Abstract
Exposure of keratinocytes (KC) to ultraviolet (UV) radiation results in the initiation of apoptosis, a protective mechanism that eliminates cells harboring irreparable DNA damage. Hence, a manipulation of UV-induced apoptosis may significantly influence photocarcinogenesis. We have discovered that the aryl hydrocarbon receptor (AHR), a key regulator of drug metabolism and an UVB-sensitive transcription factor, serves an anti-apoptotic function in UVB-irradiated human KC. Chemical and shRNA-mediated inhibition of AHR signaling sensitized KC to UVB-induced apoptosis by decreasing the expression of E2F1 and its target gene checkpoint kinase 1 (CHK1). The decreased expression of these cell-cycle regulators was due to an enhanced expression of p27KIP1 and an associated decrease in phosphorylation of both cyclin-dependent kinase 2 and its substrate molecule retinoblastoma protein. The subsequent inhibition of E2F1 autoregulation and downstream CHK1 expression resulted in an enhanced susceptibility of damaged cells to undergo apoptosis. Accordingly, ectopic overexpression of either E2F1 or CHK1 in AHR-knockdown KC attenuated the observed sensitization to UVB-induced apoptosis. Using an AHR-knockout SKH-1 hairless mouse model, we next demonstrated the physiological relevance of the anti-apoptotic function of AHR. In contrast to their AHR-proficient littermates, the constitutive expression of E2F1 and CHK1 was significantly reduced in the skin of AHR-knockout mice. Accordingly, a single exposure of the animals to UVB resulted in an enhanced cleavage of caspase-3 in the skin of AHR-knockout mice. These results identify for the first time the AHR-E2F1-CHK1 axis as a novel anti-apoptotic pathway in KC, which may represent a suitable target for chemoprevention of non-melanoma skin cancer.
Keywords: aryl hydrocarbon receptor, apoptosis, chemoprevention, skin cancer, UVB radiation
With an annual incidence of two to three million new cases worldwide, non-melanoma skin cancer (NMSC) is one of the most frequent forms of cancer in humans (World Health Organization; http://www.who.int/uv/faq/skincancer/en/index1.html). Typically, NMSCs, such as basal cell and squamous cell carcinomas, occur on sun-exposed areas of the skin, indicating that solar ultraviolet (UV) radiation, especially its UVB part (290–320 nm), is the most important etiological factor.1, 2 In fact, it is well established that UVB radiation gives rise to skin cancer without additional initiators or promoters and thus is a complete carcinogen.1 Because of the thinning of the ozone layer, the increase in recreational sunbathing and the enhanced usage of UV tanning beds, the incidence of NMSC is expected to increase in future years,1, 2 underscoring the urgent need for novel preventives against UV-induced skin malignancies.
When skin is exposed to solar radiation, high-energy UVB photons penetrate into the epidermis and initiate the so-called UVB response.3 The main trigger of this stress response is the absorbance of UVB rays by DNA, which leads to the generation of highly mutagenic DNA photoproducts.3, 4 In addition, UVB exposure results in the formation of reactive oxygen species (ROS), which may cause spontaneous single- and double-strand breaks due to random oxidation of DNA bases.4 Occurrence of DNA damage activates the DNA damage response network, which initiates cell-cycle arrest to enable DNA repair or, in case damage is too severe, apoptosis to avoid mitotic manifestation of mutations.4, 5 Besides its role in epidermal development, apoptosis is probably the most important mechanism protecting the organism against genotoxic hazards.4, 6 UVB irradiation initiates apoptosis via (i) damage of nuclear DNA,5 (ii) activation of cell surface death receptors,7 and (iii) intracellular formation of ROS accompanied by mitochondrial dysfunction and cytochrome c release.8 The importance of UVB-induced apoptotic cell death as a protective mechanism becomes evident by the fact that the reduced risk of dark-skinned individuals to develop skin cancer is, to a major part, due to facilitation of UVB-induced epidermal apoptosis by melanin.9 In addition, the property of several plant polyphenols to prevent UVB-induced skin carcinogenesis is also attributed to their ability to increase apoptosis in epidermal cells.6
We have previously shown that UVB exposure of keratinocyte (KC) resulted in the activation of the aryl hydrocarbon receptor (AHR),10 a ligand-activated transcription factor and central regulator of drug metabolism.11 More precisely, UVB rays are absorbed by free tryptophan in the cytosol of KC, resulting in the formation of the tryptophan-photoproduct 6-formylindolo[3,2-b]carbazole (FICZ),10 a high-affinity ligand of the AHR.12 Alike polycyclic aromatic hydrocarbons (PAHs) and dioxins, FICZ binds to the AHR and initiates the dissociation of the cytosolic AHR multiprotein complex, consisting of heat-shock protein 90, tyrosine kinase c-src, and other co-chaperones, thereby enabling the nuclear translocation of AHR.10, 11 In the nucleus, the AHR dimerizes with ARNT (AHR nuclear translocator) and binds to xenobiotic-responsive elements (XRE) in the promoter region of genes to enforce their transcription. The AHR gene battery encodes for drug-metabolizing enzymes, such as cytochrome P450 (CYP) 1A1, as well as for proteins involved in the regulation of cell growth and differentiation.11, 13 Beside this AHR/ARNT-triggered pathway, the ligand-driven release of c-src in the cytoplasm initiates so-called ‘non-genomic' AHR signaling that sequentially comprises c-src translocation from cytosol to cell membrane, phosphorylation of the epidermal growth factor receptor, activation of downstream MAPK, and induction of another set of target genes, including cyclooxygenase-2 (COX-2).10, 13 As an induction of CYP activity can lead to ROS formation and the role of COX-2 in inflammation and cancer is well-known, the AHR is regarded as a crucial regulator of the UVB stress response.14
Although it was reported that, depending on cell-type, tissue, and applied stressor, the AHR can act either pro-apoptotic or anti-apoptotic,15 it is up-to-now not clear to what extent AHR signaling influences apoptosis in UVB-exposed KC. Here we asked if the AHR influences apoptosis in human NCTC 2544 KC, an established non-tumorigenic in vitro model for KC biology.16 We found that chemical- and shRNA-mediated inhibition of AHR sensitized human KC to UVB-induced apoptosis by increasing the expression of p27KIP1, which subsequently repressed the autoregulatory expression of E2F1 and downstream expression of checkpoint kinase 1 (CHK1). Recovery of E2F1 or CHK1 expression abrogated apoptosis susceptibility of AHR-knockdown KC, indicating that the AHR-E2F1-CHK1 axis is a promising target for prevention of UVB-induced NMSC. Studies on AHR-proficient and AHR-knockout mice confirmed the in vivo relevance of this novel anti-apoptotic pathway in skin.
Results
Inhibition of AHR signaling increases the rate of UVB-induced apoptosis in human KC
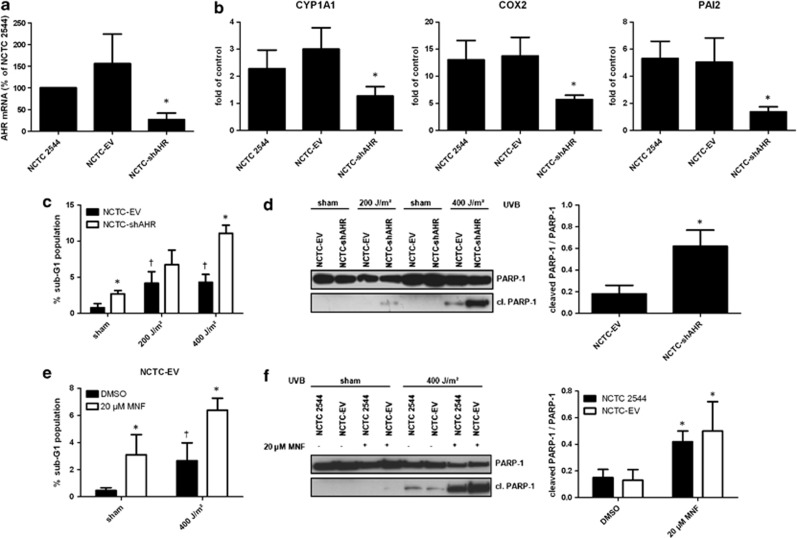
In order to investigate whether AHR signaling has an impact on UVB-induced apoptosis in NCTC 2544 KC, we generated stable AHR-knockdown NCTC 2544 cells (NCTC-shAHR) via lentiviral shRNA transduction. In contrast to NCTC 2544 and empty vector control cells (NCTC-EV), the basal mRNA expression of AHR in NCTC-shAHR KC was decreased by approximately 80% (Figure 1a). Irradiation with 200 J/m2 UVB, equivalent to the minimal erythema dose of fair-skinned individuals,17 led to a significantly lower induction of the AHR target genes CYP1A1, COX-2, and plasminogen activator inhibitor-2 (PAI-2; involved in regulation of extracellular matrix degradation18) in NCTC-shAHR cells compared with NCTC 2544 and NCTC-EV cells (Figure 1b), providing evidence that AHR signaling is significantly suppressed in these KC.
