Abstract
Hoxb8 overexpression immortalises haematopoietic progenitor cells in a growth-factor-dependant manner and co-operates with interleukin-3 (IL-3) to cause acute myeloid leukaemia. To further understand how Hoxb8 contributes to myeloid cell immortalisation, we generated IL-3-dependant myeloid cells expressing Hoxb8 under the control of an inducible promoter. Downregulation of Hoxb8, in the presence of IL-3, caused cell-cycle arrest and apoptosis in the majority of cells. Apoptosis was dependant on Bax and Bak and, in part, on Bim, which was repressed by Hoxb8. Deletion of the miR-17∼92 seed sequences in the Bim 3′UTR abolished Hoxb8-dependant regulation of Bim reporter constructs. Expression of all six miRNAs from this cluster were elevated when Hoxb8 was overexpressed. The miR-17∼92 cluster was required for repression of Bim in Hoxb8-immortalised cells and deletion of the miR-17∼92 cluster substantially inhibited Hoxb8, but not Hoxa9, mediated survival and proliferation. Hoxb8 appears to promote miR-17∼92 expression through c-Myc, a known transcriptional regulator of the miR-17∼92 cluster. We have uncovered a previously unrecognised link between Hoxb8 expression and microRNAs that provides a new insight into the oncogenic functions of Hoxb8.
Keywords: Apoptosis, Hoxb8, interleukin-3, microRNA
Hox genes are a family of transcription factors characterised by a conserved homeodomain motif.1 Hox genes regulate transcription as part of a complex with homeodomain-containing proteins of the Pre-B cell/CEH-20 family (PBC proteins, Pbx proteins in vertebrates) and the Meis family.2, 3 Hox genes are critical for the development of body patterning, and several Hox genes from the A and B clusters are important for normal haematopoiesis.4 For example, transgenic expression of Hoxb4, Hoxb8 or Hoxa9 results in expansion of the haematopoietic stem cell compartment,5, 6, 7 whereas deletion of Hoxa9 results in leukopaenia as a consequence of stem cell depletion.8, 9
Deregulated Hox gene expression is linked to leukaemia. Hoxb8, the first Hox gene unequivocally demonstrated to be an oncogene, co-operates with interleukin-3 (IL-3) to cause acute myeloid leukaemia (AML).6, 10, 11, 12 In human AML, HoxB8 is upregulated as a consequence of overexpression of another homeobox protein, CDX2.13 Overexpression of Hoxb8 in haematopoietic progenitor cells, in the presence of high concentrations of IL-3, permits the generation of growth factor-dependant myeloid cell lines capable of self-renewal,6, 14, 15 combining the proliferative signal from IL-3 with the function of Hoxb8 overexpression to block myeloid differentiation.16, 17 Some evidence suggests that Hox genes capable of immortalising haematopoietic cells, such as Hoxb8, may have additional functions to control apoptosis. For example, AML cell lines harbouring mixed lineage leukaemia (MLL) rearrangements undergo apoptosis when HoxA9 expression is silenced,18 and Hoxa9-deficient mice have increased lymphocyte apoptosis.9 In Caenorhabditis elegans, Hox genes regulate the viability of specific neurones by directly regulating the expression of a proapoptotic Bcl-2 family member, EGL-I.19, 20 Until now, a direct link between Hoxb8 and cell death pathways has not been identified.
We have used a 4-hydroxytamoxifen (4-OHT)-inducible lentiviral expression system to regulate Hoxb8 expression in IL-3-dependant myeloid progenitor cells. In response to Hoxb8 downregulation, cells arrest in the G1 phase of the cell cycle and the fate of most cells was to undergo apoptosis, even in the presence of IL-3. This cell death was absolutely dependant on the Bcl-2-regulatable pathway, because it was completely blocked by deletion of both Bax and Bak. Downregulation of Hoxb8 resulted in elevated Bim expression, which was required for efficient apoptosis after Hoxb8 withdrawal. Hoxb8-dependant regulation of Bim expression required the Bim 3′UTR (untranslated region) and, specifically, seed sequences of the miR-17∼92 cluster. We show that Hoxb8 expression resulted in higher expression of all microRNAs (miRNAs) from the miR-17∼92 cluster. The ability of Hoxb8-immortalised cells (which we refer to as FDM cells for factor-dependant myeloid cells) to clonogenically proliferate was dependant on the presence of at least one allele of miR-17∼92. We have gone on to identify a subset of miRNAs that are regulated in a Hoxb8-dependant manner. These findings link, for the first time in mammalian cells, Hox gene expression with the regulation of miRNAs and apoptosis and provide a new insight into the oncogenic functions of Hoxb8.
Results
Hoxb8 overexpression is required to maintain IL-3 FDM cells
We previously generated IL-3-dependant myeloid cell lines using retroviral overexpression of Hoxb8 to study the genes required for apoptosis induced by IL-3 deprivation.14, 15, 21 To study the response of cells when IL-3 is maintained but Hoxb8 expression is varied, we cloned Hoxb8 into a lentiviral expression system in which expression is induced by 4-OHT.22 In this system, Hoxb8 is not fused to an estrogen-receptor tag.23 C-kit-positive, lineage-negative (c-kit+ve/lin−ve) haematopoietic progenitor cells (HPC) from C57BL/6 E14.5 embryos were infected with Hoxb8 lentivirus in the presence of 4-OHT and IL-3 to generate IL-3-dependant myeloid progenitor FDM cells. Hoxb8 expression declined to undetectable levels 96 h after 4-OHT removal and re-addition of 4-OHT restored expression of Hoxb8 within 48 h (Figure 1a and Supplementary Figure S1a). We also used a tetracycline-repressible lentiviral expression system in which Hoxb8 expression was also tightly regulated (Supplementary Figures S1b and c).
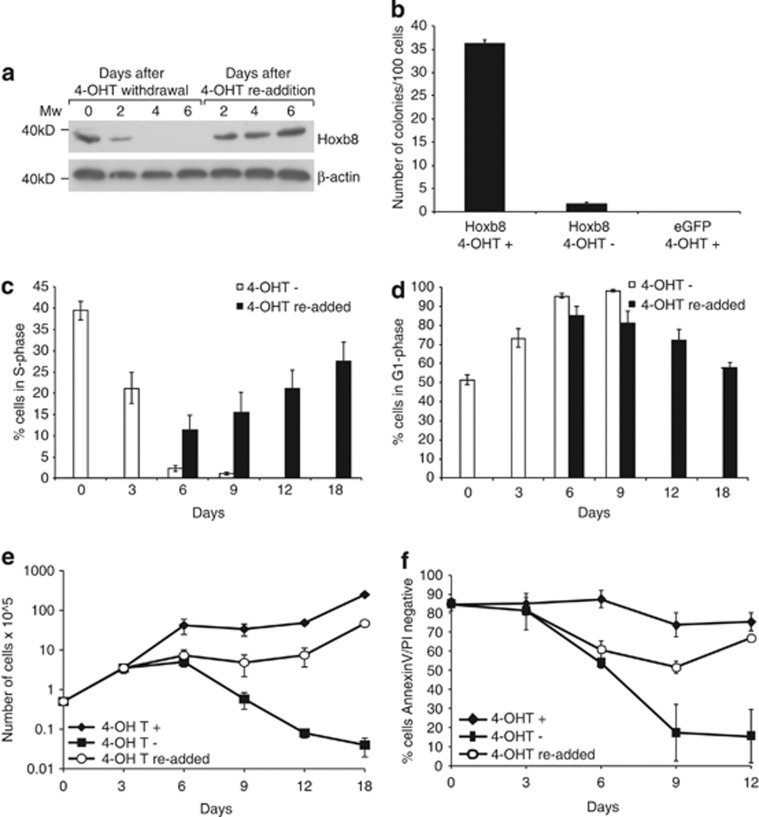
Figure 1.
