GBM [14]. Several laminins are expressed at various stages of glomerular development, but only laminin-521 is expressed in the adult GBM [15]. Laminin-521 is critical for the glomerular function, as patients with mutations in the laminin β2 gene develop Pierson syndrome, a genetic disease characterized by glomerulosclerosis and defects of the GBM [16, 17] (Figure 1). Similarly, mice in which the laminin β2 chain is deleted develop nephrotic syndrome [18]. Deletion of the laminin α5 chain selectively in podocytes results in proteinuria that progresses to nephrotic syndrome [19] (Figure 1).
In glomerulosclerosis, changes in the levels of glomerular matrix components include increased synthesis and deposition of collagen IV [20, 21] as well as ectopic expression of laminin chains. In this regard, in membranous glomerulonephritis increased expression of laminin β1 (only expressed during kidney development) in the GBM is evident [22]. Changes in levels or de-novo expression of matrix components have pathological consequences for the progression of glomerulosclerosis, as they could modify the filtering properties of the GBM or affect cell-matrix interactions and subsequent glomerular cellular function.
Integrins and glomerulosclerosis
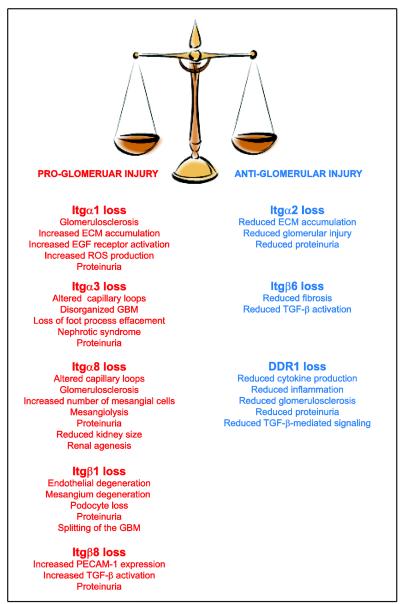
Integrins are transmembrane receptors for ECM components that consist of two non-covalently associated α and β subunits. Both integrin subunits have large extracellular domains which contain the ligand binding site and confer ligand specificity, a single transmembrane domain, and a cytoplasmic domain which interacts with the cytoskeleton and regulates intracellular signaling [23, 24]. Integrins influence critical cell functions including proliferation, survival, migration, as well as matrix homeostasis [1]. In mammals, 18 α subunits associate with 8 β subunits to form 24 distinct integrins. The most widely expressed integrin subunit is the β1 subunit, which associates with α1-11 and αv subunits [23]. Global deletion of the integrin β1 subunit results in embryonic lethality [25], thus making it impossible to determine the role of this receptor in glomerular homeostasis. The development of conditionally null mice has allowed the selective deletion of integrin subunits in various cells, including glomerular cells. Selective deletion of the integrin β1 subunit in podocytes using podocin-cre mice results in proteinuria at birth, podocytes loss, capillary loop and mesangium degeneration, followed by end stage renal disease at 6 weeks of age [26] (Figure 2). Deletion of the same integrin subunit in podocytes using nephrin-cre results in a more severe phenotype, including splitting of the GBM [27] (Figure 2). These findings indicate that β1-containing integrins are required for regulating glomerular cell functions. In addition to β1, integrin β6, β8 and several α subunits play a role in glomerular homeostasis and their loss is associated with either progression to or protection from glomerulosclerosis. The contribution of some of these subunits is described in detail below.
Figure 2.
List of matrix receptors whose loss has been shown to either promote or protect from the development of glomerular injury. See text for details.
Integrin α3β1
Integrin α3β1 is the main integrin expressed on podocytes and function as the major GBM receptor in these cells [28]. Global deletion of the integrin α3 subunit in mice results in death within 24 hours after birth because of severe developmental abnormalities, including alterations in glomerular capillary loops, a disorganized GBM, and inability of the foot processes of the podocytes to mature properly [29] (Figure 2). Selective deletion of the integrin α3 subunit in podocytes by crossing the integrin α3 flox mice with podocin-cre leads to massive proteinuria within a week after birth, followed by nephrotic syndrome at 5-6 weeks, and glomerulosclerosis [30]. Interestingly, integrin α3β1 binds CD151 [31], a member of tetraspanin family and this interaction is proposed to increase the strength of integrin α3β1-GBM interaction. In support of this hypothesis, patients with a nonsense mutation in CD151 show glomerular abnormalities, develop glomerulosclerosis, and progress to end stage kidney disease [32]. In addition, mice globally deleted of CD151 develop GBM abnormalities, podocytes dysfunction and glomerulosclerosis, which results in renal failure [30, 33] (Figure 2). Finally, it has been recently shown that selective deletion of CD151 in podocytes reduces integrin α3β1-mediated adhesive strength to laminin in vitro and leads to glomerular nephropathy in vivo [34]. All together, these studies show that 1) integrin α3β1 is the major GBM receptor in podocytes; 2) integrin α3β1 is critical for interactions with matrices (i.e. laminin) or tetraspanin proteins (i.e. CD151); 3) interaction of integrin α3β1 with CD151 is important for regulating the strength of adhesion to laminin; and 4) loss of the integrin α3 subunit or CD151 in podocytes leads to severe glomerular injury and end stage renal disease.
Although integrin α3β1 is the major laminin receptor in podocytes, other laminin receptors are expressed by glomerular cells, including integrins α6β1 and α6β4. However, the role of the integrin α6 or β4 subunit in glomerular homeostasis is difficult to determine, as global integrin α6-null or β4-mice die at birth due to severe skin blistering [35, 36]. The recent generation of mice lacking the integrin β4 subunit specifically in podocytes has ruled out a potential role of this subunit in glomerular homeostasis, as these mice do not have any kidney defects nor show kidney failure [34]. Thus, generation of mice lacking the β4 subunit in other glomerular cells is therefore necessary to address the potential function of this laminin receptor in glomerular homeostasis.