Figure 1.
Inhibition of AHR enhances UVB-induced apoptosis in human KC. (a) Real-time PCR analyses revealed a reduced AhR mRNA expression in NCTC-shAHR KC compared with NCTC-EV and NCTC 2544 cells. AHR transcripts are shown as fold of NCTC 2544. *Significantly reduced compared with NCTC 2544; (b) NCTC 2544, NCTC-EV, and NCTC-shAHR KC were irradiated with 200 J/m2 UVB. After 6 h, KC were harvested and CYP1A1, COX-2, and PAI-2 mRNA were quantified by real-time PCR. Expression rates are given as fold of non-irradiated control. *Significantly reduced compared with NCTC-EV; (c) NCTC-EV and NCTC-shAHR KC were irradiated with 200 or 400 J/m2 UVB. After 24 h, percentage of sub-G1 DNA content was measured by PI staining and flow cytometry. *Significantly increased compared with NCTC-EV, †significantly increased compared with sham-irradiated NCTC-EV; (d) NCTC-EV and NCTC-shAHR KC were irradiated with 200 or 400 J/m2 UVB. After 24 h, protein was isolated and western blots were done using an anti-PARP-1 antibody that detects both PARP-1 and cleaved (cl.) PARP-1. *Significantly increased compared with NCTC-EV exposed to 400 J/m2 UVB; (e) NCTC-EV and NCTC-shAHR KC were treated with 0.1% DMSO or 20 μM MNF 1 h prior exposure to 400 J/m2 UVB, and 24 h later the percentage of sub-G1 DNA content was measured. *Significantly increased compared with DMSO-treated NCTC-EV, †significantly increased to sham-irradiated solvent control cells. (f) NCTC 2544 and NCTC-EV KC were treated with 0.1% DMSO or 20 μM MNF 1 h prior exposure to 400 J/m2 UVB, and 24 h later cleavage of caspase-3 was analyzed. *Significantly increased compared with DMSO-treated NCTC 2544 and NCTC-EV cells, respectively
Exposure of NCTC-EV cells to 200 and 400 J/m2 UVB significantly elevated the rate of apoptosis after 24 h, as shown by both increasing sub-G1 fraction (Figure 1c; for representative cell-cycle profiles see Supplementary Figure 1) and cleavage of the caspase substrate poly(ADP-ribose)-polymerase-1 (PARP-1; Figure 1d). Identical UVB exposure of NCTC-shAHR KC resulted in a significantly higher apoptosis rate, suggesting that AHR-knockdown sensitizes KC to UVB stress (Figures 1c and d). This observation was further confirmed by analyses of caspase-3 cleavage (see Supplementary Figure 2). Noteworthy, sham-irradiated NCTC-shAHR cells also exhibited a slight but significant enhancement of apoptosis compared with NCTC-EV cells. To verify the AHR-dependency of these results, we treated NCTC-EV cells with 20 μM of the specific AHR antagonist 3′-methoxy-4′-nitroflavone (MNF)10 for 1 h before UVB exposure. As expected, chemical AHR antagonism also led to an increase of UVB-induced apoptosis, as indicated by an augmented sub-G1 population (Figure 1e). Accordingly, a 1-h pre-treatment of NCTC 2544 and NCTC-EV cells with 20 μM MNF resulted in an enhanced cleavage of both PARP-1 (Figure 1f) and caspase-3 (see Supplementary Figure 2) upon exposure to 400 J/m2 UVB.
Enhanced apoptosis susceptibility of KC with impaired AHR signaling is accompanied by a loss of E2F1 expression
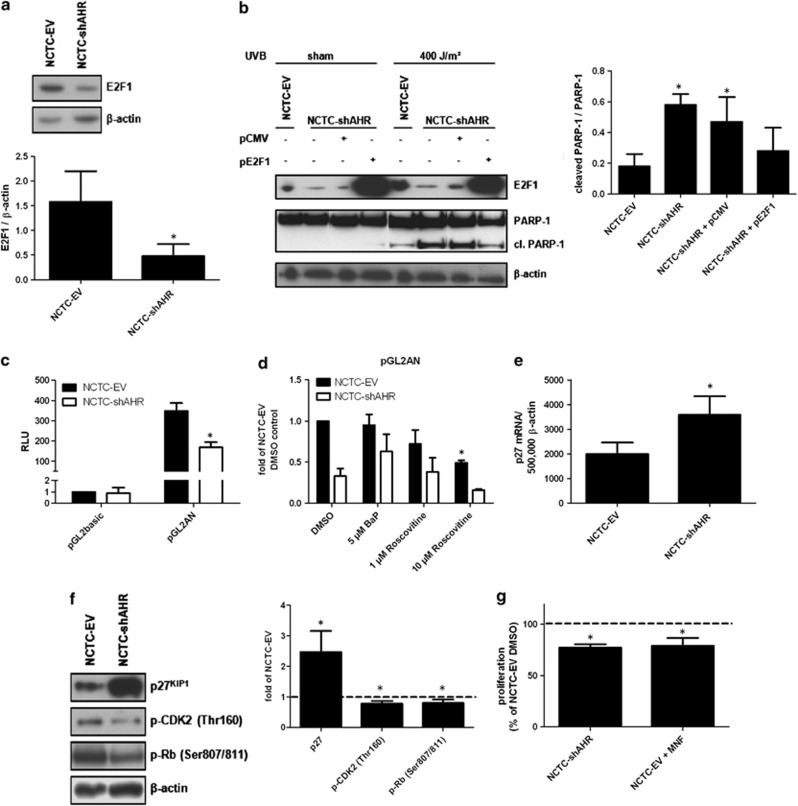
To identify the molecular targets responsible for the enhanced susceptibility of NCTC-shAHR cells to UVB radiation, we analyzed the expression of proteins involved in DNA damage responses. The expression of E2F1 was found to be dramatically repressed in NCTC-shAHR cells (Figure 2a). To test if the reduced E2F1 level was causative for sensitization of NCTC-shAHR cells to UVB-induced apoptosis, we transiently overexpressed E2F1 in these cells. 24 h later, KC were irradiated with 400 J/m2 UVB and apoptosis was analyzed by PARP-1/cleaved PARP-1 western blotting. Recovery of E2F1 expression abrogated the sensitization to UVB-induced apoptosis in NCTC-shAHR cells (Figure 2b).
Figure 2.