Hoxb8 FDM cells stop proliferating and undergo apoptosis in the absence of Hoxb8 expression. (a) Lysates from Hoxb8 FDM cells were prepared at the indicated times after 4-OHT withdrawal and following 4-OHT re-addition after 4 days of withdrawal. Membranes were probed with antibodies to detect Hoxb8 and beta-actin as a loading control. (b) Hoxb8 expression is required for colony formation. Viable c-kit+ve/lin−ve cells were infected with Hoxb8 or eGFP encoding lentivirus, in the presence (+) or absence (−) of 4-OHT. Infected cells were single-cell sorted into 96-well plates and the number of colonies counted 14 days later. Results represent means±S.E.M. of four independent infections of four independent pools of c-kit+ve/lin−ve cells. (c) Cells in S-phase diminish after Hoxb8 downregulation. Hoxb8 FDM cells were cultured in IL-3 without 4-OHT (−) or following re-addition of 4-OHT after a 3-day period of withdrawal (re-added). At the indicated time points, cell-cycle analysis was performed using hypotonic PI buffer staining and flow cytometric analysis. The percentage of cells in S-phase was determined using the cell-cycle analysis software ModFit. Results indicate means±S.E.M. of seven independent clones in three independent experiments. (d) Cells arrest in G1 after Hoxb8 downregulation. Hoxb8 FDM cells were prepared as described in (c) and the percentage of cells in G1 phase was determined using Modfit. Results indicate means±S.E.M. of seven independent clones in three independent experiments. (e) Hoxb8 expression maintains proliferation in IL-3. Hoxb8 FDM cells were cultured in IL-3 with 4-OHT (+), without 4-OHT (−) or following re-addition of 4-OHT after a 3-day period of withdrawal (re-added). At the indicated time points, cell number was counted (see Materials and Methods). Results are means±S.E.M. of seven independent clones in three independent experiments. (f) Hoxb8 expression maintains viability in IL-3. Cell viability was determined by PI exclusion and FITC-conjugated AnnexinV staining from the same samples as described in (e). Results are means±S.E.M. of seven independent clones in three independent experiments
We tested the requirement for Hoxb8 in immortalisation using colony assays to compare c-kit+ve/lin−ve progenitors infected with inducible enhanced green fluorescent protein (eGFP) or Hoxb8 (Figure 1b). Colonies were efficiently generated in the presence of Hoxb8 expression but not in the presence of GFP. The few colonies generated from progenitor cells infected with Hoxb8 in the absence of 4-OHT probably arose as a result of background Hoxb8 expression. Cytokines other than IL-3 could not maintain colony growth in soft agar or viability in liquid culture (Supplementary Figures S1d and e). These data demonstrate that c-kit+ve/lin−ve progenitors immortalised with Hoxb8 in the presence of IL-3 are strictly dependant on both Hoxb8 and IL-3 for survival and proliferation.
Following Hoxb8-dependant immortalisation, all FDM clones expressed lineage markers and no longer expressed c-kit. Surface antigen expression remained largely unchanged in the presence or absence of 4-OHT or following the addition of GM-CSF. Some cells expressed higher levels of CD11b and F4/80 after 4-OHT withdrawal (Supplementary Figure S2a) and the morphology of a population of cells altered, changing from small cells with relatively large nuclei (promyelocytes) to larger, non-adherent cells resembling monocytes, with occasional granulocytes (Supplementary Figure S2b). These data show that Hoxb8 imposes a block on differentiation after commitment to a myeloid lineage, as previously observed,23 that is released in a proportion of cells when Hoxb8 expression is downregulated.
Reduced Hoxb8 expression caused cells to exit S-phase and accumulate in G1 despite the continued presence of abundant IL-3 in the culture (Figures 1c and d). Virtually no cells remained in S-phase 6 days after 4-OHT removal. Thus Hoxb8 overexpression was required for continued proliferation in response to IL-3 receptor signalling. This was not a result of diminished expression of the IL-3 receptor components as IL-3 beta common, IL-3-specific beta chain and IL-3 alpha-chain remained detectable after 4-OHT withdrawal (Supplementary Figure S3a and b) and re-addition of 4-OHT was sufficient to initiate re-entry into S-phase of at least a proportion of cells, accompanied by concomitant increase in the number of viable cells (Figures 1c, d and e). The cell counts in these same cultures showed that cells with uninterrupted Hoxb8 expression steadily increased in number, although a ‘plateau' effect was observed when cells required replating at day 6. After 4-OHT was removed, the rate of cell division declined between days 3 and 6, and total cell number fell thereafter. 4-OHT re-addition after 3 days restored proliferation. Together, these data showed that maintenance of Hoxb8 overexpression was required for cells to respond to proliferative signals transduced by IL-3.
Hoxb8 withdrawal activates Bax and Bak-dependant apoptosis pathways
The ultimate fate of most cells after Hoxb8 downregulation was to undergo apoptosis (Figure 1f). The decline in cell viability was concomitant with the decline of Hoxb8 to undetectable levels. Cells surviving 4-OHT withdrawal remained IL-3 dependant, eliminating the possibility of selection of IL-3-independent populations (Supplementary Figure S3c). Moreover, cells could not be rescued from apoptosis by the addition of differentiation-associated cytokines M-CSF or GM-CSF (Supplementary Figure S3d).
In FDM cells, apoptosis following IL-3 deprivation is regulated by the Bcl-2 family of proteins and is critically dependant on two pro-apoptotic Bcl-2 family members, Bax and Bak.14, 24 To determine whether apoptosis induced by Hoxb8 downregulation was also regulated by Bcl-2 family proteins, we generated Hoxb8 FDM cells from E13.5 Bax−/−;Bak−/− HPC. When Bax−/−;Bak−/− Hoxb8 FDM cells were cultured in the absence of 4-OHT for 9 days, no decline in cell viability was observed (Figure 2a). Further, apoptosis induced by Hoxb8 downregulation in wild-type FDMs was blocked by the caspase inhibitor Q-VD-Oph (Supplementary Figure S4a), indicating that a Bax and Bak, caspase-dependant apoptotic pathway was activated by downregulated Hoxb8 expression. We next determined whether regulated Hoxb8 expression was associated with changes in the expression of other Bcl-2 family members. We analysed the protein expression of Bcl-2 family proteins by western blot in wild-type Hoxb8 FDM cells after 4-OHT withdrawal and following 4-OHT re-addition (Figure 2b and Supplementary Figure S4b). The most consistent finding, across multiple independently generated lines, was reduced or absent Bim protein expression in the presence of Hoxb8. Thus, independent of the baseline expression of Bim in any clone, as Hoxb8 expression declined, Bim expression increased. This was also observed in Bax−/−;Bak−/− FDM cells (Supplementary Figure S4e). Bim expression remained elevated after Hoxb8 restoration over the time course examined, consistent with ongoing apoptosis. Reduced Bim expression was also observed in tetracycline-repressible Hoxb8 FDM cells (Supplementary Figure S4c). Subtle variations in the expression of other Bcl-2 family members were observed. For example, in some clones, Bmf levels increased, Bcl-xL levels declined and Mcl-1 increased over the time course, even after 4-OHT re-addition (Supplementary Figure S4b). However, with the exception of Bim, these variations were not consistently observed in all the clones tested. qRT-PCR analysis demonstrated elevated Bim mRNA over a time course of Hoxb8 downregulation (Figure 2c), indicating that increased Bim protein expression resulted from increased transcription or stability of Bim mRNA.
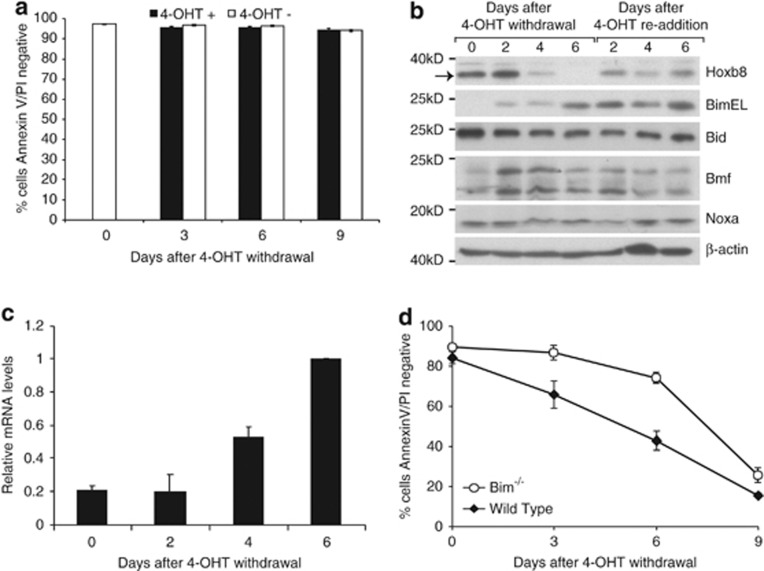
Figure 2.
