Abstract
Intestinal infections by attaching and effacing (A/E) bacterial pathogens cause severe colitis and bloody diarrhea. Although p38α in intestine epithelial cells (IEC) plays an important role in promoting protection against A/E bacteria by regulating T cell recruitment, its impact on immune responses remains unclear. In this study, we show that activation of p38α in T cells is critical for the clearance of the A/E pathogen Citrobacter rodentium. Mice deficient of p38α in T cells, but not in macrophages or dendritic cells, were impaired in clearing C. rodentium. Expression of inflammatory cytokines such as IFN-γ by p38α-deficient T cells was reduced, which further reduced the expression of inflammatory cytokines, chemokines and anti-microbial peptide by IECs and led to reduced infiltration of T cells into the infected colon. Administration of IFN-γ activated the mucosal immunity to C. rodentium infection by increasing the expression of inflammation genes and the recruitment of T cells to the site of infection. Thus, p38α contributes to host defense against A/E pathogen infection by regulating the expression of inflammatory cytokines that activate host defense pathways in IECs.
Introduction
Infection by attaching and effacing (A/E) bacterial pathogens including enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic E. coli (EHEC) is common and potentially serious causes of gastroenteritis around the world. EHEC O157:H7 (E. coli O157:H7) infection causes severe colitis and bloody diarrhea due to the production of shiga-like toxins, while EPEC causes diarrhea in millions of children in developing countries. Since EPEC and EHEC are human-specific and do not infect mice efficiently, infection of the natural mouse pathogen Citrobacter rodentium is a commonly used model, which has provided information about the A/E bacterial pathogenesis and the host immune response (1). Both innate and adaptive immune responses contribute to host defense against C. rodentium infection (2-6). Toll-like receptors (TLRs) have been demonstrated to play a major role in the recognition of C. rodentium infection and in initiating the inflammatory immune responses (4,7). Additionally, intracellular innate NOD-like receptors (NLRs) participate in host defense by inducing TH1 and TH17 and responses in the gastrointestinal tract (8,9). Meanwhile, CD4+ T cells are the critical mediators for the adaptive immune response to C. rodentium in the murine colonic mucosa (3,10), and a TH1/ TH17-mediated response is associated with host defense against C. rodentium infection (6,11). Additionally, certain cytokines such as IFN-γ and TNF-α from lymphocytes play a critical role in host defense in C. rodentium infection (12,13). These cytokines stimulate not only the innate immune response of the infected epithelial cells but also the inflammatory phenotypes of lymphocytes (11).
p38α is a member of the serine-threonine mitogen-activated protein kinase (MAPK) family and regulates numerous biological processes including immune responses and inflammation (14-16). p38α-mediated expression of pro-inflammatory cytokines and chemokines is initiated by TLR responses in innate immune cells (17), and p38α also plays an important role in the pathology of skin and gut inflammation (18-20). p38α regulates the development of T cells in thymus, differentiation of naïve T cells into TH effector cells, and production of cytokines that contribute to inflammation and host defense (16,21-23).
We previously demonstrated that expression of inflammatory cytokines and chemokines was reduced, and infiltration of T cells was impaired in the colon of C. rodentium-infected intestinal epithelial cell (IEC)-specific p38α-deficient mice, which resulted in the failure of bacterial clearance (24). However, the role of p38α expression in immune cells in controlling the host response to A/E pathogen infection is unknown. In this study, we generated immune cell-specific p38α deficient mouse strains and investigated the role of p38α in controlling the immune response against C. rodentium infection. We observed that p38α expression by T cells is critical for host clearance of C. rodentium by producing the inflammatory cytokines that activate IEC defense mechanisms.
Method and materials
Mice
p38αfl/fl mice were described previously (17). To generate macrophage-, dendritic cell-, or T cell-specific p38α-deficient mice, p38αfl/fl mice were bred with LysM, CD11c, or Lck promoter-driven Cre transgenic mice (The Jackson Laboratory, Bar Harbor, ME). C56Bl/6J wildtype mice were obtained from Institutional Breeding Colony at The Scripps Research institute. Animal studies were performed using sex-matched 8 to 10-week old mice and conducted according to the guideline and approval of the Institutional Animal Care and Use Committee.
Bacterial infection and bacterial antigen preparation
C. rodentium strain DBS 100 (American Type Culture Collection, Manassas, VA) in a volume of 200 μl (2 × 109 CFU) was orally inoculated into each mouse after fasting for 8 hours. IFN-γ (10 μg per mouse, R&D systems) was injected intraperitoneally at the indicated time points after C. rodentium infection. C. rodentium lysate was prepared as previously described (24,25).
Colony-forming units count, colon tissue collection, and cell isolation
To assess the level of C. rodentium infection, in colonic tissues, a distal piece (~ 1 cm) of colon was removed, weighed, and homogenized in sterile PBS. Homogenates were serially diluted in PBS and plated on MacConkey agar. The number of colonies was counted after 18 hours of incubation at 37°C. To obtain RNA of colon tissues, a piece of colon (~ 0.5 cm) was collected and kept in RNAlater (Qiagen) at −80°C until the RNA preparation. IECs and lamina propria lymphocytes were obtained as previously described (24). Briefly, the colon was removed and opened longitudinally, then washed with ice-cold PBS to remove debris and mucus. The tissue was cut into small pieces (~ 1 cm) and further incubated at 37°C for 15 min under gentle shaking in HBSS supplemented with 5mM EDTA and 2% FBS. The epithelial cells in the supernatant were collected spun down at 150g for 5 min. The cell pellets were resuspended in 40% Percoll solution and spun down again. The epithelial cells at the top layer were collected. The purity was assessed by staining the cells with epithelial cell specific markers anti-cytokeratin-18 (C-04, Santa Cruz Biotechnology) and anti-EpCAM (G8.8, eBioscience) (>95% purity). The remaining tissue pellets after the gentle shaking in HBSS with 5mM EDTA and 2% FBS were further incubated at 37°C for 2 hours in RPMI-1640 medium containing 20% FBS, 2 mg/ml of collagenase type III (Worthington Biochemical Corp), and 15 mg/ml of DNase I (Roche, Switzerland). The cells were further washed in HBSS and passed through a 40 μm cell strainer to obtain lamina propria (LP) lymphocytes. Dendritic cells were obtained from mesenteric lymph nodes (MLNs). MLNs were disaggregated through a 70 μm cell strainer to remove debris.