A reduced E2F1 level is responsible for sensitization of NCTC-shAHR cells to UVB-induced apoptosis. (a) Protein expression of E2F1 and β-actin in NCTC-EV and NCTC-shAHR KC. *Significantly reduced compared with NCTC-EV; (b) NCTC-shAHR KC were transiently transfected with 0.75 μg of the E2F1 expression vector (pE2F1) or empty vector pCMV. After 24 h, cells were irradiated with 400 J/m2 UVB, additional 24 h later western blotting was performed using the indicated antibodies. *Significantly increased compared with NCTC-EV; (c) NCTC-EV and NCTC-shAHR KC were transiently transfected with the E2F1 promoter-driven reporter construct pGL2AN or empty vector pGL2basic. Luciferase activities were measured after 48 h. Results are shown as fold of NCTC-EV empty vector controls. *Significantly reduced compared with NCTC-EV; (d) NCTC-EV and NCTC-shAHR KC were transiently transfected with pGL2AN. After 24 h, KC were treated with 5 μM BaP, 1 and 10μM Roscovitine or 0.1% DMSO, and luciferase activities were measured after additional 24 h. Results are shown as fold of DMSO-treated NCTC-EV samples. *Significantly reduced compared with NCTC-EV. (e) Basal expression of p27KIP1 mRNA in NCTC-EV and NCTC-shAHR KC 24 h after seeding. *Significantly increased compared with NCTC-EV KC; (f) basal expression of p27KIP1 protein in NCTC-EV and NCTC-shAHR KC 24 h after seeding. *Significantly altered compared with NCTC-EV; (g) proliferation rates of growing NCTC-EV (treated with 0.1% DMSO or 20 μM MNF) and NCTC-shAHR cells were measured by BrdU incorporation for 24 h and are given as fold of DMSO-treated NCTC-EV. *Significantly reduced compared with DMSO-treated NCTC-EV
To investigate the mechanism responsible for the decreased E2F1 expression in NCTC-shAHR KC, we performed comparative analyses of E2F1 promoter-driven reporter gene (pGL2AN; encompassing an approximately 275 bp fragment directly located 5′-upstream of the E2F1 transcription start site19) activities. As shown in Figure 2c, NCTC-shAHR cells revealed an approximately 50% reduction in basal luciferase activity, despite the lack of putative XRE sequences. Accordingly, treatment of pGL2AN-transfected NCTC-EV cells with 5 μM of the AHR agonist benzo(a)pyrene (BaP) did not alter luciferase activity (Figure 2d). Exposure of both transfected KC lines to the src-kinase inhibitor PP2 and the MEK inhibitor PD98059 did also not affect reporter gene activity, excluding the involvement of non-genomic AHR signaling in E2F1 gene regulation (see Supplementary Figure 3). Extensive inhibitor studies also excluded the involvement of NF-κB, Sp1, and activating transcription factor, an effector of activated protein kinase A, in E2F1 regulation (see Supplementary Figure 3), although respective putative-binding motifs are located in the human E2F1 promoter.19 In addition, the E2F1 promoter contains E2F-binding sites, which enable E2F1 autoregulation.19, 20 This E2F1 autoregulatory loop strongly depends on the release of E2F1 from retinoblastoma protein (Rb), which is triggered by the balance between the Rb-phosphorylating cyclin-dependent kinase-2 (CDK2) and the inhibitory p27KIP1 protein.21 As expected, exposure of pGL2AN-transfected NCTC-EV KC to 10 μM Roscovitine, a CDK2 inhibitor (that notably also inhibits CDK1 and CDK522), resulted in a 50% reduction of basal E2F1 promoter activity (Figure 2d), implying that CDK2 activity and thus E2F1 autoregulation is most probably repressed in NCTC-shAHR KC. Accordingly, we observed an increased expression of p27KIP1 mRNA and protein in NCTC-shAHR KC (Figures 2e and f), implying a repressive function of the AHR on p27KIP1 expression in NCTC-EV KC. Importantly, (i) Roscovitine exposure was sufficient to sensitize NCTC-EV, but not NCTC-shAHR, cells to UVB-induced apoptosis (see Supplementary Figure 4), and (ii) the repressive function of AHR on p27KIP1 expression was also confirmed in stable HaCaT-shAHR KC (see Supplementary Figure 5).
It is worth mentioning that we observed a slight reduction of E2F1 promoter activity in Roscovitine-treated NCTC-shAHR KC (Figure 2d), which was probably due to an inhibition of the residual CDK2 activity in these cells. The enhanced p27KIP1 expression in NCTC-shAHR KC was accompanied by an approximately 20% reduced phosphorylation of CDK2 and Rb (Figure 2f), which may explain the reduced E2F1 expression as well as the anti-proliferative effects of AHR inhibition, which became evident by an approximately 20% reduced proliferation rate of NCTC-shAHR and MNF-exposed NCTC-EV KC compared with NCTC-EV cells (Figure 2g). These data provide compelling evidence that the AHR maintains E2F1 expression in KC, most probably via repression of p27KIP1 expression.
Reduction of CHK1 expression is causative for the enhanced apoptosis susceptibility of KC with impaired AHR signaling
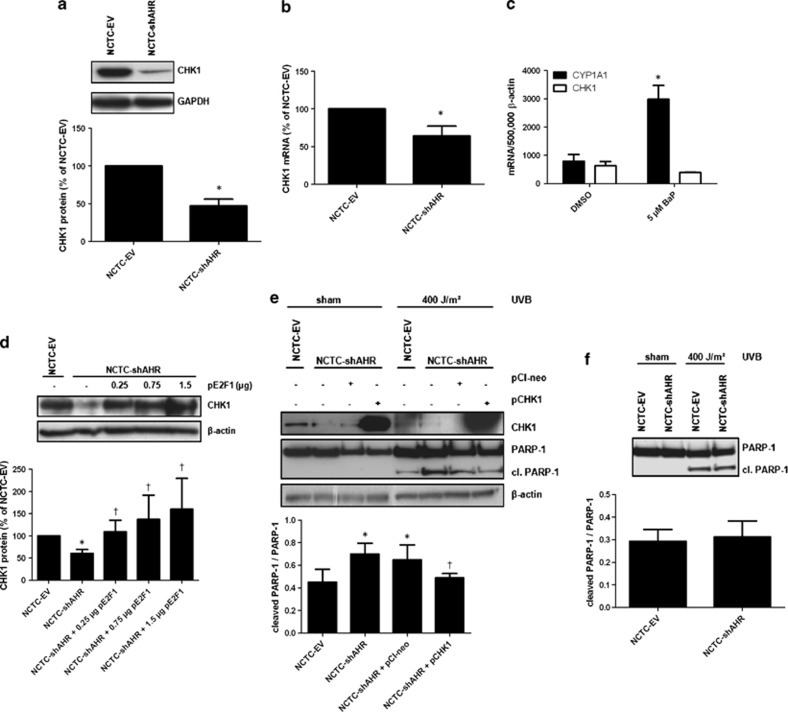
We next addressed the molecular mechanism by which the loss of E2F1 expression sensitizes KC to UVB-induced apoptosis. A prominent target gene of E2F1 is CHK1, a critical regulator of cell cycle that induces cell-cycle arrest in response to DNA damage.23, 24 To investigate a potential involvement of CHK1 in the observed sensitization to apoptosis, we compared CHK1 expression in NCTC-EV and NCTC-shAHR KC. We found that CHK1 expression was clearly reduced in NCTC-shAHR cells (Figure 3a), and this decline in protein level was accompanied by a significantly decreased expression of the corresponding mRNA (Figure 3b). Exposure of NCTC-EV cells to 5 μM BaP did not alter CHK1 expression, indicating that CHK1 is not part of the XRE-dependent AHR gene battery (Figure 3c). Accordingly, the characterization of the human CHK1 gene promoter did not reveal the existence of putative XRE, but the presence of several responsive E2F1 binding sites.23 As expected, E2F1 overexpression in NCTC-shAHR cells restored CHK1 expression in a gene dose-dependent manner, providing evidence that the loss of E2F1 was causative for the decrease of CHK1 expression (Figure 3d). To test if the loss of CHK1 expression in NCTC-shAHR cells was responsible for the increase in UVB-induced apoptosis, we performed ectopic overexpression of human CHK1 protein in these cells. Twenty-four hours after transient transfection, cells were irradiated with 400 J/m2 UVB and apoptosis was measured by analysis of PARP-1 cleavage. Indeed, recovery of CHK1 expression attenuated the previously observed sensitization to UVB-induced apoptosis in NCTC-shAHR cells (Figure 3e). These data strongly indicate that inhibition of AHR signaling in KC leads to a p27KIP1-mediated decrease in expression of E2F1 and its target gene CHK1, which results in an enhanced elimination of UVB-damaged cells.