Hoxb8-withdrawal-induced cell death is completely blocked by deletion of Bax and Bak and partially blocked by Bim. (a) Hoxb8 downregulation induces Bax/Bak-dependant apoptosis. Bax−/−;Bak−/− Hoxb8 FDM cells were cultured with IL-3 in the presence or absence of 4-OHT. At the indicated times, viability was determined by PI exclusion and FITC-conjugated AnnexinV staining. Results are means±S.E.M. of five independent clones in two independent experiments. (b) Bim expression is repressed by Hoxb8. Western blot analysis of Bcl-2 family proteins from lysates of wild-type Hoxb8 FDM cells cultured in IL-3 after 4-OHT withdrawal and following 4-OHT re-addition on day 4 of 4-OHT deprivation. Membranes were probed with antibodies against Hoxb8, Bim, Bid, Bmf and Noxa. Arrow indicates Hoxb8. Bmf protein runs as a doublet. (c) Bim mRNA increases after Hoxb8 downregulation. Real-time PCR analysis of RNA harvested from wild-type Hoxb8 FDM cells 0, 2, 4 and 6 days after 4-OHT withdrawal. All samples were normalised against Sdh2a and Polr2a. Bim mRNA levels are expressed relative to the Bim mRNA level at 6 days after 4-OHT withdrawal (highest Bim mRNA level). Results show means±S.E.M. of three independent pools in three independent experiments. (d) Deletion of Bim reduces apoptosis after Hoxb8 downregulation. Wild-type and Bim−/− Hoxb8 FDM cells were cultured in IL-3 in the absence of 4-OHT. Viability was determined by PI exclusion and FITC-conjugated AnnexinV staining at the indicated time points. Results show means±S.E.M. of eight independent clones in three independent experiments
We tested the requirement for Bim in apoptosis induced by Hoxb8 downregulation using Hoxb8 FDM cells from Bim-deficient mice.25 Hoxb8 expression was regulated by 4-OHT in Bim−/− Hoxb8 FDM cells as in wild-type cells (Supplementary Figure S4d). We compared the viability of Bim−/− with wild-type Hoxb8 FDM cells over 9 days following 4-OHT removal (Figure 2d). Significantly, fewer Bim−/− Hoxb8 FDM cells underwent apoptosis, particularly during the first 6 days. Thereafter, the viability of Bim−/− Hoxb8 FDM cells declined to approach, but not reach, that of wild-type cells. Thus, Bim is required for the efficient apoptosis that follows Hoxb8 downregulation, and repression of Bim expression is an important mechanism by which Hoxb8 maintains viable, IL-3-dependant cells. However, as many Bim−/− Hoxb8 FDM cells eventually undergo apoptosis after Hoxb8 downregulation, other Bax and Bak-dependant, Bim-independent apoptosis pathways must also be activated.
Proliferative arrest following Hoxb8 withdrawal is independent of apoptosis
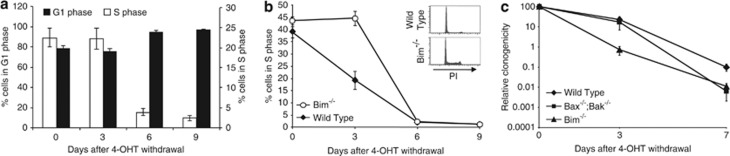
The survival of more Bim−/− and Bax/Bak/FDM cells after Hoxb8 downregulation raised the possibility that this experiment selected cells that proliferated independently of Hoxb8 expression. To test this possibility, we analysed whether Bim−/− and Bax−/−;Bak−/− Hoxb8 FDM cells still underwent cell cycle arrest after Hoxb8 downregulation. After 9 days of 4-OHT withdrawal, Bax−/−;Bak−/− Hoxb8 FDM cells stopped proliferating with similar kinetics to wild-type cells (Figure 3a compared with Figures 1c and d). Cell-cycle analysis comparing wild-type and Bim−/− Hoxb8 FDM cells showed that Bim−/− FDM cells were slower to enter G1 arrest than wild-type cells, but by day 6 of 4-OHT deprivation, virtually no wild-type or Bim−/− Hoxb8 FDM cells remained in S-phase (Figure 3b). These data demonstrate that blocking apoptosis does not result in the accumulation of cells that proliferate independently of Hoxb8 expression. We next determined whether Bax−/−;Bak−/− and Bim−/− Hoxb8 FDM cells surviving Hoxb8 downregulation were able to form colonies in soft agar when Hoxb8 expression was restored by replating in soft agar, in the presence of abundant IL-3 and 4-OHT (Figure 3c). The number of colonies generated after restoration of Hoxb8 expression steadily declined over time in all clones tested. Deletion of Bim or of both Bax and Bak offered no advantage in colony formation. In fact, some Bim−/− Hoxb8 FDM clones had a lower relative clonogenicity than wild-type or Bax−/−;Bak−/− Hoxb8 FDM clones 3 days after 4-OHT withdrawal. These data indicate that the function of Hoxb8 to maintain cell viability and self-renewal can be separated genetically. Maintaining cell viability after Hoxb8 downregulation is not equivalent to accumulating cells capable of continued self-renewal. Our data suggest that the subpopulation of cells that retain the capacity for clonogenic proliferation is not expanded in vitro by blocking intrinsic apoptosis pathways.
Figure 3.
Blocking apoptosis does not alter cell-cycle arrest after Hoxb8 withdrawal. (a) Bax−/−;Bak−/− Hoxb8 FDM cells arrest in G1 after Hoxb8 downregulation. Bax−/−;Bak−/− Hoxb8 FDM cells were cultured in the presence of IL-3 and absence of 4-OHT and, at the indicated times were subjected to cell-cycle analysis using hypotonic PI staining. Results were analysed using Modfit and show means±S.E.M. of five independent clones in two independent experiments. (b) Bim−/− Hoxb8 FDM cells undergo cell-cycle arrest after Hoxb8 downregulation. Wild-type and Bim−/− Hoxb8 FDM cells were cultured for the indicated times without 4-OHT and then subjected to cell-cycle analysis using hypotonic PI staining. The inset shows representative histograms of hypotonic PI staining 3 days after 4-OHT withdrawal of wild-type and Bim−/− Hoxb8 FDM cells. Results were analysed using Modfit and show means±S.E.M. of eight independent clones in three independent experiments. (c) Clonogenicity declines after Hoxb8 downregulation. Wild-type, Bim−/− and Bax−/−;Bak−/− Hoxb8 FDM cells were cultured for the indicated times without 4-OHT before being plated in soft agar in the presence of 4-OHT. Colonies were counted after 14 days and relative clonogenicity determined. Results shown are means±S.E.M. of four independent clones of each genotype in three independent experiments
Hoxb8-dependant regulation of Bim expression requires the Bim 3′UTR
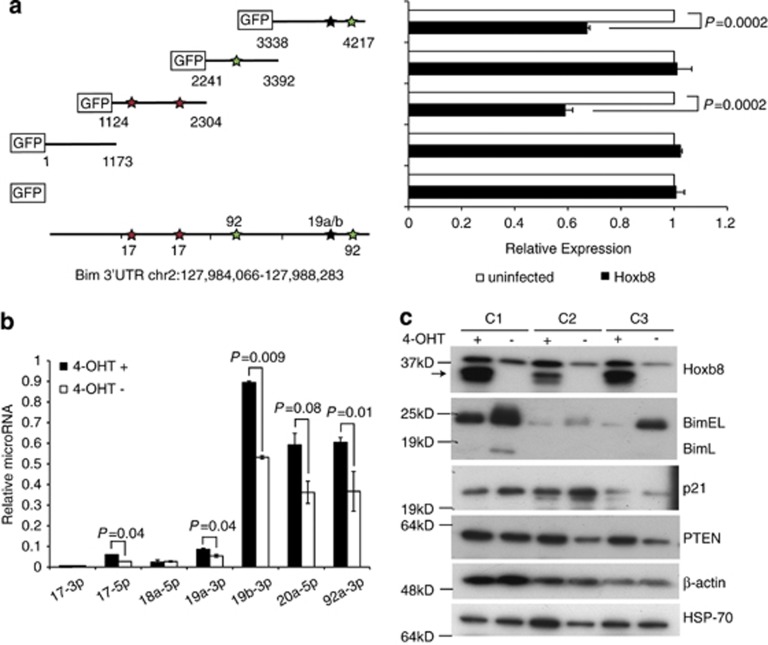
Our data suggested that Bim is a target of a Hoxb8-dependant gene repression mechanism. To define the region of the Bim gene required for Hoxb8-dependant repression, we used luciferase reporter constructs that encoded 3.6 kB of the murine Bim promoter region upstream of Exon 1, the first intron of Bim and the 3′UTR of Bim. These constructs were transiently transfected into 293T cells stably overexpressing GFP or Hoxb8. Expression of Hoxb8 halved luciferase activity only in cells expressing the Bim 3′UTR construct (Figure 4a). This result suggested that Hoxb8-dependant Bim repression involved mRNA stability and possibly miRNAs. Several miRNAs can repress Bim expression, in particular miRNAs from the miR-17∼92 cluster.26, 27, 28, 29 To determine whether any miRNAs predicted to bind the Bim 3′UTR were also regulated in a Hoxb8-dependant manner, we profiled the changes in expression levels of all murine miRNAs after Hoxb8 withdrawal by qPCR arrays (Figure 4b). Most notable among the miRNAs expressed in the presence of Hoxb8 were all members of the miR-17∼92 cluster, miR-17, miR-18, miR-19a, miR-20, miR-19b and miR-92 (Figure 4c). This significant upregulation of all members of the cluster strongly suggests that Hoxb8 transcriptionally regulates the cluster. Of the various miRNAs induced by Hoxb8, miR-17, miR-19a/b, miR-92, miR-214 and miR-9 are predicted by Targetscan (http://www.targetscan.org/) to target the Bim 3′UTR, although miR-9 is given an exceedingly low ranking.30, 31
Figure 4.