Integrin α8β1
Integrin α8β1 is highly expressed by mesangial cells, binds with high affinity to nephronectin [37] and plays an important role in kidney development and glomerular homeostasis. In this context, loss of the integrin α8 subunit in mice results in different renal phenotypes ranging from renal agenesis to slightly reduced kidney size [38]. Examination of kidneys from integrin α8-null mice revealed hypercellular glomeruli with an increased number of mesangial cells, increased mesangial matrix deposition, and abnormalities in the glomerular capillary networks [39]. The evidence that integrin α8β1 might play a protective role in glomerular injury comes from the observation that hypertensive integrin α8-null mice show more mesangiolysis than hypertensive wild type mice, suggesting that integrin α8β1 is important for glomerular capillary stability [40]. Moreover, diabetic integrin α8-null mice develop more pronounced proteinuria, glomerulosclerosis, mesangial expansion, and glomerular expression of fibrillar collagens compared to diabetic wild type mice [41] (Figure 2). In addition to these in vivo findings, in vitro studies suggest that engagement of integrin α8β1 by fibronectin and vitronectin promotes mesangial cell adhesion, but prevents migration and proliferation of mesangial cells [42]. Thus, integrin α8β1 could play an important role in maintaining glomerular tissue integrity by preventing unwanted mesangial cell proliferation in the course of glomerular injury. Genetic analysis in two different ethnic groups (European and African descent) has been conducted with the hope to understand not only the genomic structure, localization and sequence variation of the integrin α8 gene, but also to possibly enable genetic association studies of integrin α8β1 in kidney disease [43].
Integrins α1β1 and α2β1
Integrins α1β1 and α2β1 are the two major glomerular collagen receptors and they are highly expressed by mesangial cells, endothelial cells and podocytes. Integrin α1β1 binds collagen IV with high affinity, while integrin α2β1 binds preferentially fibrillar collagen, like collagen I [44]. Loss of the integrin α1 or α2 subunit in mice does not affect renal development, as integrin α1-null and integrin α2-null mice are born alive with no obvious renal phenotype [45-47]. However, integrins α1β1 and α2β1 play an important role in the response of the glomerulus to injury. In this context, integrin α1β1 is overexpressed in the proliferating mesangium in glomerulonephritis [48, 49]. In addition, anti-integrin α1 antibodies have been successfully used to reduce scarring in rat models of glomerular injury. This protective effect is achieved by primarily inhibiting integrin α1β1-dependent (VLA-1) leukocyte function with consequent immune response dampening [50]. In contrast to these results, integrin α1-null mice develop more severe glomerulosclerosis following injury characterized by excessive collagen IV deposition and reactive oxygen species (ROS) generation [20, 21]. This data seems to agree with the finding that in non-glomerular cells, integrin α1β1 is required to sense extracellular collagen levels and to downregulate endogenous collagen I synthesis [51]. Using mesangial cell cultures, we showed that integrin α1β1 is a negative regulator of collagen IV synthesis and it does this by downregulating the activation of the pro-fibrotic EGF receptor [52]. In addition, integrin α1β1 exerts its anti-fibrotic role by regulating the level and phosphorylation state of caveolin-1, a scaffolding protein that negatively regulates EGF receptor activation [53, 54].
Similar to integrin α1β1, the function of the major collagen I receptor in the glomerulus, integrin α2β1, in glomerulosclerosis is also controversial. Expression of integrin α2β1 increases in the kidneys of patients with diabetic nephropathy [55] and rapidly progressive glomerulonephritis [56]. However, whether increased expression of this collagen receptor contributes to or it counteracts the development of glomerulosclerosis, is unclear. Integrin α2-null mice develop mild proteinuria at 6 months of age and mild glomerular damage due to increased expression of the pro-fibrotic transforming growth factor (TGF)-β and connective tissue growth factor (CTGF) [57]. Although this result suggests that integrin α2β1 is a negative regulator of glomerulosclerosis, in vitro studies with non-renal cells suggest that integrin α2β1 is a positive regulator collagen I and ROS synthesis [58, 59]. Furthermore, crossing the COL4A3-null mice, a mouse model of Alport disease, with the integrin α2-null mouse results in increased survival, improved renal function and decreased glomerular matrix deposition and scarring [O. Gross, J. Reinhardt, M. Martin, S. Koschnick, M. Weber, G.A. Mueller, R. Girgert. Poster Session: Extracellular Matrix Biology, Fibrosis, and Cell Adhesion, Poster number [TH-PO447], American Society of Nephrology, Philadelphia, 2008]. Recently, we examined the role of integrin α2β1 in regulating ROS-mediated glomerulosclerosis and found that integrin α2-null mice developed significantly less proteinuria and glomerulosclerosis than wild type mice following adriamycin injection (Borza and Pozzi, under revision). In agreement with the observation that loss of integrin α2β1 plays a protective role in glomerular injury, treatment of wild type mice with a selective integrin α2β1 inhibitor [60], decreases albuminuria and glomerular injury following adriamycin injection (Borza and Pozzi, under revision).
Taken together, these studies suggest that the collagen receptors integrin α1β1 and α2β1 exert opposite effects in glomerulosclerosis. Integrin α1β1 negatively regulates collagen synthesis thus preventing excessive glomerular injury, while integrin α2β1 positively regulates collagen synthesis thus contributing to glomerular injury (Figure 2).