Histological analysis
To compare the degree of inflammation, colon tissues were fixed in 10% formalin, and paraffin sections were stained with hematoxylin and eosin (H&E) for microscopic analysis. The histological scoring was assessed to determine the degree of inflammatory cell infiltration and tissue damage (26). The cell infiltration score was defined as a scale of 0–3 of inflammatory cell infiltration (0, no or occasional inflammatory cells in the LP; 1, slightly increased number of inflammatory cells; 2, moderate infiltration of inflammatory cells; 3, extensive infiltration of inflammatory cells). The histological tissue damage score was determined (0, no damage; 1, mild hyperplasia with superficial epithelial injury; 2, moderate hyperplasia, with focal erosions; 3, severe hyperplasia with multifocal erosions).
Immunohistochemistry
Immunostaining of C. rodentiun and CD4 T cells was performed as follows. Paraffin section slides were deparaffinized and rehydrated prior to antigen retrieval by boiling in 10 mM sodium citrate buffer (pH 6.0). Sections were blocked in blocking buffer (3% BSA and animal-free blocker (Vector Labs, Burlingame, CA)), and stained with rabbit anti-C. rodentium antibody followed by Alexa Fluor 488 anti-rabbit IgG (Molecular Probe, Eugene, OR), or stained with FITC-conjugated anti-mouse CD4 (Biolegend, San Diego, CA). Slides were counterstained with VECTASHIELD mounting media with DAPI (Vector Labs, Burlingame, CA) prior to visualization.
Preparation and stimulation of T cells
T cells were purified using Pan T Cell Isolation Kit II (Miltenyi Biotec Inc., Auburn, CA) from spleen. To induce T cell activation, cells were seeded on the plates coated with anti-CD3/CD28 antibodies. After 3 days, culture supernatants were collected to determine IL-2 and IFN-γ levels by ELISA. Cell proliferation was tested using CFSE Cell Proliferation kit (Invitrogen). Cells were labeled with CFSE and cultured in the plates coated with anti-CD3/CD28 antibodies for 3 days. Degree of proliferation was measured using flow cytometer according to the manufacturer’s protocol.
Ex vivo colon culture and cytokine measurement
Colon fragments (~ 1cm) was obtained aseptically and weighed. The pieces were washed three times in ice-cold PBS, and incubated in DMEM supplemented with 10% FBS and antibiotics for 24 hours (24,25). Culture supernatants were obtained and cytokine levels were measured by ELISA.
Preparation of bone marrow-derived DCs
Bone marrow cells were obtained and cultured in RPMI 1640 supplemented with 10% FBS and GM-CSF (20 ng/ml) for 6 days.
Preparation of FITC-conjugated C. rodentium and analysis of phagocytic activity of macrophages and DCs
Labeling of C. rodentium and phagocytosis analysis was as previously described (27). Cultured C. rodentium was labeled with 1 mg/ml of FITC solution in PBS for 15 min. Peritoneal macrophages from control or macrophage-specific p38α-deficient mice, or BM-derived DCs from control or DC-specific p38α-deficient mice were co-cultured with FITC-conjugated C. rodentium for 6 hours. Cells were washed and harvested in ice-cold PBS. DCs were stained with anti-CD11c-PE antibody. Intracellular level of FITC-labeled C. rodentium was measured by flow cytometry.
Flow cytometry of intracellular cytokine and surface marker expressions
Isolated cells from LP were treated with Brefeldin A (10 μg/ml), PMA (50 ng/ml), and ionomycin (1 μg/ml) for 4 hours before intracellular cytokine staining of IL-17 and IFN-γ. Intracellular staining of cytokines was performed using Cytofix/Cytoperm Fixation/Permeabilization Solution kit (BD Bioscience, CA). LP and MLN cells were suspended in FACS buffer and incubated with the indicated antibodies for FACS analysis. Fc Block (anti-CD16/CD32), anti-CD4-APC, anti-IL-17A-FITC, anti-IFN-γ-PE, anti-CD11c-APC, anti-TNFα-FITC, anti-MHCII-PE, anti-CD80-PE, and anti-CD86–FITC (eBiosciences) antibodies were used as indicated. Stained cells were analyzed by LSR-II (BD Biosciences) and using FlowJo (version 3.6; TreeStar) software.
RNA isolation and quantitative PCR analysis
Total RNA from the colon tissues or LP lymphocytes was isolated using RNeasy kit (Qiagen) and cDNA was synthesized by reverse transcription. The mRNA levels of indicated mouse genes were determined by quantitative PCR analysis using the SYBR Green/ROX qPCR Master Mix (Thermo Scientific). All values were normalized to the housekeeping gene actin mRNA, and relative expressions were calculated by the ΔΔCt method. Fold induction of genes was compared to the gene expression levels of uninfected mice.
Statistical analysis
Differences were tested using the Student t test. The p values are shown and <0.05 were considered statistically significant.
Results
p38α in T cells, not in macrophages or DCs, plays an essential role in protection against C. rodentium infection
We previously reported that p38α in IECs promotes T cell recruitment to provide protection against C. rodentium infection (24). To test the role of p38α in immune cells against C. rodentium infection, we generated macrophage-specific (p38αΔMAC) (17), dendritic cell-specific (p38αΔDC) or T cell-specific (p38αΔT) p38α-deficient mouse strains. Expression of p38α was defective in thymocytes, purified splenic T cells and LP T cells, but was intact in other tissues and cells including B cells and macrophages in p38αΔT mice (Supplemental Fig. S1A & B). Flow cytometry analysis also confirmed the deletion of p38α in DCs of p38αΔDC mouse (Supplemental Fig. S1C). The ratio of CD4+ and CD8+ cells among CD3+ T cells in the thymus, spleen, and LP was comparable between control (p38αfl/fl) and p38αΔT mice (Supplemental Fig. S1D), indicating that deletion of p38α does not affect the development of T cells. Also, p38α-deficient T cells did not show any significant differences in TCR-mediated responses such as proliferation and IL-4 production compared with the control cells while production of IFN-γ was significantly reduced in p38α-deficient T cells (Supplemental Fig. S2). This observation is consistent with other reports using inhibitors or kinase-dead knock-in mutant mice (21,28).