Bim repression by Hoxb8 is mediated at the Bim 3′UTR with possible regulation by miRNAs. (a) Reporter activity generated by different segments of the Bim promoter and 3′UTR. 293T cells stably expressing GFP or Hoxb8 were transiently transfected with luciferase reporter constructs containing either the 3.6 kB Bim promoter upstream of Exon 1, Intron 1 or the 3′UTR. Cells were co-transfected with renilla luciferase to control for transfection efficiency. Luciferase levels are expressed relative to the GFP-expressing 293T cells. Results are mean±S.E.M. of three independent experiments. I: Intron, E: Exon, L: Luciferase. Structure of murine Bim gene is shown (b) Wild-type Hoxb8-FDM cells were cultured in IL-3 and in the presence (+) or 4-day absence of 4-OHT (−). The left panel shows the fold change in expression in response to 4-OHT for the most affected miRNAs. For each miRNA, the change in Ct value in response to 4-OHT is shown relative to the average Ct of all the six samples. This scheme allows visualisation of the degree of change for all the levels of expression. The heat map on the right shows the actual Ct values, averaged across the three biological replicates for each treatment. Highest expression is represented as red and the lowest expression as blue. Red arrows indicate guide strands from the miRNAs of the miR-17∼92 cluster of miRNAs. (c) Table of miRNAs with significant fold changes as observed from (b) that are members of the miR-17∼92 cluster on chromosome 13 and related genes on chromosomes 7 and X. P-values of fold changes were calculated using a Student's t-test (independent, two-tailed). Indicated are both the -5p and -3p strands of each miRNA corresponding to the fold change observed for each in the qPCR array. This includes miRNAs previously known as the star forms
To determine the region of the Bim 3′UTR required for Hoxb8-dependant repression, we used a GFP reporter system in which GFP is fused to the regions of murine Bim 3′UTR extending from the stop codon for 4217 bases (corresponding to chr2:127,984,066-127,988,283). The reporter constructs contain several predicted miR-17∼92 cluster binding sites. Reporter plasmids were infected into 293T cells stably expressing Hoxb8 and flow cytometry used to measure mean GFP fluorescence intensity relative to cells expressing a reporter containing GFP alone (Figure 5a). Hoxb8 overexpression decreased GFP expression in cells transfected with the Bim 3′UTR reporter construct retaining binding sites for miR-17 as well as miR-19a/b and miR-92. No change in GFP expression was observed in cells expressing reporter constructs with other segments of the 3′UTR. This data, together with the changes of miRNA expression observed after downregulation of Hoxb8, supports the conclusion that miRNAs from the miR-17∼92 cluster, in particular miR-17, miR-19a/b and miR-92, mediate Hoxb8-dependant repression of Bim.
Figure 5.
Bim 3′UTR segments, which contain miR-17, miR-19a/b and miR-92 binding sites, are required for Hoxb8-mediated repression of Bim. (a) Murine Bim 3′UTR reporter activity in segments containing binding sites for miR-17, miR-19a/b and miR-92. 293T cells stably expressing the indicated GFP reporter plasmids or GFP alone were left uninfected or infected with Hoxb8 and GFP fluorescence analysed by flow cytometry. Fluorescence is relative to the uninfected cell population for each GFP reporter construct. Results are mean±S.E.M. of three independent experiments. P-values were derived using Student's t-test (two-tailed, equal variance). The star symbols indicate miRNA binding sites shown in the representation of the full Bim 3′UTR at the bottom of the panel. These sites were derived from the target prediction software, TargetScan, and are representative of a number of miRNAs with identical seed regions. These include miR17-5p/20ab/20b-5p/92/106ab/427/518-3p/519d for the miR-17 site and miR25/32/363/363-3p/367 for the mir-92 site. (b) qRT-PCR analysis of expression levels of miR-17-3p, miR-17-5p, miR-18a-5p, miR-19a-3p, miR-19b-3p, miR-20a-5p and miR-92a-3p in wild-type Hoxb8 FDM cells in the presence (+) and 4-day absence (−) of 4-OHT. All miRNA levels are normalised to U6 and expressed relative to miR-16. Results are mean±S.E.M. of three independent clones analysed in triplicate in two independent experiments. P-values were derived using Student's t-test (two-tailed, equal variance). (c) Western blot of other predicted miR-17∼92 targets in Hoxb8 FDM cells. Lysates of Hoxb8 FDMs cultured in the presence (+) and 4-day absence (−) of 4-OHT were analysed by western blotting for antibodies against Hoxb8, Bim, p21 and PTEN. Beta-actin is the loading control for all antibodies excluding PTEN for which HSP-70 is shown. Three individual clones, C1, C2 and C3, are shown. Arrow indicates Hoxb8
We independently validated the changes in miR-17∼92 expression observed after Hoxb8 downregulation. We measured the abundance of mature miRNAs from this cluster in the presence or absence of induced Hoxb8 expression in independent Hoxb8 FDM cell lines using Taqman microRNA assays. As observed in the qPCR array, the expression of miR-17-5p, miR-19a-3p, miR-19b-3p and miR-92-3p decreased significantly after 4-OHT withdrawal (Figure 5b). No decrease in miR-17-3p and miR-18a-5p was observed, with miR-17-3p being at almost undetectable levels. miR-18a-5p expression remained stable.
We then determined whether other predicted targets of the miR-17∼92 cluster were also differentially expressed in the presence and absence of Hoxb8. Cyclin-dependant kinase inhibitor 21 is described as regulated by the miR-17∼92 cluster and PTEN expression is repressed by miR-17-5p and miR-19 (ref 32, 33, 34). Western blots of lysates from three independent Hoxb8 FDM clones cultured in the presence or absence of 4-OHT were probed with antibodies against Bim, p21 and the phosphatase PTEN (Figure 5c). The baseline expression of Bim varied from clone to clone, but as we have observed in numerous independent clones and again in these, reduced Hoxb8 expression is always associated with elevated Bim expression. We observed variation in p21 expression across the three lines, with a subtle increase in p21 expression in the absence of Hoxb8 in two of three lines. This may reflect the proportion of cells within each clone that have recently entered G1 arrest, which we have previously observed is associated with transient p21 expression in these cells.35 PTEN expression was consistently reduced in all the three clones after Hoxb8 expression was downregulated (Figure 5c). As miR-17-5p and miR-19a/b expression levels decline after Hoxb8 withdrawal, the diminished expression of PTEN is probably independent of the miR-17∼92 cluster. The data suggest that the principal putative miR-17∼92 target that is repressed in these cells is Bim.
