Abstract
A healthy 34-year-old man presented with Ochrobactrum intermedium endophthalmitis due to a metallic intraocular foreign body. After vitrectomy, lensectomy, removal of the metallic intraocular foreign body, intravitreal vancomycin and ceftazidime, and systemic ciprofloxacin, intraocular inflammation worsened. Repeat vitreous culture confirmed persistent endophthalmitis due to multidrug-resistant O. intermedium. The endophthalmitis successfully resolved after the administration of intravitreal moxifloxacin.
Keywords: moxifloxacin, Ochrobactrum intermedium, endophthalmitis, intraocular foreign body
Introduction
Endophthalmitis can cause severe visual loss following open-globe injuries, and occurs in 3%–30% of retained intraocular foreign body (IOFB) cases.1,2 Gram-negative bacteria are a frequent cause of endophthalmitis in IOFB cases.3 Multidrug resistance among endophthalmitis isolates is rare, but more common in Gram-negative organisms.4Ochrobactrum species are aerobic, nonfermenting, Gram-negative rods associated with opportunistic infections. O. intermedium is an emerging human pathogen difficult to identify by conventional methods and associated with multidrug resistance.5 In the present case, persistent O. intermedium endophthalmitis successfully resolved after the administration of intravitreal moxifloxacin.
Case report
A healthy 34-year-old emmetropic man presented with a 2-day history of pain and worsening vision due to metallic IOFB of the right eye. The metallic IOFB was part of a mechanical wire brush contaminated with soil. A computed tomography scan confirmed metallic IOFB (Figure 1). Visual acuity (VA) was hand motion in the right eye and 20/20 in the left eye. Intraocular pressures were 24 mmHg in the right eye and 11 mmHg in the left eye. A 3+ relative afferent pupillary defect of the right eye was present. Examination was notable for temporal subconjunctival hemorrhage, 3+ cell and fibrin of the anterior chamber with 0.5 mm hypopyon, 3+ vitreous cell, and poor view posteriorly. Urgent vitrectomy, lensectomy, removal of the IOFB, and intravitreal injection of vancomycin and ceftazidime were performed. Intraoperatively, vitreous cells were heavily concentrated around the foreign body, and retinal whitening and hemorrhages were present in all four quadrants. An inferior retinal tear was treated with endolaser photocoagulation and fluid–gas exchange. Postoperatively, the patient was treated with systemic ciprofloxacin and topical gentamicin, moxifloxacin, prednisolone acetate, and atropine. An initial Gram stain was notable for Gram-negative rods.
Figure 1.
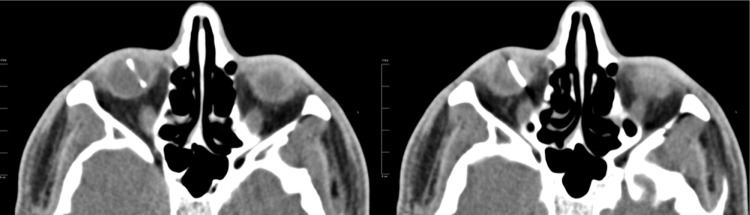
Axial computed tomography scan of the orbits shows metallic intraocular foreign body of the right eye measuring 14 mm in length.
Intraocular inflammation worsened over the next 2 days as the hypopyon increased to 2 mm, fibrin reaccumulated in the anterior chamber, and VA remained hand motion. A vitreous tap and injection of vancomycin, ceftazidime, and dexamethasone was performed. Two days later, intraocular inflammation continued to worsen, and the hypopyon increased to 3 mm.
The first vitreous culture showed nonfermenting, Gramnegative rods resistant to amikacin, ampicillin, ampicillin/sulbactam, ceftazidime, ceftriaxone, gentamicin, piperacillin/tazobactam, and tobramycin, but sensitive to ciprofloxacin, levofloxacin, and trimethoprim/sulfamethoxazole. The subsequent vitreous culture confirmed persistent endophthalmitis due to a multidrug-resistant Gram-negative rod sensitive to fluoroquinolones. Studies with intravitreal moxifloxacin were reviewed. The benefits, risks, and alternatives, including in vitro safety and potential toxicity of intravitreal moxifloxacin, were discussed thoroughly with the patient. The patient freely consented and received intravitreal moxifloxacin (Vigamox) 0.1 mL of 500 μg/0.1 mL.