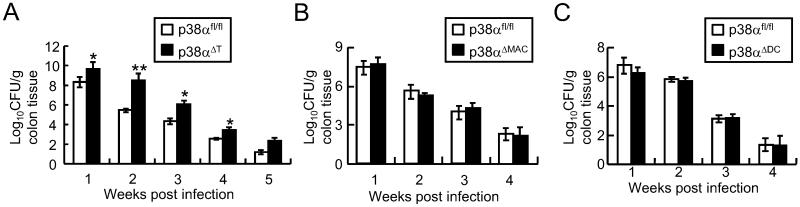
To test the role of p38α in immune cells against C. rodentium infection, p38αfl/fl, p38αΔMAC, p38αΔDC or p38αΔT mice were orally inoculated with C. rodentium, and the pathogen burden in their colons was measured. We found that p38αΔT mice were impaired at clearing the C. rodentium infection, unlike p38αfl/fl, p38αΔMAC and p38αΔDC mice (Fig. 1). The difference in bacterial clearance was moderate but statistically significant after 1 week of infection in the colon tissues of p38αΔT mice, and the pathogen burden was much worse in p38αΔT compared with control mice at 2 weeks post-infection. Moreover, the bacterial clearance in p38αΔT mice was still defective at the later phase of infection (Fig. 1A), and the development of transmissible colonic hyperplasia, the pathologic hallmark of C. rodentium infection, was more significant in p38αΔT mice (data not shown). Clearance of bacterial burdens and the degree of inflammation did not significantly differ in p38αΔMAC and p38αΔDC from control mice, indicating that p38α was not involved in innate immune cell-mediated host defense against C. rodentium infection (Fig. 1 B & C, and Supplemental Fig. S3 A & B). Involvement of p38α in macrophages or DCs against C. rodentium infection was further tested in vitro. Macrophages or BM-derived DCs were incubated with FITC-labeled C. rodentium, and phagocytic activity was measured by FACS analysis of the intracellular C. rodentium. Phagocytosis of C. rodentium was comparable between control, and p38α-deficient macrophages or DCs, indicating that p38α did not regulate the phagocytosis of C. rodentium by the innate immune cells (Supplemental Fig. S3 C & D). Next, we tested whether p38α deficiency affected TNF production by macrophages in C. rodentium infection. LPS-induced TNF-α production in p38α-deficient macrophages was significantly lower than the control cells, but production of TNF-α was comparable between control and p38α-deficient macrophages exposed to live C. rodentium or C. rodentium lysates, confirming that p38α is not a significant player in innate immune cells against C. rodentium infection (Supplemental Fig. S3E), supporting that p38α in macrophages or DCs was not involved in the regulation of host defense against the A/E pathogen infection.
Figure 1. p38α in T lymphocytes, not in macrophages or dendritic cells, plays an essential role in host defense against C. rodentium.
Bacterial loads in the colon tissues of tissue-specific p38α-deficient mice. Control (p38αfl/fl), or (A) T cell-specific (p38αΔT, n = 6), (B) macrophage- (p38αΔMAC, n = 6), or (C) DC-specific (p38αΔDC, n = 5-6) mice were orally inoculated with C. rodentium. Colon tissues were collected to measure C. rodentium CFU after 1, 2, 3 or 4 weeks after infection. *, p<0.05, and **, p<0.01. Error bars indicate s.d. The results shown are representative of 2-3 experiments.
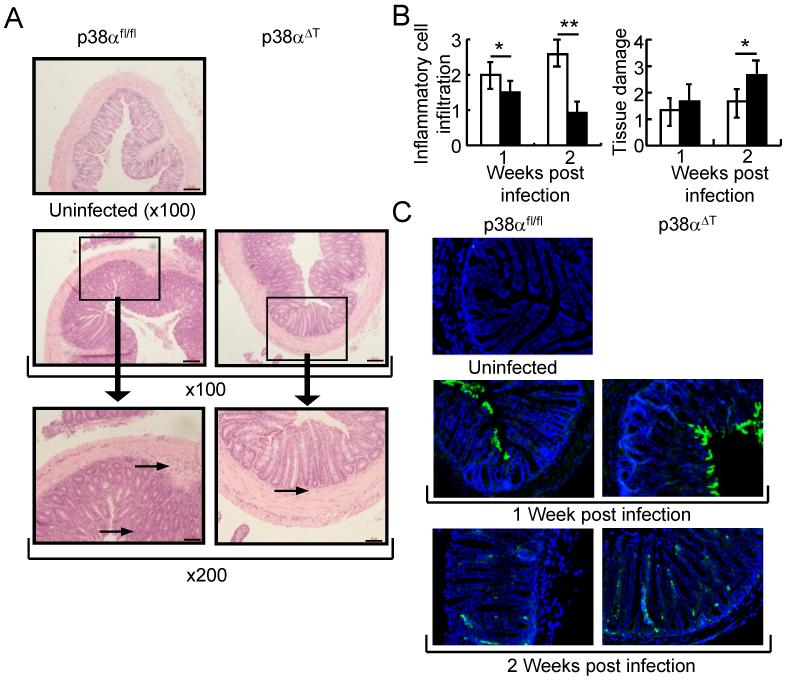
Hematoxylin/eosin staining and histological analysis of the C. rodentium-infected colon tissues revealed significantly more infiltrating lymphocytes in the tissue of p38αfl/fl mice compared to the p38αΔT mice, supporting the suggestion that the infiltrating immune cells play a primary role in C. rodentium clearance. The colonic mucosa was deregulated with more severe hyperplasia in p38αΔT mice, indicating that abrogation of p38α in T cells resulted in the impaired immune response against C. rodentium infection (Fig. 2 A & B and Supplemental Fig. S4). Immunostaining of colon tissues showed that more C. rodentium were localized in close proximity to the colonic epithelium in p38αΔT than in p38αfl/fl mice after 1 week of infection (Fig. 2C). At 2 weeks after infection, very little staining for C. rodentium on the colon surface was observed in p38αfl/fl mice, whereas more C. rodentium were found on the colonic surface of p38αΔT mice.
Figure 2. p38α in T lymphocytes regulates the bacterial clearance of C. rodentium-infected colon tissues.
