Abstract
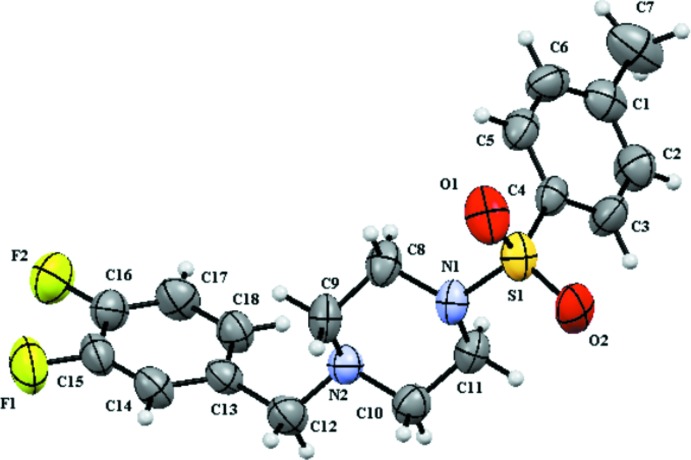
In the title compound, C18H20F2N2O2S, the central piperazine ring adopts a chair conformation. The dihedral angle between the two benzene rings is 40.20°, whereas those between the piperazine ring (considering the best fit plane through all the non-H atoms) and the sulfonyl-bound benzene and difluorobenzene rings are 74.96 and 86.16°, respectively. In the crystal, molecules are stacked along the a axis through weak C—H⋯O and C—H⋯F interactions.
Related literature
For similar structures, see: Sreenivasa et al. (2013a
▶,b
▶,c
▶).
Experimental
Crystal data
C18H20F2N2O2S
M r = 366.42
Monoclinic,
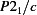
a = 6.6680 (2) Å
b = 36.0404 (8) Å
c = 7.6093 (2) Å
β = 99.728 (2)°
V = 1802.35 (8) Å3
Z = 4
Mo Kα radiation
μ = 0.21 mm−1
T = 298 K
0.28 × 0.24 × 0.20 mm
Data collection
Bruker APEXII diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.943, T max = 0.959
9583 measured reflections
2434 independent reflections
1910 reflections with I > 2σ(I)
R int = 0.025
θmax = 22.8°
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.114
S = 1.02
2434 reflections
227 parameters
H-atom parameters constrained
Δρmax = 0.19 e Å−3
Δρmin = −0.25 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: APEX2 and SAINT-Plus (Bruker, 2009 ▶); data reduction: SAINT-Plus and XPREP (Bruker, 2009 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813016462/sj5330sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813016462/sj5330Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813016462/sj5330Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯O1i | 0.93 | 2.67 | 3.380 (4) | 134 |
| C7—H7A⋯O1ii | 0.96 | 2.66 | 3.400 (4) | 134 |
| C10—H10B⋯F1iii | 0.97 | 2.66 | 3.585 (3) | 160 |
Symmetry codes: (i) 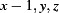 ; (ii)
; (ii) 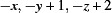 ; (iii)
; (iii) 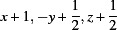 .
.
Acknowledgments
The authors thank Dr S. C. Sharma, Ex-Vice Chancellor, Tumkur University, for his constant encouragement. JT also thanks the DST, New Delhi, for the SCXRD facility under the PURSE Grant (SR/S9/Z-23/2008/11, 2009) at USIC, Karnatak University.
supplementary crystallographic information
Comment
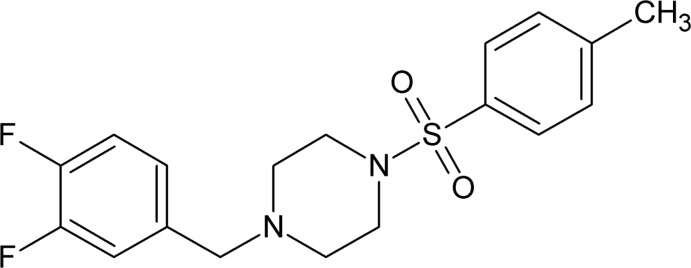
As a part of our continued efforts to study the crystal structures of N-(aryl)(4-tosylpiperazin-1-yl)methanone derivatives (Sreenivasa et al., 2013a,b,c), we report herein the crystal structure of the title compound.
