Abstract
Heterochromatin assembly in fission yeast depends on the Clr4 histone methyltransferase, which targets H3K9. We show that the histone deacetylase Sir2 is required for Clr4 activity at telomeres, but acts redundantly with Clr3 histone deacetylase to maintain centromeric heterochromatin. However, Sir2 is critical for Clr4 function during de novo centromeric heterochromatin assembly. We identified new targets of Sir2 and tested if their deacetylation is necessary for Clr4-mediated heterochromatin establishment. Sir2 preferentially deacetylates H4K16Ac and H3K4Ac, but mutation of these residues to mimic acetylation did not prevent Clr4-mediated heterochromatin establishment. Sir2 also deacetylates H3K9Ac and H3K14Ac. Strains bearing H3K9 or H3K14 mutations exhibit heterochromatin defects. H3K9 mutation blocks Clr4 function, but why H3K14 mutation impacts heterochromatin was not known. Here, we demonstrate that recruitment of Clr4 to centromeres is blocked by mutation of H3K14. We suggest that Sir2 deacetylates H3K14 to target Clr4 to centromeres. Further, we demonstrate that Sir2 is critical for de novo accumulation of H3K9me2 in RNAi-deficient cells. These analyses place Sir2 and H3K14 deacetylation upstream of Clr4 recruitment during heterochromatin assembly.
Keywords: centromere, fission yeast, heterochromatin, histone deacetylase, histone methyltransferase
Introduction
In fission yeast, repressive chromatin assembles at the centromeres, mating type locus and at telomeres (Klar and Bonaduce, 1991; Thon and Klar, 1992; Allshire et al, 1994; Nimmo et al, 1994; Allshire et al, 1995). This heterochromatin is critical for the maintenance of genomic integrity and to determine cell type identity, and is characterized by both hypoacetylation of the histone tails (Ekwall et al, 1997; Bjerling et al, 2002; Gomez et al, 2005) and by methylation of histone H3 on lysine 9 (Bannister et al, 2001; Nakayama et al, 2001; Cam et al, 2005). Several deacetylases contribute to histone hypoacetylation, including Clr3 and Clr6 (Bjerling et al, 2002), as well as members of a distinct class of deacetylases; the Sir2 family (Shankaranarayana et al, 2003; Freeman-Cook et al, 2005).
The Sir2 family is a group of NAD+-dependent lysine deacetylases that are conserved among eukaryotes and archaea (Brachmann et al, 1995; Imai et al, 2000; Landry et al, 2000; Smith et al, 2000). Sir2 homologues are important for silencing of constitutive heterochromatin, and have both histone and non-histone substrates (Bell et al, 2002; Yang et al, 2005). In budding yeast, heterochromatin assembly at telomeres and at the mating type locus is reliant on Sir2p and other components of the SIR complex, and the role of these proteins in the assembly of silent chromatin has been well studied (Johnson et al, 1990; Aparicio et al, 1991; Braunstein et al, 1993; Johnson et al, 2009; Hickman et al, 2011; Oppikofer et al, 2011). In the simplest model, silencer proteins bind to specific DNA sequences located within the telomere repeat sequences or at silencers within the mating type region and directly recruit the SIR silencing complex to these initiation sites. Next, histone deacetylation by Sir2 facilitates binding of Sir3 and Sir4 to proximal nucleosomes, which in turn allows spreading of the repressive complex (reviewed in Rusche et al (2003)).
In contrast to budding yeast, how fission yeast homologues of Sir2 promote heterochromatin assembly is comparatively understudied. For example, it is not known whether Sir2 proteins in fission yeast are components of structural silencing complexes like the SIR complex, or whether these proteins are recruited to genomic regions by specific binding factors to promote heterochromatin initiation. The Sir2 family in fission yeast comprises three members, namely Sir2, Hst2 and Hst4. Fission yeast Sir2 is a primarily nuclear protein that is important for the silencing of telomeres and the mating type locus, and also plays a role in centromeric silencing (Shankaranarayana et al, 2003; Freeman-Cook et al, 2005), although the extent of its influence at centromeres is less clear. The fission yeast Sir2 homologue, Hst4, also has roles in heterochromatic silencing (Freeman-Cook et al, 1999). Hst4 has deacetylase activity on H3K56Ac (Haldar and Kamakaka, 2008), and is involved in repair of DNA damage during S phase, while Hst2 is generally less well characterized.
Confusion persists about the role of fission yeast Sir2 in centromeric silencing. While it is clear that Sir2 plays an important role at telomeres and at the mating type locus, the silencing functions of Sir2 at centromeres are less pronounced and are dependent on the location of the reporter gene (Shankaranarayana et al, 2003; Freeman-Cook et al, 2005). H3K9Ac peptides have been shown to serve as Sir2 targets in vitro (Shankaranarayana et al, 2003). Consistent with this observation, H3K9Ac is increased at heterochromatic loci in sir2 mutants, which may or may not lead to a reduction in H3K9me2 and a reduction in Swi6 localization to centromeres (Shankaranarayana et al, 2003; Freeman-Cook et al, 2005).
In this study, we have evaluated centromeric functions of Sir2 in greater detail. We show that Sir2 functions upstream of and is required to promote de novo Clr4-mediated H3K9 methyltransferase activity, and that disruption of this pathway has physiological consequences consistent with the loss of centromere function. Further, we have probed the specific catalytic role of the Sir2 histone deacetylase activity in promoting Clr4 activity. We created a novel mutant of fission yeast Sir2 (sir2N247A) that is defective for deacetylase activity. We show that this mutant lacks deacetylase activity in vitro and in vivo, and largely fails to promote de novo function of Clr4 at centromeres. In addition, we examine the substrate specificity of Sir2 in vitro, and use this analysis to identify specific targets of Sir2 HDAC activity. We test whether these targets are important for establishing centromeric silencing.
Results
Sir2 HDAC mutants show a complete loss of Clr4 function at telomeres
To determine the contribution of Sir2’s deacetylase activity to heterochromatin maintenance, we generated a mutation within the catalytic domain of Sir2 that changes a conserved asparagine to an alanine (N247A). The mutation resides in a conserved series of amino acids that make up the catalytic core of the enzyme, and is analogous to the N345A mutation in Saccharomyces cerevisiae Sir2p, which is a catalytically inactive protein both in vivo and in vitro (Imai et al, 2000; Armstrong et al, 2002; Oppikofer et al, 2011) (Figure 1A). The N247A mutation was engineered into the genomic sir2 locus that we additionally marked with the his3 allele and into strains that express a fully functional TAP-tagged allele of sir2 at the endogenous locus. Western analyses showed that expression of the wild-type (WT) and N247A mutant Sir2–TAP proteins was equivalent (Figure 1B), suggestive that the N247A mutation does not alter Sir2 protein stability.
Figure 1.
The presumed catalytic mutant, sir2N247A, causes defects in the maintenance of subtelomeric heterochromatin. (A) Alignment of fission yeast Sir2 with nearest homologues from budding yeast and man. The fission yeast sir2N247A mutant is analogous to the budding yeast Sir2pN345A, which lacks catalytic activity. Position of the N247A substitution is indicated. (B) The predicted catalytic mutant Sir2N247A–TAP is stably expressed. Western blot of extracts from Sir2–TAP and sir2N247A–TAP expressing cells, probed for TAP with tubulin as loading control. (C) Subtelomeric transcripts accumulate in sir2Δ and sir2N247A mutant cells. mRNA transcripts from subtelomeric tlh genes were quantified by quantitative real-time PCR (qRT–PCR) amplification of cDNA, and normalized to transcript levels of the adh1+ (alcohol dehydrogenase) control. Graphic data present the average of two distinct experimental replicates and error bars depict s.e.m. (D) H3K9 acetylation is increased and (E) H3K9me2 methylation is abolished at subtelomeres in sir2 deletion and sir2N247A mutants. qRT–PCR analysis of ChIP experiments monitoring relative enrichment of tlh sequences over adh1+ control euchromatic locus in immunoprecipitated samples. Graphs represent average of data obtained from two biological samples, with error bars depicting s.e.m.
We first analysed the effects of sir2 deletion and the presumed catalytically dead sir2N247A mutation on gene silencing in subtelomeric regions (Figure 1C). In WT cells, subtelomeric heterochromatin coats and silences transcription from the tlh genes that are located close to the telomeres of the left arm of chromosome 1 and right arm of chromosome 2 (Hansen et al, 2006). Cells that lack sir2 show accumulation of transcripts from tlh genes compared with WT cells, but this is not to the extent seen in clr4Δ cells, which exhibit very high levels of tlh transcripts. Importantly, the level of tlh transcript accumulation in the sir2N247A strain was similar to that in sir2Δ cells, suggesting that the sir2N247A mutant is defective for sir2 function at telomeres.