(A) Inflammation in C. rodentium-infected mice. Colon tissues of C. rodentium-infected p38αfl/fl or p38αΔT mice were obtained after 1 week of infection and stained with hematoxylin and eosin. Boxed area is X200 of the original X100 magnification. Arrows in the figure indicate inflammatory cell infiltration. Scale bar = 20 μm. (B) Histological analysis of the colon tissues of C. rodentium-infected p38αfl/fl or p38αΔT mice. After 1 or 2 weeks of infection, histological scoring of the infiltration of inflammatory cells and tissue damage was assessed (n=6). *, p<0.05, and **, p<0.01. Error bars indicate s.d. (C) Detection of C. rodentium in the colon tissues. Colon tissues specimens of infected mice were stained with anti-C. rodentium antibody (green) and nuclei were counterstained with DAPI (blue). Inflammation and bacterial staining of colon tissue of uninfected mouse are shown. Original magnification X100.
p38α regulates the production of inflammatory cytokines and the recruitment of T cells
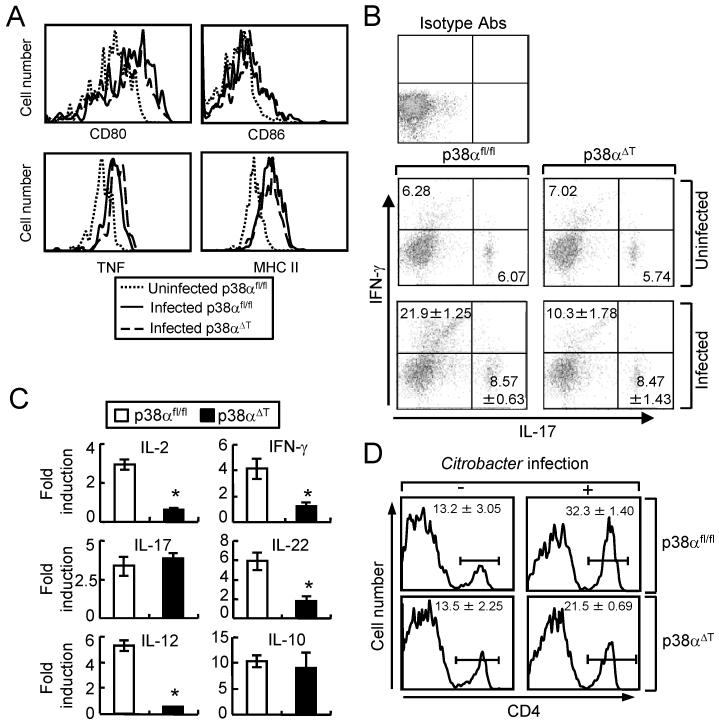
We further investigated the mechanism of p38α-mediated T cell responses against C. rodentium infection. Because DCs are on the front line of host defense against the bacterial infection, we tested whether the activation of DCs was affected in p38αΔT mice. DCs were obtained from p38αfl/fl and p38αΔT mice after 1 week of C. rodentium infection. No significant differences in the expression of surface markers like CD80, CD86 or MHC II, and production of the inflammatory cytokine TNF-α were observed in CD11c+ DCs in MLNs of p38αfl/fl and p38αΔT mice (Fig. 3A). Also, activation of DCs in LP was comparable between p38αfl/fl and p38αΔT mice (data now shown). These results indicated deletion of p38α in T cells did not overtly affect the function of DCs during C. rodentium infection.
Figure 3. p38α promotes the activation and recruitment of T cells to the site of C. rodentium infection.
(A) Activation of DC is not affected in p38αΔT mice. DCs from MLN of C. rodentium-infected p38αfl/fl or p38αΔT mice were obtained after 1 week of infection, and stained with the indicated antibodies for FACS analysis. DCs from uninfected p38αfl/fl were used as the control. (B) Inflammatory cytokine expression in LP T cells. LP lymphocytes from C. rodentium-infected p38αfl/fl or p38αΔT mice were obtained after 1 week of infection, and stained with anti-CD3-PerCP and anti-CD4-APC Abs. Cells were further permeabilized and stained with anti-IL-17A-FITC and anti-IFN-γ-PE Abs. LP T cells from uninfected mice were used for the staining of isotype antibodies. The numbers indicate the percentage of the cells. (C) The mRNAs levels of inflammatory cytokines of enriched T lymphocytes from LP were measured by the quantitative PCR. Cells were obtained from uninfected or infected mice after 1 week of C. rodentium infection, and total RNA was prepared for quantitative PCR. Values are shown as the fold changes of each gene from the cells of infected mice compared to the uninfected control. Actin level was measured as an internal control. *, p<0.05, and error bars indicate s.d. (D) Recruitment of T cells to the LP. LP lymphocytes were obtained from uninfected mice or mice after 1 week of infection, and the proportion of CD4 cells was analyzed by FACS analysis. Numbers indicate the percentage of CD4-positive cells. Data are representatives of 2-3 experiments.
We next examined the function of T cells in C. rodentium-infected p38αfl/fl and p38αΔT mice. Since the expression of IFN-γ and IL-17 is critical for the mucosal immunity against C. rodentium infection (6,11), the intracellular levels of IFN-γ and IL-17 in the intestine-associated LP T lymphocytes were examined by flow cytometry. Expression of IFN-γ was significantly reduced in CD4+ T cells of p38αΔT mice after infection compared with p38αfl/fl mice. However, abrogation of p38α in T cells did not affect the expression of IL-17, indicating that p38α regulates TH1 responses in bacterial infection (Fig. 3B). We further analyzed the expression of pro-inflammatory cytokines in LP lymphocytes in C. rodentium-infected p38αfl/fl and p38αΔT mice by quantitative PCR method. Consistent with the flow cytometry results, induction of IFN-γ was reduced in T cells from p38αΔT mice while that of IL-17 was similar between p38αfl/fl and p38αΔT mice (Fig. 3C). Inflammatory cytokines such as IL-2, IL-12, and IL-22 are important components of T cell-mediated host defense against the enteric bacterial infections. Expression levels of IL-2, IL-12, and IL-22 were significantly reduced, whereas IL-10 level was not affected in p38αΔT mice, indicating that p38α regulated the production of inflammatory cytokines by T cells that are known to provide host protection against C. rodentium infection. Since the infiltration of inflammatory cells was reduced in the colon tissues of p38αΔT mice (Fig. 2A and Supplemental Fig. S3), the recruitment of immune cells into the colonic mucosa was further tested. Flow cytometry analysis of CD4+ T cells in isolated LP lymphocytes showed that recruitment of CD4+ T cells into the colonic mucosa in p38αΔT mice was decreased (Fig. 3D), which indicated that p38α in T cells also affected the infiltration of CD4+ T cells to the site of C. rodentium infection. These results suggested that p38α in T cells regulated the activation and infiltration of T cells to protect the host from infection by A/E bacterial pathogens.