Figure 3.
Expression of CHK1 is diminished in NCTC-shAHR cells and causative for the enhanced susceptibility to UVB-induced apoptosis. (a) Protein expression of CHK1 and GAPDH in NCTC-EV and NCTC-shAHR KC. *Significantly reduced compared with NCTC-EV; (b) CHK1 mRNA expression was quantified by real-time PCR. Expression levels are given as fold of NCTC-EV. *Significantly reduced compared with NCTC-EV cells; (c) NCTC-EV KC were treated for 6 h with 5 μM BaP or 0.1% DMSO. CYP1A1 and CHK1 transcripts were determined by real-time PCR. *Significantly different from DMSO-treated NCTC-EV; (d) NCTC-shAHR KC were transiently transfected with indicated amounts of pE2F1. After 48 h, CHK1 and β-actin protein expression were determined. *Significantly reduced compared with NCTC-EV, †significantly increased compared with NCTC-shAHR; (e) NCTC-shAHR KC were transfected with 0.75 μg of the CHK1 expression vector (pCHK1) or empty vector pCI-neo. After 24 h, KC were irradiated with 400 J/m2 UVB, and additional 24 h later western blotting was performed using indicated antibodies. *Significantly increased compared with UVB-exposed NCTC-EV, †significantly decreased compared with UVB-irradiated pCI-neo-transfected NCTC-shAHR KC; (f) NCTC-EV and NCTC-shAHR KC were cultured in FCS-free medium for 24 h prior UVB irradiation (400 J/m2) to stop proliferation. After 24 h, protein was isolated and western blots were done using an anti-PARP-1 antibody that detects both PARP-1 and cleaved (cl.) PARP-1
As CHK1-triggered checkpoint control occurs in proliferating cells, we compared the apoptosis sensitivity of serum-starved, non-proliferative NCTC-EV and NCTC-shAHR cells. As expected, we detected equal amounts of cleaved PARP-1 in both cell lines 24 h after UVB irradiation, indicating that the identified molecular mechanism depends on cell-cycle progression (Figure 3f).
The anti-apoptotic AHR-E2F1-CHK1 axis is present in vivo
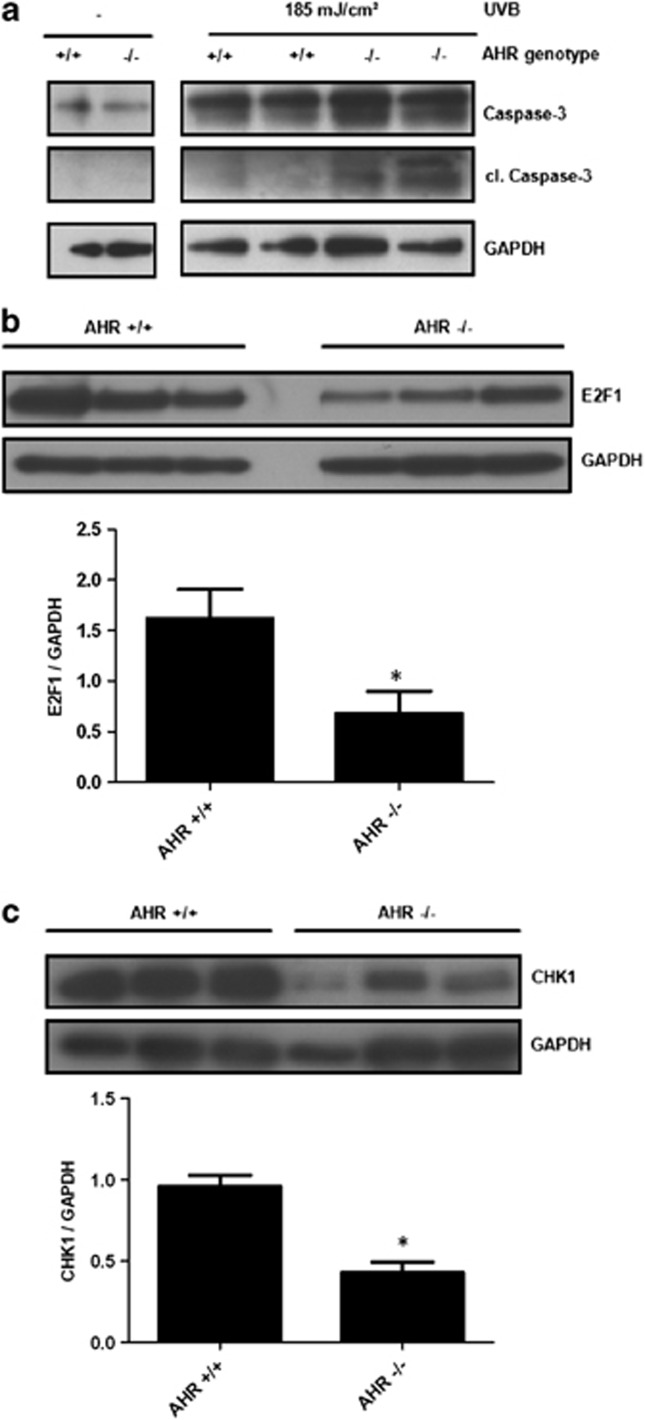
Next, we investigated if the anti-apoptotic function of the AHR was also present in mouse skin. Therefore, male AHR-proficient and AHR-deficient SKH-1 hairless mice were irradiated with a single dose of 185 mJ/cm2 UVB, and 48 h later the dorsal skin of the animals was prepared and protein was isolated. In contrast to AHR-proficient SKH-1 mice, we observed an enhanced cleavage of caspase-3 in the skin of AHR-knockout SKH-1 mice (Figure 4a), indicating that the identified AHR-dependent anti-apoptotic pathway is of in vivo relevance. Expression analyses from skin samples of unexposed AHR-proficient and AHR-knockout SKH-1 mice revealed a reduced expression of E2F1 and CHK1 (Figures 4b and c), further emphasizing the importance of the AHR-E2F1-CHK1 axis for the cutaneous DNA damage response.
Figure 4.
Presence of the anti-apoptotic AHR-E2F1-CHK1 axis in skin of SKH-1 hairless mice. (a) AHR-proficient and AHR-knockout mice were irradiated once with 185 mJ/cm2 UVB and 48 h later the mice were killed, the dorsal skin was prepared and total protein was isolated. Expression and cleavage of caspase-3 was determined by western blot analyses; (b) expression of E2F1 in the skin of unexposed AHR-proficient and AHR-knockout SKH-1 mice was determined by western blot analyses. *Significantly reduced compared with AHR-proficient SKH-1 mice. (c) Expression of CHK1 in the skin of unexposed AHR-proficient and AHR-knockout SKH-1 mice was determined by western blot analyses. *Significantly reduced compared with AHR-proficient SKH-1 mice
Discussion
The AHR is an intracellular chemosensor, whose activity can be easily manipulated by either agonistic or antagonistic ligands.11 AHR-deficient mice are protected against PAH carcinogenicity,25 transgenic overexpression of a constitutive active AHR induces tumorigenesis,26 and immortalized AHR-deficient mouse fibroblasts have impaired tumorigenicity in a subcutaneous mouse xenograft model.27 Thus, antagonism of the AHR may prevent tumor initiation and promotion. The cancer preventive properties of AHR antagonists are often attributed to their ability to decrease the expression and activity of enzymes involved in metabolic activation of procarcinogens.28, 29, 30 Interestingly, some AHR-antagonizing compounds, such as resveratrol,30 were also identified to protect mice against UVB-induced skin carcinogenesis by enhancing apoptosis.31 Thus, it is tempting to speculate that AHR antagonism may counteract tumor initiation by increasing epidermal cell death. Indeed, the AHR is known to modulate apoptosis in many different cell types and tissues in both pro-apoptotic and anti-apoptotic manners.15 Here, we demonstrate for the first time that AHR inhibition leads to an increase in apoptosis of UVB-exposed human KC. Moreover, we validate this in vitro observation by showing an enhanced occurrence of UVB-induced apoptosis in the skin of SKH-1 AHR knockout mice.