We further tested the effects of mutation of Sir2 on subtelomeric heterochromatin maintenance. Sir2 has been reported to have H3K9 deacetylase activity in fission yeast (Shankaranarayana et al, 2003). Chromatin immunoprecipitation (ChIP) experiments performed with antibodies specific for H3K9Ac revealed that H3K9Ac levels are elevated on subtelomeric sequences in cells lacking sir2, and that the sir2N247A mutant causes the same enrichment of H3K9Ac as does loss of sir2 (Figure 1D). We note that the level of enrichment for H3K9Ac on subtelomeric sequences is similar between clr4Δ and sir2Δ cells. Next, we examined H3K9me2 levels within the subtelomeric regions by ChIP (Figure 1E). Cells that lack clr4 show a complete loss of H3K9me2 signal. We found that sir2Δ and the sir2N247A mutant both resemble clr4Δ cells, and appear to have complete loss of H3K9me2 from subtelomeric sequences. From these experiments, we conclude that the N247A mutant of Sir2 is impacting the catalytic activity of Sir2 in vivo. Critically, the loss of Sir2 or Sir2 HDAC activity results in a complete loss of H3K9me2 signal from the tlh genes, suggesting that Clr4 function is inhibited at subtelomeres.
Loss of Sir2 HDAC activity does not affect maintenance of centromeric heterochromatin
Next, we examined the effect of sir2Δ and the sir2N247A mutant on centromeric heterochromatin. sir2-deficient fission yeast have been reported to exhibit different levels of defects in the maintenance of centromeric heterochromatin (Shankaranarayana et al, 2003; Freeman-Cook et al, 2005). We assessed the state of centromeric heterochromatin in strains lacking sir2 or bearing the sir2N247A allele using a strain background that carries a ura4+ transgene inserted within the outer repeats of the centromere (cen::ura4+) (Allshire et al, 1995). WT cells exhibit robust silencing of this centromeric transgene, allowing growth of cells on media that contains 5-fluoro-orotic acid (FOA), which is toxic to cells that express ura4+ (Figure 2A). Cells that have defective centromeric heterochromatin, such as clr4-deficient cells, fail to grow on FOA. sir2 null cells showed a mild defect in silencing of the transgene, such that while sufficient ura4+ silencing occurs for cells to grow on FOA, there was enhanced growth on media lacking uracil, indicating some increase in the expression of the centromeric reporter. The sir2N247A mutant showed a minimal defect in silencing of the centromeric reporter similar to the sir2 null.
Figure 2.
Sir2 functions redundantly with Clr3 for centromeric heterochromatin maintenance. (A) Deletion of sir2 or sir2N247A does not impact centromeric transgene silencing. Serial dilutions of WT, clr4Δ, sir2Δ, and sir2N247A yeast strains bearing the cen::ura4+ transgene were assayed for growth on nonselective (complete) medium, as well as selective medium lacking uracil (−URA), and complete medium additionally containing +FOA. (B) Centromeric transcripts do not appreciably accumulate in sir2 mutants. Centromeric dh, dg, and ura4+mRNA transcripts were quantified by quantitative real-time PCR amplification of cDNA, and normalized to transcript levels of the adh1+ control. Graphic data present the average of two distinct experimental replicates and error bars depict s.e.m. (C) siRNA production is unaffected in sir2 mutants. Centromeric dh siRNA production was evaluated by northern blot relative to snoRNA controls in WT, clr4Δ, and sir2Δ mutants. (D) H3K9 acetylation at centromeres is increased in sir2 mutants, while H3K9 dimethylation, (E) is unaffected. ChIP experiments were performed using antibodies against the acetylated or dimethylated H3K9 epitope. Specific enrichment of immunoprecipitated centromeric dh sequences was determined relative to the adh1+ euchromatic locus by real-time PCR. Graphical data present the average of two biological replicates, with error bars depicting the s.e.m. (F) sir2Δ and (G) sir2N247A mutants function redundantly with Clr3 to maintain centromeric transgene silencing. Serial dilutions of yeast strains bearing the cen::ura4+ transgene were assayed for growth on nonselective (complete) medium, as well as selective medium lacking uracil (−URA), and complete medium additionally containing +FOA. (H) Sir2 and Clr3 function redundantly to maintain centromeric heterochromatin. Swi6 recruitment to centromeres is lost in sir2 clr3 compound mutants. ChIP experiments were conducted using antibodies specific for Swi6, and enrichment of centromeric dh sequences relative to adh1+ was quantified by real-time PCR and normalized to input DNA. Data represent the average of two biological replicates, with error bars depicting the s.e.m.
Analysis of cen::ura4+transcripts and transcripts from the endogenous centromeric repeats (dg and dh) indicated that there was a very minor accumulation of transcripts in cells lacking sir2, especially when compared with clr4Δ cells, which accumulate high levels of centromeric transcripts (Figure 2B). Consistent with the maintenance of centromeric silencing, the RNAi pathway appears intact in sir2Δ cells since the processing of centromeric transcripts into siRNAs occurs efficiently (Figure 2C). In keeping with a lack of effect of sir2 mutation on centromeric heterochromatin maintenance, we saw little evidence of sir2Δ cells displaying defects in chromosome segregation when cells were assessed for chromosome segregation defects during anaphase (Supplementary Figure S1). Loss of sir2 does, however, impact the levels of H3K9 acetylation at centromeres. ChIP analysis revealed a three-fold increase in the levels of H3K9Ac on centromeric repeats in sir2Δ or the sir2N247A mutant cells, supporting a role for Sir2 in deacetylation of K9 of histone H3 at centromeres (Figure 2D) (Shankaranarayana et al, 2003).
Intriguingly, when we monitored H3K9me2 on centromeric repeats, we saw no loss of H3K9 methylation at centromeres in sir2Δ or sir2N247A cells (Figure 2E). This observation is in stark contrast to the effects at subtelomeres, where loss of sir2 function caused a complete loss of H3K9me2. It suggests that although H3K9Ac is increased at centromeres, there is still sufficient H3K9 available for methylation by Clr4 at centromeres in sir2-deficient cells. In addition, while Sir2 and its HDAC activity are critical for Clr4 activity at subtelomeres, at centromeres, Sir2 is either not important for Clr4 function or acts redundantly with additional pathways to maintain centromeric heterochromatin.
We tested whether Sir2 acts redundantly in heterochromatin maintenance at centromeres with another histone deacetylase, Clr3 (Grewal et al, 1998). clr3Δ alone led to a minor defect in heterochromatin maintenance, but combining clr3Δ with either sir2Δ or sir2N247A led to a complete loss of centromeric silencing, and loss of centromeric heterochromatin as monitored by loss of recruitment of the Swi6 chromodomain protein to centromeres (Figure 2F–H). These data suggest that Sir2 and Clr3 play redundant roles in the maintenance of centromeric heterochromatin.
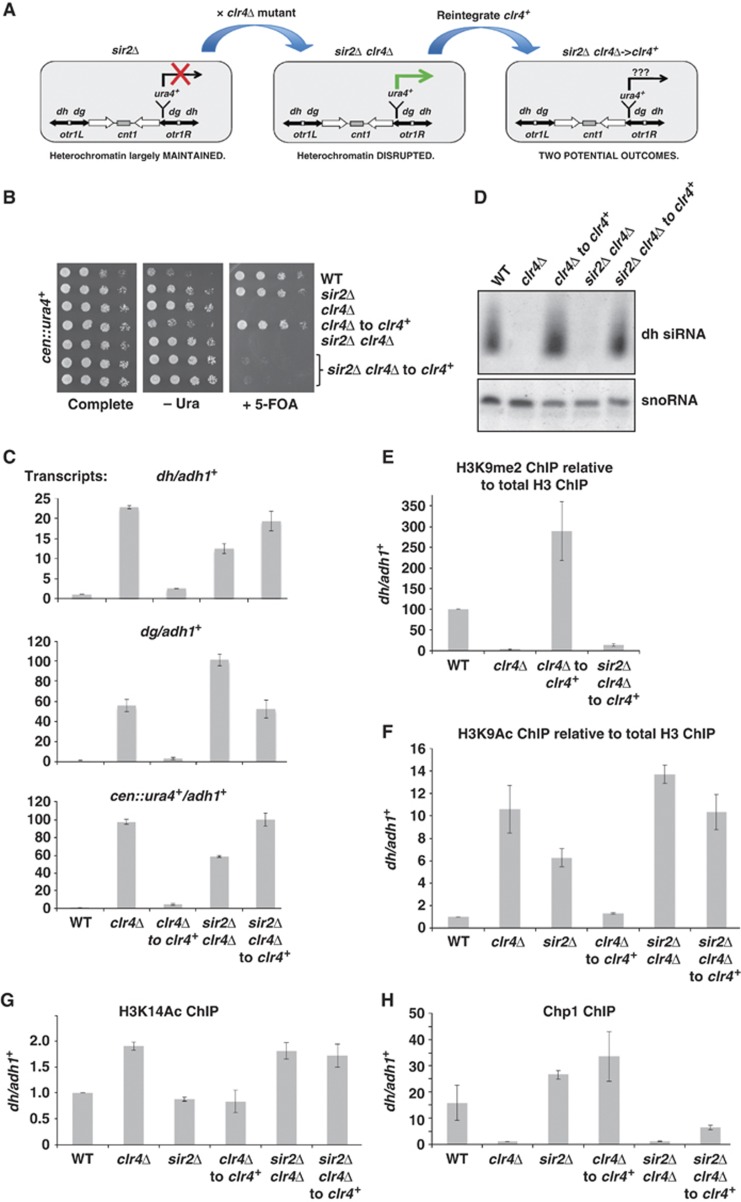
We have adopted a strategy to determine whether genes with minor roles in centromeric heterochromatin maintenance function upstream of Clr4 during heterochromatin initiation (Sadaie et al, 2004; Partridge et al, 2007). Removal of clr4+ from WT cells causes complete loss of centromeric heterochromatin, with complete loss of H3K9me2, accumulation of high levels of centromeric transcripts, and high rates of chromosome missegregation. On reintegration of clr4+ into its normal genomic location, there is a rapid and efficient establishment or initiation of heterochromatin, with resumption of silencing of centromeric transcripts and reassembly of H3K9me2 chromatin. However, if this clr4 withdrawal and reintegration is performed in a genetic background lacking a factor required for the recruitment or de novo activity of Clr4 at centromeres, a defect in centromeric heterochromatin assembly is seen.