Integrins αvβ6 and αvβ8
Integrins αvβ6 and αvβ8 regulate glomerular matrix homeostasis by mediating the activation state of TGF-β. TGF-β is a pro-fibrotic cytokine that influences glomerular cells survival, proliferation and matrix production, thus leading to mesangial expansion and, ultimately, glomerulosclerosis. TGF-β is secreted by podocytes and mesangial cells and its expression is upregulated in various glomerular diseases [61]. Although overexpression of TGF-β alone is sufficient to induce glomerulosclerosis [62], TGF-β has to be activated in order to exert its biological activities, as the levels of active rather than total TGF-β are predictive of fibrosis. TGF-β is usually sequestered in the extracellular matrix in the inactive form and is tightly bound to latency associated peptide (LAP), which alters its conformation and blocks the growth factor from interacting with its receptors [63]. One mechanism of latent TGF-β activation involves interaction of integrins with an RGD sequence in LAP [64]. Although, several αv containing integrins bind LAP in vitro, the phenotypes of the integrin β6-null and β8-null mice indicate that αvβ6 and αvβ8 are the main integrins that activate TGF-β in vivo. TGF-β activation by integrin αvβ6 and αvβ8 is distinct. In the case of integrin αvβ6, TGF-β release and activation from the LAP/TGF-β complex requires binding of integrin αv to the RGD sequence in LAP, association of the integrin β cytoplasmic domain with the cytoskeleton, and contractile force that exposes the active TGF-β [65]. In contrast, integrin αvβ8 activation of TGF-β requires proteolytic cleavage of LAP by membrane-type matrix metalloproteinases (i.e. MT1-MMP) which results in the release of active TGF-β in the surrounding tissue [66].
Integrin αvβ6 is expressed at low levels in epithelial cells, but its expression increases following injury in various organs including the lungs and the kidneys [67]. Mice that lack the integrin β6 subunit have significant inflammation in skin and lungs, but no renal abnormalities [68]. This result suggests that loss of integrin αvβ6 might protect the mice from renal injury. Consistent with this hypothesis, mice that lack the integrin β6 subunit are protected from bleomycin-mediated pulmonary fibrosis [65]. In addition, integrin β6-null mice show reduced renal fibrosis following unilateral ureteral obstruction with concomitant decreased levels of activated TGF-β [69]. Finally, either blocking integrin αvβ6 or β6 deficiency reduces renal fibrosis in the COL4A3 Alport mice [70]. Overall these studies suggest that integrin αvβ6, via activation of TGF-β, contributes to fibrosis and blocking this integrin may be a valuable strategy for the treatment of kidney fibrosis (Figure 2).
In contrast to integrin αvβ6 whose expression increases following renal injury, the glomerular expression of integrin αvβ8 decreases in mouse models of glomerulosclerosis [71]. However, whether its downregulation contributes to or it counteracts the disease is unclear. The observation that integrin β8-null mice develop albuminuria over time suggests that decreased expression of integrin αvβ8 indeed contributes to glomerular injury [72]. The increased albuminuria can be explained by the fact that in the absence of integrin αvβ8, mesangial cells fail to bind and sequester latent TGF-β, thus resulting in increased levels of secreted active TGF-β. Mesangial cells can prevent activation of TGF-β in two different ways: 1) they can bind latent TGF-β via integrin αvβ8; and 2) due to the fact that, when quiescent, they do not express MT1-MMP, they prevent TGF-β activation and release [72]. Despite integrin β8-null mice show increased levels of activated TGF-β and proteinuria, they do not develop glomerular cell damage. This is because these mice can adapt to increased levels of activated TGF-β by overexpressing PECAM-1 in glomerular endothelial cells, which protects them from apoptosis and damage [72] (Figure 2).
In conclusion, in vivo activation of TGF-β by αv containing integrins is complex, tissue and cell specific, thus making it difficult to design selective anti-integrin and/or anti-TGF-β therapy for the treatment of renal disease [73].
Non-integrin receptors and glomerulosclerosis
Non-integrin matrix receptors expressed in the glomerulus include DDRs and dystroglycan. DDR1 and DDR2 constitute a subfamily of receptor tyrosine kinases that function as fibrillar and non-fibrillar collagen receptors [74]. DDRs are single-span transmembrane proteins, with an extracellular domain consisting of an N-terminal discoidin homology domain [75] followed by a region of ~200 amino acids unique to DDRs. The cytoplasmic domain contains an unusually large juxtamembrane domain followed by the C-terminal catalytic tyrosine kinase domain. The DDRs are unique among receptor tyrosine kinases as 1) they are activated by an extracellular matrix component, rather than by growth factors; and 2) unlike traditional receptor tyrosine kinases, DDR autophosphorylation upon ligand binding is unusually slow and sustained [76-78]. DDRs display a broad collagen specificity: whereas both receptors bind fibrillar collagen I, DDR1 preferentially interacts with the non-fibrillar collagen IV [6, 76, 77], while DDR2 preferentially interacts with fibrillar collagens II and X [79, 80].
Several tyrosine residues that are phosphorylated upon collagen binding to DDRs serve as docking sites for adaptor molecules such as Shc and Nck2 [81, 82]. DDRs, like other receptor tyrosine kinases, regulate multiple cellular processes including proliferation, migration and survival and extracellular matrix synthesis [83].
In the kidneys DDR1 is expressed in basolateral membranes of specific nephron segments, from the connecting tubule to the renal papilla [84]. DDR2 is expressed in apical membranes of specific nephron segments, from the loop of Henle to the macula densa [84]. Interestingly, DDR1 is not detectable in the glomeruli of healthy adult kidney, but its expression is upregulated in the glomeruli of rodents undergone partial renal ablation [84]. In contrast, the distribution of DDR2 in remnant kidneys is similar to that in controls [84].
