Abstract
Costameres are sub-membranous, Z-line associated structures found in striated muscle. They have been shown to have important roles in transmission of force from the sarcomere to the sarcolemma and extracellular matrix, maintaining mechanical integrity of the sarcolemma, and orchestrating mechanically related signaling. The costamere is akin to the more well-known focal adhesion complex present in most cells. The Z-line is a critical structural anchor for the sarcomere, but it is also a hot-spot for muscle cell signaling. Therefore functionally, the costamere represents a two-way signaling highway tethered between the Z-line and the extracellular matrix, relaying mechanical stress signals from outside the cell to intracellular signaling networks. In this role it can modulate myofibril growth and contraction. The major force generated by sarcomeres is transduced in the lateral direction from the sarcomere to the extracellular matrix through the costamere.
Two major protein complexes have been described at the costamere: the dystrophin–glycoprotein complex and the integrin–vinculin–talin complex. The importance of these two protein complexes in striated muscle function has between demonstrated both in human disease and mouse models. Members of the dystrophin glycoprotein complex and integrins have both been reported to interact directly with filamin-C, thus linking costameric complexes with those present at the Z-line. Moreover, studies from our labs and others have shown that the Z-line proteins belonging to the PDZ-LIM domain protein family, enigma homolog (ENH) and cypher, may directly or indirectly be involved in this linkage. The following review will focus on the protein components of this linkage, their function in force transmission, and how the dysfunction or loss of proteins within these complexes contributes to muscular disease.
Keywords: Z-line, Cypher/ZASP, Costamere, Mechanotransduction, Integrin, Vinculin
1. Introduction
The mechanical force we use to move our bodies is generated by the contraction of skeletal muscle fibers, while at the same time our hearts keep pumping blood through our circulatory system by way of cardiac muscle contraction. At the ultrastructural level, the force necessary for both cardiac and skeletal muscle contraction is generated by the shortening of individual sarcomeres. Force transmission in muscle can occur either parallel to or lateral to the long axis of the sarcomere [1]. In the longitudinal direction, force is transduced from one sarcomere to the next within the same fiber until the end of fiber is reached. Perpendicular, or lateral force transmission, allows for transduction from one myofibril to a neighboring myofibril until it reaches the costameric complex which channels the intracellular force across the sarcolemma to the extracellular matrix (ECM). Recent experiments have shown that longitudinal force transmission accounts for only 20– 30% of force generated by sarcomeres, indicating that the major force vector occurs laterally within striated muscle1. This suggests that the location of the costamere makes it critical for its central role in force transmission. Moreover, signals which produce physiological or pathological growth of myocytes following alterations of mechanical load are received by the cellular membrane and subsequently transmitted to subcellular domains including costameres [2,3]. As evidence of the importance of the costamere in muscle function, recent data has linked mutations in a large number of costameric proteins to cardiomyopathy or myofibrillar myopathy in humans and mice [4–9]. This review will provide detailed information regarding the communi-cation of mechanical forces and signals from the sarcomere to the ECM, through this important submembranous structure, the costamere.
2. The Z-line and cardiomyopathy
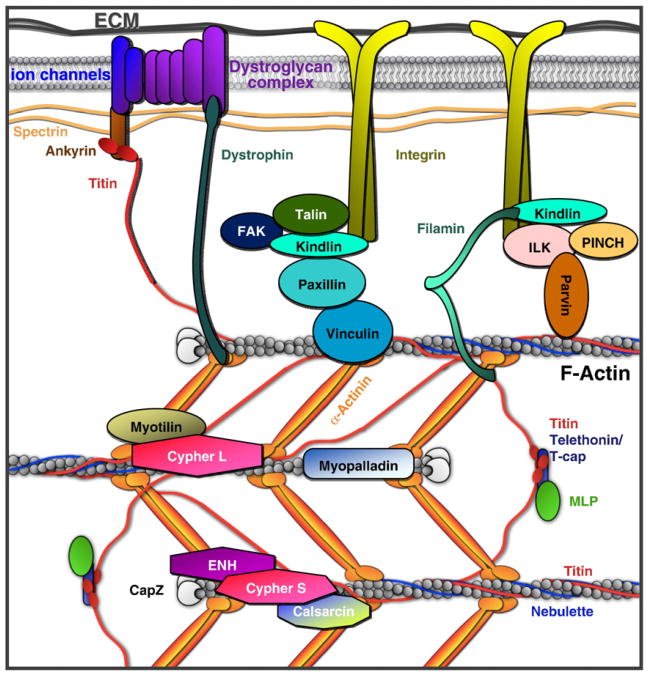
Cardiomyopathy is a general term used to describe heart disease caused by any cardiac muscle defect. Hypertrophic, dilated, and restrictive cardiomyopathies are the major forms of cardiomyopathy. Mutations in sarcomeric and cytoskeletal proteins have been linked to all types of cardiomyopathies in both humans and mice. This review will focus primarily on cardiomyopathies associated with the Z-line and costameric proteins. A detailed description of all sarcomeric/cytoskeletal-linked cardiomyopathies is outside the scope of the current review article, but we refer the reader to some recent review papers on this topic [4–9] (Fig. 1).
Fig. 1.