Intestinal epithelial cell function was affected by reduced T cell activation in C. rodentium-infected p38αΔT mice
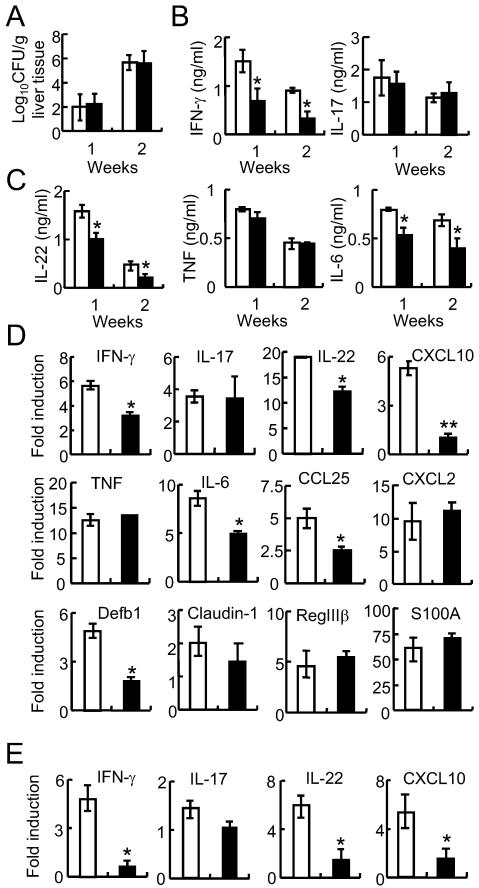
One of the functions of IECs is to maintain a protective barrier against luminal pathogens. The tight junctions between epithelial cells are known to play an important role in protecting against the translocation and escape of the enteric bacteria from the intestinal lumen (29). We tested the integrity of epithelial cells in p38αfl/fl and p38αΔT mice by measuring C. rodentium CFUs in liver tissues following infection (30). Bacterial counts in liver tissues were comparable between p38αfl/fl and p38αΔT mice after 1 or 2 weeks of infection (Fig. 4A), suggesting that IEC barrier function was not affected by reduced activation of T cells in p38αΔT mice.
Figure 4. Function of intestinal epithelial cells (IECs) was affected by reduced activation and recruitment of T cells in C. rodentium-infected p38αΔT mice.
(A) Epithelial cell integrity is normal. Liver tissues were obtained from C. rodentium-infected p38αfl/fl (□) or p38αΔT (■) mice after 1 or 2 weeks of infection, and C. rodentium CFU was measured. (B &C) Ex vivo culture of IECs to measure the production of inflammatory cytokines. Colon tissues of p38αfl/fl (□) or p38αΔT (■) mice were obtained after 1 week of C. rodentium infection, and incubated for 24 hours. Levels of cytokines in culture supernatants were measured by ELISA. (D & E) Expression of inflammation genes in the colon tissues (D) or purified IECs (E) of C. rodentium-infected p38αfl/fl (□) or p38αΔT (■) mice. Total RNAs were obtained from colon tissues or IECs after 1 week of infection. RNA samples were also prepared from uninfected mice as a control. Quantitative PCR analysis was performed to measure the expression of target genes. Values are shown as the fold changes of each gene in infected samples compared with the uninfected control. Actin level was measured to normalize the values. *, p<0.05, and error bars indicate s.d. Results are representatives of 2-3 experiments.
Next, we tested whether reduced T cell activation resulted in changes of mucosal defense function in p38αΔT mice by comparing the expression levels of pro-inflammatory cytokines, chemokines, and anti-microbial peptides in colon tissues. Ex vivo production of cytokines was measured by incubating colon tissue fragments from C. rodentium-infected mice, and we found that the levels of IFN-γ, IL-22, and IL-6 were lower in the colon of p38αΔT mice compared with control mice while IL-17 and TNF levels did not differ (Fig. 4, B & C). Expression of pro-inflammatory cytokines, chemokines and anti-microbial peptides from colonic tissues of C. rodentium-infected p38αfl/fl and p38αΔT mice was further examined by quantitative PCR analysis (Fig. 4D). The mRNA levels of inflammatory cytokines in the colonic tissues were similar; IFN-γ, IL-22, and IL-6 levels were lower in the colon of p38αΔT mice while IL-17 and TNF levels were comparable. We previously reported that p38α in IECs promotes the expression of chemokines such as CXCL10 and CCL25 that recruit T cells (24). In the colon tissue of p38αΔT mice, expression of CXCL10 and CCL25 was significantly reduced whereas CXCL2 levels were comparable (Fig. 4D). The expression of intestinal antimicrobial peptides did not differ between C. rodentium-infected p38αfl/fl and p38αΔT mice, except that expression of β-defensin 1 (Defb1) was significantly reduced in p38αΔT mice, indicating that Defb1 expression was regulated by inflammatory cytokines produced by T cells (Fig. 4D).
Induction of chemokine expression by IECs contributes to host defense against C. rodentium infection (4) and expression of chemokines in the intestinal mucosa regulates the recruitment of effector lymphocytes to the intestine (31,32). Therefore, we examined the expression of cytokines and chemokines by IECs of C. rodentium-infected p38αfl/fl and p38αΔT mice. Similar to the colon tissues, expression level of IFN-γ and IL-22 was significantly lower in the IECs of p38αΔT mice. Also, expression of CXCL10 was significantly reduced in IECs of p38αΔT mice (Fig. 4E), indicating that p38α-mediated T cell activation regulated the expression of inflammatory cytokines and chemokines in the IECs of C. rodentium-infected mice, which further recruited T cells to the site of bacterial infection. These results suggested that p38α-mediated T cell activation limited the A/E bacterial burden by promoting defense mechanisms within the intestinal mucosa.