AHR-knockdown in human KC was accompanied by a decreased expression of E2F1, and experimental recovery of E2F1 expression attenuated the responsiveness to UVB-induced apoptosis. E2F1 is a transcription factor involved in cell-cycle control and apoptosis, whose expression is often dysregulated in human tumors.32, 33 Upon DNA damage, E2F1 is phosphorylated by CHK1 and CHK2 and subsequently induces transcription of p73 and its target genes to initiate p53-independent apoptosis.34 Studies on murine embryonic fibroblasts indicated that the AHR inhibits E2F1-induced apoptosis, probably due to a physical interaction of both transcription factors.35 However, the observed downregulation of E2F1 protein expression (Figure 2a) contradicts an involvement of a putative AHR/E2F1 protein interaction in enhancing the sensitivity of NCTC-shAHR KC to UVB-induced apoptosis.
Noteworthy, the inhibition of either E2F1 expression or E2F1 transcriptional activity was also reported to enhance the sensitivity of several cell types to apoptotic stimuli,36, 37 and blocking of E2F1 expression in vivo led to an increase of p53-independent apoptosis in UVB-irradiated mouse epidermis.38
Puga and colleagues observed a reduced E2F1 expression in livers of AHR-deficient mice, indicating that the AHR is required for basal E2F1 expression.39 This is confirmed by our data, demonstrating that E2F1 protein expression as well as promoter activity is reduced in NCTC-shAHR KC. Interestingly, E2F1 promoter activity did not change in response to BaP treatment in AHR-proficient KC, strongly indicating that E2F1 is not a member of the XRE-dependent AHR gene battery. The negative effect of Roscovitine on E2F1 promoter activity in NCTC-EV cells, combined with the divergent proliferation rates of NCTC-EV and KC with impaired AHR signaling, point to AHR-dependent differences in CDK2 activity. The enhanced expression of p27KIP1, combined with the reduced phosphorylation of CDK2 and Rb, observed in NCTC-shAHR cells further strengthen this idea. p27KIP1 is known to block CDK2 activation and subsequent Rb phosphorylation, and thereby inhibits the release of E2F1 from Rb resulting in an impaired E2F1 autoregulation.19, 20, 21 In contrast to an earlier study showing that dioxin exposure induces p27KIP1 expression in murine thymus and hepatoma cells in an AHR-dependent manner,40 recently published studies confirmed a higher basal expression of this protein in AHR-knockdown HaCaT KC41 and AHR-knockdown medulloblastoma cells.42 Although a comparison between receptor activation and loss-of-function experiments has to be interpreted carefully, the observed repressive action of AHR on gene expression in the absence of any exogenous ligands may point to the potential presence of endogenous AHR ligands.12 Noteworthy, a nuclear-localized constitutively active AHR that blocks gene expression, probably by inducing local epigenetic modifications, has also been previously described.43, 44 Whether the AHR directly binds the p27KIP1 promoter to repress transcription is not known up-to-now. Alternatively, the AHR may affect p27KIP1 expression indirectly by modulating other transcription factors, such as HES-1 or c-myc, which are known to inhibit p27KIP1 expression.45, 46 Even though the precise molecular mechanism remains to be elucidated, our data indicate that the AHR is a critical regulator of p27KIP1 in human KC. Ectopic overexpression of p27KIP1 was reported to initiate apoptosis in several mammalian cells.47 Accordingly, an increased expression of p27KIP1 was previously linked to the anti-proliferative and pro-apoptotic properties of the chemopreventive agent epigallocatechin-3-gallate,48, 49 a known antagonist of AHR signaling.29 The repressive influence of an enhanced p27KIP1 level on E2F1 expression, observed in our study, may contribute to these beneficial effects.
Our E2F1 rescue experiments revealed that the decrease in E2F1 expression was causative for the reduced CHK1 expression in NCTC-shAHR KC. Furthermore, BaP exposure did not affect CHK1 transcription, providing evidence that CHK1 expression is not directly regulated via the AHR. The CHK1 gene encodes for a serine/threonine protein kinase responsible for induction of cell-cycle arrest upon DNA damage.50, 51 More precisely, DNA damage-activated ATR kinase phosphorylates CHK1, which subsequently phosphorylates CDC25 phosphatase. This phosphorylation targets CDC25 to 14-3-3-mediated nuclear export and subsequent proteolysis and thus prevents CDC25-mediated activation of CDC2, which is critical for progression into M-phase. Recently, it was shown that both siRNA-triggered inhibition of CHK1 expression as well as caffeine-mediated inhibition of CHK1 activation abrogated checkpoint control and augmented UVB-induced apoptosis in human primary KC.52 Accordingly, pre-exposure of SKH-1 hairless mice to caffeine resulted in an enhanced rate of UVB-induced apoptosis in the epidermis53 and protected the animals against photocarcinogenesis.54 In addition, epidemiological data imply that daily caffeinated coffee consumption decreases the incidence and prevalence of NMSC in Caucasians,55, 56 indicating that CHK1 inhibition is indeed effective in terms of cancer chemoprevention in humans. As overexpression of CHK1 attenuated the enhanced apoptosis rate of UVB-exposed NCTC-shAHR KC, it is highly likely that the loss of checkpoint control drives damaged KC into the suicide program (Figure 5). This notion is further confirmed by the missing difference in apoptosis susceptibility of UVB-exposed non-proliferative NCTC-EV and NCTC-shAHR KC (Figure 3f).
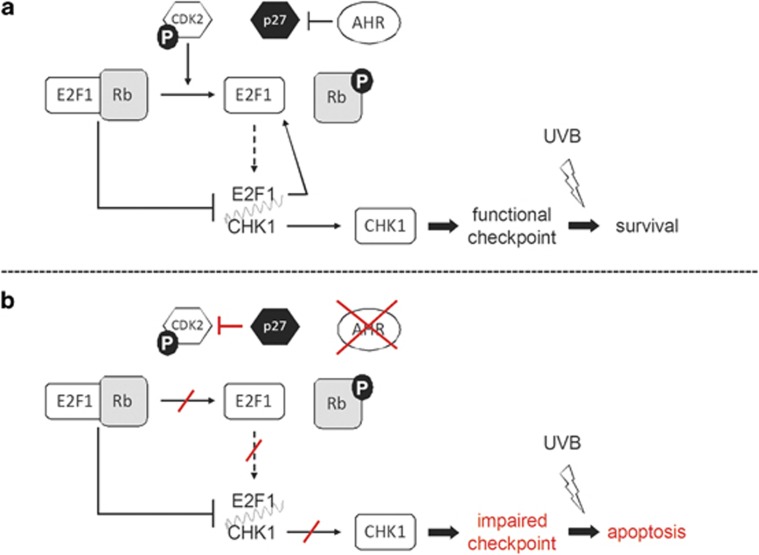
Figure 5.
Scheme of the AHR-E2F1-CHK1 axis, a novel anti-apoptotic signaling cascade in KC. (a) The AHR represses the expression of p27KIP1 and thereby maintains CDK2-dependent Rb phosphorylation. This in turn enables autoregulatory E2F1 expression as well as CHK1 expression. CHK1 ensures checkpoint control and enables cellular survival upon UVB exposure. (b) AHR inhibition results in an increased expression of CDK2-inhibitory p27KIP1 and the subsequent repression of E2F1 and CHK1 expression, respectively. The loss of CHK1 impairs proper checkpoint control and sensitizes UVB-damaged KC to apoptotic cell death
In this study, we identify the AHR as a crucial mediator of apoptosis in UVB-irradiated KC that suppresses cell death via a newly discovered AHR-E2F1-CHK1 axis, which is triggered in a p27KIP1-/CDK2-dependent manner (Figure 5). We also demonstrated an increase in apoptosis in the skin of UVB-exposed AHR-deficient SKH-1 mice, accompanied by a reduced expression of E2F1 and CHK1, providing evidence that the AHR-E2F1-CHK1 axis is present in vivo. Based on our previous findings that UVB exposure of KC increases the expression of CYP1A1 and COX-2 in an AHR-dependent manner, it was postulated that AHR signaling contributes to the development of UVB-induced NMSC,4, 14 an idea that is further strengthened by the results of our present study. As the UVB doses used in this study, with 200 J/m2 being equivalent to the minimal erythema dose of fair-skinned human individuals,17 can be easily reached during recreational sunbathing, we propose that a transient antagonism of this signaling pathway, especially in times of extensive UV exposure, will be beneficial for the prevention of NMSC. It will be interesting to see, if this newly described anti-apoptotic pathway is also present in other epidermal cell populations harboring functional AHR signaling, such as Langerhans cells57 and melanocytes.58 However, as the AHR was also described to act pro-apoptotic, for instance in PAH-exposed oocytes,59 cell- and tissue-specific alterations of this pathway may be expected.