Costameric proteins associated with Z-lines. Dystrophin glycoprotein complex and integrin–vinculin–talin complex are two major costameric protein complexes. Members of the dystrophin glycoprotein complex and integrin were reported to directly interact with protein components at the Z-line, such as filamin-C. Filamin-C (γ-filamin, filamin2) physically links the costamere and the sarcomere by interacting with two major costameric protein complexes: the sarcoglycans in the dystrophin glycoprotein complex and integrin. In addition, filamin-C interacts with the Z-line proteins calsarcin-1 and myotilin. Vinculin, the founding member of the costamere, interacts with multiple proteins including talin, paxillin, and α-actinin. Integrin interacts with the ILK-pinch-parvin complex and FAK. Dystrophin binds to ankyrin B and G, which are essential to organize the dystrophin and dystroglycan complex. Titin is the largest known protein and crosses longitudinally from the Z-line to the M-line. The N-terminus of titin at the Z-line binds to ankyrin. Not all costameric proteins or Z-line proteins are illustrated in this figure for simplicity. Key proteins are shown to highlight the bridges between the sarcomere and the costamere.
The sarcomere is the basic structural unit of striated muscle [10]. There are three major components important for proper functioning of the contractile machinery of the sarcomere: the thick filament, the thin filament, and titin. The thick filament is composed of myosin and other accessory proteins, such as myosin binding protein C (MyBPc). The thin filament is assembled mainly from actin monomers, though other proteins such as troponin and tropomyosin are also essential for this system. The thin filaments connect to the Z-line which constitutes the border between two sarcomeres [11,12]. The giant protein titin crosses from Z-line to M-line and functions as a spring and ruler for the contractile apparatus. Titin is associated with another large protein, nebulin in skeletal muscle [13].
When viewed by electron microscopy (EM), the Z-line, also referred to as the Z-band or Z-disc, appears as an electron dense structure due to its multiple highly ordered protein complexes. Recent research has shown the Z-line is not only a structural anchor responsible for transmitting force between sarcomeres during muscular contraction, but is also a hot-spot for cellular signaling [14,15]. Moreover, the Z-line connects myofibrils to the sarcolemmal membrane via the costamere and ultimately transmits information to or from the ECM [16–18]. A large number of Z-line, Z-line associated, and costameric proteins have been identified and mutations in many of these have been linked to cardiac and skeletal myopathies in humans and mice [4,6,17,19]. One such protein is cypher.
Cypher is a member of the PDZ-LIM domain protein family [20,21]. It localizes to the Z-line through direct protein interactions with proteins such as α-actinin, calsarcin, and myotilin [20,22,23]. Through generation of several cypher gene targeted mouse lines, we have shown cypher is essential for the integrity of Z-line structure [21,22,24]. In man, the cypher gene is also known as Z-band alternatively spliced PDZ-motif protein, ZASP. As an extension of the data obtained from the various mouse models, more than sixteen ZASP mutations have been associated with cardiomyopathy or myofibril myopathy. The disease associated with these mutations has been termed ZASPopathy or ZASP-related myofibril myopathy [17,25–29]. However, the mechanism by which cypher mutations lead to cardio- or skeletal-myopathies is still unclear [5,30].
Similar to cypher, another member of PDZ-LIM domain protein family, enigma homolog protein (ENH) (also called PDZ and LIM domain 5 protein, PDLIM5), also localizes to the Z-line [31–33]. Recently, we showed ENH forms a protein complex at Z-lines with calsarcin-1 and cypher, and that this complex is destabilized in ENH-null mouse hearts [34,35]. Loss of the ENH-cypher-calsarcin protein complex further disturbed costameric protein complexes as shown by the compensatory increased expression of both integrin and members of dystrophin–glycoprotein complex. In the future, it will be very interesting to see if mutations in ENH are associated with human cardiomyopathies.
3. Costamere and cardiomyopathy
The costamere is a submembranous structure in striated muscle, composed of two major protein complexes: the dystrophin–glycoprotein complex and the integrin-vinculin-talin complex. It has characteristics of the focal adhesion of other cell types [36]. The costamere was originally described as an “orthogonal lattice” using immunofluorescence images of skeletal muscle which had been stained with an antibody specific for the focal-adhesion protein, vinculin [36]. Since their discovery, numerous proteins have been found to localize to the costamere [16]. In general, costameres are thought to serve a mechanical role by transmitting forces bidirectionally from the sarcomere to the sarcolemma. They also act as important sites of cellular signaling transmitting signals from the extracellular environment to intracellular signaling networks (outside-in signaling) [37] while also functioning to transmit signals to the extracellular environment from inside the cell (inside-out signaling)[38]. Moreover, costameric proteins such as the dystrophin-glycoprotein complex are thought to have an important role in maintaining the structural integrity of the sarcolemma during muscular contraction.