Treatment of IFN-γ activates the host defense against C. rodentium infection in vivo
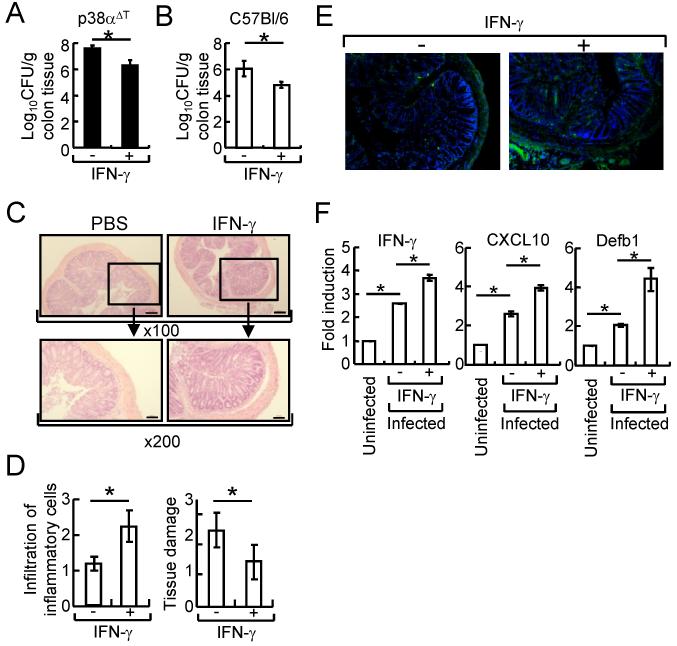
Given the results that reduced expression of inflammatory cytokines such as IFN-γ by p38α-deficient T cells resulted in the impaired host defense against C. rodentium infection, we tested the recovery/promotion of host defense by IFN-γ treatment in C. rodentium infection. First, we examined whether INF-γ administration recovered the reduced host defense activity of p38αΔT mice. Bacterial counts in colon tissues were lower and the development of transmissible colonic hyperplasia was less significant in IFN-γ-treated p38αΔT mice compared with the mice treated with PBS (Fig. 5A, and data not shown), indicating that IFN-γ recovered the reduced host defense against the enteric bacterial infection that was impaired by p38α deletion in T cells. We further evaluated whether administration of IFN-γ enhanced the immune response of C. rodentium-infected wildtype mice. The bacterial CFU was significantly lower in the colon tissues by IFN-γ administration (Fig. 5 B). Also, treatment of IFN-γ increased the recruitment of inflammatory cells and ameliorated the colonic tissue damage (Fig. 5 C & D). Recruitment of T cell to the site of bacterial infection and the expression of some essential genes for host defense were significantly up-regulated in the C. rodentium-infected wildtype mice treated with IFN-γ (Fig. 5 E&F), suggesting that administration of IFN-γ activated the mucosal immune response against C. rodentium infection.
Figure 5. Administration of IFN-γ enhanced host defense activity of C. rodentium-infected mice.
(A) p38αΔT mice were orally inoculated with C. rodentium, and intraperitoneally injected with IFN-γ (10 μg/mouse) or PBS on days 0, 2, and 4 after infection. C. rodentium CFU in colon tissues was measured after 10 days. (B-F) The same as (A) except wildtype mice were used and colon tissues were obtained after 10 days. Bacterial counts in colon tissues were measured (B). (C & D) H & E staining (C) and histological scoring of the infiltration of inflammatory cells and tissue damage (D, n=6) was assessed. Boxed area is X200 of the original X100 magnification. Scale bar = 20 μm. (E) Recruitment of T cells was examined by immunostaining using anti-CD4 Ab (green). Nuclei were counterstained with DAPI (blue). Original magnification X100. (F) Expression of IFN-γ, CXCL10, and Defb1 in IECs. Actin level was used as an internal normalization control, and fold induction of each gene was compared to that of uninfected mice. *, p<0.05, and error bars indicate s.d. Representative results of 2-3 experiments are shown.
In this study, we showed that p38α was essential for the T cell-mediated immune response against C. rodentium infection. Although p38α has been previously reported to play an important role in innate immune responses against many different types of microbial infections, we found that activation of p38α did not limit C. rodentium infection in macrophages and dendritic cells. In line with the role of p38α in IECs regulating the recruitment of T cells, our current study suggests that p38α regulates T cell- and IEC-mediated host defense against A/E pathogen infection.
Discussion
NF-κB and p38α MAPK signaling pathways are critical in the development of host defense against pathogenic enteric bacterial infections. The NF-κB pathway is essential for maintaining immune homeostasis in IECs, as abrogation of NF-κB signaling within IECs dramatically impaired mucosal immune responses, dysregulated IEC integrity, and led to the subsequent failure to clear bacterial pathogen burdens (12,33,34). Using IEC-specific p38α-deficient mice, we previously showed that p38α in IECs plays a protective role in host defense against C. rodentium infection by recruiting T cells to the site of infection while the immune functions were not affected (24). In this study, we demonstrated that mice lacking p38α in T cells, but not in macrophages or DCs, failed to clear C. rodentium infection, indicating that p38α regulates the adaptive immunity to limit the degree of A/E pathogen infection. In TLR-mediated innate immune responses, p38α regulates the activation of inflammatory signaling pathways (17). TLR2 is required in maintaining mucosal integrity and MyD88-mediated signaling pathway is essential for a protective innate immunity by neutrophils in C. rodentium infection (4,7). However, TLR4 deficiency showed a delayed spread and colonization of C. rodentium, indicating that TLR4-mediated responses against this A/E pathogen are not host protective despite that C. rodentium is a Gram-negative bacterium (35). Although p38α in IECs is important, p38α does not significantly affect the functions of macrophages and DCs against C. rodentium infection.
Abrogation of p38α in T cells resulted in impaired pathogen clearance from colon tissues due to the reduced production of inflammatory cytokines by T cells. While the involvement of TH1 and TH17 T cells is a critical process in host defense against C. rodentium infection (3,11,36-38), TH1 responses such as IFN-γ production were regulated by p38α in T cells whereas IL-17 expression was unaffected by the deletion of p38α. However, the role of p38α in TH17 differentiation is arguable. While studies using pharmacological and dominant-negative approaches showed the role of p38a in T cell–intrinsic IL-17 expression (22,39,40), a recent report excluded the involvement of p38 signaling in TH17 differentiation (41). The underlying mechanism of the selective cytokine expression by p38α is unclear. Since activation of STAT1 is involved in IFN-γ production and STAT3 regulates the expression of IL-17 in TCR-mediated activation (42), the selective phosphorylation of STATs by p38α may regulate the cytokine production. We observed that phosphorylation of STAT1 was reduced in p38α-deficient T cells while STAT3 activation was not affected (unpublished data), suggesting that p38α may control the activation of STAT1, but not STAT3 in T cell-mediated host defense against C. rodentium infection.