The generation of DDR1-null mice has revealed a pro-fibrotic function for this receptor. In this context, DDR1-null mice are protected from angiotensin II-mediated proteinuria, glomerular fibrosis, and renal inflammation [85]. Moreover, loss of DDR1 delays renal fibrosis and inflammation in a mouse model of Alport syndrome by decreasing TGF-β mediated signaling and reducing the levels of the pro-inflammatory cytokine IL6 [86]. Finally, DDR1-null mice show reduced fibrosis, macrophage infiltration, and pro-inflammatory cytokine production following unilateral ureteral obstruction-mediated tubulointerstitial injury [87]. Since kidneys from injured DDR1-null show reduced levels of pro-inflammatory cytokines and reduced numbers of infiltrating macrophage, it is conceivable that DDR1 might contribute to glomerular fibrosis either directly by controlling matrix homeostasis or indirectly by stimulating renal inflammation. In addition, it is also possible that DDR1 might exert its deleterious functions via cross-talk with integrins. In this regard, DDR-1 has been reported to inhibit integrin α2β1 function in MDCK cells [88], but to augment integrin α2β1 function in pancreatic cancer cells [89], suggesting that the cross-talk between these two receptors is cell type dependent. Whether DDR1 cross-talks with integrin α2β1 in the kidney and whether this cross-talk contributes to glomerular injury is at present unexplored. Analysis of double DDR1/integrin α2-null mice should provide the answer to this question. In conclusion, the studies highlighted above demonstrate that DDR1 activation contributes to kidney injury and suggests that blocking this receptor may have beneficial therapeutic effects.
Another non-integrin matrix receptor is dystroglycan, which consists of an α and β subunit and is an integral membrane component of the dystrophin–glycoprotein complex. The β subunit interacts with the intracellular cytoskeletal proteins, while the α subunit binds to several extracellular ligands such as laminin, agrin, and perlecan. Dystroglycan has been shown to play a major role in the assembly and maintenance of basement membranes [90] and in vitro studies suggest that preventing dystroglycan-laminin interactions prevent branching morphogenesis [91]. In the glomerulus, dystroglycan is highly expressed in podocytes where it facilitates podocyte binding to laminins and agrin in the GBM [92]. As the expression of dystroglycan decreases in minimal change disease, but is unchanged in focal segmental glomerulosclerosis [93], it was thought that the expression of this podocyte receptor could be used as a diagnostic marker to differentiate between glomerular diseases. However, a more recent study comparing the renal expression of dystroglycan in healthy individuals and patients with minimal change disease, membranous glomerulopathy, and lupus nephritis, show no differences in expression between patients vs. control groups [94].
The contribution of this laminin receptor in kidney functions has been recently explored. Mice lacking fukutin, a glycosyltransferase required for the post-translational modification of α-dystroglycan, show flattening of podocyte foot processes, and decreased number of podocytes compared to wild type controls [95]. Although these mice do not develop a severe kidney phenotype (i.e. focal segmental glomerulosclerosis, proteinuria), this study indicates that glycosylation of α-dystroglycan is important for the maintenance of podocyte architecture [95]. This finding agrees with the observation that in two different in vivo models of podocyte-mediated injury, the levels of α-dystroglycan on podocytes decrease with concomitant changes in the fibrillar components of the GBM [96]. As this study suggests that decreased levels of α-dystroglycan might lead to structural changes in the GBM, dystroglycan flox mice have been used to address this issue. Crossing the mice with podocin-cre (to delete dystrogycan selectively in podocytes) or with Pax2-cre mice (to delete dystroglycan in all renal epithelial cells) resulted in mice with no significant renal morphological or functional abnormalities at baseline or following injury [97]. Although surprising, this study suggests that integrins, rather than dystroglycans, are responsible for renal cell stability.
Conclusions
Interactions between glomerular cell receptors with the extracellular matrix are important modulators of glomerular cell function and glomerular response to injury. Glomerular cells express matrix receptors that can either promote or suppress matrix synthesis, thus controlling matrix homeostasis. Although the availability of transgenic mice has allowed us to identify receptors that predispose to or protect from the development of glomerulosclerosis, it is quite difficult to target these receptors in renal disease. One example is offered by integrin α1β1.
Although antibody to integrin α1β1 ameliorates immunologically-mediated glomerular injury [50], we showed that loss of this receptor predisposes mice to ROS-mediated or diabetes-mediated glomerular injury [20, 21]. Thus, the cell type expressing integrin α1β1 (immune cells vs. renal resident cells) dictates the severity of injury after insult. In addition, matrix receptors can cross-talk with growth factor receptors, including the EGF receptor. Blocking and/or activating a matrix receptor might result in activation of the EGF receptor. Although EGF receptor-mediated functions are beneficial in acute kidney injury as they facilitate renal epithelial cell proliferation [98], EGF receptor-mediated functions within the glomerulus promote a pro-fibrotic response by increasing ROS production and subsequent collagen synthesis [20, 52]. Finally, depending on the cell type, a matrix receptor can either up or downregulate another matrix receptor. DDR1, for example, negatively regulates the pro-fibrotic receptor integrin α2β1 in MDCK cells [88], but enhances integrin α2β1-mediated functions in pancreatic cancer cells [89]. Thus, is it conceivable that blocking DDR1 function in the kidney might play both anti- and/or pro-fibrotic action depending on the renal cell type targeted. Despite these obstacles, a better understanding of how cell-matrix interactions regulate glomerulosclerosis is critical for designing strategies to selectively reduce and ideally prevent this devastating disease.