Materials and Methods
Cell culture, UVB irradiation, and treatment
The KC cell line NCTC 2544 was cultured in MEM medium (PAA, Coelbe, Germany) supplemented with 10% FCS Gold (PAA) and penicillin/streptomycin in a humidified atmosphere of 5% CO2 at 37 °C. AHR-knockdown and empty vector control HaCaT (HaCaT-shAHR; HaCaT-EV) KC10 were cultured in DMEM medium (PAA) supplemented with 10% FCS Gold (PAA), penicillin/streptomycin, and 0.68 mg/ml G418 (AppliChem, Darmstadt, Germany) in a humidified atmosphere of 5% CO2 at 37 °C. The source for UVB irradiation was a TL20W/12RS lamp, four tubes in parallel connection (Philips, Eindhoven, the Netherlands), which emits most of its energy in the UVB range (290–320 nm) with an emission peak at 310 nm. For UVB exposure, culture medium was replaced by warm PBS. Afterwards, fresh medium was immediately added to the cells. Sham-irradiated cells were subjected to the identical procedure without being UVB exposed. For AHR antagonism, cells were 1 h pretreated with the specific antagonist MNF (or DMSO) before UVB irradiation. BaP, WP631, PP2, PD98059 (Sigma-Aldrich, Munich, Germany), KT5720, Roscovitine (Enzo Life Science, Loerrach, Germany), and BAY 11-7085 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were dissolved in DMSO, LPS (Sigma-Aldrich) in water.
Generation of NCTC-shAHR and NCTC-EV cells
For stable AHR knockdown, NCTC 2544 were lentiviral transduced with a shRNA-encoding vector targeting human AHR or empty control vector, using the protocol previously described for human immortalized HaCaT KC.10 NCTC-shAHR and NCTC-EV KC were cultured as described above, but medium was supplemented with 0.84 mg/ml G418 (AppliChem).
Quantitative real-time PCR
For quantitative gene expression analyses, 3 × 105 cells/well were seeded into six-well plates, cultured overnight and treated at a confluence of approximately 70% as indicated. Total RNA was isolated using the Gold RNA kit (Peqlab, Erlangen, Germany). For each sample, 0.5 μg of total RNA was reverse transcribed using MMLV reverse transcriptase (Invitrogen, Karlsruhe, Germany) in a total volume of 20 μl. Three microliters of cDNA of a 1 : 3 dilution were used for real-time PCR with SYBR Fast Reagent (Qiagen, Hilden, Germany) in a Corbett-Rotor Gene 300 light cycler (Qiagen). Gene expression was normalized to β-actin. The sequences of the oligonucleotides used were: 5′-CCCCAGGCACCAGGGCGTGAT-3′ and 5′-GGTCATCTTCTCGCGGTTGGCCTTGGGGT-3′ for β-actin, 5′-TGGTCTCCCCCAGACAGTAG-3′ and 5′-TTCATTGCCAGAAAACCAGA-3′ for AHR, 5′-TAGACACTGATCTGGCTGCAG-3′ and 5′-GGGAAGGCTCCATCAGCATC-3′ for CYP1A1, 5′-CCCTTGGGTGTCAAAGGTAA-3′ and 5′-AACTGATGCGTGAAGTGCTG-3′ for COX-2, 5′-TGTTCTTGTTGCTTCCAGATGAAATTGCCG-3′ and 5′-CATTCCTCTCCGACATCCCTGAGAAATTGG-3′ for PAI-2; 5′-CTTTGGCTTGGCAACAGT-3′ and 5′-CCAGTCAGAATACTCCTG-3′ for CHK1, and 5′-AGATGTCAAACGTGCGAGTG-3′ and 5′-TCTCTGCAGTGCTTCTCCAA-3′ for p27KIP1.
SDS-PAGE and western blot analyses
For western blot analyses, cells were seeded as described under ‘Quantitative Real-Time PCR'. Cells were lysed in RIPA buffer (25 mM Tris-HCl, pH 7.4; 150 mM NaCl; 0.1 mM EDTA, pH 8.0; 1% Nonidet P-40; 1% desoxycholate; 0.1% SDS; 0.025% NaN3; protease inhibitors) on ice and subsequently centrifuged for 5 min at 4 °C at maximum speed. Protein samples were separated by 10% SDS-polyacrylamide or NUPAGE Novex 3–8% Tris-acetate (Invitrogen) gel electrophoresis and blotted onto PVDF membranes (GE Healthcare, Freiburg, Germany). Blots were blocked with 5% skim milk in TBS-Tween-20 0.1% (TBS-T) for 1 h and with 5% BSA in TBS-T for 30 min at room temperature. Blots were incubated overnight at 4 °C with antibodies against CHK1, E2F1, p27KIP1, P-CDK2 (Thr160), P-Rb (Ser807/811), GAPDH, or β-actin (Cell Signaling Technology, Danvers, MA, USA), caspase-3 (Santa Cruz Biotechnology) and PARP-1 (Epitomics, Burlingame, CA, USA) according to the manufacturer's instructions. Blots were washed and subsequently incubated for 1 h with a 1 : 5000 dilution of HRP-conjugated anti-rabbit (Cell Signaling Technology) or anti-mouse (GE Healthcare) secondary antibody in 5% skim milk in TBS-T at room temperature. Bands were visualized using the chemiluminescence ECL Prime detection system (GE Healthcare) and X-ray films. Densitometry was carried out using the AlphaEaseFC Software (Alpha Innotech, San Leandro, CA, USA).
Flow cytometry analysis
For FACS analyses, 1 × 105 cells/well were seeded into six-well plates and cultured overnight to reach a confluence of approximately 50%. Analysis of apoptosis by propidium iodide (PI; Sigma-Aldrich) staining and flow cytometry was performed as described in reference.60 Briefly, trypsinized cells plus medium were centrifuged at 200 × g for 5 min at room temperature, followed by washing with PBS. Pellets were resuspended in 150 μl of fluorochrome solution containing 0.1% sodium citrate, 0.1% Triton X-100, and 50 mg/l PI. After incubation in the dark at 4 °C for at least 1 h, cells were analyzed by flow cytometry. The acquired data were analyzed using the FlowJo software package (Tree Star Inc., Ashland, OR, USA).
Overexpression experiments
For overexpression experiments, 1.5 × 105 cells/well were seeded into six-well plates and cultured overnight to reach a confluence of approximately 60%. Transient transfection of KC with pCI-neo FLAG-CHK1 (referred to as pCHK1), pCMVHA E2F1 (referred to as pE2F1; Addgene, Cambridge, MA, USA; plasmid #24225), or respective empty vectors was done using JetPEI transfection reagent (Polyplus Transfection, Illkirch, France) according to the manufacturer's instructions. Twenty-four hours after transfection, cells were exposed to UVB and 24 h later western blot analyses were performed as described above.