miR-17∼92 is required for the efficient survival and proliferation of Hoxb8 FDM cells
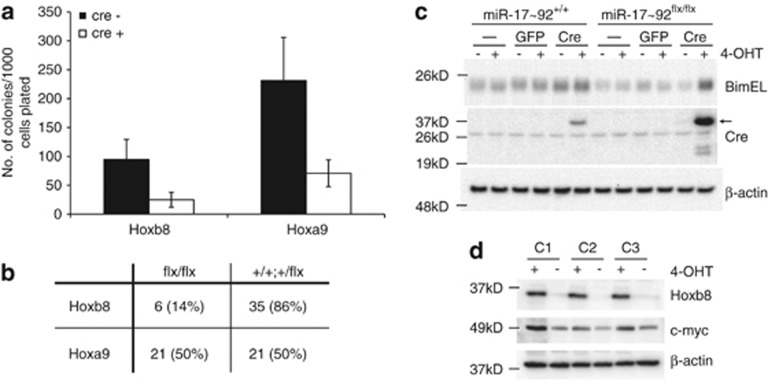
To determine whether miR-17∼92 was also required for Hoxb8-dependant progenitor cell survival and proliferation, we took advantage of mice in which the miR-17∼92 alleles are flanked by LoxP sites (miR-17∼92flx/flx), permitting deletion of the miR-17∼92 cluster following cre-recombinase expression.29 We utilised miR-17∼92flx/flx constitutive Hoxb8 FDM cell lines and included FDMs immortalised with Hoxa9 as a control. We reasoned that if Hoxb8 did not require miR-17∼92, deletion of the cluster would have no effect on Hoxb8-dependant immortalisation. miR-17∼92flx/flx Hoxb8 and Hoxa9 FDM cells were cultured in semi-solid agar after infection with cre-recombinase lentivirus and the number of colonies counted after 14 days. Both Hoxb8 and Hoxa9 miR-17∼92flx/flx FDM cells formed colonies after cre-recombinase expression (Figure 6a), despite an anticipated cre-recombinase-associated reduction in colony formation. Random colonies were selected from all independent experiments and genotyped to establish deletion of miR-17∼92 alleles. We found that 85% of miR-17∼92flx/flx Hoxb8 colonies retained at least one miR-17∼92 allele while 50% of Hoxa9-immortalised miR-17∼92flx/flx colonies had deleted both alleles (Figure 6b). This result established that while colonies could be generated from miR-17∼92flx/flx cells, a strong selection bias against deletion of miR-17∼92 was evident in Hoxb8 FDM cells.
Figure 6.
Hoxb8 FDM cells require the miR-17∼92 cluster for efficient survival and proliferation. (a) Clonogenic potential of miR-17∼92flx/flx Hoxb8- and Hoxa9-immortalised FDM cells after infection with lentiviral constructs encoding constitutive cre-recombinase. Cells were plated in soft agar and the number of colonies counted after 14 days. Results are mean±S.E.M. of two independent pools in three independent experiments. (b) Deletion of miR-17∼92 is selected against in Hoxb8 FDM cells. Colonies derived from (a) infected with cre-recombinase expressing lentivirus were transferred to liquid culture and genomic DNA harvested for genotyping by PCR. Colonies were scored depending on whether cells had deleted both miR-17∼92 alleles (flx/flx) or had retained at least one allele (+/++/flx). Values in brackets indicate percentage of overall colonies genotyped. At least 41 miR-17∼92flx/flx Hoxb8 FDM and 42 miR-17∼92flx/flx Hoxa9 FDM colonies were randomly selected and genotyped across the three independent experiments. Chi-square test P-value of 0.00058. (c) Western blot of Bim expression in miR-17∼92flx/flx Hoxb8 FDM cells after miR-17∼92 deletion. Cells were left uninfected or infected with either a 4-OHT-inducible GFP or cre-recombinase encoding lentivirus. After selection, cells were induced with 4-OHT for 14 hours before lysates were probed with antibodies against Bim, Cre and beta-actin as a loading control. Arrow indicates cre (d) Western blot of c-Myc expression in three independent clones of WT Hoxb8 FDM cells. Cells were cultured in the presence (+) or 4-day absence (−) of 4-OHT. Membrane was probed with antibodies against Hoxb8, c-myc and beta-actin as a loading control
To explore the possibility that deletion of the miR-17∼92 cluster in Hoxb8 FDMs resulted in increased levels of Bim, western blots of miR-17∼92−/− Hoxb8 FDM cells before and after treatment with cre-recombinase were probed with antibodies against Bim and cre-recombinase. The cre-recombinase-induced deletion of the miR-17∼92 cluster resulted in an increase in Bim expression which was not observed when cre-recombinase was expressed in WT cells or when GFP was expressed in miR-17∼92flx/flx cells (Figure 6c). This result clearly indicates that Bim repression by Hoxb8 is mediated by the miR-17∼92 cluster.
Upregulated expression of miR-17∼92 may be a direct or indirect consequence of Hoxb8 expression. A region 2 kB upstream of the transcriptional start site of the MIR17HG locus contains a putative Hox binding site. We therefore immortalised progenitor cells with a Flag-tagged Hoxb8 construct and performed a chromatin immunoprecipitation (ChIP)-PCR experiment to detect any direct binding of Hoxb8 within the miR-17∼92 promoter (Supplementary Figure S5a and b). We selected primers that covered the main promoter regions of MIR17HG, as well as regions up-and downstream. We found no significant enrichment for Hoxb8 binding above that observed for beta-actin, a negative control. This strongly suggests Hoxb8 does not bind within the promoter region of MIR17HG. The best described transcriptional regulator of the miR-17∼92 cluster is c-Myc.36 We speculated that Hoxb8 expression may be associated with c-Myc expression. We used western blotting to visualise c-Myc expression levels in three independently derived Hoxb8 FDM cell lines in the presence or absence of 4-OHT (to regulate Hoxb8 expression). In each clone, we observed a reduction in c-Myc expression when Hoxb8 expression declined (Figure 6d). These data support the hypothesis that miR-17∼92 expression, in the presence of Hoxb8, is a consequence of c-Myc expression. The mechanism by which Hoxb8 maintains c-Myc levels remains to be determined.
Discussion
Hoxb8 was the first Hox gene demonstrated to function as an oncogene, contributing to the development of AML.6, 10, 12 When overexpressed, Hoxb8, like other oncogenic Hox genes, imposes a block on differentiation. However, the molecular targets regulated by Hoxb8 remain largely unknown. Here we show that an additional critical role of Hoxb8 overexpression is suppression of apoptosis, achieved in part by repression of Bim. Furthermore, we have identified the miR-17∼92 cluster as a mediator of Hoxb8-dependant Bim repression. Our data strongly suggest that a larger subset of miRNAs are also involved in the block to differentiation and apoptosis imposed by Hoxb8 overexpression and the response when that block is released. The link between Hoxb8 and miR-17∼92 expression has not previously been described. Hoxb8 and paralogues Hoxa7, Hoxc8 and Hoxd8 are themselves regulated by the miR-196 miRNAs.37 However, the concept that some or all of the oncogenic functions of Hoxb8 might be mediated through the expression of other miRNAs is a novel finding for which, to our knowledge, no precedent exists.
miR-17-92 is a polycistronic miRNA cluster located in the human genome at chromosome 13q31.3. The cluster contains six mature miRNAs, miR-17, miR-18a, miR-19a, miR-20a, miR-19b and miR-92. Strong evidence supports the oncogenic effects of overexpression of miR-17∼92. Genomic amplification and elevated expression of miR-17∼92 expression is recognised in several tumour types, including diffuse large B-cell lymphoma,38 Mantle Cell Lymphoma39 and several types of solid tumours.40, 41 In the absence of copy number changes, other transcription factors, notably c-Myc, directly upregulate miR-17∼92 expression.36 Co-expression of c-Myc and miR-17∼92 cooperate to promote lymphomagenesis.42 In the absence of any direct binding by Hoxb8 in the miR-17∼92 genomic locus, we observed a correlation between Hoxb8 expression, miR-17∼92 elevation and c-Myc expression. This suggests a pathway in which c-Myc, expressed at elevated level in the presence of Hoxb8, drives expression of miR-17∼92. The mechanism by which Hoxb8 maintains c-Myc levels remains to be determined. Our success in immunoprecipitating Hoxb8 under ChIP conditions makes it now possible to undertake a ChIP-Seq approach to identify bona fide Hoxb8 targets, including miRNA genes and possibly c-Myc.
Our data shows that Hoxb8 specifically requires miR-17∼92 to repress Bim expression and efficiently maintain the survival and proliferation of hematopoietic cells. This requirement was evident in the strong selection against deletion of miR-17∼92 in Hoxb8-immortalised FDM cells and suggests that elevated miR-17∼92 expression, driven by Hoxb8, is an important mechanism of Hoxb8 oncogenic activity. This may also be true of other Hox genes, as half the Hoxa9-immortalised colonies from miR-17∼92flx/flx FDM cells also retained at least one miR-17∼92 allele. This is supported by observations that rearrangements of the MLL locus cause AML that is dependant on endogenous Hoxa9 (ref 43, 44) and that leukaemic stem cells from MLL-associated leukaemias express higher levels of miR-17∼92 than normal progenitor cells.32 However, our data also clearly show that miR-17∼92 is not absolutely required for Hoxa9-dependant immortalisation.