The title compound, Fig. 1, crystallizes in the monoclinic crystal system and P21/c space group. The piperazine ring in the title compound adopts a chair conformation. The dihedral angle between the two benzene rings is 40.20°, compared to the observed dihedral angles of 72.2 (12)°, 76.86° and 30.97 (2)° respectively in (I), 1-(2,4-dichlorobenzyl)-4-[(4-methyl-phenyl)sulfonyl]-piperazine (Sreenivasa et al., 2013a), (II) 1-tosyl-4-[2-(trifluoromethyl)-benzyl]piperazine (Sreenivasa et al., 2013b) and (III) (2,3-difluorophenyl)(4-tosylpiperazin-1-yl)methanone (Sreenivasa et al., 2013c). Further, the dihedral angles between the piperazine ring (considering the best fit plane through all the non-hydrogen atoms) and the sulfonyl bound benzene and difluorobenzene rings are 74.96° and 86.16° respectively, compared to 74.16 (2)° and 2.44 (13)° in I, 74.36° and 68.29 (3)° in II, and 69.4 (2)° and 75.98 (2)° in III.
In the crystal structure, the molecules are stacked along the a axis through weak C–H···O and C–H···F interactions, Fig. 2.
Experimental
A mixture of 1-tosylpiperazine (0.01 mmol), potassium carbonate (0.03 mmol) and 3,4-difluorobenzyl bromide (0.01 mmol) was added to dry acetonitrile (5 ml). The mixture was stirred at 85°C for 8 h. The reaction was monitored by TLC. Solvent was removed by vacuum distillation and the crude product obtained was purified by column chromatography using 230–400 silica gel and petroleum ether/ethyl acetate as eluent.
Colourless prisms were obtained from a mixture of dichloromethane/methanol (7:3) by slow evaporation.
Refinement
H atoms were positioned with idealized geometry using a riding model with C—H = 0.93 - 0.96 Å. The isotropic displacement parameters for all H atoms were set to 1.2 times Ueq of the parent atom or 1.5 times that of the parent atom for CH3.
Crystals were small and very weakly diffracting, with no significant data obtained beyond θ = 22.8° hence the low values of sin(θ/λ). However the structure solved and refined satisfactorily and gave acceptable residuals and su values.
Figures
Fig. 1.
Molecular structure of the title compound, showing the atom-labeling scheme. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
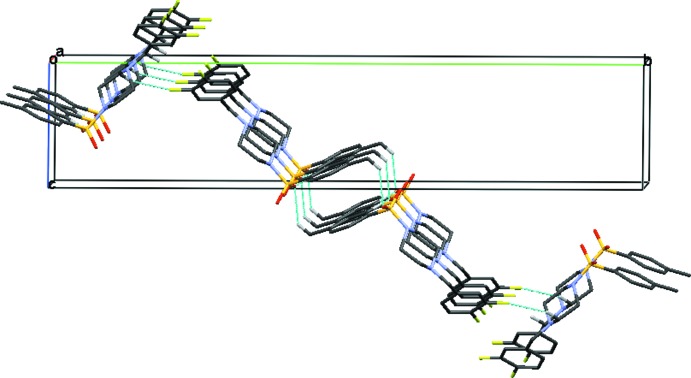
A packing diagram of the title compound, showing C–H···O and C–H···F interactions (dotted lines). Hydrogen atoms not involved in hydrogen bonding are omitted.