Reporter gene assay
Cells were seeded as described under ‘Overexpression Experiments' and subsequently transiently transfected with E2F1 reporter construct pGL2AN (Addgene plasmid #20950) or empty pGL2basic vector (Promega, Mannheim, Germany) by using JetPEI transfection reagent. After 24 h, cells were treated with the indicated substances for additional 24 h. E2F1 promoter-driven luciferase activity was measured in a Multi-Bioluminat LB 9505C (Berthold Technologies, Bad Wildbad, Germany) using a luciferase assay system (Promega). Firefly luciferase activities were normalized to protein concentration.
Proliferation assay
KC proliferation was measured 24 h after seeding (2.5 × 104 cells/well in 24-well plates to reach a confluence of approximately 50%) using the BrdU labeling and detection kit III (Roche, Penzberg, Germany), according to the manufacturer's instructions.
Animals and western blot analyses
The AHR exon-2 deletion of B6.AHRtmIbra mice, originally generated by C Bradfield61 and obtained from the Jackson Laboratory (Bar Harbor, ME, USA), was backcrossed into the SKH-1 hairless background for more than nine generations. All animals were housed in our specific pathogen-free animal facility. Genotyping was performed using the oligonucleotides 5′-CTGAATGAACTGCAGGACGA-3′ and 5′-ATACTTTCTCGGCAGGAGCA-3′ for the AHR-knockout allele, and 5′-GGATTTGACTTAATTCCTTCAGCGG-3′ and 5′-TCTTGGGCTCGATCTTGTGTCAGGAACAGG-3′ for the AHR wild-type allele.
For western blot analyses, male SKH-1 mice (except the control animals) were exposed to a single dose of 185 mJ/cm2 UVB, and 48 h later mice were killed. The dorsal skin of each animal was prepared and protein was isolated from two 4 mm punch biopsies. SDS-PAGE and western blot analyses were carried out as described above using antibodies detecting E2F1 (Santa Cruz Biotechnology), CHK1, GAPDH, β-actin, and caspase-3/cleaved caspase-3 (Cell Signaling). The animal experiments were performed according to the national animal care guidelines.
Statistical analyses
All data shown are mean (±standard deviation) or representative results (western blots) from three or more independent experiments, if not indicated otherwise. Data were analyzed using two-sided Student's t-test. P-values below 0.05 were considered as significant.
Acknowledgments
We thank Jiri Lukas (Danish Cancer Society, Copenhagen, Denmark) for providing the CHK1 expression plasmid and Gabriele Vielhaber (Symrise GmbH & Co.KG, Holzminden, Germany) for providing MNF. The E2F1 expression construct and the E2F1 reporter plasmid were gifts from Kristian Helin (University of Copenhagen, Denmark) and William Kaelin (Dana Farber Cancer Institute, Boston, MA, USA), respectively. We thank Roland Piekorz (University of Düsseldorf, Germany), Birgit Neumann, and Ingo Uthe for excellent technical support. This work was supported by grants of the Jürgen Manchot Foundation (to T.H.-S.), the Medical Faculty of the University of Düsseldorf (to T.H.-S.), the DFG SPP1230 (to H.H.), and the BMBF networks bmfs and FoneFA (to H.H.).
Glossary
- AHR
aryl hydrocarbon receptor
- ARNT
AHR nuclear translocator
- CHK1
checkpoint kinase 1
- CDK2
cyclin-dependent kinase-2
- CYP
cytochrome P450
- COX2
cyclooxygenase-2
- FICZ
6-formylindolo[3,2-b]carbazole
- KC
keratinocyte
- MNF
3′-methoxy-4′-nitroflavone
- NMSC
non-melanoma skin cancer
- PAH
polycyclic aromatic hydrocarbons
- PAI-2
plasminogen activator inhibitor-2
- PARP-1
poly(ADP-ribose)-polymerase-1
- PI
propidium iodide
- Rb
retinoblastoma protein
- ROS
reactive oxygen species
- UV
ultraviolet
- XRE
xenobiotic-responsive element
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by RA Knight
Supplementary Material
References
- Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010;375:673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- Herrlich P, Karin M, Weiss C. Supreme EnLIGHTenment: damage recognition and signaling in the mammalian UV response. Mol Cell. 2008;29:279–290. doi: 10.1016/j.molcel.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippens S, Hoste E, Vandenabeele P, Agostinis P, Declercq W. Cell death in skin. Apoptosis. 2009;14:549–569. doi: 10.1007/s10495-009-0324-z. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Stander S, Berneburg M, Bohm M, Kulms D, van Steeg H, et al. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat Cell Biol. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- Sun SY, Hail NJr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004;96:662–672. doi: 10.1093/jnci/djh123. [DOI] [PubMed] [Google Scholar]
- Kulms D, Poppelmann B, Yarosh D, Luger TA, Krutmann J, Schwarz T. Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation. Proc Natl Acad Sci USA. 1999;96:7974–7979. doi: 10.1073/pnas.96.14.7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulms D, Zeise E, Poppelmann B, Schwarz T. DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way. Oncogene. 2002;21:5844–5851. doi: 10.1038/sj.onc.1205743. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, et al. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006;20:1486–1488. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]
- Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci USA. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem. 2010;391:1235–1248. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- Wincent E, Bengtsson J, Mohammadi BA, Alsberg T, Luecke S, Rannug U, et al. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2012;109:4479–4484. doi: 10.1073/pnas.1118467109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarmann-Stemmann T, Bothe H, Abel J. Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways. Biochem Pharmacol. 2009;77:508–520. doi: 10.1016/j.bcp.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Agostinis P, Garmyn M, Van Laethem A. The Aryl hydrocarbon receptor: an illuminating effector of the UVB response. Sci STKE. 2007;2007:e49. doi: 10.1126/stke.4032007pe49. [DOI] [PubMed] [Google Scholar]
- Dietrich C.The AHR in the Control of Cell Cycle and ApoptosisIn: Pohjanvirta R (ed).The AH Receptor in Biology and Toxicology1 edn.John Wiley & Sons, Inc.: Hoboken, New Jersey; 2012,467–483. [Google Scholar]
- Bakken P, Evans V, Earle W, Stevenson R. Establishment of a strain of human skin cells on chemically defined medium NCTC 109. Am J Hyg. 1961;73:96–104. doi: 10.1093/oxfordjournals.aje.a120170. [DOI] [PubMed] [Google Scholar]
- Autier P, Dore JF, Reis AC, Grivegnee A, Ollivaud L, Truchetet F, et al. Sunscreen use and intentional exposure to ultraviolet A and B radiation: a double blind randomized trial using personal dosimeters. Br J Cancer. 2000;83:1243–1248. doi: 10.1054/bjoc.2000.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter T, Guzman K, Dold K, Greenlee W. Targets for dioxin: genes for plasminogen activator inhibitor-2 and interleukin-1 beta. Science. 1991;254:415–418. doi: 10.1126/science.1925598. [DOI] [PubMed] [Google Scholar]
- Neuman E, Flemington EK, Sellers WR, Kaelin WGJr. Transcription of the E2F-1 gene is rendered cell cycle dependent by E2F DNA-binding sites within its promoter. Mol Cell Biol. 1994;14:6607–6615. doi: 10.1128/mcb.14.10.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DG, Ohtani K, Nevins JR. Autoregulatory control of E2F1 expression in response to positive and negative regulators of cell cycle progression. Genes Dev. 1994;8:1514–1525. doi: 10.1101/gad.8.13.1514. [DOI] [PubMed] [Google Scholar]
- Vairo G, Soos TJ, Upton TM, Zalvide J, DeCaprio JA, Ewen ME, et al. Bcl-2 retards cell cycle entry through p27Kip1, pRB relative p130, and altered E2F regulation. Mol Cell Biol. 2000;20:4745–4753. doi: 10.1128/mcb.20.13.4745-4753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Carrassa L, Broggini M, Vikhanskaya F, Damia G. Characterization of the 5'flanking region of the human Chk1 gene: identification of E2F1 functional sites. Cell Cycle. 2003;2:604–609. [PubMed] [Google Scholar]