Sir2 is required for heterochromatin reinitiation at centromeres
To test whether Sir2 plays a role in the initiation of centromeric heterochromatin, we asked whether clr4+ could function when reintegrated back into its genomic locus in sir2Δclr4Δ cells (Figure 3A). As a control, clr4+ was reintegrated into cells lacking clr4+ alone. Southern analysis was performed to confirm single-copy reintegration into the correct locus, and multiple independent reintegrant strains were analysed and data pooled.
Figure 3.
Sir2 is required for de novo silencing and establishment of Clr4-dependent H3K9 methylation at centromeres. (A) Schematic of assay used to evaluate the requirement for sir2+ in promoting de novo clr4+-dependent silencing activity at centromeres. (B) Sir2 is required for de novo centromeric silencing upon reintroduction of the clr4+ methyltransferase. Serial dilutions of yeast bearing the cen::ura4+ transgene were assayed for growth on complete medium, as well medium lacking uracil (−URA), and complete medium containing +FOA. (C) Reintroduction of the clr4+ methyltransferase in sir2Δ clr4Δ cells reveals defective establishment of transcriptional silencing at centromeres. Centromeric dh, dg, and ura4+ mRNA transcripts were quantified by quantitative real-time PCR amplification of random primed cDNA and normalized to transcript levels for the euchromatic adh1+ control. Experimental data present the average of two experimental replicates. (D) siRNA production occurs in cells defective for de novo centromeric silencing. Centromeric dh siRNA production was evaluated by northern blot relative to snoRNA controls. (E–H) Changes in chromatin structure underlie transcriptional silencing defects in cells defective for de novo centromeric silencing. ChIP experiments were performed using antibodies specific for dimethylated (E) or acetylated H3K9 (F), acetylated H3K14 (G) and Chp1 (H), and data represent the average of two experimental replicates, including a total of four biological replicates from two distinct sir2Δ clr4Δ to clr4+ reintegrant strains.
In contrast to the efficient assembly of heterochromatin in clr4Δ to clr4+ reintegrants, demonstrated by the resumption of silencing of the centromeric transgene (Figure 3B), sir2Δ clr4Δ cells into which clr4+ was reintegrated (sir2Δ clr4Δ to sir2Δ clr4+ reintegrants) did not re-establish cen::ura4+transgene silencing, and grew on media lacking uracil, but not on media containing FOA. Analysis of centromeric transcripts confirmed that there was a failure to fully silence both transcription from the transgene and the endogenous centromeric repeats following reintegration of clr4+ into sir2Δclr4Δ-deficient cells (Figure 3C). In contrast, in the controls, normal silencing of transcripts was reinstated on reintegration of clr4+ into clr4Δsir2+ backgrounds. We monitored clr4+ transcripts following clr4+ reintegration into the distinct backgrounds, and saw no evidence of reduction of clr4+ transcript levels in sir2Δ backgrounds (Supplementary Figure S2A).
Frequently, failure to silence centromeric transcripts correlates with a defect in the RNAi pathway (Volpe et al, 2002). We analysed siRNAs derived from processing of centromeric dh transcripts by northern analysis (Figure 3D). Cells deficient in clr4 largely lack siRNAs (Buhler et al, 2006; Halic and Moazed, 2010), but following reintegration of clr4+, siRNAs accumulate to normal levels, demonstrating that the RNAi pathway is efficiently restored. Interestingly, centromeric siRNAs were also present following reintroduction of clr4+ into clr4Δsir2Δ cells, suggesting that the failure to establish centromeric silencing in these cells is not caused by a defect in the initiation of the RNAi pathway. We confirmed that the defect is attributable to sir2Δ, since re-expression of sir2+ in the clr4+ reintegrant clr4Δsir2Δ cells fully compensated for the silencing defect (Supplementary Figure S2B and C).
We next assessed heterochromatin assembly by ChIP. We found that whereas robust H3K9me2 accumulates at centromeres on reintegration of clr4+ into clr4Δ cells, reintegration of clr4+ into clr4Δsir2Δ cells does not lead to enrichment of H3K9me2 on centromeric repeats above the background seen in clr4Δ strains (Figure 3E). In addition, levels of H3K9Ac and H3K14Ac on centromeric sequences remain high on reintegration of clr4+ into clr4Δsir2Δ cells, similar to levels in clr4Δ cells (which are elevated above those seen in sir2Δ cells) (Figure 3F and G). In contrast, reintegration of clr4+ into control clr4Δ cells permits deacetylation of K9Ac and K14Ac to levels found in WT cells. In addition, consistent with the low levels of centromeric H3K9me2, levels of Chp1 at centromeres on reintegration of clr4+ into sir2Δclr4Δ cells remained low (Figure 3H). These results demonstrate that in cells lacking Sir2, there is a defect in the ability of Clr4 to direct the de novo assembly of centromeric heterochromatin.
We analysed the efficiency of chromosome segregation, and found that as expected for strains with defective centromeric heterochromatin, sir2Δ clr4+ reintegrant strains accumulated lagging chromosomes during anaphase (Supplementary Figure S1). We also tested the stability of the defect in heterochromatin initiation. The inability of clr4Δsir2Δ to clr4+ reintegrant cells to establish centromeric heterochromatin was perpetuated over more than 100 generations, demonstrating that this is a stably inherited epigenetic defect (Supplementary Figure S3).
Sir2 HDAC activity contributes to the establishment of centromeric heterochromatin
To test the role of Sir2’s deacetylase activity in heterochromatin initiation, we generated the compound clr4Δ sir2N247A strain, and performed reintegration of clr4+. Analysis of these strains demonstrated that the sir2N247A mutant showed an intermediate defect in the initiation of heterochromatin. Instead of a complete absence of growth on FOA, as seen in clr4+ reintegrants into clr4Δsir2Δ, some clr4Δsir2N247A clr4+ reintegrant cells grew on FOA (Figure 4A). Consistent with this, levels of transcripts arising from dg and dh regions of the centromere were reduced on reintegration of clr4+ into clr4Δsir2N247A compared with levels seen in clr4Δ sir2N247A, but were still substantially elevated above those in the sir2N247A mutant background alone (Figure 4B). Monitoring the production of siRNAs in these strains revealed that siRNA production was efficiently restored on reintegration of clr4+ into sir2N247A cells (Figure 4C). Together, these results suggest that the heterochromatin initiation is not caused by a defect in the RNAi pathway, and is largely dependent on the deacetylase function of Sir2.
Figure 4.
The sir2N247A mutant exhibits defects in de novo assembly of heterochromatin at centromeres. (A) sir2N247A cells show some defect in establishment of centromeric silencing. Serial dilution assays were performed to monitor silencing of cen::ura4 transgene. (B) De novo silencing defects observed in yeast expressing sir2N47A reflect defective transcriptional silencing at centromeres. Centromeric dh, dg, and ura4+ mRNA transcripts were quantified by quantitative real-time PCR and normalized to levels of the euchromatic adh1+control. Data represent the average of two experimental replicates, including a total of four biological replicates from two distinct sir2N247A-his3 clr4Δ to clr4+ reintegrant strains. (C) sir2N247A mutation does not disrupt siRNA production during maintenance or establishment. Centromeric dh siRNA production was evaluated by northern blot relative to that of a snoRNA control in each of the cell backgrounds indicated.