Crystal data
| C18H20F2N2O2S | prism |
| Mr = 366.42 | Dx = 1.350 Mg m−3 |
| Monoclinic, P21/c | Melting point: 501 K |
| Hall symbol: -P 2ybc | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.6680 (2) Å | Cell parameters from 227 reflections |
| b = 36.0404 (8) Å | θ = 2.3–22.8° |
| c = 7.6093 (2) Å | µ = 0.21 mm−1 |
| β = 99.728 (2)° | T = 298 K |
| V = 1802.35 (8) Å3 | Prism, colourless |
| Z = 4 | 0.28 × 0.24 × 0.20 mm |
| F(000) = 768 |
Data collection
| Bruker APEXII diffractometer | 2434 independent reflections |
| Radiation source: fine-focus sealed tube | 1910 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.025 |
| Detector resolution: 1.03 pixels mm-1 | θmax = 22.8°, θmin = 2.3° |
| φ and ω scans | h = −7→7 |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | k = −39→37 |
| Tmin = 0.943, Tmax = 0.959 | l = −7→8 |
| 9583 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.045 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.114 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0512P)2 + 0.6421P] where P = (Fo2 + 2Fc2)/3 |
| 2434 reflections | (Δ/σ)max = 0.028 |
| 227 parameters | Δρmax = 0.19 e Å−3 |
| 0 restraints | Δρmin = −0.25 e Å−3 |
| 32 constraints |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | −0.3654 (5) | 0.51803 (9) | 0.7226 (4) | 0.0763 (8) | |
| C2 | −0.4516 (5) | 0.48860 (11) | 0.7956 (4) | 0.0850 (9) | |
| H2 | −0.5880 | 0.4901 | 0.8070 | 0.102* | |
| C3 | −0.3451 (5) | 0.45692 (9) | 0.8529 (4) | 0.0761 (8) | |
| H3 | −0.4088 | 0.4376 | 0.9028 | 0.091* | |
| C4 | −0.1454 (4) | 0.45409 (7) | 0.8359 (3) | 0.0598 (7) | |
| C5 | −0.0564 (4) | 0.48283 (9) | 0.7599 (4) | 0.0821 (9) | |
| H5 | 0.0790 | 0.4811 | 0.7455 | 0.099* | |
| C6 | −0.1666 (6) | 0.51438 (9) | 0.7045 (4) | 0.0870 (9) | |
| H6 | −0.1034 | 0.5336 | 0.6535 | 0.104* | |
| C7 | −0.4821 (6) | 0.55287 (10) | 0.6662 (5) | 0.1135 (13) | |
| H7A | −0.4283 | 0.5729 | 0.7431 | 0.170* | |
| H7B | −0.6229 | 0.5492 | 0.6742 | 0.170* | |
| H7C | −0.4697 | 0.5587 | 0.5455 | 0.170* | |
| C8 | −0.2454 (4) | 0.36712 (8) | 0.6955 (4) | 0.0737 (8) | |
| H8A | −0.3419 | 0.3850 | 0.6347 | 0.088* | |
| H8B | −0.2926 | 0.3592 | 0.8033 | 0.088* | |
| C9 | −0.2310 (5) | 0.33415 (7) | 0.5761 (4) | 0.0738 (8) | |
| H9A | −0.1406 | 0.3157 | 0.6400 | 0.089* | |
| H9B | −0.3644 | 0.3230 | 0.5431 | 0.089* | |