4. The dystrophin-glycoprotein complex
Integral and peripheral membrane proteins of the dystrophin–glycoprotein complex, or DGC, function to provide a physical linkage across the muscle membrane to connect the ECM with the F-actinbased cytoskeleton [39,40]. The proteins classified as core components of the DGC include dystrophin, the sarcoglycans, sarcospan, dystroglycan, and syntrophin [41–45]. Laminin in the ECM binds to α-dystroglycan, a peripheral membrane protein located on the extracellular face of the sarcolemma [46]. In addition to its interaction with β-dystroglycan, α-dystroglycan is stabilized at the membrane by the sarcoglycan-sarcospan subcomplex [47]. Dystrophin binds both actin and β-dystroglycan thus anchoring the transmembrane components of the DGC to the cytoskeleton [48–50]. The sarcoglycan subcomplex is comprised of four single-pass transmembrane glycoproteins, referred to as α-, β- γ-, and δ-sarcoglycans [51]. Sarcospan, a tetraspan-like protein, forms a tight subcomplex with the sarcoglycans and together the sarcoglycan-sarcospan subcomplex serves to stabilize α-dystroglycan's association with the β-dystroglycan [39,52,53].
Loss of DGC core components alters the structural integrity of the sarcolemma, resulting in progressive contraction induced damage. Skeletal- and cardio-myopathies associated with mutations in core DGC components and DGC associated proteins, underscore the importance of maintaining stable protein-protein interactions within this complex. Duchenne muscular dystrophy is caused by mutations in the dystrophin gene resulting in progressive muscle wasting and eventually can result in cardiac and/or respiratory failure [54,55]. Loss of functional dystrophin results in destabilization of the other DGC components and ultimately alters the sarcolemmal localization of the entire complex. In addition to mutations in the dystrophin protein, its proteolytic cleavage by enteroviral protease 2A, has been implicated in the pathophysiology of viral myocarditis [56]. Also, the absence of dystrophin from muscle cells increases sarcolemmal permeability and subsequent susceptibility to viral infection [57]. These findings further underscore the importance of this protein in the cardiovascular system. Autosomal recessive limb-girdle muscular dystrophy types 2D-2F are caused by primary mutations in α-, β-, γ-, and δ-sarcoglycans genes, respectively [51]. Genetic mutation of any one of the sarcoglycans generally results in loss of the entire sarcoglycan-sarcospan subcomplex, destabilization of α-dystroglycan from the sarcolemma, and contraction induced damage to the myofiber [47,52]. The genetic and acquired disorders caused by disruption of members of the dystrophin–glycoprotein complex highlight the importance of this complex in maintaining the structural integrity of both skeletal and cardiac muscle.
In addition to the core components of the DGC, abundant DGC-associated proteins have been discovered based on immunofloures-cence colocalization, co-immunoprecipitation, and in vitro binding assays. A growing number of these proteins localize to the costamere and Z-disk. One such Z-disk protein, filamin-C, has been shown to directly interact with both δ- and -γ-sarcoglycan [58]. Similar to dystrophin, filamin-C is an actin-binding protein which has been shown to interact with many muscle proteins involved in muscular dystrophies. Mutations discovered in filamin-C have also been linked to myofibrillar myopathy in patients [59–61]. Disruption of filamin-C in mice results in respiratory failure and death shortly after birth with notable defects in myogenesis [62]. Although filamin-C has been shown to directly interact with myotilin, δ- and γ-sarcoglycans, and myozenin, localization of these proteins is rarely altered in the absence of filamin-C thus indicating localization of these costameric and Z-disk proteins is independent of their interaction with filamin-C. The number of DGC associated proteins, including filamin-C, which are important for maintaining myofibrillar structure is increasing rapidly and we are only beginning to understand the importance of many of these interactions.
5. Integrin-vinculin-talin complex
The second major complex present at the costamere in striated muscle is the integrin-vinculin-talin complex. Both vinculin and talin are cytoskeletal proteins which are tethered at the costamere through their interaction with integrin. As mentioned above, it was the staining pattern of the focal adhesion marker vinculin in skeletal muscle, which first defined the costamere in striated muscle [36]. While costameres appear as doublets flanking the Z-line in skeletal muscle cells [36], in cardiomyocytes they lie directly above the Z-line therefore giving the appearance of a single band [63]. Since the initial characterization of vinculin, many studies have been conducted analyzing the function of this protein in striated muscle. It has been demonstrated that vinculin and vinculin-containing complexes are essential for the normal hemodynamic stress response of heart. Mice with global ablation of the vinculin gene died by embryonic day 10, while work by our group showed that heterozygous vinculin knockout mice survived to adulthood and were basally normal but were more susceptible to mechanically-mediated dysfunction, such as that produced by pressure overload of the left ventricle [64,65]. Furthermore, when we constructed mice with cardiomyocyte-specific deletion of the vinculin gene, they displayed a dilated cardiomyopathy with conduction defects developing in early adulthood without provocation [66]. In humans, patients with both dilated and hypertrophic cardiomyopathy were found to have mutations in the vinculin muscle-specific isoform, metavinculin [67]. Together these data support that vinculin is important for normal heart function.
Integrins are glycosylated, heterodimeric, transmembrane proteins which function as bidirectional signal transducers within the cell membrane [68]. In eukaryotic organisms, there are eighteen α- and eight β-integrin subunits which non-covalently heterodimerize to form twenty-four distinct integrin complexes [68]. The extracellular domains of integrin complexes interact with ligands present in the ECM while their short cytoplasmic tails interact with actin associated adaptor proteins, such as talin, vinculin, and kindlin. Similar to the DGC, integrins have also been shown to interact directly with Z-disk proteins such as filamin-C [69]. Since integrins do not themselves possess kinase activity, they signal through several downstream cytoplasmic kinases. These include focal adhesion kinase (FAK) as well as integrin-linked kinase (ILK) [68,70,71]. Thus, integrin complexes are poised to serve as critical mediators for a variety of cellular processes including cell adhesion, migration, and survival.