How Sir2 promotes Clr4 function is not known. Sir2’s H3K9 deacetylase activity could be necessary to provide the deacetylated H3K9 substrate to allow Clr4 to methylate H3K9. However, under steady-state conditions, we have demonstrated that cells lacking Sir2 show elevation of H3K9Ac at centromeres, without impacting H3K9 methylation (Figure 2D and E). We therefore questioned whether H3K9Ac is the critical target of Sir2, or whether Sir2 targets other acetyl moieties to promote heterochromatin initiation.
Substrate specificity of Sir2 in vitro
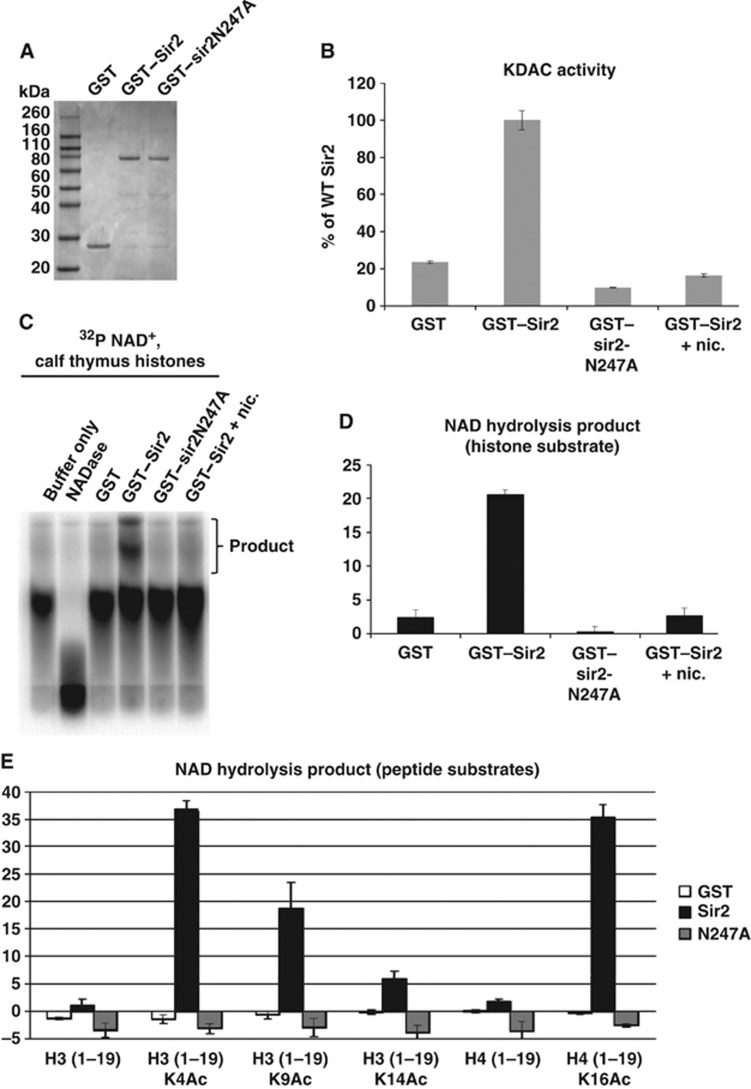
We purified recombinant GST fusion proteins of WT and N247A mutant Sir2 (Figure 5A), and tested these proteins on different acetylated or non-acetylated peptides in deacetylase assays. Using a fluorogenic substrate (Figure 5B), we demonstrated that GST–Sir2 is a catalytically active lysine deacetylase. In contrast, the GST–sir2N247A mutant showed no deacetylase activity above background in these assays. Consistent with prior studies (Bitterman et al, 2002; Avalos et al, 2005), we further demonstrated that the deacetylase activity of GST–Sir2 is blocked by addition of nicotinamide, a recognized sirtuin inhibitor.
Figure 5.
Sir2, but not the catalytic point mutant sir2N247, is catalytically active in vitro, and exhibits preference for deacetylation of H3K4Ac and H4K16Ac peptides. (A) Affinity purified recombinant GST–Sir2 and GST–sir2N247A are stable proteins. Equal amounts (∼1 μg) of GST, GST–Sir2, and GST–sir2N247A were resolved by SDS–PAGE and stained using Coomassie brilliant blue. (B) GST–Sir2, but not GST–sir2N247A, is catalytically active and sensitive to inhibition by nicotinamide. Catalytic activity of GST fusion proteins was evaluated by fluorogenic deacetylation assay. GST–Sir2 activity was also evaluated in the presence 2 mM nicotinamide, a sirtuin inhibitor. (C, D) GST–Sir2, but not GST–sir2N247A, promotes 32P NAD+ cofactor hydrolysis in the presence of calf thymus histones. (C) The ability of GST fusions to promote hydrolysis of 32P NAD+ was appraised in the presence of acetylated calf thymus histones by TLC and autoradiography. GST–Sir2 activity was also evaluated in the presence 5 mM nicotinamide. (D) Graphical representation of data from panel C, one of the two experimental replicates, following analysis by quantitative densitometry. (E) Sir2 exhibits preference for deacetylation of H3KAc and H4K16Ac peptides, but also deacetylates H3K9Ac and H3K14Ac peptides. Evaluation of 32P NAD+ hydrolysis products was performed following the coupled NAD+ hydrolysis/lysine deacetylation reaction, in the presence of differentially acetylated or unacetylated peptides derived from the N-terminal 19 amino acids of Schizosaccharomyces pombe histones H3 or H4. TLC autoradiographs were evaluated by computational densitometry. Graphs represent the mean of two experimental replicates, for which representative autoradiographs are presented in Supplementary Figure S2A–C.
Sirtuins are NAD+-dependent deacetylases. Lysine deacetylation by Sir2 is coupled to the obligate hydrolysis of the NAD+ cofactor and, conversely, NAD+ hydrolysis by Sir2 requires coupled lysine deacetylation. Quantitative analysis of radiolabelled 32P NAD+ hydrolysis in the presence of acetylated histone or peptide substrates has therefore been reported to provide a measure of the degree to which putative sirtuin substrates serve as deacetylation targets (Tanny and Moazed, 2001). By evaluating coupled 32P NAD+ hydrolysis, we demonstrated that GST–Sir2, but not GST–sir2N247A, promotes lysine deacetylation and thus 32P NAD+ hydrolysis in the presence of acetylated histones from calf thymus, and that this activity is abrogated in the presence of nicotinamide (Figures 5C and D).
To explore the specificity of Sir2’s deacetylase activity in greater detail, we generated histone tail peptides for histone H3(aa 1–19) and H4(aa 1–19) that bear different acetyl marks. Specifically, we made peptides bearing H4K16Ac (an extensively studied target of budding yeast Sir2) (Tanny and Moazed, 2001), H3K9Ac (previously reported as a substrate for fission yeast Sir2; Shankaranarayana et al, 2003), H3K14Ac, and H3K4Ac—a newly reported acetyl mark on H3 that appears to be regulated by Sir2 in vivo (Garcia et al, 2007; Xhemalce and Kouzarides, 2010). As can be seen in Figure 5E and Supplementary Figure S4, Sir2 displayed robust NAD+-dependent deacetylase activity when supplied with the canonical sirtuin target H4K16Ac, while sir2N247A lacked such activity. Similar to H4K16Ac, H3K4Ac provided an excellent substrate for Sir2 deacetylase activity. Both H4K16Ac and H3K4Ac appear to be better targets than H3K9Ac. Interestingly, we show that Sir2 can directly deacetylate H3K14Ac. Previously, it had been shown that the total cellular level of H3K14Ac was not altered in histones isolated from sir2Δ cells but that levels of H3K4Ac were increased (Xhemalce and Kouzarides, 2010). Increased levels of H3K14Ac have been documented at heterochromatic loci in sir2Δ cells and were attributed to loss of heterochromatin in these cells (Shankaranarayana et al, 2003). We asked whether H4K16Ac levels were affected by sir2+ deletion, and found a small but reproducible increase in total cellular H4K16Ac in sir2Δ cells (Supplementary Figure S4D). Here, we demonstrate that fission yeast Sir2 can directly deacetylate both H3K14Ac and H3K4Ac in vitro.
H3K4 and the canonical Sir2 target H4K16 do not contribute to heterochromatin initiation
Our in vitro assays suggested that H3K4Ac, H4K16Ac, H3K9Ac, and H3K14Ac are all substrates for Sir2 deacetylase activity. We therefore attempted to assess the in vivo relevance of these histone targets for centromeric heterochromatin initiation. To do this, we used fission yeast bearing lysine mutations in analogous positions of the H3 and H4 histone tails.