| C10 | 0.0484 (4) | 0.36109 (8) | 0.4655 (4) | 0.0734 (8) | |
| H10A | 0.1008 | 0.3680 | 0.3587 | 0.088* | |
| H10B | 0.1392 | 0.3427 | 0.5292 | 0.088* | |
| C11 | 0.0404 (4) | 0.39451 (7) | 0.5809 (4) | 0.0680 (7) | |
| H11A | 0.1762 | 0.4046 | 0.6154 | 0.082* | |
| H11B | −0.0443 | 0.4134 | 0.5148 | 0.082* | |
| C12 | −0.1536 (5) | 0.31498 (8) | 0.2910 (4) | 0.0801 (9) | |
| H12A | −0.0854 | 0.2938 | 0.3529 | 0.096* | |
| H12B | −0.0773 | 0.3224 | 0.1990 | 0.096* | |
| C13 | −0.3660 (4) | 0.30386 (8) | 0.2051 (3) | 0.0629 (7) | |
| C14 | −0.4280 (5) | 0.26741 (8) | 0.2015 (4) | 0.0737 (8) | |
| H14 | −0.3405 | 0.2492 | 0.2572 | 0.088* | |
| C15 | −0.6186 (5) | 0.25787 (8) | 0.1158 (4) | 0.0759 (8) | |
| C16 | −0.7479 (5) | 0.28422 (10) | 0.0346 (4) | 0.0772 (8) | |
| C17 | −0.6903 (5) | 0.32020 (9) | 0.0356 (4) | 0.0834 (9) | |
| H17 | −0.7788 | 0.3381 | −0.0212 | 0.100* | |
| C18 | −0.4993 (5) | 0.33006 (8) | 0.1215 (4) | 0.0729 (8) | |
| H18 | −0.4596 | 0.3548 | 0.1231 | 0.088* | |
| N1 | −0.0432 (3) | 0.38434 (6) | 0.7410 (3) | 0.0646 (6) | |
| N2 | −0.1548 (3) | 0.34542 (6) | 0.4167 (3) | 0.0627 (6) | |
| O1 | 0.2056 (3) | 0.42331 (6) | 0.9324 (3) | 0.0918 (7) | |
| O2 | −0.0916 (4) | 0.39768 (6) | 1.0463 (2) | 0.0989 (7) | |
| F1 | −0.6798 (3) | 0.22224 (5) | 0.1108 (3) | 0.1225 (8) | |
| F2 | −0.9357 (3) | 0.27392 (6) | −0.0489 (3) | 0.1200 (7) | |
| S1 | −0.00612 (12) | 0.41390 (2) | 0.90583 (9) | 0.0734 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.080 (2) | 0.089 (2) | 0.0536 (17) | 0.0093 (19) | −0.0063 (15) | −0.0117 (15) |
| C2 | 0.0544 (19) | 0.114 (3) | 0.086 (2) | 0.001 (2) | 0.0093 (16) | −0.022 (2) |
| C3 | 0.074 (2) | 0.087 (2) | 0.0703 (19) | −0.0205 (18) | 0.0190 (15) | −0.0075 (16) |
| C4 | 0.0567 (18) | 0.0677 (18) | 0.0527 (15) | −0.0136 (13) | 0.0024 (12) | −0.0043 (13) |
| C5 | 0.0567 (18) | 0.077 (2) | 0.114 (3) | −0.0079 (16) | 0.0178 (17) | 0.0131 (18) |
| C6 | 0.092 (3) | 0.071 (2) | 0.099 (2) | −0.0020 (18) | 0.0179 (19) | 0.0170 (17) |
| C7 | 0.128 (3) | 0.116 (3) | 0.082 (2) | 0.047 (2) | −0.023 (2) | −0.014 (2) |
| C8 | 0.082 (2) | 0.079 (2) | 0.0601 (17) | −0.0242 (16) | 0.0110 (15) | 0.0036 (15) |
| C9 | 0.084 (2) | 0.0610 (17) | 0.0711 (19) | −0.0216 (15) | −0.0014 (15) | 0.0047 (14) |
| C10 | 0.0626 (19) | 0.076 (2) | 0.0795 (19) | −0.0024 (15) | 0.0056 (14) | 0.0011 (16) |