Bim deletion protected cells from apoptosis after downregulation of Hoxb8, showing that repression of Bim expression is one mechanism by which Hoxb8 maintains cell viability. Bim is required for normal haematopoiesis and deletion of Bim results in leukocytosis and diminished apoptosis in response to a range of stimuli.25 It is well established that Bim mRNA is regulated by miR-17∼92. It is tempting to conclude that Bim regulation by Hoxb8 is a key molecular mechanism underpinning Hoxb8 onocgenic function, as both deletion of Bim and enforced expression of miR17∼92 can drive the generation of lymphoma and lymphoproliferative disease.42, 45, 46 However, deletion of Bim (or Bax and Bak) was not sufficient to promote clonogenic survival or growth-factor-independent proliferation in our experiments. In contrast, deletion of miR-17∼92 significantly diminished the capacity of Hoxb8 to maintain clonogenic potential, indicating that miR-17∼92-regulated targets other than Bim are equally, if not more, important in Hoxb8-dependant immortalisation. Although other published data suggest these targets include p21 or PTEN (ref 32, 33, 34, 36, 47), our data do not support the hypothesis that p21 or PTEN are directly regulated by Hoxb8 in our cells. Full characterisation of bone fide miRNA target mRNAs remains a considerable experimental challenge.48 Other approaches, which capture and sequence mRNAs in complex with the RNAi-induced silencing complex, may provide a more robust approach.
We have identified that the ability of Hoxb8 overexpression to maintain viability and proliferation of haematopoietic progenitor cells relies crucially on the capacity of Hoxb8 to maintain miR-17∼92 expression, and for this miRNA cluster to regulate critical targets that include Bim. The regulation of miR-17∼92 and other miRNAs in a Hoxb8-dependant manner provides a novel insight into the oncogenic functions of Hoxb8 and identifies a subset of miRNAs that may crucially regulate the block in differentiation and maintenance of self-renewal potential that contribute to leukaemiagenesis. Identification of other key targets regulated by miR-17∼92 and other miRNAs in our model may provide the necessary means to specifically target critical deregulated pathways in these malignancies.
Materials and Methods
Cloning
The mouse Hoxb8 cDNA was amplified using PCR from pET Hoxb8 HIS-tag kana and cloned into the 4-OHT inducible, pF 5xUAS eGFP SV40 puro GEV16 lentiviral plasmid49 and the doxycycline repressible, pF 7xTetOP GSlinker RS PGK Hygro TetRVP16 lentiviral plasmid as described in Supplementary Materials and Methods and Supplementary Tables 1 and 2. Both plasmids were kind gifts from Dr. John Silke (Walter and Eliza Hall Institute, Parkville, VIC, Australia). Constitutive expression of Hoxb8 as well as Hoxa9 was achieved by cloning Hoxb8 or HoxA9 into the lentiviral vector, pFU SV40 puro W. Cre-recombinase was also cloned into this vector system.
The Bim 3.6 kB promoter region upstream of Exon 1 as well as full-length Intron 1 were cloned into the pGL2 basic plasmid using restriction enzyme sites HindII and KpnI while the Bim 3′UTR was cloned into the pGL3 promoter plasmid using restriction enzyme sites SpeI and BamHI. All plasmids were a kind gift from Hamsa Puthalakath (LaTrobe University, Melbourne, VIC, Australia). The various Bim 3′UTR segments were cloned into the FUGW lentiviral plasmid using an EcoRI/EcoRI site. All constructs were a kind gift from Dr. Marco Herold (Walter and Eliza Hall Institute).
Constitutive cre-recombinase was expressed using the pFU cre PGK Hygro W plasmid, a kind gift from Dr. John Silke (Walter and Eliza Hall Institute). Doxycycline inducible cre-recombinase expression was achieved using the pFTREtight MCS rtTAadvanced GFP lentiviral vector (Kahn, Okamoto and Huang—manuscript in preparation). Cre-recombinase was digested from the pFU cre-PGK Hygro W plasmid using BamHI/NheI and ligated into the BamHI/NheI digested pFTREtight MCS rtTAadvanced GFP lentiviral vector. Successful ligation was confirmed by diagnostic digest.
Mice
All wild-type cell lines were derived from C57BL/6 E14.5 embryos. The Bim−/−, Bax−/−; Bak−/− and miR-17∼92flx/flx mice have been previously described.25, 29, 50
Hoxb8 FDM cell line generation
IL-3-dependant Hoxb8 FDM cells were generated by isolating c-kit-positive, lineage-negative, haematopoietic progenitors from E14.5 fetal livers by flow cytometry using an antibody cocktail mix of c-kit-APC, Gr-1-FITC, NK1.1-FITC, B220-FITC and TER-119-FITC (All from BD Pharmingen, North Ryde, NSW, Australia). Cells were infected via spinoculation with 4-OHT-inducible or doxycycline-repressible Hoxb8 lentivirus and 5 μg/ml polybrene at 30 °C for 90 min at 2500 r.p.m. 4-OHT-inducible cell lines were selected with 0.5 μg/ml of puromycin (Sigma-Aldrich, St. Louis, MO, USA) for 10 days while doxycycline-repressible cell lines were selected with 200 μg/ml of hygromycin (Roche, Dee Why, NSW, Australia) for 14 days. After selection, 4-OHT-inducible cell lines were continuously maintained in low glucose DMEM supplemented with 10% fetal calf serum, 0.25 ng/ml IL-3 (R&D Systems, Minneapolis, MN, USA) and 0.1 μM 4-OHT (Sigma-Aldrich) to induce Hoxb8 expression. Doxycycline-repressible cell lines were cultured as per 4-OHT-inducible cell lines, however, in the absence of 4-OHT. Repression was achieved with 0.5 μg/ml of doxycycline (Sigma-Aldrich). Individual clones were selected using soft agar cloning as previously described.15
Reverse transcription and real-time PCR
RNA was extracted from 5.5 × 106 cells using the RNeasy RNA extraction kit (Qiagen, Chadstone, VIC, Australia), as per the manufacturer's instructions. In all, 1.5 μg of RNA was reverse transcribed using H-HLMV (Promega, South Sydney, NSW, Australia) and random primers (Promega, South Sydney, NSW, Australia). Levels of Bim mRNA were quantitated using the Universal Probe Library (Roche, Melbourne, VIC, Australia) and Faststart Taqman Probe Mastermix (Roche, Melbourne, VIC, Australia). Experiments were run using the ABI 7900 HT instrument (Applied Biosystems, Foster City, CA, USA), and results analysed using the LightCycle 480 software (Roche, Hawthorne, VIC, Australia).
Cell lysis and western blotting
A total of 5 × 104 cells/μl were lysed in RIPA buffer (150 mM NaCl, 50 mM TrisHCl pH7.4, 0.5% sodium deoxycholate, 0.1% SDS, 1% NP40) with protease inhibitor cocktail (Merck, Kilsyth, VIC, Australia) and phosphatase inhibitors (5 mM β-glycerophosphate, 1 mM Na Molybdate, 2 mM Na Pyrophosphate, 10 mM NaF). Lysates were centrifuged at 10 000 r.p.m., supernatant collected, diluted 1 : 5 with 5 × SDS-PAGE loading buffer (250 mM Tris.Cl pH 6.8, 10% 2-mercaptoethanol, 10% SDS, 0.2% Bromophenol blue and 50% glycerol) and boiled for 10 min. Lysates were run on either 10 or 12% SDS-PAGE gels, loaded equally by cell number and transferred to nitrocellulose membranes (Hydrobond, Amersham Biosciences, Little Chalfont, Buckinghamshire, UK) and western blot reactions were detected using Super Signal West Dura chemiluminescence reagent (Thermo Scientific, Rockford, IL, USA). Membranes were probed with the following antibodies: anti-Hoxb8 (Abnova, Neihu District. Taipei City, Taiwan), anti-Bim (Stressgen, Waterloo, NSW, Australia), anti-Bid (kind gift from Andreas Strasser), anti-Bax (Sigma-Aldrich), anti-Bak (Sigma-Aldrich), anti-Puma (ProSci, Poway, CA, USA), anti-Mcl-1 (Rockland, Gilbertsville, PA, USA), anti-Bcl-xL (R&D Systems), anti-Bcl-2 (BD Pharmingen), anti-Bmf (kind gift from Lorraine O-Reilly, Walter and Eliza Hall Institute), anti-Noxa (Millipore, Billerica, MA, USA), anti-IL-3 alpha chain and anti-IL-3 ß-specific chain (R&D Systems), anti-p21 (Abcam, Waterloo, NSW, Australia), anti-PTEN (Cell Signalling, Danvers, MA, USA), anti-HSP-70 (Cell Signalling), anti-c-Myc (Santa Cruz, Dallas, Texas, USA), anti-Flag and anti-β-actin (Sigma-Aldrich), anti-rat-HRP (Amersham, Rydalmere, NSW, Australia), anti-rabbit-HRP (Amersham), anti-mouse-HRP (Sigma-Aldrich) and anti-goat-HRP (Sigma-Aldrich).