Fission yeast encode three copies each of histones H3 and H4, but strains have been developed that retain just one copy of histones H3 and H4, which appear relatively WT (Mellone et al, 2003). Several mutants have been generated in these strains in which lysine residues have been mutated to mimic the charge of an acetylated lysine (K to G and A or Q) as a proxy for loss of specific HDAC activity on a particular residue.
Mutation of H4K16 to G has been shown to have no impact on centromeric heterochromatin maintenance in fission yeast (Mellone et al, 2003). H3K9A and H3K14A mutants, however, exhibit loss of heterochromatin ((Mellone et al, 2003) and Figures 6A and B) and so cannot be tested for effects on heterochromatin initiation via the clr4+ withdrawal/ reintegration assay. To our knowledge, no mutants in fission yeast H3K4 had been reported apart from a H3K4 to R mutant, which maintains this residue’s basic charge (Xhemalce and Kouzarides, 2010). We therefore generated amino-acid substitutions at K4 to mimic the lack of charge of an acetylated residue at this position. We found that H3K4G mutants are not viable when serving as the only source of histone H3 expressed in cells. H3K4A mutants are viable and show little defect in centromeric heterochromatin maintenance (Figure 6A and B). H3K4Q mutant strains are also viable, and maintain silencing of centromeric dg transcripts but exhibit elevated levels of centromeric dh transcripts (Supplementary Figure S5A and B).
Figure 6.
H3K4 and H4K16 do not contribute to de novo centromeric silencing. (A, B) The integrity of H3K9 and H3K14, but not H3K4 or H4K16, is critical to maintenance of centromeric silencing. Accumulation of centromeric transcripts from the dh (A) and dg (B) repeats was evaluated by quantitative real-time PCR of cDNA derived from yeast expressing sole copies of histone H3 and histone H4 with the mutations indicated. Euchromatic act1+ transcripts serve as a normalization control. Neither H3K4 (C, D) nor H4K16 (E, F) are critical for de novo centromeric silencing mediated by clr4+. Experimental data present the average of two experimental replicates, including a total of four biological replicates from two distinct H3K4A clr4Δ to clr4+ and H4K16G clr4Δ to clr4+ reintegrant strains. (G) Flag–Clr4 is equivalently expressed in cells bearing mutations in histone H3. Western analysis of clr4Δ cells bearing different histone H3 mutations and transformed with episomal Flag–Clr4 expression vector demonstrates equivalent Flag–Clr4 expression compared with tubulin loading control. (H) Flag–Clr4 is efficiently recruited to centromeres, but fails to localize in H3K9A and H3K14A mutant cells. Q–PCR analysis of ChIP for Flag–Clr4 recruitment to centromeric dh sequences compared with adh1+ euchromatic control. Data averaged from five experimental replicates, with s.e.m. shown.
To address the role of these residues in centromeric heterochromatin initiation, we made compound mutant strains with clr4Δ, and performed clr4+ reintegration experiments in clr4Δ H4K16G, clr4Δ H3K4A, and clr4Δ H3K4Q mutants, alongside clr4Δ strains bearing single-copy WT H3 and H4 genes. Analysis of centromeric transcripts demonstrated that on reintegration of clr4+ into single-copy WT histone H3 and H4 clr4Δ strains (Figure 6C and D), both dg and dh transcripts are reduced to background levels. Somewhat surprisingly, we found that dg and dh transcripts were also reduced to near background levels on reintegration of clr4+ into the clr4Δ H3K4A strain (Figure 6C and D). Similar results were obtained for dg transcripts on reintegration of clr4+ into the clr4Δ H3K4Q strains (Supplementary Figure S5C). When clr4+ was reintegrated into the clr4Δ H4K16G strain (Figure 6E and F), dg and dh transcripts were also downregulated to near WT levels.
Together, these data demonstrate that the substitutions at two of Sir2’s targets: H3K4 and H4K16, with residues that mimic the lack of charge of an acetylated residue, do not impede the ability of Clr4 to initiate assembly of centromeric heterochromatin. This leads us to conclude that either another acetylated histone target of Sir2, such as H3K9Ac or H3K14Ac, a combination of acetylated histone targets, or perhaps an acetylated non-histone chromatin target contributes to the centromeric heterochromatin establishment defect seen in cells lacking Sir2 activity.
H3K14 is critical for Clr4 recruitment to centromeres
Testing the role of H3K14 and H3K9 during centromeric heterochromatin assembly is difficult, since mutations at K9 or K14 of histone H3 cause disruption of centromeric heterochromatin. We did attempt to address whether H3K14A mutation blocked heterochromatin initiation (Supplementary Figure S6), but interpretation was hampered by the high levels of centromeric transcripts that accumulate under normal growth of this strain. That mutation of H3K9 phenocopies loss of clr4 function is not unexpected, since K9 is the target for methylation by Clr4. However, it is presently unclear why mutation of K14 should disrupt centromeric heterochromatin. In vitro studies have demonstrated that a peptide bearing H3K14Ac modification can be efficiently methylated on K9 by Clr4 or its human homologue Suv39h1 (Nakayama et al, 2001; Rea et al, 2000), and binding of H3K9me2 ‘reader’ proteins, such as Swi6, is not altered by H3K14Ac on peptide substrates (Yamada et al, 2005). We therefore questioned whether in vivo recruitment of Clr4 to centromeres might be impacted by acetylation of K14 within the histone H3 tail. We used the H3K14A acetyl mimic strain to address this question.
Compound mutant strains were generated from strains bearing single-copy mutant H3 (K9A or K14A) and clr4Δ, and were transformed with a plasmid expressing 3xFlag-clr4+ under control of its native regulatory sequences. This episomal Flag–Clr4 fully complements for clr4 function at centromeres (Supplementary Figure S7A). Western analyses demonstrated that expression of Flag–Clr4 protein was similar between these strains (Figure 6G). ChIP experiments revealed that whereas Flag–Clr4 was efficiently recruited to centromeres in clr4Δ strains expressing single-copy WT H3, Flag–Clr4 association with centromeres was reduced to background levels in cells expressing H3K9A or H3K14A (Figure 6H). Consistent with this result, Swi6 was not recruited to centromeres in cells expressing H3K14A (Supplementary Figure S7B).
These experiments suggest that the presence of amino-acid substitutions at K9 or K14 of histone H3 that mimic the charge of an acetylated lysine cause a defect in the recruitment of Clr4 to centromeres. Since Sir2 can deacetylate K9 and K14 of H3, we might infer that deacetylation of these residues by Sir2 is necessary for Clr4 recruitment to centromeres to initiate heterochromatin assembly. Alternatively, the lack of recruitment to centromeres in the H3K9A and H3K14A mutant strains could be an indirect effect of the high levels of transcription at centromeres in these backgrounds. Counter to this argument, previously we had analysed the ability of Clr4 to mediate H3K9 methylation when overexpressed in mutant backgrounds that exhibit high levels of transcriptional activity at centromeres. We found that in cells lacking clr4 and dcr1 or clr4 and ago1, low levels of H3K9 methylation could be detected on centromeric repeats following reintroduction of episomal genomic clr4+ (Shanker et al, 2010). To further probe the role of Sir2 in heterochromatin assembly, we asked whether Sir2 was necessary for accumulation of this methyl mark in RNAi-deficient cells.
Sir2 is required for the de novo deposition of H3K9me2 in RNAi-deficient cells
To test whether Sir2 contributes to the ability of Clr4 to methylate H3 in RNAi-deficient backgrounds, we generated strains triply deleted for clr4, dcr1, and sir2. We then expressed clr4+ from an episomal vector, and monitored by ChIP whether H3K9me2 and Swi6 could accumulate at centromeres in these strains (Figure 7A). Whereas significant accumulation of H3K9me2 was detected on overexpression of clr4+ in clr4Δ or even clr4Δdcr1Δ cells, levels of H3K9me2 in clr4+-transformed clr4Δdcr1Δsir2Δ cells did not differ from clr4Δ cells transformed with empty vector.
Figure 7.
De novo H3K9 methylation and Swi6 recruitment in RNAi-deficient backgrounds is dependent on Sir2. (A) Sir2 is required for centromeric H3K9 methylation upon clr4+ expression in RNAi-defective cells. Quantitative real-time PCR analysis of ChIP experiments performed with anti-H3K9me2 antibody, monitoring relative enrichment of centromeric dg and dh sequences over adh1+ euchromatic locus following episomal clr4+ expression in clr4Δ, clr4Δdcr1Δ, and clr4Δdcr1Δsir2Δ strains. Results are s.e.m. for duplicate independent ChIP experiments, with inclusion of duplicate data for two biological replicates for clr4Δdcr1Δsir2Δ strains. (B) Sir2 is required for centromeric Swi6 recruitment upon clr4+ expression in RNAi-defective cells. Swi6 ChIP experiments performed on strains outlined in (A), assessing relative enrichment at centromeric dg and dh sequences relative to adh1+ control.