Acknowledgments
These studies were in part supported by a Merit Review from the Department of Veterans Affairs (AP); and the NIH grants: 2P01DK065123 (AP); and the O’Brien P30DK79341-01 (AP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pozzi A, Voziyan PA, Hudson BG, Zent R. Regulation of matrix synthesis, remodeling and accumulation in glomerulosclerosis. Curr Pharm Des. 2009;15:1318–1333. doi: 10.2174/138161209787846748. [DOI] [PubMed] [Google Scholar]
- [2].Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- [5].Calderwood DA, Tuckwell DS, Eble J, Kuhn K, Humphries MJ. The integrin alpha1 A-domain is a ligand binding site for collagens and laminin. J Biol Chem. 1997;272:12311–12317. doi: 10.1074/jbc.272.19.12311. [DOI] [PubMed] [Google Scholar]
- [6].Xu H, Raynal N, Stathopoulos S, Myllyharju J, Farndale RW, Leitinger B. Collagen binding specificity of the discoidin domain receptors: binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1. Matrix Biol. 2011;30:16–26. doi: 10.1016/j.matbio.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- [8].Plaisier E, Chen Z, Gekeler F, Benhassine S, Dahan K, Marro B, Alamowitch S, Paques M, Ronco P. Novel COL4A1 mutations associated with HANAC syndrome: a role for the triple helical CB3[IV] domain. Am J Med Genet A. 2010;152A:2550–2555. doi: 10.1002/ajmg.a.33659. [DOI] [PubMed] [Google Scholar]
- [9].Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, Marro B, Desmettre T, Cohen SY, Roullet E, Dracon M, Fardeau M, Van Agtmael T, Kerjaschki D, Antignac C, Ronco P. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- [10].Van Agtmael T, Schlotzer-Schrehardt U, McKie L, Brownstein DG, Lee AW, Cross SH, Sado Y, Mullins JJ, Poschl E, Jackson IJ. Dominant mutations of Col4a1 result in basement membrane defects which lead to anterior segment dysgenesis and glomerulopathy. Hum Mol Genet. 2005;14:3161–3168. doi: 10.1093/hmg/ddi348. [DOI] [PubMed] [Google Scholar]
- [11].Cosgrove D. Glomerular pathology in Alport syndrome: a molecular perspective. Pediatr Nephrol. 2011 doi: 10.1007/s00467-011-1868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, Wieslander J, Kashtan C, Borza DB, Neilson EG, Wilson CB, Hudson BG. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med. 2010;363:343–354. doi: 10.1056/NEJMoa0910500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miner JH. Laminins and their roles in mammals. Microsc Res Tech. 2008;71:349–356. doi: 10.1002/jemt.20563. [DOI] [PubMed] [Google Scholar]
- [14].Miner JH. Building the glomerulus: a matricentric view. J Am Soc Nephrol. 2005;16:857–861. doi: 10.1681/ASN.2004121139. [DOI] [PubMed] [Google Scholar]
- [15].St John PL, Wang R, Yin Y, Miner JH, Robert B, Abrahamson DR. Glomerular laminin isoform transitions: errors in metanephric culture are corrected by grafting. Am J Physiol Renal Physiol. 2001;280:F695–705. doi: 10.1152/ajprenal.2001.280.4.F695. [DOI] [PubMed] [Google Scholar]
- [16].Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- [17].Matejas V, Hinkes B, Alkandari F, Al-Gazali L, Annexstad E, Aytac MB, Barrow M, Blahova K, Bockenhauer D, Cheong HI, Maruniak-Chudek I, Cochat P, Dotsch J, Gajjar P, Hennekam RC, Janssen F, Kagan M, Kariminejad A, Kemper MJ, Koenig J, Kogan J, Kroes HY, Kuwertz-Broking E, Lewanda AF, Medeira A, Muscheites J, Niaudet P, Pierson M, Saggar A, Seaver L, Suri M, Tsygin A, Wuhl E, Zurowska A, Uebe S, Hildebrandt F, Antignac C, Zenker M. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat. 2010;31:992–1002. doi: 10.1002/humu.21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat Genet. 1995;10:400–406. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- [19].Goldberg S, Adair-Kirk TL, Senior RM, Miner JH. Maintenance of glomerular filtration barrier integrity requires laminin alpha5. J Am Soc Nephrol. 2010;21:579–586. doi: 10.1681/ASN.2009091004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen X, Moeckel G, Morrow JD, Cosgrove D, Harris RC, Fogo AB, Zent R, Pozzi A. Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury. Am J Pathol. 2004;165:617–630. doi: 10.1016/s0002-9440(10)63326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zent R, Yan X, Su Y, Hudson BG, Borza DB, Moeckel GW, Qi Z, Sado Y, Breyer MD, Voziyan P, Pozzi A. Glomerular injury is exacerbated in diabetic integrin alpha1-null mice. Kidney Int. 2006;70:460–470. doi: 10.1038/sj.ki.5000359. [DOI] [PubMed] [Google Scholar]
- [22].Fischer E, Mougenot B, Callard P, Ronco P, Rossert J. Abnormal expression of glomerular basement membrane laminins in membranous glomerulonephritis. Nephrol Dial Transplant. 2000;15:1956–1964. doi: 10.1093/ndt/15.12.1956. [DOI] [PubMed] [Google Scholar]
- [23].Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- [24].Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- [26].Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol. 2008;316:288–301. doi: 10.1016/j.ydbio.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, Lebleu VS, Gattone VH, Jr., Kalluri R. Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol. 2008;313:584–593. doi: 10.1016/j.ydbio.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Adler S. Characterization of glomerular epithelial cell matrix receptors. Am J Pathol. 1992;141:571–578. [PMC free article] [PubMed] [Google Scholar]