Production of inflammatory cytokines and recruitment of T cells are important for host defense against C. rodentium infection (3,6,43,44). C. rodentium infection induces the expression of inflammatory cytokines, chemokines and anti-microbial peptides that participate in the host defense mediated by IECs against C. rodentium. In IECs, p38α regulates the expression of chemokines such as CXCL10 and CCL25 that are important for the recruitment of T cells to the site of infection to protect the host (24,31). In the IECs of p38αΔT mice, the expression of several cytokines and chemokines was reduced, most notably CXCL-10, IFN-γ, and IL-22. IFN-γ and IL-22 are known to induce the expression of CXCL10 and CCL25 in IECs, both of which are involved in the attraction of immune cells (45,46). Importantly, similar cytokines and chemokines were down-regulated in IECs of C. rodentium-infected p38αΔIEC and p38αΔT mice, indicating their significance in host defense against A/E pathogen infection for both cell types. Therefore, it is suggested that p38α plays a prominent role in both T cells and IECs against C. rodentium infection by promoting the production of pro-inflammatory cytokines and chemokines.
Production of IFN-γ was significantly reduced in C. rodentium-infected p38αΔT mice and the frequency of T cells in the colonic mucosa was lower, indicating that p38α regulates not only cytokine production but also recruitment of T cells to the site of infection by regulating the expression of chemokines by the IECs. Impaired bacterial clearance was recovered by IFN-γ administration in C. rodentium-infected p38αΔT mice, indicating the role of IFN-γ in mucosal immunity against the enteric bacterial infection. Also, administration of IFN-γ increased the expression of CXCL10 to enhance the host defense mechanism of C. rodentium-infected wildtype mice by recruiting T cells to the site of bacterial infection. Expression of IFN-γ and Defb1 was also increased, indicating that IFN-γ activated the mucosal immune response to protect a host from the enteric bacterial infection. Our data has suggested that a strategy that activates the host mucosal immunity can be utilized as a treatment of A/E pathogen infection. Treatment of the A/E pathogen infection such as E. coli O157:H7 is limited to the replacement of fluids and electrolytes to prevent dehydration since antibiotics may increase the chance of developing Hemolytic Uremic Syndrome (HUS), a potentially fatal complication caused by Shiga Toxin-mediated kidney failure (47). Therefore, activation of host mucosal immune response such as targeting the p38α signaling can be utilized as a potential future method for the treatment of A/E infections.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institutes of Health (AI088229) to Y. J. K. and by grants from the Crohn’s and Colitis Foundation of Canada (CCFC) and the Canadian Institutes of Health Research (CIHR) to B.A.V. M.S. was funded by a CIHR/CCFC fellowship. J.H. was supported by NSF China grant number 30830092.
References
- 1.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 2.Bry L, Brenner MB. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J. Immunol. 2004;172:433–441. doi: 10.4049/jimmunol.172.1.433. [DOI] [PubMed] [Google Scholar]
- 3.Bry L, Brigl M, Brenner MB. CD4+-T-cell effector functions and costimulatory requirements essential for surviving mucosal infection with Citrobacter rodentium. Infect. Immun. 2006;74:673–681. doi: 10.1128/IAI.74.1.673-681.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J. Immunol. 2007;179:566–577. doi: 10.4049/jimmunol.179.1.566. [DOI] [PubMed] [Google Scholar]
- 5.Maaser C, Housley MP, Iimura M, Smith JR, Vallance BA, FInlay BB, Schreiber JR, Varki NM, Kagnoff MF, Eckmann L. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect. Immun. 2004;72:3315–3324. doi: 10.1128/IAI.72.6.3315-3324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons CP, Goncalves NS, Ghaem-Maghami M, Bajaj-Elliott M, Clare S, Neves B, Frankel G, Dougan G, MacDonald TT. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-gamma. J. Immunol. 2002;168:1804–1812. doi: 10.4049/jimmunol.168.4.1804. [DOI] [PubMed] [Google Scholar]
- 7.Gibson DL, Ma C, Rosenberger CM, Bergstrom KS, Valdez Y, Huang JT, Khan MA, Vallance BA. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell Microbiol. 2008;10:388–403. doi: 10.1111/j.1462-5822.2007.01052.x. [DOI] [PubMed] [Google Scholar]
- 8.Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le BL, Cho JH, Robertson SJ, Kim CJ, Kaul R, Philpott DJ, Girardin SE. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat. Med. 2011;17:837–844. doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- 9.Kim YG, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, Nunez G. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity. 2011;34:769–780. doi: 10.1016/j.immuni.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDonald TT, Frankel G, Dougan G, Goncalves NS, Simmons C. Host defences to Citrobacter rodentium. Int. J. Med. Microbiol. 2003;293:87–93. doi: 10.1078/1438-4221-00247. [DOI] [PubMed] [Google Scholar]
- 11.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 12.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 13.Goncalves NS, Ghaem-Maghami M, Monteleone G, Frankel G, Dougan G, Lewis DJ, Simmons CP, MacDonald TT. Critical role for tumor necrosis factor alpha in controlling the number of lumenal pathogenic bacteria and immunopathology in infectious colitis. Infect. Immun. 2001;69:6651–6659. doi: 10.1128/IAI.69.11.6651-6659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 15.Rincon M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol. Rev. 2009;228:212–224. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 16.Cook R, Wu CC, Kang YJ, Han J. The role of the p38 pathway in adaptive immunity. Cell Mol. Immunol. 2007;4:253–259. [PubMed] [Google Scholar]
- 17.Kang YJ, Chen J, Otsuka M, Mols J, Ren S, Wang Y, Han J. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J. Immunol. 2008;180:5075–5082. doi: 10.4049/jimmunol.180.7.5075. [DOI] [PubMed] [Google Scholar]