| C11 | 0.0652 (18) | 0.0661 (18) | 0.0705 (18) | −0.0125 (14) | 0.0051 (14) | 0.0079 (14) |
| C12 | 0.073 (2) | 0.077 (2) | 0.086 (2) | 0.0074 (16) | 0.0020 (16) | −0.0156 (17) |
| C13 | 0.0687 (19) | 0.0607 (18) | 0.0582 (16) | 0.0072 (15) | 0.0069 (13) | −0.0111 (13) |
| C14 | 0.086 (2) | 0.0618 (19) | 0.0689 (18) | 0.0114 (16) | 0.0011 (16) | −0.0066 (14) |
| C15 | 0.093 (2) | 0.0558 (19) | 0.080 (2) | −0.0109 (18) | 0.0167 (18) | −0.0134 (15) |
| C16 | 0.066 (2) | 0.087 (2) | 0.075 (2) | −0.0040 (18) | −0.0003 (15) | −0.0177 (17) |
| C17 | 0.082 (2) | 0.079 (2) | 0.083 (2) | 0.0152 (18) | −0.0043 (17) | −0.0005 (17) |
| C18 | 0.078 (2) | 0.0592 (18) | 0.0782 (19) | 0.0012 (15) | 0.0042 (16) | −0.0011 (15) |
| N1 | 0.0693 (15) | 0.0594 (13) | 0.0599 (13) | −0.0137 (11) | −0.0038 (11) | 0.0045 (10) |
| N2 | 0.0631 (15) | 0.0628 (14) | 0.0602 (13) | −0.0042 (11) | 0.0047 (11) | −0.0009 (11) |
| O1 | 0.0685 (14) | 0.0894 (15) | 0.1015 (16) | −0.0033 (11) | −0.0311 (11) | −0.0102 (12) |
| O2 | 0.148 (2) | 0.0899 (15) | 0.0536 (12) | −0.0194 (13) | 0.0032 (12) | 0.0131 (11) |
| F1 | 0.1360 (18) | 0.0734 (13) | 0.154 (2) | −0.0270 (12) | 0.0134 (14) | −0.0162 (12) |
| F2 | 0.0804 (14) | 0.1292 (17) | 0.1398 (18) | −0.0121 (11) | −0.0119 (12) | −0.0334 (13) |
| S1 | 0.0845 (6) | 0.0688 (5) | 0.0590 (5) | −0.0099 (4) | −0.0110 (4) | 0.0051 (4) |
Geometric parameters (Å, º)
| C1—C6 | 1.362 (4) | C10—H10A | 0.9700 |
| C1—C2 | 1.368 (4) | C10—H10B | 0.9700 |
| C1—C7 | 1.501 (4) | C11—N1 | 1.469 (3) |
| C2—C3 | 1.376 (4) | C11—H11A | 0.9700 |
| C2—H2 | 0.9300 | C11—H11B | 0.9700 |
| C3—C4 | 1.363 (4) | C12—N2 | 1.457 (3) |
| C3—H3 | 0.9300 | C12—C13 | 1.510 (4) |
| C4—C5 | 1.369 (4) | C12—H12A | 0.9700 |
| C4—S1 | 1.754 (3) | C12—H12B | 0.9700 |
| C5—C6 | 1.381 (4) | C13—C14 | 1.376 (4) |
| C5—H5 | 0.9300 | C13—C18 | 1.377 (4) |
| C6—H6 | 0.9300 | C14—C15 | 1.371 (4) |
| C7—H7A | 0.9600 | C14—H14 | 0.9300 |
| C7—H7B | 0.9600 | C15—F1 | 1.346 (3) |
| C7—H7C | 0.9600 | C15—C16 | 1.359 (4) |
| C8—N1 | 1.472 (3) | C16—C17 | 1.352 (4) |
| C8—C9 | 1.508 (4) | C16—F2 | 1.356 (3) |
| C8—H8A | 0.9700 | C17—C18 | 1.377 (4) |
| C8—H8B | 0.9700 | C17—H17 | 0.9300 |
| C9—N2 | 1.450 (3) | C18—H18 | 0.9300 |
| C9—H9A | 0.9700 | N1—S1 | 1.632 (2) |
| C9—H9B | 0.9700 | O1—S1 | 1.432 (2) |