- [29].Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- [30].Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol. 2006;175:33–39. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sterk LM, Geuijen CA, van den Berg JG, Claessen N, Weening JJ, Sonnenberg A. Association of the tetraspanin CD151 with the laminin-binding integrins alpha3beta1, alpha6beta1, alpha6beta4 and alpha7beta1 in cells in culture and in vivo. J Cell Sci. 2002;115:1161–1173. doi: 10.1242/jcs.115.6.1161. [DOI] [PubMed] [Google Scholar]
- [32].Crew V. Karamatic, Burton N, Kagan A, Green CA, Levene C, Flinter F, Brady RL, Daniels G, Anstee DJ. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood. 2004;104:2217–2223. doi: 10.1182/blood-2004-04-1512. [DOI] [PubMed] [Google Scholar]
- [33].Baleato RM, Guthrie PL, Gubler MC, Ashman LK, Roselli S. Deletion of CD151 results in a strain-dependent glomerular disease due to severe alterations of the glomerular basement membrane. Am J Pathol. 2008;173:927–937. doi: 10.2353/ajpath.2008.071149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sachs N, Claessen N, Aten J, Kreft M, Teske GJ, Koeman A, Zuurbier CJ, Janssen H, Sonnenberg A. Blood pressure influences end-stage renal disease of Cd151 knockout mice. J Clin Invest. 2011 doi: 10.1172/JCI58878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- [36].van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- [37].Sato Y, Uemura T, Morimitsu K, Sato-Nishiuchi R, Manabe R, Takagi J, Yamada M, Sekiguchi K. Molecular basis of the recognition of nephronectin by integrin alpha8beta1. J Biol Chem. 2009;284:14524–14536. doi: 10.1074/jbc.M900200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Muller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997;88:603–613. doi: 10.1016/s0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Haas CS, Amann K, Schittny J, Blaser B, Muller U, Hartner A. Glomerular and renal vascular structural changes in alpha8 integrin-deficient mice. J Am Soc Nephrol. 2003;14:2288–2296. doi: 10.1097/01.asn.0000082999.46030.fe. [DOI] [PubMed] [Google Scholar]
- [40].Hartner A, Cordasic N, Klanke B, Muller U, Sterzel RB, Hilgers KF. The alpha8 integrin chain affords mechanical stability to the glomerular capillary tuft in hypertensive glomerular disease. Am J Pathol. 2002;160:861–867. doi: 10.1016/s0002-9440(10)64909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hartner A, Cordasic N, Menendez-Castro C, Volkert G, Yabu JM, Kupraszewicz-Hutzler M, Rascher W, Hilgers KF. Lack of {alpha}8-integrin aggravates podocyte injury in experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2010;299:F1151–1157. doi: 10.1152/ajprenal.00058.2010. [DOI] [PubMed] [Google Scholar]
- [42].Bieritz B, Spessotto P, Colombatti A, Jahn A, Prols F, Hartner A. Role of alpha8 integrin in mesangial cell adhesion, migration, and proliferation. Kidney Int. 2003;64:119–127. doi: 10.1046/j.1523-1755.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- [43].Ekwa-Ekoka C, Diaz GA, Carlson C, Hasegawa T, Samudrala R, Lim KC, Yabu JM, Levy B, Schnapp LM. Genomic organization and sequence variation of the human integrin subunit alpha8 gene (ITGA8) Matrix Biol. 2004;23:487–496. doi: 10.1016/j.matbio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- [44].Heino J. The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biol. 2000;19:319–323. doi: 10.1016/s0945-053x(00)00076-7. [DOI] [PubMed] [Google Scholar]
- [45].Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- [46].Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Holtkotter O, Nieswandt B, Smyth N, Muller W, Hafner M, Schulte V, Krieg T, Eckes B. Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 2002;277:10789–10794. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- [48].Kuhara T, Kagami S, Kuroda Y. Expression of beta 1-integrins on activated mesangial cells in human glomerulonephritis. J Am Soc Nephrol. 1997;8:1679–1687. doi: 10.1681/ASN.V8111679. [DOI] [PubMed] [Google Scholar]
- [49].Shikata K, Makino H, Morioka S, Kashitani T, Hirata K, Ota Z, Wada J, Kanwar YS. Distribution of extracellular matrix receptors in various forms of glomerulonephritis. Am J Kidney Dis. 1995;25:680–688. doi: 10.1016/0272-6386(95)90542-1. [DOI] [PubMed] [Google Scholar]
- [50].Cook HT, Khan SB, Allen A, Bhangal G, Smith J, Lobb RR, Pusey CD. Treatment with an antibody to VLA-1 integrin reduces glomerular and tubulointerstitial scarring in a rat model of crescentic glomerulonephritis. Am J Pathol. 2002;161:1265–1272. doi: 10.1016/S0002-9440(10)64403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gardner H, Broberg A, Pozzi A, Laato M, Heino J. Absence of integrin alpha1beta1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci. 1999;112(Pt 3):263–272. doi: 10.1242/jcs.112.3.263. [DOI] [PubMed] [Google Scholar]