To confirm and extend these results, we monitored enrichment of Swi6 on centromeric repeats in these strain backgrounds (Figure 7B). Similar to the results obtained for H3K9me2, we did not detect Swi6 at centromeres on overexpression of clr4+ in clr4Δdcr1Δsir2Δ cells, whereas Swi6 was enriched at centromeres in clr4Δdcr1Δ cells overexpressing clr4+. These data reinforce that Clr4 activity and the H3K9 methylation seen in RNAi-defective cells is dependent on Sir2.
Discussion
The assembly of heterochromatin relies on a complex interplay between factors that are important for the establishment of heterochromatin and for its maintenance. Here, we have uncovered novel roles for the Sir2 histone deacetylase in the establishment of heterochromatin in fission yeast. Our studies reveal that Sir2 is necessary for promoting Clr4-mediated H3K9 methyltransferase activity and establishment of transcriptional silencing at centromeres. We further demonstrate that de novo recruitment of H3K9 methylation and transcriptional silencing mediated by Sir2 is highly dependent on the enzyme’s deacetylase activity. This is supported by our observation that at centromeres, de novo silencing upon clr4+ reintroduction is largely lost in sir2N247A mutant backgrounds.
How does Sir2 promote Clr4 activity? We hypothesize that Sir2 may act to create a nucleosomal environment that is permissive to Clr4 binding and activity. Here, we provide the first direct evidence that H3K4Ac and K14Ac are indeed bona fide targets of the fission yeast Sir2 activity. We further demonstrate that the enzyme preferentially deacetylates H3K4Ac and H4K16Ac, and to a lesser degree H3K9Ac and H3K14Ac peptides in vitro. Surprisingly, despite the apparent in vitro specificity of Sir2 deacetylase activity towards H3K4Ac and H4K16Ac, these substrates do not appear to be critical to the establishment of centromeric heterochromatin. Indeed, H3K4A and H4K16G mutants intended to mimic the charge of constitutively acetylated residues, as might be expected upon sir2 deletion or catalytic mutation, were found to have little impact on either maintenance or establishment of centromeric silencing.
However, we cannot rule out contributions to our centromeric heterochromatin establishment phenotype from two other histone targets of Sir2: namely, H3K9Ac and H3K14Ac. H3K9A and H3K14A mutants are known to profoundly affect centromeric silencing (Mellone et al, 2003). Loss of heterochromatin is expected in cells bearing H3K9A mutations, since H3K9 is the critical target of Clr4 histone methyltransferase activity (Rea et al, 2000). It is unclear, however, why mutation of H3K14 should cause heterochromatin loss similar to that of cells lacking clr4, with commensurate loss of chromatin association by Swi6 (Mellone et al, 2003).
In vitro studies have demonstrated that recombinant Clr4 can methylate H3K9 even on peptides bearing H3K14Ac, suggesting that the Clr4 H3K9 methyltransferase activity is not abrogated by charge differences at H3K14 (Nakayama et al, 2001). In addition, the interaction of the Swi6 HP1-like chromodomain protein with the histone H3K9me2 tail is not affected by K14Ac in vitro (Yamada et al, 2005). However, we note that these experiments are performed under conditions of enzyme or effector excess, and use histone tail peptides rather than nucleosomal substrates. To our knowledge, the impact of H3K9 or H3K14 mutation on Clr4 association with nucleosomal targets has not been previously evaluated.
Because the epitope for commercially available H3K9me2 antibodies overlies the K14 residue, we could not directly test whether Clr4 promoted H3K9 methylation at centromeres in H3K14A mutants by ChIP experiments. Instead, we monitored the association of Clr4 with centromeres in cells bearing histone H3 mutant chromatin. We found that in H3K14A or H3K9A mutant cells, Clr4 association with centromeres was lost. This failure to recruit Clr4 to chromatin would cause loss of H3K9me2 and mislocalization of H3K9me2-binding proteins such as Swi6 (shown in Supplementary Figure S7B) and could explain the profound loss of centromeric heterochromatin in H3K14A mutant cells.
Why do H3K9A and H3K14A mutations result in loss of localization of Clr4? One clear possibility is that in these mutant backgrounds there are high levels of transcription through the centromeric repeats, which may perturb the ability of Clr4 to be recruited. However, our experiments comparing Clr4 function in cells bearing multiple mutations in components required for centromeric heterochromatin assembly reveal differential Clr4 function in cells that all express high levels of centromeric transcripts (Figure 7). These data suggest that it is not the level of transcription per se that is blocking Clr4 function, but that the Clr4 recruitment defect is specifically linked to loss of Sir2 function. Clr4 is a member of the Clr–C complex, and in vivo the function of Clr4 depends on all components of Clr–C (Hong et al, 2005; Horn et al, 2005; Jia et al, 2005; Li et al, 2005; Thon et al, 2005). Little is known about how this complex associates with chromatin, but it is possible that H3K14 acetylation or H3K14A mutation blocks recruitment of another Clr–C component to chromatin, and thereby indirectly blocks localization of Clr4. In agreement with this, H3K14R mutant cells also display disruption of heterochromatin, and a defect in Clr4 recruitment to centromeres (Mellone et al, 2003, Supplementary Figure S7C and D).
For H3K9A mutants, another possible explanation stems from loss of identity of the H3K9 residue itself. The N-terminal chromodomain of Clr4 has been shown to bind H3K9me2, resulting in a positive feedforward mechanism to amplify the H3K9me2 signal (Zhang et al, 2008). H3K9me2 appears critical for recruitment of Clr4, since mutation of Clr4’s histone methyltransferase domain to cause loss of centromeric H3K9me2 leads to a defect in Clr4 recruitment (Supplementary Figure S8).
Several histone deacetylases in fission yeast can deacetylate H3K14. These include Clr3, which is thought to be specific for H3K14 deacetylation, and Clr6, which is a broad spectrum deacetylase (Bjerling et al, 2002). One might predict that strains bearing mutations in these enzymes would show similar phenotypes to sir2 mutants. Indeed, similar to sir2Δ, cells lacking Clr3 and mutant for Clr6 show a profound loss of silencing of subtelomeric sequences (Hansen et al, 2006) and at centromeres, a redundant role for Clr3 in maintenance of H3K9me2 heterochromatin is revealed in cells additionally mutated for the RNAi pathway (Yamada et al, 2005). Sir2 HDAC activity is clearly required for Clr4 function to maintain heterochromatin within subtelomeric domains, but loss of sir2 has little impact on the maintenance of centromeric heterochromatin. We note that the RNAi pathway plays a critical role in the maintenance of centromeric heterochromatin (Volpe et al, 2002), but is relatively unimportant for maintenance of subtelomeric heterochromatin (Petrie et al, 2005). It is possible, and has very recently been demonstrated (Buscaino et al, 2013) that, similar to Clr3 (Yamada et al, 2005), Sir2 functions redundantly with RNAi in centromeric heterochromatin maintenance. However, Sir2’s role in promotion of Clr4 activity is revealed at subtelomeres where heterochromatin is maintained through an RNAi-independent pathway. Indeed, we present here that Sir2 and Clr3 play redundant roles in the maintenance of centromeric heterochromatin (Figure 3), as has recently been reported (Buscaino et al 2013). Additionally, we demonstrate an important dependence on Sir2 for Clr4 function during the RNAi-independent establishment of heterochromatin at centromeres (Figure 7; Partridge et al, 2007; Shanker et al, 2010). We note that Sir2 enzymatic activity has recently been reported as sufficient and necessary for maintenance of heterochromatin in RNAi-deficient yeast (Buscaino et al, 2013), although this study did not reveal a dependence on Sir2 for heterochromatin initiation.
Why does sir2N247A promote limited de novo silencing of centromeres upon clr4+ reintroduction? One possibility is that the enzyme retains a low level of histone deacetylase activity. Though our data clearly demonstrate that GST–sir2N247A is catalytically inactive in vitro, it is conceivable that this enzyme may have very low levels of activity that suffice to promote limited H3K9 and H3K14 deacetylation, and thereby limited clr4+ activity and recruitment. We speculate that sir2N247A may serve in a structural capacity that is sufficient to nucleate low-level silencing at centromeres. An intriguing possibility is that sir2N247A may serve as a scaffold that functions in concert with other histone deacetylases, such as Clr3, which we have demonstrated to act redundantly with sir2N247A (Figure 3). A wholly distinct possibility is that Sir2 physically interacts with Clr4 to directly recruit its activity to centromeres and other loci, and that this physical association is required for Clr4 function. In mammals, the Sir2 homologue, SirT1, has been shown to deacetylate the homologue of Clr4, Suv39h1, to promote its methyltransferase activity (Vaquero et al, 2007). In addition, binding of SirT1 to Suv39h1 masks a site of ubiquitination on Suv39h1, such that in cells lacking SirT1 protein, Suv39h1 protein levels are decreased (Bosch-Presegue et al, 2011). However, we have conclusively demonstrated that loss of Sir2 protein does not impact the transcription of clr4+ or steady-state levels of expression of endogenous Clr4 protein (Supplementary Figure S9). While it remains possible that Sir2 deacetylates Clr4 to promote its activity, the deacetylation site is not conserved between Suv39h1 and Clr4, and experiments to test this possibility lie outside the scope of the current work.