The function of integrin complexes in striated muscle, specifically in the myocyte, has been analyzed by several groups using both in vitro [72] and in vivo [72–74] studies. In adult cardiomyocytes, α7β1 heterodimers are dominantly expressed. In the developing myocardium, α5β1 and α6β1 are also expressed [75]. In vivo analysis revealed global deletion of the β1-integrin in mice resulted in lethality shortly after implantation [76,77], while our own work showed cardiac-specific deletion of β1-integrin lead to the development of dilated cardiomyopathy and a marked increase in DGC protein expression [78]. Interestingly, cardiac-specific ablation of β1-integrin in dystrophin-deficient (mdx) mice leads to female peri- and postpartum mice having increased mortality with increased myocardial necrosis, fibrosis, and calcification [79].
As mentioned above, integrins transduce signals through a range of signaling molecules, including FAK and ILK. In addition to its interaction with integrin, ILK has been shown to interact with pinch and parvin to form the important ILK-pinch-parvin (IPP) protein complex [80]. The importance of this complex is evident in the numerous mouse models which have been developed. ILK global knockout mice die around the time of implantation [81], similar to the global β1-integrin KO mice. In addition, cardiac-specific ablation of ILK causes dilated cardiomyopathy and sudden death [82]. In contrast, cardiac-restricted transgenic overexpression of ILK induces hypertrophy [83]. In our laboratory, we have demonstrated that pinch1 is essential for embryonic development, and in cardiomyocytes, the function of pinch1 and pinch2 are redundant but indispensable for the structure of costamere [84–87].
Another important integrin-associated kinase is FAK. Several mouse models have been used to analyze the function of FAK [88–94]. Global ablation of FAK results in early embryonic lethality [95]. When cardiac-specific ablation of FAK was accomplished using either a myosin light chain-2a (MLC2a) or a Nxk2.5 Cre-recombinase, mice displayed early embryonic lethality marked by thin ventricular walls and ventricular septal defects (MLC-2a Cre) or perinatal mortality with subaortic VSDs and outflow tract malalignment (Nkx2.5 Cre) [89,92]. In contrast, when ablation was effected with Cre-recombinase producing mice which caused later or less potent gene excision, mice were liveborn but had impaired responses to hemodynamic loading or eccentric hypertrophy when stimulated by angiotensin II [88,92].
6. ENH-cypher-calsarcin protein complex
Recently, our laboratory has characterized a newly emerging Z-line protein complex which may directly interact with the costamere: the ENH-cypher-calsarcin protein complex. In order to examine the function of ENH, we generated both global and cardiac-specific ENH knockout mouse models. Both of these mouse models developed dilated cardiomyopathy and were associated with widening of the Z-disk. Because of the disruption of the Z-disk, we began analyzing closely associated Z-disk proteins. In wild-type muscle, we found ENH is associated with cypher and calsarcin. Notably, the short and long cypher isoforms were present in different protein complexes and are thus differentially altered upon ENH disruption. The short cypher isoform was found to be associated with ENH and calsarcin while the long cypher isoform was found to be associated with myotilin. While the protein expression of the short cypher isoform and calsarcin was decreased in ENH-null mice, the expression of the long cypher isoform and myotilin were increased [34]. Calsarcin interacts with filamin-C [69], which has been shown interacts with integrin [69] and with both -γ and δ sarcoglycan [58]. Thus, the ENH-cypher-calsarcin complex at the Z-line is likely to play an important role in linking the Z-line to the extracellular matrix via filamin-C. The observed up-regulation of filamin-C, members of the DGC, and integrin in ENH mutants are likely a consequence of a compensatory mechanism due to disruption of the connection between the Z-line and the extracellular matrix [34].
Interestingly, Jani and Schock showed disruption of dZASP, the only Drosophila Alp/Enigma PDZ-LIM domain protein, depletes integrin adhesion sites [96]. In addition, they showed dZASP co-localizes with integrins in Drosophila tissues and directly binds to α-actinin. Most recently, Rui and his colleagues found that in Drosophila dZASP, similar to integrin, is highly enriched at muscle attachment sites where they serve as tension sensors between the sarcolemma and the sarcomere [97]. Fly larvae lacking functional dZASP do not form Z-lines or successfully recruit α-actinin to the Z-line. This phenotype is similar to that the phenotype that of integrin-deficient flies [96]. While this data does not show a direct interaction between integrin and dZASP, it does implicate both integrin and ZASP as crucial mediators for maintaining muscle integrity. Only future experiments will be able to determine if there is indeed a direct interaction between integrin and members of the PDZ-LIM domain family.
7. Perspectives
Continuing advances in the identification and characterization of Z-line and/or costameric proteins is increasing our knowledge of the importance of force and signal transmission between the sarcomere, sarcolemma, and the ECM. In this review, we have highlighted the critical roles of Z-line and costamere-associated protein complexes. Future experiments will focus on understanding the role of the growing number of known and yet unidentified Z-line and costamere-associated proteins in myofibrillogenesis, muscle function, and disease.