- [52].Chen X, Abair TD, Ibanez MR, Su Y, Frey MR, Dise RS, Polk DB, Singh AB, Harris RC, Zent R, Pozzi A. Integrin alpha1beta1 controls reactive oxygen species synthesis by negatively regulating epidermal growth factor receptor-mediated Rac activation. Mol Cell Biol. 2007;27:3313–3326. doi: 10.1128/MCB.01476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chen X, Whiting C, Borza C, Hu W, Mont S, Bulus N, Zhang MZ, Harris RC, Zent R, Pozzi A. Integrin alpha1beta1 regulates epidermal growth factor receptor activation by controlling peroxisome proliferator-activated receptor gamma-dependent caveolin-1 expression. Mol Cell Biol. 2010;30:3048–3058. doi: 10.1128/MCB.00892-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Borza CM, Chen X, Mathew S, Mont S, Sanders CR, Zent R, Pozzi A. Integrin {alpha}1{beta}1 promotes caveolin-1 dephosphorylation by activating T cell protein-tyrosine phosphatase. J Biol Chem. 2010;285:40114–40124. doi: 10.1074/jbc.M110.156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jin DK, Fish AJ, Wayner EA, Mauer M, Setty S, Tsilibary E, Kim Y. Distribution of integrin subunits in human diabetic kidneys. J Am Soc Nephrol. 1996;7:2636–2645. doi: 10.1681/ASN.V7122636. [DOI] [PubMed] [Google Scholar]
- [56].Baraldi A, Zambruno G, Furci L, Ballestri M, Tombesi A, Ottani D, Lucchi L, Lusvarghi E. Beta 1 and beta 3 integrin upregulation in rapidly progressive glomerulonephritis. Nephrol Dial Transplant. 1995;10:1155–1161. [PubMed] [Google Scholar]
- [57].Girgert R, Martin M, Kruegel J, Miosge N, Temme J, Eckes B, Muller GA, Gross O. Integrin alpha2-deficient mice provide insights into specific functions of collagen receptors in the kidney. Fibrogenesis Tissue Repair. 2010;3:19. doi: 10.1186/1755-1536-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ivaska J, Reunanen H, Westermarck J, Koivisto L, Kahari VM, Heino J. Integrin alpha2beta1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the alpha2 cytoplasmic tail. J Cell Biol. 1999;147:401–416. doi: 10.1083/jcb.147.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Honore S, Kovacic H, Pichard V, Briand C, Rognoni JB. Alpha2beta1-integrin signaling by itself controls G1/S transition in a human adenocarcinoma cell line (Caco-2): implication of NADPH oxidase-dependent production of ROS. Exp Cell Res. 2003;285:59–71. doi: 10.1016/s0014-4827(02)00038-1. [DOI] [PubMed] [Google Scholar]
- [60].Miller MW, Basra S, Kulp DW, Billings PC, Choi S, Beavers MP, McCarty OJ, Zou Z, Kahn ML, Bennett JS, DeGrado WF. Small-molecule inhibitors of integrin alpha2beta1 that prevent pathological thrombus formation via an allosteric mechanism. Proc Natl Acad Sci U S A. 2009;106:719–724. doi: 10.1073/pnas.0811622106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lee HS. Pathogenic role of TGF-beta in the progression of podocyte diseases. Histol Histopathol. 2011;26:107–116. doi: 10.14670/HH-26.107. [DOI] [PubMed] [Google Scholar]
- [62].Isaka Y, Fujiwara Y, Ueda N, Kaneda Y, Kamada T, Imai E. Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest. 1993;92:2597–2601. doi: 10.1172/JCI116874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-beta structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- [66].Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, Gillett N, Sheppard D, Matthay MA, Albelda SM, Kramer RH, Pytela R. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108(Pt 6):2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- [68].Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr., Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(−/−) mice. Am J Pathol. 2003;163:1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Wang L. Chun, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF, 3rd, Sheppard D, Gardner HA, Violette SM. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol. 2007;170:110–125. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lakhe-Reddy S, Khan S, Konieczkowski M, Jarad G, Wu KL, Reichardt LF, Takai Y, Bruggeman LA, Wang B, Sedor JR, Schelling JR. Beta8 integrin binds Rho GDP dissociation inhibitor-1 and activates Rac1 to inhibit mesangial cell myofibroblast differentiation. J Biol Chem. 2006;281:19688–19699. doi: 10.1074/jbc.M601110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Khan S, Lakhe-Reddy S, McCarty JH, Sorenson CM, Sheibani N, Reichardt LF, Kim JH, Wang B, Sedor JR, Schelling JR. Mesangial cell integrin alphavbeta8 provides glomerular endothelial cell cytoprotection by sequestering TGF-beta and regulating PECAM-1. Am J Pathol. 2011;178:609–620. doi: 10.1016/j.ajpath.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pozzi A, Zent R. TGF-beta Sequestration by Mesangial Cell Integrin alphavbeta8 A Novel Mechanism of Glomerular Endothelial Cell Regulation. Am J Pathol. 2011;178:485–489. doi: 10.1016/j.ajpath.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26:146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- [75].Baumgartner S, Hofmann K, Chiquet-Ehrismann R, Bucher P. The discoidin domain family revisited: new members from prokaryotes and a homology-based fold prediction. Protein Sci. 1998;7:1626–1631. doi: 10.1002/pro.5560070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- [77].Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- [78].C G. L’Hote, Thomas PH, Ganesan TS. Functional analysis of discoidin domain receptor 1: effect of adhesion on DDR1 phosphorylation. FASEB J. 2002;16:234–236. doi: 10.1096/fj.01-0414fje. [DOI] [PubMed] [Google Scholar]