Over the next week, the hypopyon retracted and the intraocular inflammation resolved. The clear view posteriorly permitted visualization of a macula-involving retinal detachment and choroidal detachment associated with the initial inferior retinal tear. The patient underwent scleral buckle procedure with vitrectomy and silicone oil with repeat injection of intravitreal moxifloxacin 0.1 mL of 500 μg/0.1 mL. Culture results obtained at the time of retinal detachment repair were negative, confirming resolution of the endophthalmitis after the administration of intravitreal moxifloxacin. Seven weeks after initial presentation, VA improved to 20/200, and examination showed a quiet anterior chamber with clear view posteriorly (Figure 2). The Mayo Clinic in Rochester identified O. intermedium by performing DNA sequencing of the 16S ribosomal DNA (16S rDNA) genes with the first vitreous culture.
Figure 2.
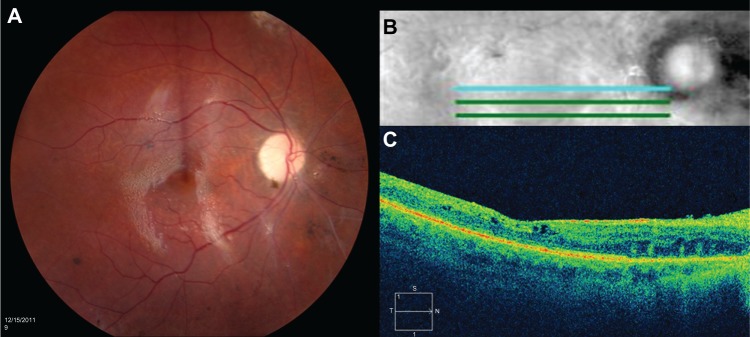
(A) Color fundus photograph of the right eye shows a clear view posteriorly with silicone oil present, sharp disc with 1+ pallor, normal vessels, flat macula, and periphery with preserved pigmentation and scattered focal areas of pigment clumping common after retinal detachment. (B) Five-line optical coherence tomography horizontal raster. (C) High-definition optical coherence tomography horizontal image shows preserved foveal contour, intact inner segment–outer segment junction, and trace intraretinal microcysts.
Discussion
Ochrobactrum anthropi has been identified as a rare cause of postoperative endophthalmitis.6,7O. anthropi is the only species of Ochrobactrum in the database of API 20NE, making differentiation of O. anthropi and O. intermedium difficult. 16S rDNA gene sequencing has been adopted to differentiate O. anthropi from O. intermedium.8 Reported cases of O. anthropi endophthalmitis presented 6–9 weeks after cataract extraction, with decreased VA ranging from 20/200 to count fingers, hypopyon, and vitritis. The organisms were resistant to β-lactams and aminoglycosides. Successful management required vitrectomy in all cases, with some cases also requiring the removal of the intraocular lens and capsule.6,7 The present case of Ochrobactrum endophthalmitis differed from previously reported cases, as the presentation occurred 2 days after penetrating injury by contaminated IOFB rather than postcataract extraction. The acute onset of endophthalmitis may be due to the high bacterial load introduced directly into the vitreous at the time of the penetrating injury.
The O. intermedium isolate in the present case was multidrug-resistant to both amikacin and ceftazidime, which are commonly used for empiric intravitreal Gram-negative coverage. The O. intermedium isolate was sensitive to fluoroquinolones. Moxifloxacin is a fourth-generation fluoroquinolone commonly prescribed topically for endophthalmitis prophylaxis after cataract surgery.9
To determine the effective dose of moxifloxacin for intravitreal injection, the half-life and toxicity of moxifloxacin, 90% minimum inhibitory concentration (MIC90) of the causative organism, and vitreous volume of the patient should be considered. Previous studies with moxifloxacin have established 150 μg/mL as a safe concentration for the rabbit eye and human retinal pigment epithelial cells. Intravitreal moxifloxacin caused no electroretinographic or retinal histologic abnormality in rabbit eyes at a concentration of 150 μg/mL (200 μg/0.1 mL injected into a measured vitreous volume of 1.2 mL).10 In human retinal pigment epithelial cells, there was no evidence of toxicity with a moxifloxacin concentration of 150 μg/mL.11 Intravitreal moxifloxacin did demonstrate toxicity in rabbit eyes at a concentration of 213 μg/mL (320 μg/0.1 mL injected into an estimated vitreous volume of 1.5 mL).12
The vitreous volume of an adult emmetropic human eye is approximately 4 mL, giving an empiric concentration of 125 μg/mL when 500 μg/0.1 mL moxifloxacin is injected intravitreally. Intravitreal moxifloxacin has shown an exponential decay, with a half-life of 1.72 hours. The mean vitreous concentration decreased from 120.5 μg/mL 1 hour after injection to 1.1 μg/mL at 12 hours, when 200 μg/0.1 mL was administered in rabbit eyes.13 The MIC90 in the present case was low, ≤1 μg/mL and ≤2 μg/mL for ciprofloxacin and levofloxacin, respectively, which may explain the clinical resolution of infection despite the short half-life of intravitreal fluoroquinolones.