Acknowledgments
Work cited from the authors' laboratories was supported by NIH (J.C., K.U.K., and R.S.R.) and MDA (J.C.) grants. A.K.P. is a recipient of the AHA Postdoctoral Fellowship grant.
Contributor Information
Robert S. Ross, Email: rross@ucsd.edu.
Kirk U. Knowlton, Email: kknowlton@ucsd.edu.
Ju Chen, Email: juchen@ucsd.edu.
References
- 1.Bloch RJ, Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev. 2003;31(2):73–8. doi: 10.1097/00003677-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hoshijima M. Mechanical stress–strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290(4):H1313–25. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell B, Curtis MW, Koshman YE, Samarel AM. Mechanical stress-induced sarcomere assembly for cardiac muscle growth in length and width. J Mol Cell Cardiol. 2010;48(5):817–23. doi: 10.1016/j.yjmcc.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank D, Kuhn C, Katus HA, Frey N. Role of the sarcomeric Z-disc in the pathogenesis of cardiomyopathy. Future Cardiol. 2007;3(6):611–22. doi: 10.2217/14796678.3.6.611. [DOI] [PubMed] [Google Scholar]
- 5.Selcen D, Carpen O. The Z-disk diseases. Adv Exp Med Biol. 2008;642:116–30. doi: 10.1007/978-0-387-84847-1_10. [DOI] [PubMed] [Google Scholar]
- 6.Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchi-tecture: an intricate web of form and function. Annu Rev Cell Dev Biol. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto S. Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc Res. 2008;77(4):659–66. doi: 10.1093/cvr/cvm084. [DOI] [PubMed] [Google Scholar]
- 8.Bowles NE, Bowles K, Towbin JA. Prospects for gene therapy for inherited cardiomyopathies. Prog Pediatr Cardiol. 2000;12(1):133–45. doi: 10.1016/s1058-9813(00)00065-5. [DOI] [PubMed] [Google Scholar]
- 9.Marian AJ. Hypertrophic cardiomyopathy: from genetics to treatment. Eur J Clin Invest. 2010;40(4):360–9. doi: 10.1111/j.1365-2362.2010.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottenheijm CA, Heunks LM, Dekhuijzen RP. Diaphragm adaptations in patients with COPD. Respir Res. 2008;9:12. doi: 10.1186/1465-9921-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanger JM, Sanger JW. The dynamic Z bands of striated muscle cells. Sci Signal. 2008;1(32):Pe37. doi: 10.1126/scisignal.132pe37. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein MA, Schroeter JP, Michael LH. Role of the Z band in the mechanical properties of the heart. FASEB J. 1991;5(8):2167–74. doi: 10.1096/fasebj.5.8.2022313. [DOI] [PubMed] [Google Scholar]
- 13.Bang ML, Li X, Littlefield R, et al. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J Cell Biol. 2006;173(6):905–16. doi: 10.1083/jcb.200603119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruger M, Linke WA. Titin-based mechanical signalling in normal and failing myocardium. J Mol Cell Cardiol. 2009;46(4):490–8. doi: 10.1016/j.yjmcc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Luther PK. The vertebrate muscle Z-disc: sarcomere anchor for structure and signalling. J Muscle Res Cell Motil. 2009;30(5–6):171–85. doi: 10.1007/s10974-009-9189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ervasti JM. Costameres: the Achilles' heel of Herculean muscle. J Biol Chem. 2003;278(16):13591–4. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- 17.Sheikh F, Bang ML, Lange S, Chen J. “Z”eroing in on the role of Cypher in striated muscle function, signaling, and human disease”. Trends Cardiovasc Med. 2007;17(8):258–62. doi: 10.1016/j.tcm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Chien KR. Complexity in simplicity: monogenic disorders and complex cardiomyopathies. J Clin Invest. 1999;103(11):1483–5. doi: 10.1172/JCI7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng M, Cheng H, Banerjee I, Chen J. ALP/Enigma PDZ-LIM domain proteins in the heart. J Mol Cell Biol. 2010;2(2):96–102. doi: 10.1093/jmcb/mjp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q, Ruiz-Lozano P, Martone ME, Chen J. Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to alpha-actinin-2 and protein kinase C. J Biol Chem. 1999;274(28):19807–13. doi: 10.1074/jbc.274.28.19807. [DOI] [PubMed] [Google Scholar]
- 21.Huang CQ, Zhou Q, Liang PH, et al. Characterization and in vivo functional analysis of splice variants of cypher. J Biol Chem. 2003;278(9):7360–5. doi: 10.1074/jbc.M211875200. [DOI] [PubMed] [Google Scholar]
- 22.Zheng M, Cheng H, Li X, et al. Cardiac-specific ablation of Cypher leads to a severe form of dilated cardiomyopathy with premature death. Hum Mol Genet. 2009;18(4):701–13. doi: 10.1093/hmg/ddn400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Nandelstadh P, Ismail M, Gardin C, et al. A class III PDZ binding motif in the myotilin and FATZ families binds enigma family proteins: a common link for Z- Disc myopathies. Mol Cell Biol. 2009;29(3):822–34. doi: 10.1128/MCB.01454-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q, Chu PH, Huang C, et al. Ablation of cypher, a PDZ-LIM domain Z-line protein, causes a severe form of congenital myopathy. J Cell Biol. 2001;155(4):605–12. doi: 10.1083/jcb.200107092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arimura T, Hayashi T, Terada H, et al. A Cypher/ZASP mutation associated with dilated cardiomyopathy alters the binding affinity to protein kinase C. J Biol Chem. 2004;279(8):6746–52. doi: 10.1074/jbc.M311849200. [DOI] [PubMed] [Google Scholar]