- Verlinden L, Vanden BI, Eelen G, Drijkoningen M, Verlinden I, Marchal K, et al. The E2F-regulated gene Chk1 is highly expressed in triple-negative estrogen receptor/progesterone receptor /HER-2 breast carcinomas. Cancer Res. 2007;67:6574–6581. doi: 10.1158/0008-5472.CAN-06-3545. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, et al. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson P, McGuire J, Rubio C, Gradin K, Whitelaw ML, Pettersson S, et al. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci USA. 2002;99:9990–9995. doi: 10.1073/pnas.152706299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulero-Navarro S, Pozo-Guisado E, Perez-Mancera PA, Alvarez-Barrientos A, Catalina-Fernandez I, Hernandez-Nieto E, et al. Immortalized mouse mammary fibroblasts lacking dioxin receptor have impaired tumorigenicity in a subcutaneous mouse xenograft model. J Biol Chem. 2005;280:28731–28741. doi: 10.1074/jbc.M504538200. [DOI] [PubMed] [Google Scholar]
- Puppala D, Gairola CG, Swanson HI. Identification of kaempferol as an inhibitor of cigarette smoke-induced activation of the aryl hydrocarbon receptor and cell transformation. Carcinogenesis. 2007;28:639–647. doi: 10.1093/carcin/bgl169. [DOI] [PubMed] [Google Scholar]
- Palermo CM, Hernando JI, Dertinger SD, Kende AS, Gasiewicz TA. Identification of potential aryl hydrocarbon receptor antagonists in green tea. Chem Res Toxicol. 2003;16:865–872. doi: 10.1021/tx025672c. [DOI] [PubMed] [Google Scholar]
- Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58:5707–5712. [PubMed] [Google Scholar]
- Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease. FASEB J. 2005;19:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- Bell LA, Ryan KM. Life and death decisions by E2F-1. Cell Death Differ. 2004;11:137–142. doi: 10.1038/sj.cdd.4401324. [DOI] [PubMed] [Google Scholar]
- Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer. 2009;9:738–748. doi: 10.1038/nrc2718. [DOI] [PubMed] [Google Scholar]
- Urist M, Tanaka T, Poyurovsky MV, Prives C. p73 induction after DNA damage is regulated by checkpoint kinases Chk1 and Chk2. Genes Dev. 2004;18:3041–3054. doi: 10.1101/gad.1221004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlowe JL, Fan Y, Chang X, Peng L, Knudsen ES, Xia Y, et al. The aryl hydrocarbon receptor binds to E2F1 and inhibits E2F1-induced apoptosis. Mol Biol Cell. 2008;19:3263–3271. doi: 10.1091/mbc.E08-04-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Nadiminty N, Fitzpatrick JE, Alworth WL, Slaga TJ, Kumar AP. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J Biol Chem. 2005;280:5812–5819. doi: 10.1074/jbc.M411429200. [DOI] [PubMed] [Google Scholar]
- Spyridopoulos I, Principe N, Krasinski KL, Xu Sh, Kearney M, Magner M, et al. Restoration of E2F expression rescues vascular endothelial cells from tumor necrosis factor-alpha-induced apoptosis. Circulation. 1998;98:2883–2890. doi: 10.1161/01.cir.98.25.2883. [DOI] [PubMed] [Google Scholar]
- Wikonkal NM, Remenyik E, Knezevic D, Zhang W, Liu M, Zhao H, et al. Inactivating E2f1 reverts apoptosis resistance and cancer sensitivity in Trp53-deficient mice. Nat Cell Biol. 2003;5:655–660. doi: 10.1038/ncb1001. [DOI] [PubMed] [Google Scholar]
- Fan Y, Boivin GP, Knudsen ES, Nebert DW, Xia Y, Puga A. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res. 2010;70:212–220. doi: 10.1158/0008-5472.CAN-09-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri SK, Weiss C, Koff A, Gottlicher M. p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev. 1999;13:1742–1753. doi: 10.1101/gad.13.13.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmes M, Hennen J, Clemens J, Blomeke B. Impact of aryl hydrocarbon receptor (AhR) knockdown on cell cycle progression in human HaCaT keratinocytes. Biol Chem. 2011;392:643–651. doi: 10.1515/BC.2011.067. [DOI] [PubMed] [Google Scholar]
- Dever DP, Opanashuk LA. The aryl hydrocarbon receptor contributes to the proliferation of human medulloblastoma cells. Mol Pharmacol. 2012;81:669–678. doi: 10.1124/mol.111.077305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Duran A, Ballestar E, Carvajal-Gonzalez JM, Marlowe JL, Puga A, Esteller M, et al. Recruitment of CREB1 and histone deacetylase 2 (HDAC2) to the mouse Ltbp-1 promoter regulates its constitutive expression in a dioxin receptor-dependent manner. J Mol Biol. 2008;380:1–16. doi: 10.1016/j.jmb.2008.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Liu D, Murray TJ, Mitchell GC, Hesterman EV, Karchner SI, et al. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells. Oncogene. 2005;24:7869–7881. doi: 10.1038/sj.onc.1208938. [DOI] [PubMed] [Google Scholar]
- Murata K, Hattori M, Hirai N, Shinozuka Y, Hirata H, Kageyama R, et al. Hes1 directly controls cell proliferation through the transcriptional repression of p27Kip1. Mol Cell Biol. 2005;25:4262–4271. doi: 10.1128/MCB.25.10.4262-4271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Shen J, Wu M, Arsura M, FitzGerald M, Suldan Z, et al. Repression of transcription of the p27(Kip1) cyclin-dependent kinase inhibitor gene by c-Myc. Oncogene. 2001;20:1688–1702. doi: 10.1038/sj.onc.1204245. [DOI] [PubMed] [Google Scholar]
- Wang X, Gorospe M, Huang Y, Holbrook NJ. p27Kip1 overexpression causes apoptotic death of mammalian cells. Oncogene. 1997;15:2991–2997. doi: 10.1038/sj.onc.1201450. [DOI] [PubMed] [Google Scholar]
- Gupta S, Hussain T, Mukhtar H. Molecular pathway for (-)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch Biochem Biophys. 2003;410:177–185. doi: 10.1016/s0003-9861(02)00668-9. [DOI] [PubMed] [Google Scholar]
- Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114:513–521. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- Ma CX, Janetka JW, Piwnica-Worms H. Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends Mol Med. 2011;17:88–96. doi: 10.1016/j.molmed.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan TP, Kawasumi M, Blasina A, Anderes K, Conney AH, Nghiem P. ATR-Chk1 pathway inhibition promotes apoptosis after UV treatment in primary human keratinocytes: potential basis for the UV protective effects of caffeine. J Invest Dermatol. 2009;129:1805–1815. doi: 10.1038/jid.2008.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YP, Lou YR, Peng QY, Xie JG, Nghiem P, Conney AH. Effect of caffeine on the ATR/Chk1 pathway in the epidermis of UVB-irradiated mice. Cancer Res. 2008;68:2523–2529. doi: 10.1158/0008-5472.CAN-07-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YP, Lou YR, Xie JG, Peng QY, Liao J, Yang CS, et al. Topical applications of caffeine or (-)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc Natl Acad Sci USA. 2002;99:12455–12460. doi: 10.1073/pnas.182429899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel EL, Hendrix SO, McNeeley SG, Johnson KC, Rosenberg CA, Mossavar-Rahmani Y, et al. Daily coffee consumption and prevalence of nonmelanoma skin cancer in Caucasian women. Eur J Cancer Prev. 2007;16:446–452. doi: 10.1097/01.cej.0000243850.59362.73. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Bjelke E, Kvale G, Heuch I. Coffee drinking, mortality, and cancer incidence: results from a Norwegian prospective study. J Natl Cancer Inst. 1986;76:823–831. [PubMed] [Google Scholar]
- Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J Immunol. 2009;182:6709–6717. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- Jux B, Kadow S, Luecke S, Rannug A, Krutmann J, Esser C. The aryl hydrocarbon receptor mediates UVB radiation-induced skin tanning. J Invest Dermatol. 2011;131:203–210. doi: 10.1038/jid.2010.269. [DOI] [PubMed] [Google Scholar]
- Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu HY, et al. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28:355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.