Intravitreal moxifloxacin was effective in the management of the present case of bacterial endophthalmitis. In an era of increasing multidrug resistance, intravitreal moxifloxacin may play a role in the management of bacterial endophthalmitis.
Footnotes
Disclosure
The authors in this paper have no financial or proprietary interests in products, methods, or materials published in this paper.
References
- 1.Mieler WF, Ellis MK, Williams DF, Han DP. Retained intraocular foreign bodies and endophthalmitis. Ophthalmology. 1990;97(11):1532–1538. doi: 10.1016/s0161-6420(90)32381-3. [DOI] [PubMed] [Google Scholar]
- 2.Parke DW, 3rd, Pathengay A, Flynn HW, Jr, Albini T, Schwartz SG. Risk factors for endophthalmitis and retinal detachment with retained intraocular foreign bodies. J Ophthalmol. 2012;2012:758526. doi: 10.1155/2012/758526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang CS, Lu CK, Lee FL, Hsu WM, Lee YF, Lee SM. Treatment and outcome of traumatic endophthalmitis in open globe injury with retained intraocular foreign body. Ophthalmologica. 2010;224(2):79–85. doi: 10.1159/000235725. [DOI] [PubMed] [Google Scholar]
- 4.Pathengay A, Moreker MR, Puthussery R, et al. Clinical and microbiologic review of culture-proven endophthalmitis caused by multidrug-resistant bacteria in patients seen at a tertiary eye care center in southern India. Retina. 2011;31(9):1806–1811. doi: 10.1097/IAE.0b013e31820f4b9d. [DOI] [PubMed] [Google Scholar]
- 5.Apisarnthanarak A, Kiratisin P, Mundy LM. Evaluation of Ochrobactrum intermedium bacteremia in a patient with bladder cancer. Diagn Microbiol Infect Dis. 2005;53(2):153–155. doi: 10.1016/j.diagmicrobio.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Song S, Ahn JK, Lee GH, Park YG. An epidemic of chronic pseudophakic endophthalmitis due to Ochrobactrum anthropi: clinical findings and managements of nine consecutive cases. Ocul Immunol Inflamm. 2007;15(6):429–434. doi: 10.1080/09273940701798546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greven CM, Nelson KC. Chronic postoperative endophthalmitis secondary to Ochrobactrum anthropi. Retina. 2001;21(3):279–280. doi: 10.1097/00006982-200106000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Kämpfer P, Citron DM, Goldstein EJ, Scholz HC. Difficulty in the identification and differentiation of clinically relevant Ochrobactrum species. J Med Microbiol. 2007;56(Pt 11):1571–1573. doi: 10.1099/jmm.0.47350-0. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MK, Fiscella RG, Moshirfar M, Mooney B. Third- and fourth-generation fluoroquinolones: retrospective comparison of endophthalmitis after cataract surgery performed over 10 years. J Cataract Refract Surg. 2008;34(9):1460–1467. doi: 10.1016/j.jcrs.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 10.Gao H, Pennesi ME, Qiao X, et al. Intravitreal moxifloxacin: retinal safety study with electroretinography and histopathology in animal models. Invest Ophthalmol Vis Sci. 2006;47(4):1606–1611. doi: 10.1167/iovs.05-0702. [DOI] [PubMed] [Google Scholar]
- 11.Kernt M, Neubauer AS, Ulbig MW, Kampik A, Welge-Lüssen U. In vitro safety of intravitreal moxifloxacin for endophthalmitis treatment. J Cataract Refract Surg. 2008;34(3):480–488. doi: 10.1016/j.jcrs.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Aydin E, Kazi AA, Peyman GA, Esfahani MR. Intravitreal toxicity of moxifloxacin. Retina. 2006;26(2):187–190. doi: 10.1097/00006982-200602000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Iyer MN, He F, Wensel TG, Mieler WF, Benz MS, Holz ER. Clearance of intravitreal moxifloxacin. Invest Ophthalmol Vis Sci. 2006;47(1):317–319. doi: 10.1167/iovs.05-1124. [DOI] [PubMed] [Google Scholar]