- 26.Vatta M, Mohapatra B, Jimenez S, et al. Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J Am Coll Cardiol. 2003;42(11):2014–27. doi: 10.1016/j.jacc.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Moric-Janiszewska E, Markiewicz-Loskot G. Genetic heterogeneity of left- ventricular noncompaction cardiomyopathy. Clin Cardiol. 2008;31(5):201–4. doi: 10.1002/clc.20202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xing Y, Ichida F, Matsuoka T, et al. Genetic analysis in patients with left ventricular noncompaction and evidence for genetic heterogeneity. Mol Genet Metab. 2006;88(1):71–7. doi: 10.1016/j.ymgme.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Zheng M, Cheng H, Banerjee I, Chen J. ALP/Enigma PDZ-LIM domain proteins in the heart. J Mol Cell Biol. 2010;2(2):96–102. doi: 10.1093/jmcb/mjp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griggs R, Vihola A, Hackman P, et al. Zaspopathy in a large classic late-onset distal myopathy family. Brain. 2007;130:1477–84. doi: 10.1093/brain/awm006. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa N, Hoshijima M, Oyasu M, et al. ENH, containing PDZ and LIM domains, heart/skeletal muscle-specific protein, associates with cytoskeletal proteins through the PDZ domain. Biochem Biophys Res Commun. 2000;272(2):505–12. doi: 10.1006/bbrc.2000.2787. [DOI] [PubMed] [Google Scholar]
- 32.Ueki N, Seki N, Yano K, et al. Isolation, tissue expression, and chromosomal assignment of a human LIM protein gene, showing homology to rat Enigma homologue (ENH) J Hum Genet. 1999;44(4):256–60. doi: 10.1007/s100380050155. [DOI] [PubMed] [Google Scholar]
- 33.Niederlander N, Fayein NA, Auffray C, Pomies P. Characterization of a new human isoform of the enigma homolog family specifically expressed in skeletal muscle. Biochem Biophys Res Commun. 2004;325(4):1304–11. doi: 10.1016/j.bbrc.2004.10.178. [DOI] [PubMed] [Google Scholar]
- 34.Cheng H, Kimura K, Peter AK, et al. Loss of enigma homolog protein results in dilated cardiomyopathy. Circ Res. 2010;107(3):348–56. doi: 10.1161/CIRCRESAHA.110.218735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Su H. Unraveling enigma in the z-disks. Circ Res. 2010;107(3):321–3. doi: 10.1161/CIRCRESAHA.110.225615. [DOI] [PubMed] [Google Scholar]
- 36.Pardo JV, Siliciano JD, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci USA. 1983;80(4):1008–12. doi: 10.1073/pnas.80.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansour H, de Tombe PP, Samarel AM, Russell B. Restoration of resting sarcomere length after uniaxial static strain is regulated by protein kinase Cepsilon and focal adhesion kinase. Circ Res. 2004;94(5):642–9. doi: 10.1161/01.RES.0000121101.32286.C8. [DOI] [PubMed] [Google Scholar]
- 38.Danowski BA, Imanaka-Yoshida K, Sanger JM, Sanger JW. Costameres are sites of force transmission to the substratum in adult rat cardiomyocytes. J Cell Biol. 1992;118(6):1411–20. doi: 10.1083/jcb.118.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crosbie RH, Heighway J, Venzke DP, Lee JC, Campbell KP. Sarcospan: the 25 kDa transmembrane component of the dystrophin-glycoprotein complex. J Biol Chem. 1997;272(50):31221–4. doi: 10.1074/jbc.272.50.31221. [DOI] [PubMed] [Google Scholar]
- 40.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122(4):809–23. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell KP, Kahl SD. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989;338(6212):259–62. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- 42.Ervasti JM, Kahl SD, Campbell KP. Purification of dystrophin from skeletal muscle. J Biol Chem. 1991;266(14):9161–5. [PubMed] [Google Scholar]
- 43.Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990;345(6273):315–9. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- 44.Ervasti JM, Campbell KP. Membrane organization of the dystrophin–glycoprotein complex. Cell. 1991;66(6):1121–31. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida M, Ozawa E. Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem. 1990;108(5):748–52. doi: 10.1093/oxfordjournals.jbchem.a123276. [DOI] [PubMed] [Google Scholar]
- 46.Ervasti JM, Campbell KP. Dystrophin-associated glycoproteins: their possible roles in the pathogenesis of Duchenne muscular dystrophy. Mol Cell Biol Hum Dis Ser. 1993;3:139–66. doi: 10.1007/978-94-011-1528-5_6. [DOI] [PubMed] [Google Scholar]
- 47.Holt KH, Lim LE, Straub V, et al. Functional rescue of the sarcoglycan complex in the Bio 14.6 hamster using Ö-sarcoglycan gene transfer. Mol Cell. 1998;1:841–8. doi: 10.1016/s1097-2765(00)80083-0. [DOI] [PubMed] [Google Scholar]
- 48.Jung D, Yang B, Meyer J, Chamberlain JS, Campbell KP. Identification and characterization of the dystrophin anchoring site on beta-dystroglycan. J Biol Chem. 1995;270(45):27305–10. doi: 10.1074/jbc.270.45.27305. [DOI] [PubMed] [Google Scholar]