Assays for cell survival, cell number and cell cycle
As a measure of cell death, cells were stained with Propidium Iodide (Sigma-Aldrich) and FITC-coupled, Annexin V (Invitrogen, Mulgrave, VIC, Australia) in a balanced salt solution with 5 mM CaCl2 and analysed by flow cytometry. To analyse cell proliferation by cell counts, Fluorescent Microsphere beads (Sigma-Aldrich) were added at a 1 : 10 dilution to the same samples stained with FITC-coupled AnnexinV and PI and analysed by flow cytometry. The number of cells was calculated as the number of beads/μl detected. To analyse cell cycle, the individual cell nuclear content was measured by incubating cells for 1 h at 4 °C with a hypotonic Propidium Iodide buffer (0.1% Na3Citrate in ddH20, 0.1% TritonX-100, 50 μg/ml PI, 25 μg/ml RNaseA (Sigma-Aldrich)). Histogram plots generated from flow cytometry were analysed using the ModFit software programme (Verity Software House, Topsham, ME, USA).
Clonogenic assays
Cells were washed three times in PBS to remove 4-OHT and single-cell sorted by flow cytometry for PI-negative cells into round-bottom 96-well plates containing DMEM with 10% FCS, 0.25 ng/ml IL-3 with or without 0.1 μM 4-OHT. Growth supplements were replenished by adding 100 μl of DMEM plus 10% FCS, 0.25 ng/ml IL-3 and 0.1 μM 4-OHT if cells were initially sorted with 4-OHT. After 14 days, the number of colonies were counted.
For clonogenic assays in soft agar culture, cells were washed three times in PBS to remove 4-OHT and known numbers of cells were replated into a soft agar master mix with DMEM, 20% FCS and 0.3% Agar. 4-OHT was used at 0.1 μM and IL-3 at 0.3 ng/ml if required in soft agar.
Luciferase reporter assays
105 293T cells were transfected using the Effectene Transfection Kit (Qiagen) with 1.5 μg of firefly luciferase plasmid and 0.5 μg of renilla luciferase plasmid. Twenty-four hours after transfection, culture medium was replaced. Seventy-two hours after initial transfection, cells were lysed and analysed on a FLUOstar Optima (BMG Labtechnologies, Ortenberg, Germany) using the Dual Luciferase Reporter Assay System (Promega, Auburn, VIC, Australia).
miRNA PCR array
A total of 300 ng of total RNA extracted using Trizol was reverse transcribed using TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Mulgrave, VIC, Australia) and Megaplex RT Primers, Rodent Pool A and Pool B v3.0 (Applied Biosystems, Australia). cDNA was then pre-amplified using Taqman PreAmp Master Mix (Applied Biosystems, Australia) and Megaplex PreAmp Primers, Rodent Pool A nd Pool B v3.0 (Applied Biosystems, Australia). Amplified Product was then loaded onto Taqman Array Rodent v3.0 miRNA (A+B) cards (Applied Biosystems, Australia) and PCR performed on the 7900-HT real-time PCR system (Applied Biosystems, Australia). The array cards contain Taqman assays for 641 unique mouse miRNAs. Ct values were extracted for each miRNA using RQ Manager software (Applied Biosystems, Australia). miRNA data were expressed as dCT relative to U6snRNA and then normalised between samples based on global miRNA expression of the 50% highest expressing miRNAs. miRNAs with primary expression of Ct>35 and differential miRNA expression of >1.5-fold with P values ≤0.05 (Student's t-test, two-tailed, independent) were considered to be significant. Fold change is the ΔΔCt of the difference in the average normalised ΔCt across three biological samples for both +4-OHT and −4-OHT.
miRNA qRT-PCR
RNA from 5 × 106 cells was extracted using Trizol (Sigma-Aldrich). In all, 10 ng of RNA was reverse transcribed as per the manufacture's instructions using a Taqman miRNA Reverse Transcription Kit (Applied Biosystems, Australia) with the RT-specific primers for age-miR-17-3p, age-miR-17-5p, hsa-miR-16-5p, hsa-miR-20a-5p, hsa-miR-19b-3p, hsa-miR-92a-3p, age-miR-18, hsa-miR-19a-3p and U6 snRNA (all from Applied Biosystems, Australia). miRNA levels were quantitated using the Taqman Small RNA assay kit (Applied Biosystems, Australia) with miRNA-specific primers as per the manufacture's instructions. qPCR was performed using the Viia7 Real-Time PCR system (Applied Biosystems, Australia). U6 were used for normalisation.
Acknowledgments
We thank Dr. J Silke for the provision of lentiviral vectors and valuable advise, Dr. Lorraine O'Reilly, Professor Andreas Strasser and Dr. David Huang for antibodies and Bax−/−; Bak−/− mice. Dr. Matt Burton for assistance with flow cytometry. This work was supported by the National Health and Medical Research Council of Australia. PGE is supported by the Sylvia and Charles Viertel Senior Medical Fellowship. MS is supported by the National Health and Medical Research Council (NHMRC) postgraduate scholarship. This work was supported by operational infrastructure grants through the Australian Government IRISS and the Victorian State Government OIS.