How Sir2 is itself recruited to centromeres remains an open question. We have demonstrated previously that the initial recruitment of Clr4 activity to centromeres occurs independent of the RNAi pathway (Partridge et al, 2007; Shanker et al, 2010). By extension, centromeric Sir2 recruitment may also occur independently of RNAi. Indeed, our experiments demonstrating a dependence on Sir2 for Clr4 function at centromeres in RNAi-deficient yeast (Figure 7) would strongly argue that Sir2 localization occurs independently of RNAi. This is supported by recent experiments revealing that Sir2 localization to centromeres is independent of both Clr4 and RNAi (Buscaino et al, 2013). In budding yeast, the initial recruitment of Sir2 to telomeres is mediated via association of the SIR complex with silencing factors that bind to specific DNA sequences within telomeric repeats such as Rap1p (Hickman et al, 2011). Given the recent findings concerning the role of transcription factors in the assembly of H3K9me2 chromatin at mammalian centromeres (Bulut-Karslioglu et al, 2012), we think it likely that Sir2 is recruited via DNA-binding factors to initiate the assembly of centromeric heterochromatin in fission yeast.
We note that while this paper was in revision, an elegant paper was published in EMBO J from the Allshire group that has identified regions within centromeric dg sequences that contribute to Sir2-dependent H3 K9 methylation (Buscaino et al, 2013). In summary, the combined work from the two papers has helped to reveal the mechanism (in terms of DNA sequence requirements and deacetylation targets) underlying the critical role for Sir2 in establishing and maintaining centromeric heterochromatin. Future studies will reveal how Sir2 is targeted to particular loci to establish heterochromatin, and whether similar strategies are employed to recruit Sir2 to mammalian centromeres.
Materials and methods
Strain generation
Strains used in this study are listed in Supplementary Table S1. Please see Supplementary data for additional information.
Plasmid construction
Please see Supplementary data.
Transcript analyses
These were performed essentially as described (Partridge et al, 2007; Debeauchamp et al, 2008). Real-time primers for act1 (JPO-2000, 2001) are mbp86 and 87 from (Buhler et al, 2007).
siRNA analyses
siRNA preparation was as performed previously, and siRNA from dh repeats and snoR69 as loading control were detected by hybridization (Partridge et al, 2007).
Cell growth analyses
These were performed as described previously (Debeauchamp et al, 2008). To assess the maintenance of silencing in strains transformed with his3+ plasmids, cells were grown in PMG–his medium and were plated on minimal medium lacking histidine (PMG−his), medium lacking histidine and uracil (PMG−his−ura), or minimal medium lacking histidine and supplemented with 2 g FOA per litre (PMG−his+FOA). A similar approach was taken for strains bearing LEU2+ vectors.
Western analyses
Antibodies used: ‘Anti-TAP’: HRP conjugated rabbit IgG (Jackson ImmunoResearch Laboratories #011-030-00), Flag: M2 monoclonal antibody (Sigma #F3165), Tubulin: TAT1 monoclonal from K. Gull. H4K16Ac: Rabbit polyclonal IgG (Active motif # 39929).
Chromatin immunoprecipitation
ChIP assays were performed essentially as described previously (Partridge et al, 2007), with the substitution of bead beating for enzymatic cell disruption methods. Bead beating was performed for 2 min at ambient temperature using a Biospec Products mini beadbeater. Buffers used for anti-Flag ChIPs did not contain sodium deoxycholate.
Ab for H3K9me2: Mouse monoclonal (Abcam ab1220). Ab for H3K9Ac: Rabbit polyclonal against histone H3 aa 4–14 K9ac (Millipore #07-352). Ab for H3K14Ac: Rabbit polyclonal against histone H3 aa 9–18 K14ac (Millipore#07-353). Ab for Flag: Mouse monoclonal M2 anti-Flag (Sigma #F3165). Ab for Swi6: Rabbit polyclonal Thermo Scientific PA 1-497.
Chromosome segregation assays
Rates of chromosome missegregation were obtained as previously described (Petrie et al, 2005).
Fluorogenic peptide deacetylation assay
Lysine deacetylase activity of Sir2 and other enzymes was evaluated using a fluorogenic peptide substrate (Fluor-de-Lys green HDAC drug discovery kit: BML-AK530-0001, Enzo Life Sciences). Please see Supplementary data for additional information.
32P NAD+ hydrolysis assay for Sir2 deacetylase activity
32P NAD+ hydrolysis assays were performed essentially according to the method of (Tanny and Moazed, 2001). Please see Supplementary data for exact conditions.
Supplementary Material
Acknowledgments
We thank Lorraine Pillus and Robin Allshire for strains; Keith Gull for anti-TAT1 antibody; Richard Festenstein for H3K4Ac Ab; Tony Carr, Kathy Gould, Susan Forsburg, and Rohinton Kamakaka for vectors; and the Hartwell Center of St Jude Children’s Research Hospital for peptide synthesis and DNA sequencing. Thanks to Paul Brindle, Thomas Schalch, Jim Ihle, and Kevin Creamer for thoughtful comments. Funding was provided by NIH R01 GM084045 to JFP, Cancer Center support grant CCSG 2 P30 CA21765 (St Jude), and the American Lebanese Syrian Associated Charities (ALSAC) of St Jude Children’s Research Hospital.
Author contribution: Project was conceived by JFP. BJA and JFP designed the research; BJA, GJ, RKY, SS, BRL, and JFP performed the research; JFP and BJA wrote the paper, and all authors reviewed the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Allshire RC, Javerzat JP, Redhead NJ, Cranston G (1994) Position effect variegation at fission yeast centromeres. Cell 76: 157–169 [DOI] [PubMed] [Google Scholar]
- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G (1995) Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev 9: 218–233 [DOI] [PubMed] [Google Scholar]
- Aparicio OM, Billington BL, Gottschling DE (1991) Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 66: 1279–1287 [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Kaeberlein M, Imai SI, Guarente L (2002) Mutations in Saccharomyces cerevisiae gene SIR2 can have differential effects on in vivo silencing phenotypes and in vitro histone deacetylation activity. Mol Biol Cell 13: 1427–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos JL, Bever KM, Wolberger C (2005) Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol Cell 17: 855–868 [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410: 120–124 [DOI] [PubMed] [Google Scholar]
- Bell SD, Botting CH, Wardleworth BN, Jackson SP, White MF (2002) The interaction of Alba, a conserved archaeal chromatin protein, with Sir2 and its regulation by acetylation. Science 296: 148–151 [DOI] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA (2002) Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem 277: 45099–45107 [DOI] [PubMed] [Google Scholar]
- Bjerling P, Silverstein RA, Thon G, Caudy A, Grewal S, Ekwall K (2002) Functional divergence between histone deacetylases in fission yeast by distinct cellular localization and in vivo specificity. Mol Cell Biol 22: 2170–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Presegue L, Raurell-Vila H, Marazuela-Duque A, Kane-Goldsmith N, Valle A, Oliver J, Serrano L, Vaquero A (2011) Stabilization of Suv39H1 by SirT1 is part of oxidative stress response and ensures genome protection. Mol Cell 42: 210–223 [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Sherman JM, Devine SE, Cameron EE, Pillus L, Boeke JD (1995) The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev 9: 2888–2902 [DOI] [PubMed] [Google Scholar]
- Braunstein M, Rose AB, Holmes SG, Allis CD, Broach JR (1993) Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev 7: 592–604 [DOI] [PubMed] [Google Scholar]
- Buhler M, Haas W, Gygi SP, Moazed D (2007) RNAi-dependent and -independent RNA turnover mechanisms contribute to heterochromatic gene silencing. Cell 129: 707–721 [DOI] [PubMed] [Google Scholar]
- Buhler M, Verdel A, Moazed D (2006) Tethering RITS to a nascent transcript initiates RNAi- and heterochromatin-dependent gene silencing. Cell 125: 873–886 [DOI] [PubMed] [Google Scholar]
- Bulut-Karslioglu A, Perrera V, Scaranaro M, de la Rosa-Velazquez IA, van de Nobelen S, Shukeir N, Popow J, Gerle B, Opravil S, Pagani M, Meidhof S, Brabletz T, Manke T, Lachner M, Jenuwein T (2012) A transcription factor-based mechanism for mouse heterochromatin formation. Nat Struct Mol Biol 19: 1023–1030 [DOI] [PubMed] [Google Scholar]
- Buscaino A, Lejeune E, Audergon P, Hamilton G, Pidoux A, Allshire RC (2013) Distinct roles for Sir2 and RNAi in centromeric heterochromatin nucleation, spreading and maintenance. EMBO J 32: 1250–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam HP, Sugiyama T, Chen ES, Chen X, FitzGerald PC, Grewal SI (2005) Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nat Genet 37: 809–819 [DOI] [PubMed] [Google Scholar]