- 49.Rybakova IN, Amann KJ, Ervasti JM. A new model for the interaction of dystrophin with F-actin. J Cell Biol. 1996;135(3):661–72. doi: 10.1083/jcb.135.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rybakova IN, Ervasti JM. Dystrophin-glycoprotein complex is monomeric and stabilizes actin filaments in vitro through a lateral association. J Biol Chem. 1997;272(45):28771–8. doi: 10.1074/jbc.272.45.28771. [DOI] [PubMed] [Google Scholar]
- 51.Allamand V, Campbell KP. Animal models for muscular dystrophy: valuable tools for the development of therapies. Hum Mol Genet. 2000;9(16):2459–67. doi: 10.1093/hmg/9.16.2459. [DOI] [PubMed] [Google Scholar]
- 52.Crosbie RH, Lebakken CS, Holt KH, et al. Membrane targeting and stabilization of sarcospan is mediated by the sarcoglycan subcomplex. J Cell Biol. 1999;145(1):153–65. doi: 10.1083/jcb.145.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peter AK, Miller G, Crosbie RH. Disrupted mechanical stability of the dystrophin-glycoprotein complex causes severe muscular dystrophy in sarcospan transgenic mice. J Cell Sci. 2007;120(Pt 6):996–1008. doi: 10.1242/jcs.03360. [DOI] [PubMed] [Google Scholar]
- 54.Towbin JA, Hejtmancik JF, Brink P, et al. X-linked dilated cardiomyopathy Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation. 1993;87(6):1854–65. doi: 10.1161/01.cir.87.6.1854. [DOI] [PubMed] [Google Scholar]
- 55.Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–28. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 56.Badorff C, Lee GH, Lamphear BJ, et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5(3):320–6. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- 57.Xiong D, Lee GH, Badorff C, et al. Dystrophin deficiency markedly increases enterovirus-induced cardiomyopathy: a genetic predisposition to viral heart disease. Nat Med. 2002;8(8):872–7. doi: 10.1038/nm737. [DOI] [PubMed] [Google Scholar]
- 58.Guyon JR, Kudryashova E, Potts A, et al. Calpain 3 cleaves filamin C and regulates its ability to interact with gamma- and delta-sarcoglycans. Muscle Nerve. 2003;28(4):472–83. doi: 10.1002/mus.10465. [DOI] [PubMed] [Google Scholar]
- 59.Luan X, Hong D, Zhang W, Wang Z, Yuan Y. A novel heterozygous deletion-insertion mutation (2695–2712 del/GTTTGT ins) in exon 18 of the filamin C gene causes filaminopathy in a large Chinese family. Neuromuscul Disord. 2010;20(6):390–6. doi: 10.1016/j.nmd.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Shatunov A, Olive M, Odgerel Z, et al. In-frame deletion in the seventh immunoglobulin-like repeat of filamin C in a family with myofibrillar myopathy. Eur J Hum Genet. 2009;17(5):656–63. doi: 10.1038/ejhg.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kono S, Nishio T, Takahashi Y, et al. Dominant-negative effects of a novel mutation in the filamin myopathy. Neurology. 2010;75(6):547–54. doi: 10.1212/WNL.0b013e3181ec7fbd. [DOI] [PubMed] [Google Scholar]
- 62.Dalkilic I, Schienda J, Thompson TG, Kunkel LM. Loss of FilaminC (FLNc) results in severe defects in myogenesis and myotube structure. Mol Cell Biol. 2006;26(17):6522–34. doi: 10.1128/MCB.00243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pardo JV, Siliciano JD, Craig SW. Vinculin is a component of an extensive network of myofibril-sarcolemma attachment regions in cardiac muscle fibers. J Cell Biol. 1983;97(4):1081–8. doi: 10.1083/jcb.97.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125(2):327–37. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- 65.Zemljic-Harpf AE, Ponrartana S, Avalos RT, et al. Heterozygous inactivation of the vinculin gene predisposes to stress-induced cardiomyopathy. Am J Pathol. 2004;165(3):1033–44. doi: 10.1016/S0002-9440(10)63364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zemljic-Harpf AE, Miller JC, Henderson SA, et al. Cardiac-myocyte-specific excision of the vinculin gene disrupts cellular junctions, causing sudden death or dilated cardiomyopathy. Mol Cell Biol. 2007;27(21):7522–37. doi: 10.1128/MCB.00728-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olson TM, Illenberger S, Kishimoto NY, et al. Metavinculin mutations alter actin interaction in dilated cardiomyopathy. Circulation. 2002;105(4):431–7. doi: 10.1161/hc0402.102930. [DOI] [PubMed] [Google Scholar]
- 68.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 69.Gontier Y, Taivainen A, Fontao L, et al. The Z-disc proteins myotilin and FATZ-1 interact with each other and are connected to the sarcolemma via muscle-specific filamins. J Cell Sci. 2005;118(Pt 16):3739–49. doi: 10.1242/jcs.02484. [DOI] [PubMed] [Google Scholar]
- 70.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17(5):509–16. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 72.Ross RS, Pham C, Shai SY, et al. Beta1 integrins participate in the hypertrophic response of rat ventricular myocytes. Circ Res. 1998;82(11):1160–72. doi: 10.1161/01.res.82.11.1160. [DOI] [PubMed] [Google Scholar]