- 18.Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, Lawrence T, Otsu K, Brissette JL, Arthur JS, Park JM. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat. Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritprajak P, Hayakawa M, Sano Y, Otsu K, Park JM. Cell type-specific targeting dissociates the therapeutic from the adverse effects of protein kinase inhibition in allergic skin disease. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9089–9094. doi: 10.1073/pnas.1202984109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otsuka M, Kang YJ, Ren J, Jiang H, Wang Y, Omata M, Han J. Distinct effects of p38alpha deletion in myeloid lineage and gut epithelia in mouse models of inflammatory bowel disease. Gastroenterology. 2010;138:1255–65. doi: 10.1053/j.gastro.2010.01.005. 1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rincon M, Enslen H, Raingeaud J, Recht M, Zapton T, Su MS, Penix LA, Davis RJ, Flavell RA. Interferon-gamma expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 1998;17:2817–29. doi: 10.1093/emboj/17.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noubade R, Krementsov DN, Del RR, Thornton T, Nagaleekar V, Saligrama N, Spitzack A, Spach K, Sabio G, Davis RJ, Rincon M, Teuscher C. Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood. 2011;118:3290–3300. doi: 10.1182/blood-2011-02-336552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jirmanova L, Giardino Torchia ML, Sarma ND, Mittelstadt PR, Ashwell JD. Lack of the T cell-specific alternative p38 activation pathway reduces autoimmunity and inflammation. Blood. 2011;118:3280–3289. doi: 10.1182/blood-2011-01-333039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang YJ, Otsuka M, van den Berg A, Hong L, Huang Z, Wu X, Zhang DW, Vallance BA, Tobias PS, Han J. Epithelial p38alpha controls immune cell recruitment in the colonic mucosa. PLoS. Pathog. 2010;6:e1000934. doi: 10.1371/journal.ppat.1000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CC, Louie S, McCormick B, Walker WA, Shi HN. Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice. Infect. Immun. 2005;73:5468–5481. doi: 10.1128/IAI.73.9.5468-5481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Vallance BA, Boyer L, Bergstrom KS, Walker J, Madsen K, O’Kusky JR, Buchan AM, Jacobson K. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am. J. Physiol Gastrointest. Liver Physiol. 2008;294:G295–G306. doi: 10.1152/ajpgi.00173.2007. [DOI] [PubMed] [Google Scholar]
- 27.Masuda A, Yoshida M, Shiomi H, Ikezawa S, Takagawa T, Tanaka H, Chinzei R, Ishida T, Morita Y, Kutsumi H, Inokuchi H, Wang S, Kobayashi K, Mizuno S, Nakamura A, Takai T, Blumberg RS, Azuma T. Fcgamma receptor regulation of Citrobacter rodentium infection. Infect. Immun. 2008;76:1728–1737. doi: 10.1128/IAI.01493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jirmanova L, Sarma DN, Jankovic D, Mittelstadt PR, Ashwell JD. Genetic disruption of p38alpha Tyr323 phosphorylation prevents T-cell receptor-mediated p38alpha activation and impairs interferon-gamma production. Blood. 2009;113:2229–2237. doi: 10.1182/blood-2008-04-153304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 30.Guma M, Stepniak D, Shaked H, Spehlmann ME, Shenouda S, Cheroutre H, Vicente-Suarez I, Eckmann L, Kagnoff MF, Karin M. Constitutive intestinal NF-kappaB does not trigger destructive inflammation unless accompanied by MAPK activation. J. Exp. Med. 2011;208:1889–1900. doi: 10.1084/jem.20110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell DJ, Butcher EC. Intestinal attraction: CCL25 functions in effector lymphocyte recruitment to the small intestine. J. Clin. Invest. 2002;110:1079–1081. doi: 10.1172/JCI16946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008;29:514–522. doi: 10.1016/j.it.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Dennis A, Kudo T, Kruidenier L, Girard F, Crepin VF, MacDonald TT, Frankel G, Wiles S. The p50 subunit of NF-kappaB is critical for in vivo clearance of the noninvasive enteric pathogen Citrobacter rodentium. Infect. Immun. 2008;76:4978–4988. doi: 10.1128/IAI.00736-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nenci A, Becker C, Wullaert A, Gareus R, Van LG, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 35.Khan MA, Ma C, Knodler LA, Valdez Y, Rosenberger CM, Deng W, FInlay BB, Vallance BA. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect. Immun. 2006;74:2522–2536. doi: 10.1128/IAI.74.5.2522-2536.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins LM, Frankel G, Douce G, Dougan G, MacDonald TT. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect. Immun. 1999;67:3031–3039. doi: 10.1128/iai.67.6.3031-3039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spahn TW, Ross M, von EC, Maaser C, Spieker T, Kannengiesser K, Domschke W, Kucharzik T. CD4+ T cells transfer resistance against Citrobacter rodentium-induced infectious colitis by induction of Th 1 immunity. Scand. J. Immunol. 2008;67:238–244. doi: 10.1111/j.1365-3083.2007.02063.x. [DOI] [PubMed] [Google Scholar]
- 38.Symonds EL, Riedel CU, O’Mahony D, Lapthorne S, O’Mahony L, Shanahan F. Involvement of T helper type 17 and regulatory T cell activity in Citrobacter rodentium invasion and inflammatory damage. Clin. Exp. Immunol. 2009;157:148–154. doi: 10.1111/j.1365-2249.2009.03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulen MF, Kang Z, Bulek K, Youzhong W, Kim TW, Chen Y, Altuntas CZ, Sass Bak-Jensen K, McGeachy MJ, Do JS, Xiao H, Delgoffe GM, Min B, Powell JD, Tuohy VK, Cua DJ, Li X. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32:54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Kim J, Boussiotis VA. IL-1beta-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells. J. Immunol. 2010;185:4148–4153. doi: 10.4049/jimmunol.1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang G, Wang Y, Vogel P, Kanneganti TD, Otsu K, Chi H. Signaling via the kinase p38alpha programs dendritic cells to drive TH17 differentiation and autoimmune inflammation. Nat. Immunol. 2012;13:152–161. doi: 10.1038/ni.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 43.Shiomi H, Masuda A, Nishiumi S, Nishida M, Takagawa T, Shiomi Y, Kutsumi H, Blumberg RS, Azuma T, Yoshida M. Gamma interferon produced by antigen-specific CD4+ T cells regulates the mucosal immune responses to Citrobacter rodentium infection. Infect. Immun. 2010;78:2653–2666. doi: 10.1128/IAI.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubino SJ, Geddes K, Girardin SE. Innate IL-17 and IL-22 responses to enteric bacterial pathogens. Trends Immunol. 2012;33:112–118. doi: 10.1016/j.it.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Lacroix-Lamande S, Mancassola R, Naciri M, Laurent F. Role of gamma interferon in chemokine expression in the ileum of mice and in a murine intestinal epithelial cell line after Cryptosporidium parvum infection. Infect. Immun. 2002;70:2090–2099. doi: 10.1128/IAI.70.4.2090-2099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, Leclair S, Herrmann K, Seiderer J, Ochsenkuhn T, Goke B, Auernhammer CJ, Dambacher J. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am. J. Physiol Gastrointest. Liver Physiol. 2006;290:G827–G838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 47.Serna A, Boedeker EC. Pathogenesis and treatment of Shiga toxin-producing Escherichia coli infections. Curr. Opin. Gastroenterol. 2008;24:38–47. doi: 10.1097/MOG.0b013e3282f2dfb8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.