- Debeauchamp JL, Moses A, Noffsinger VJ, Ulrich DL, Job G, Kosinski AM, Partridge JF (2008) Chp1-Tas3 interaction is required to recruit RITS to fission yeast centromeres and for maintenance of centromeric heterochromatin. Mol Cell Biol 28: 2154–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwall K, Olsson T, Turner BM, Cranston G, Allshire RC (1997) Transient inhibition of histone deacetylation alters the structural and functional imprint at fission yeast centromeres. Cell 91: 1021–1032 [DOI] [PubMed] [Google Scholar]
- Freeman-Cook LL, Gomez EB, Spedale EJ, Marlett J, Forsburg SL, Pillus L, Laurenson P (2005) Conserved locus-specific silencing functions of Schizosaccharomyces pombe sir2+. Genetics 169: 1243–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman-Cook LL, Sherman JM, Brachmann CB, Allshire RC, Boeke JD, Pillus L (1999) The Schizosaccharomyces pombe hst4(+) gene is a SIR2 homologue with silencing and centromeric functions. Mol Biol Cell 10: 3171–3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia BA, Hake SB, Diaz RL, Kauer M, Morris SA, Recht J, Shabanowitz J, Mishra N, Strahl BD, Allis CD, Hunt DF (2007) Organismal differences in post-translational modifications in histones H3 and H4. J Biol Chem 282: 7641–7655 [DOI] [PubMed] [Google Scholar]
- Gomez EB, Espinosa JM, Forsburg SL (2005) Schizosaccharomyces pombe mst2+ encodes a MYST family histone acetyltransferase that negatively regulates telomere silencing. Mol Cell Biol 25: 8887–8903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SI, Bonaduce MJ, Klar AJ (1998) Histone deacetylase homologs regulate epigenetic inheritance of transcriptional silencing and chromosome segregation in fission yeast. Genetics 150: 563–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar D, Kamakaka RT (2008) Schizosaccharomyces pombe Hst4 functions in DNA damage response by regulating histone H3 K56 acetylation. Eukaryot Cell 7: 800–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halic M, Moazed D (2010) Dicer-independent primal RNAs trigger RNAi and heterochromatin formation. Cell 140: 504–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KR, Ibarra PT, Thon G (2006) Evolutionary-conserved telomere-linked helicase genes of fission yeast are repressed by silencing factors, RNAi components and the telomere-binding protein Taz1. Nucleic Acids Res 34: 78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman MA, Froyd CA, Rusche LN (2011) Reinventing heterochromatin in budding yeasts: Sir2 and the origin recognition complex take center stage. Eukaryot Cell 10: 1183–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, Villen J, Gerace EL, Gygi SP, Moazed D (2005) A cullin E3 ubiquitin ligase complex associates with Rik1 and the Clr4 histone H3-K9 methyltransferase and is required for RNAi-mediated heterochromatin formation. RNA Biol 2: 106–111 [DOI] [PubMed] [Google Scholar]
- Horn PJ, Bastie JN, Peterson CL (2005) A Rik1-associated, cullin-dependent E3 ubiquitin ligase is essential for heterochromatin formation. Genes Dev 19: 1705–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403: 795–800 [DOI] [PubMed] [Google Scholar]
- Jia S, Kobayashi R, Grewal SI (2005) Ubiquitin ligase component Cul4 associates with Clr4 histone methyltransferase to assemble heterochromatin. Nat Cell Biol 7: 1007–1013 [DOI] [PubMed] [Google Scholar]
- Johnson A, Li G, Sikorski TW, Buratowski S, Woodcock CL, Moazed D (2009) Reconstitution of heterochromatin-dependent transcriptional gene silencing. Mol Cell 35: 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LM, Kayne PS, Kahn ES, Grunstein M (1990) Genetic evidence for an interaction between SIR3 and histone H4 in the repression of the silent mating loci in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 87: 6286–6290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar AJ, Bonaduce MJ (1991) swi6, a gene required for mating-type switching, prohibits meiotic recombination in the mat2-mat3 "cold spot" of fission yeast. Genetics 129: 1033–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R (2000) The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci USA 97: 5807–5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Goto DB, Zaratiegui M, Tang X, Martienssen R, Cande WZ (2005) Two novel proteins, dos1 and dos2, interact with rik1 to regulate heterochromatic RNA interference and histone modification. Curr Biol 15: 1448–1457 [DOI] [PubMed] [Google Scholar]
- Mellone BG, Ball L, Suka N, Grunstein MR, Partridge JF, Allshire RC (2003) Centromere silencing and function in fission yeast is governed by the amino terminus of histone H3. Curr Biol 13: 1748–1757 [DOI] [PubMed] [Google Scholar]
- Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI (2001) Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292: 110–113 [DOI] [PubMed] [Google Scholar]
- Nimmo ER, Cranston G, Allshire RC (1994) Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J 13: 3801–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppikofer M, Kueng S, Martino F, Soeroes S, Hancock SM, Chin JW, Fischle W, Gasser SM (2011) A dual role of H4K16 acetylation in the establishment of yeast silent chromatin. EMBO J 30: 2610–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JF, Debeauchamp JL, Kosinski AM, Ulrich DL, Hadler MJ, Noffsinger VJ (2007) Functional separation of the requirements for establishment and maintenance of centromeric heterochromatin. Mol Cell 26: 593–602 [DOI] [PubMed] [Google Scholar]
- Petrie VJ, Wuitschick JD, Givens CD, Kosinski AM, Partridge JF (2005) RNA interference (RNAi)-dependent and RNAi-independent association of the Chp1 chromodomain protein with distinct heterochromatic loci in fission yeast. Mol Cell Biol 25: 2331–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, Jenuwein T (2000) Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406: 593–599 [DOI] [PubMed] [Google Scholar]
- Rusche LN, Kirchmaier AL, Rine J (2003) The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu Rev Biochem 72: 481–516 [DOI] [PubMed] [Google Scholar]
- Sadaie M, Iida T, Urano T, Nakayama J (2004) A chromodomain protein, Chp1, is required for the establishment of heterochromatin in fission yeast. EMBO J 23: 3825–3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaranarayana GD, Motamedi MR, Moazed D, Grewal SI (2003) Sir2 regulates histone H3 lysine 9 methylation and heterochromatin assembly in fission yeast. Curr Biol 13: 1240–1246 [DOI] [PubMed] [Google Scholar]
- Shanker S, Job G, George OL, Creamer KM, Shaban A, Partridge JF (2010) Continuous requirement for the Clr4 complex but not RNAi for centromeric heterochromatin assembly in fission yeast harboring a disrupted RITS complex. PLoS Genet 6: e1001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD (2000) A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci USA 97: 6658–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanny JC, Moazed D (2001) Coupling of histone deacetylation to NAD breakdown by the yeast silencing protein Sir2: Evidence for acetyl transfer from substrate to an NAD breakdown product. Proc Natl Acad Sci USA 98: 415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G, Hansen KR, Altes SP, Sidhu D, Singh G, Verhein-Hansen J, Bonaduce MJ, Klar AJ (2005) The Clr7 and Clr8 directionality factors and the Pcu4 cullin mediate heterochromatin formation in the fission yeast Schizosaccharomyces pombe. Genetics 171: 1583–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G, Klar AJ (1992) The clr1 locus regulates the expression of the cryptic mating-type loci of fission yeast. Genetics 131: 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D (2007) SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature 450: 440–444 [DOI] [PubMed] [Google Scholar]
- Volpe TA, Kidner C, Hall IM, Teng G, Grewal SI, Martienssen RA (2002) Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297: 1833–1837 [DOI] [PubMed] [Google Scholar]
- Xhemalce B, Kouzarides T (2010) A chromodomain switch mediated by histone H3 Lys 4 acetylation regulates heterochromatin assembly. Genes Dev 24: 647–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Fischle W, Sugiyama T, Allis CD, Grewal SI (2005) The nucleation and maintenance of heterochromatin by a histone deacetylase in fission yeast. Mol Cell 20: 173–185 [DOI] [PubMed] [Google Scholar]
- Yang Y, Hou H, Haller EM, Nicosia SV, Bai W (2005) Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J 24: 1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Mosch K, Fischle W, Grewal SI (2008) Roles of the Clr4 methyltransferase complex in nucleation, spreading and maintenance of heterochromatin. Nat Struct Mol Biol 15: 381–388 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.