Glossary
- IL-3
interleukin-3
- UTR
untranslated region
- miRNA
microRNAs
- HPC
haematopoietic progenitor cells
- ChIP
chromatin immunoprecipitation
- AML
acute myeloid leukaemia
- MLL
mixed lineage leukaemia
- 4-OHT
4-hydroxytamoxifen
- eGFP
enhanced green fluorescent protein
- FDM
factor-dependant myeloid
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by M Piacentini
Supplementary Material
References
- McGinnis W, Garber RL, Wirz J, Kuroiwa A, Gehring WJ. A homologous protein-coding sequence in drosophila homeotic genes and its conservation in other metazoans. Cell. 1984;37:403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- Phelan M, Rambaldi I, Featherstone M. Cooperative interactions between HOX and PBX proteins mediated by a conserved peptide motif. Mol Cell Biol. 1995;15:3989–3997. doi: 10.1128/mcb.15.8.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WF, Montgomery JC, Rozenfeld S, Moskow JJ, Lawrence HJ, Buchberg AM, et al. AbdB-like Hox proteins stabilize DNA binding by the Meis1 homeodomain proteins. Mol. Cell. Biol. 1997;17:6448–6458. doi: 10.1128/mcb.17.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiropoulos B, Humphries RK. Hox genes in hematopoieis and leukemogenesis. Oncogene. 2007;26:6766–6776. doi: 10.1038/sj.onc.1210760. [DOI] [PubMed] [Google Scholar]
- Antonchuk J, Sauvageau G, Humphries RK. HoxB4 induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- Perkins AC, Cory C. Conditional immortalization of mouse myelomonocytic, megakaryocytic and mast cell progenitors by the Hox-2.4 homeobox gene. EMBO J. 1993;12:3835–3846. doi: 10.1002/j.1460-2075.1993.tb06062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteinsdottir U, Mamo A, Kroon E, Jerome L, Bijl J, Lawrence HJ, et al. Overexpression of the myeloid leukemia-associated Hoxa9 gene in bone marrow cells induces stem cell expansion. Blood. 2002;99:121–129. doi: 10.1182/blood.v99.1.121. [DOI] [PubMed] [Google Scholar]
- Lawrence HJ, Helgason CD, Sauvageau G, Fong S, Izon DJ, Humphries RK, et al. Mice bearing a targeted interruption of the homeobox gene HOXA9 have defects in myeloid, erythroid, and lymphoid hematopoiesis. Blood. 1997;89:1922–1930. [PubMed] [Google Scholar]
- Izon DJ, Rozenfeld S, Fong ST, Komuves L, Largman C, Lawrence HJ. Loss of function of the homeobox gene Hoxa-9 perturbs early T-cell development and induces apoptosis in primitive thymocytes. Blood. 1998;92:383–393. [PubMed] [Google Scholar]
- Perkins A, Kongsuwan K, Visvader J, Adams JM, Cory S. Homeobox gene expression plus autocrine growth factor production elicits myeloid leukaemia. PNAS. 1990;87:8398–8402. doi: 10.1073/pnas.87.21.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt C, Aberdam D, Schwartz R, Sachs L. DNA rearrangement of a homeobox gene in myeloid leukaemic cells. EMBO J. 1988;7:4283–4290. doi: 10.1002/j.1460-2075.1988.tb03326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsuwan K, Allen J, Adams JM. Expression of Hox2.4 homeobox gene directed by proviral insertion in myeloid leukemia. Nucleic Acids Res. 1989;17:1881–1892. doi: 10.1093/nar/17.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl C, Bansal D, Dohner D, Eiwen D, Huntly BJP, Lee BH, et al. The homeobox gene CDX2 is aberrangly expressed in most cases of acute myeloid leukemia and promotes leukemogenesis. J Clin Invest. 2007;117:1037–1048. doi: 10.1172/JCI30182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekert PG, Jabbour AM, Manoharan A, Heraud JE, Yu J, Pakusch M, et al. Cell death provoked by loss of interleukin-3 signaling is independent of Bad, Bim and PI3 kinase, but depends in part on Puma. Blood. 2006;108:1461. doi: 10.1182/blood-2006-03-014209. [DOI] [PubMed] [Google Scholar]
- Ekert PG, Read SH, Silke J, Marsden VS, Kaufmann H, Hawkins CJ, et al. Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J Cell Biol. 2004;165:835–842. doi: 10.1083/jcb.200312031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino T, Yamakazi Y, Largaespada DA, Jenkins NA, Copeland NG, Hirokawa K, et al. Inhibition of myeloid differentiation by Hoxa9, Hoxb8 and Meis homeobox genes. Exp Hematol. 2001;29:856–863. doi: 10.1016/s0301-472x(01)00655-5. [DOI] [PubMed] [Google Scholar]
- Knoepfler PS, Sykes DB, Pasillas M, Kamps MP. HoxB8 requires its Pbx-interaction motif to block differentiation of primary myeloid progenitors and of most cell line models of myeloid differentiation. Oncogene. 2001;20:5440–5448. doi: 10.1038/sj.onc.1204710. [DOI] [PubMed] [Google Scholar]
- Faber J, Krivtsov AV, Stubbs MC, Wright R, Davis TN, van den Heuvel-Eibrink M, et al. HOXA9 is required for survival in human MLL-rearranged acute leukemias. Blood. 2009;113:2375–2385. doi: 10.1182/blood-2007-09-113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts MB, Wang DP, Cameron S. Trithorax, Hox, and TALE-class homeodomain proteins ensure cell survival through repression of the BH3-only gene egl-1. Dev Biol. 2009;329:374–385. doi: 10.1016/j.ydbio.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Liu H, Strauss TJ, Potts MB, Cameron S. Direct regulation of egl-1 and of programmed cell death by the Hox protein MAB-5 and by CEH-20, a C. elegans homolog of Pbx1. Development. 2005;133:641–650. doi: 10.1242/dev.02234. [DOI] [PubMed] [Google Scholar]
- Jabbour AM, Heraud JE, Daunt CP, Kaufmann T, Sandow J, O'Reilly LA, et al. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death Differ. 2009;16:555–563. doi: 10.1038/cdd.2008.179. [DOI] [PubMed] [Google Scholar]
- Sandow JJ, Jabbour AM, Condina MR, Daunt CP, Stomski FC, Green BD, et al. Cytokine receptor signaling activates an IKK-dependent phosphorylation of PUMA to prevent cell death. Cell Death Differ. 2012;19:633–641. doi: 10.1038/cdd.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GG, Calvo KR, Pasillas MP, Sykes DB, Hacker H, Kamps MP. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat Methods. 2006;3:287–293. doi: 10.1038/nmeth865. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Thompson CB. Cell death in the absence of Bax and Bak. Cell Death Differ. 2006;13:1272–1276. doi: 10.1038/sj.cdd.4401953. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TW, Kontgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocek E, Wang X, Liu X, Liu C-G, Studzinski GP. MicroRNA-32 upregulation by 1,25-dihydroxyvitamin D3 in human myeloid leukemia cells leads to Bim targeting and inhibition of AraC-induced apoptosis. Cancer Res. 2011;71:6230–6239. doi: 10.1158/0008-5472.CAN-11-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med. 2011;208:549–560. doi: 10.1084/jem.20101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, et al. Targeted deletion reveals essential and overlapping functions of the miR-17∼92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson A, KK-H Farh, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Biol. 2011;18:1139–1146. doi: 10.1038/nsmb.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Iwasaki M, Somervaille TCP, Ficara F, Carico C, Arnold C, et al. The miR-17-92 microRNA polycistron regulates MLL leukemia stem cell potential by modulating p21 expression. Cancer Res. 2010;70:3833–3842. doi: 10.1158/0008-5472.CAN-09-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, et al. miR-19 is a key oncogenic component of mir-17-92. Genes Dev. 2009;23:2839–2849. doi: 10.1101/gad.1861409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Fiori ME, Albini S, Cifaldi L, Giovinazzi S, Forloni M, et al. Antagomir-17-5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS One. 2008;3:e2236. doi: 10.1371/journal.pone.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour AM, Daunt CP, Green BD, Vogel S, Gordon L, Lee RS, et al. Myeloid progenitor cells lacking p53 exhibit delayed up-regulation of Puma and prolonged survival after cytokine deprivation. Blood. 2010;115:344–352. doi: 10.1182/blood-2009-07-230730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- Yekta S, Shih I-h, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Seto M. A microRNA cluster as a target of genomic amplification in malignant lymphoma. Leukemia. 2005;19:2013–2016. doi: 10.1038/sj.leu.2403942. [DOI] [PubMed] [Google Scholar]
- Rao E, Jiang C, Ji M, Huang X, Iqbal J, Lenz G, et al. The miRNA17∼92 cluster mediates chemoresistance and enhances tumor growth in mantle cell lymphoma via PI3K/AKT pathway activation. Leukemia. 2005;26:1064–1072. doi: 10.1038/leu.2011.305. [DOI] [PubMed] [Google Scholar]
- Diosdado B, van de Wiel MA, Terhaar Sive Droste JS, Mongera S, Postma C, Meijerink WJHJ, et al. MiR-17-92 cluster is associated with 13q gain and c-myc expression during colorectal adenoma to adenocarcinoma progression. Br J Cancer. 2009;101:707–714. doi: 10.1038/sj.bjc.6605037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volinia S, Calin GA, Liu C-G, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemin-van der Poel S, McCabe NR, Gill HJ, Espinosa R, Patel Y, Harden A, et al. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci USA. 1991;88:10735–10739. doi: 10.1073/pnas.88.23.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton PM, Cleary ML. Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev. 2003;17:2298–2307. doi: 10.1101/gad.1111603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata M, Tagawa H, Guo Y-M, Kameoka Y, Takahashi N, Sawada K. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood. 2009;113:396–402. doi: 10.1182/blood-2008-07-163907. [DOI] [PubMed] [Google Scholar]
- Nagel S, Venturini L, Przybylski GK, Grabarczyk P, Schmidt CA, Meyer C, et al. Activation of miR-17-92 by NK-like homeodomain proteins suppresses apoptosis via reduction of E2F1 in T-cell acute lymphoblastic leukemia. Leuk Lymphoma. 2009;50:101–108. doi: 10.1080/10428190802626632. [DOI] [PubMed] [Google Scholar]
- Thomson DW, Bracken CP, Goodall GJ. Experimental strategies for microRNA target identification. Nucleic Acids Res. 2011;39:6845–6853. doi: 10.1093/nar/gkr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap YYC, Ng IHW, Badrian B, Nguyen TÄ, Yip YY, Dhillon AS, et al. c-Jun N-terminal kinase/c-Jun inhibits fibroblast proliferation by negatively regulating the levels of stathmin/oncoprotein 18. Biochem J. 2010;430:345–354. doi: 10.1042/BJ20100425. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandengerg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic, ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.