- [79].Leitinger B, Steplewski A, Fertala A. The D2 period of collagen II contains a specific binding site for the human discoidin domain receptor, DDR2. J Mol Biol. 2004;344:993–1003. doi: 10.1016/j.jmb.2004.09.089. [DOI] [PubMed] [Google Scholar]
- [80].Leitinger B, Kwan AP. The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol. 2006;25:355–364. doi: 10.1016/j.matbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- [81].Ikeda K, Wang LH, Torres R, Zhao H, Olaso E, Eng FJ, Labrador P, Klein R, Lovett D, Yancopoulos GD, Friedman SL, Lin HC. Discoidin domain receptor 2 interacts with Src and Shc following its activation by type I collagen. J Biol Chem. 2002;277:19206–19212. doi: 10.1074/jbc.M201078200. [DOI] [PubMed] [Google Scholar]
- [82].Koo DH, McFadden C, Huang Y, Abdulhussein R, Friese-Hamim M, Vogel WF. Pinpointing phosphotyrosine-dependent interactions downstream of the collagen receptor DDR1. FEBS Lett. 2006;580:15–22. doi: 10.1016/j.febslet.2005.11.035. [DOI] [PubMed] [Google Scholar]
- [83].Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: an update on discoidin domain receptor function. Cell Signal. 2006;18:1108–1116. doi: 10.1016/j.cellsig.2006.02.012. [DOI] [PubMed] [Google Scholar]
- [84].Lee R, Eidman KE, Kren SM, Hostetter TH, Segal Y. Localization of discoidin domain receptors in rat kidney. Nephron Exp Nephrol. 2004;97:e62–70. doi: 10.1159/000078407. [DOI] [PubMed] [Google Scholar]
- [85].Flamant M, Placier S, Rodenas A, Curat CA, Vogel WF, Chatziantoniou C, Dussaule JC. Discoidin domain receptor 1 null mice are protected against hypertension-induced renal disease. J Am Soc Nephrol. 2006;17:3374–3381. doi: 10.1681/ASN.2006060677. [DOI] [PubMed] [Google Scholar]
- [86].Gross O, Girgert R, Beirowski B, Kretzler M, Kang HG, Kruegel J, Miosge N, Busse AC, Segerer S, Vogel WF, Muller GA, Weber M. Loss of collagen-receptor DDR1 delays renal fibrosis in hereditary type IV collagen disease. Matrix Biol. 2010;29:346–356. doi: 10.1016/j.matbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- [87].Guerrot D, Kerroch M, Placier S, Vandermeersch S, Trivin C, Mael-Ainin M, Chatziantoniou C, Dussaule JC. Discoidin domain receptor 1 is a major mediator of inflammation and fibrosis in obstructive nephropathy. Am J Pathol. 2011;179:83–91. doi: 10.1016/j.ajpath.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wang CZ, Su HW, Hsu YC, Shen MR, Tang MJ. A discoidin domain receptor 1/SHP-2 signaling complex inhibits alpha2beta1-integrin-mediated signal transducers and activators of transcription 1/3 activation and cell migration. Mol Biol Cell. 2006;17:2839–2852. doi: 10.1091/mbc.E05-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol. 2008;180:1277–1289. doi: 10.1083/jcb.200708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- [91].Pozzi A, Zent R. Extracellular matrix receptors in branched organs. Curr Opin Cell Biol. 2011 doi: 10.1016/j.ceb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Vogtlander NP, Dijkman H, Bakker MA, Campbell KP, van derVlag J, Berden JH. Localization of alpha-dystroglycan on the podocyte: from top to toe. J Histochem Cytochem. 2005;53:1345–1353. doi: 10.1369/jhc.4A6596.2005. [DOI] [PubMed] [Google Scholar]
- [93].Regele HM, Fillipovic E, Langer B, Poczewki H, Kraxberger I, Bittner RE, Kerjaschki D. Glomerular expression of dystroglycans is reduced in minimal change nephrosis but not in focal segmental glomerulosclerosis. J Am Soc Nephrol. 2000;11:403–412. doi: 10.1681/ASN.V113403. [DOI] [PubMed] [Google Scholar]
- [94].Vogtlander NP, van derVlag J, Bakker MA, Dijkman HB, Wevers RA, Campbell KP, Wetzels JF, Berden JH. Expression of sialidase and dystroglycan in human glomerular diseases. Nephrol Dial Transplant. 2010;25:478–484. doi: 10.1093/ndt/gfp465. [DOI] [PubMed] [Google Scholar]
- [95].Kojima K, Nosaka H, Kishimoto Y, Nishiyama Y, Fukuda S, Shimada M, Kodaka K, Saito F, Matsumura K, Shimizu T, Toda T, Takeda S, Kawachi H, Uchida S. Defective glycosylation of alpha-dystroglycan contributes to podocyte flattening. Kidney Int. 2011;79:311–316. doi: 10.1038/ki.2010.403. [DOI] [PubMed] [Google Scholar]
- [96].Kojima K, Davidovits A, Poczewski H, Langer B, Uchida S, Nagy-Bojarski K, Hovorka A, Sedivy R, Kerjaschki D. Podocyte flattening and disorder of glomerular basement membrane are associated with splitting of dystroglycan-matrix interaction. J Am Soc Nephrol. 2004;15:2079–2089. doi: 10.1097/01.ASN.0000133531.43177.21. [DOI] [PubMed] [Google Scholar]
- [97].Jarad G, Pippin JW, Shankland SJ, Kreidberg JA, Miner JH. Dystroglycan does not contribute significantly to kidney development or function, in health or after injury. Am J Physiol Renal Physiol. 2011;300:F811–820. doi: 10.1152/ajprenal.00725.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wang Z, Chen JK, Wang SW, Moeckel G, Harris RC. Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J Am Soc Nephrol. 2003;14:3147–3154. doi: 10.1097/01.asn.0000098681.56240.1a. [DOI] [PubMed] [Google Scholar]