- 73.Valencik ML, Keller RS, Loftus JC, McDonald JA. A lethal perinatal cardiac phenotype resulting from altered integrin function in cardiomyocytes. J Card Fail. 2002;8(4):262–72. doi: 10.1054/jcaf.2002.127335. [DOI] [PubMed] [Google Scholar]
- 74.Valencik ML, Zhang D, Punske B, et al. Integrin activation in the heart: a link between electrical and contractile dysfunction? Circ Res. 2006;99(12):1403–10. doi: 10.1161/01.RES.0000252291.88540.ac. [DOI] [PubMed] [Google Scholar]
- 75.Zemljic-Harpf A, Manso AM, Ross RS. Vinculin and talin: focus on the myocardium. J Investig Med. 2009;57(8):849–55. doi: 10.231/JIM.0b013e3181c5e074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stephens LE, Sutherland AE, Klimanskaya IV, et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9(15):1883–95. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 77.Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9(15):1896–908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 78.Shai SY, Harpf AE, Babbitt CJ, et al. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90(4):458–64. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 79.Elsherif L, Huang MS, Shai SY, et al. Combined deficiency of dystrophin and beta1 integrin in the cardiac myocyte causes myocardial dysfunction, fibrosis and calcification. Circ Res. 2008;102(9):1109–17. doi: 10.1161/CIRCRESAHA.108.173153. [DOI] [PubMed] [Google Scholar]
- 80.Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7(1):20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 81.Sakai T, Li S, Docheva D, et al. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17(7):926–40. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.White DE, Coutu P, Shi YF, et al. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006;20(17):2355–60. doi: 10.1101/gad.1458906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu H, Fedak PW, Dai X, et al. Integrin-linked kinase expression is elevated in human cardiac hypertrophy and induces hypertrophy in transgenic mice. Circulation. 2006;114(21):2271–9. doi: 10.1161/CIRCULATIONAHA.106.642330. [DOI] [PubMed] [Google Scholar]
- 84.Liang X, Zhou Q, Li X, et al. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol. 2005;25(8):3056–62. doi: 10.1128/MCB.25.8.3056-3062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang X, Sun Y, Schneider J, et al. Pinch1 is required for normal development of cranial and cardiac neural crest-derived structures. Circ Res. 2007;100(4):527–35. doi: 10.1161/01.RES.0000259041.37059.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang X, Sun Y, Ye M, et al. Targeted ablation of PINCH1 and PINCH2 from murine myocardium results in dilated cardiomyopathy and early postnatal lethality. Circulation. 2009;120(7):568–76. doi: 10.1161/CIRCULATIONAHA.109.864686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang X, Sun Y, Chen J. Particularly interesting cysteine- and histidine-rich protein in cardiac development and remodeling. J Investig Med. 2009;57(8):842–8. doi: 10.231/JIM.0b013e3181c5e31d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DiMichele LA, Doherty JT, Rojas M, et al. Myocyte-restricted focal adhesion kinase deletion attenuates pressure overload-induced hypertrophy. Circ Res. 2006;99(6):636–45. doi: 10.1161/01.RES.0000240498.44752.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hakim ZS, DiMichele LA, Doherty JT, et al. Conditional deletion of focal adhesion kinase leads to defects in ventricular septation and outflow tract alignment. Mol Cell Biol. 2007;27(15):5352–64. doi: 10.1128/MCB.00068-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hakim ZS, DiMichele LA, Rojas M, et al. FAK regulates cardiomyocyte survival following ischemia/reperfusion. J Mol Cell Cardiol. 2009;46(2):241–8. doi: 10.1016/j.yjmcc.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peng X, Wu X, Druso JE, et al. Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc Natl Acad Sci USA. 2008;105(18):6638–43. doi: 10.1073/pnas.0802319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peng X, Kraus MS, Wei H, et al. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J Clin Invest. 2006;116(1):217–27. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shen TL, Park AY, Alcaraz A, et al. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005;169(6):941–52. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng X, Ueda H, Zhou H, et al. Overexpression of focal adhesion kinase in vascular endothelial cells promotes angiogenesis in transgenic mice. Cardiovasc Res. 2004;64(3):421–30. doi: 10.1016/j.cardiores.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 95.Ilic D, Furuta Y, Kanazawa S, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377(6549):539–44. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 96.Jani K, Schock F. Zasp is required for the assembly of functional integrin adhesion sites. J Cell Biol. 2007;179(7):1583–97. doi: 10.1083/jcb.200707045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rui Y, Bai J, Perrimon N. Sarcomere formation occurs by the assembly of multiple latent protein complexes. PLoS Genet. 2010;6(11):e1001208. doi: 10.1371/journal.pgen.1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]