| C10—N2 | 1.457 (3) | O2—S1 | 1.419 (2) |
| C10—C11 | 1.497 (4) | ||
| C6—C1—C2 | 116.7 (3) | N1—C11—C10 | 110.0 (2) |
| C6—C1—C7 | 121.2 (3) | N1—C11—H11A | 109.7 |
| C2—C1—C7 | 122.1 (3) | C10—C11—H11A | 109.7 |
| C1—C2—C3 | 122.8 (3) | N1—C11—H11B | 109.7 |
| C1—C2—H2 | 118.6 | C10—C11—H11B | 109.7 |
| C3—C2—H2 | 118.6 | H11A—C11—H11B | 108.2 |
| C4—C3—C2 | 119.5 (3) | N2—C12—C13 | 112.0 (2) |
| C4—C3—H3 | 120.3 | N2—C12—H12A | 109.2 |
| C2—C3—H3 | 120.3 | C13—C12—H12A | 109.2 |
| C3—C4—C5 | 118.9 (3) | N2—C12—H12B | 109.2 |
| C3—C4—S1 | 120.5 (2) | C13—C12—H12B | 109.2 |
| C5—C4—S1 | 120.5 (2) | H12A—C12—H12B | 107.9 |
| C4—C5—C6 | 120.4 (3) | C14—C13—C18 | 118.5 (3) |
| C4—C5—H5 | 119.8 | C14—C13—C12 | 121.2 (3) |
| C6—C5—H5 | 119.8 | C18—C13—C12 | 120.2 (3) |
| C1—C6—C5 | 121.7 (3) | C15—C14—C13 | 120.0 (3) |
| C1—C6—H6 | 119.2 | C15—C14—H14 | 120.0 |
| C5—C6—H6 | 119.2 | C13—C14—H14 | 120.0 |
| C1—C7—H7A | 109.5 | F1—C15—C16 | 119.2 (3) |
| C1—C7—H7B | 109.5 | F1—C15—C14 | 120.3 (3) |
| H7A—C7—H7B | 109.5 | C16—C15—C14 | 120.5 (3) |
| C1—C7—H7C | 109.5 | C17—C16—F2 | 120.3 (3) |
| H7A—C7—H7C | 109.5 | C17—C16—C15 | 120.6 (3) |
| H7B—C7—H7C | 109.5 | F2—C16—C15 | 119.1 (3) |
| N1—C8—C9 | 109.0 (2) | C16—C17—C18 | 119.3 (3) |
| N1—C8—H8A | 109.9 | C16—C17—H17 | 120.3 |
| C9—C8—H8A | 109.9 | C18—C17—H17 | 120.3 |
| N1—C8—H8B | 109.9 | C17—C18—C13 | 121.0 (3) |
| C9—C8—H8B | 109.9 | C17—C18—H18 | 119.5 |
| H8A—C8—H8B | 108.3 | C13—C18—H18 | 119.5 |
| N2—C9—C8 | 110.5 (2) | C11—N1—C8 | 111.8 (2) |
| N2—C9—H9A | 109.6 | C11—N1—S1 | 116.43 (17) |
| C8—C9—H9A | 109.6 | C8—N1—S1 | 118.03 (18) |
| N2—C9—H9B | 109.6 | C9—N2—C12 | 112.3 (2) |
| C8—C9—H9B | 109.6 | C9—N2—C10 | 109.7 (2) |
| H9A—C9—H9B | 108.1 | C12—N2—C10 | 110.5 (2) |
| N2—C10—C11 | 109.7 (2) | O2—S1—O1 | 120.16 (13) |
| N2—C10—H10A | 109.7 | O2—S1—N1 | 106.38 (12) |
| C11—C10—H10A | 109.7 | O1—S1—N1 | 106.30 (13) |
| N2—C10—H10B | 109.7 | O2—S1—C4 | 108.02 (14) |
| C11—C10—H10B | 109.7 | O1—S1—C4 | 107.81 (12) |
| H10A—C10—H10B | 108.2 | N1—S1—C4 | 107.58 (11) |
| C6—C1—C2—C3 | 1.5 (5) | C14—C13—C18—C17 | −0.1 (4) |
| C7—C1—C2—C3 | −178.0 (3) | C12—C13—C18—C17 | 177.0 (3) |
| C1—C2—C3—C4 | −0.6 (4) | C10—C11—N1—C8 | −56.5 (3) |
| C2—C3—C4—C5 | −0.8 (4) | C10—C11—N1—S1 | 163.77 (18) |
| C2—C3—C4—S1 | −179.4 (2) | C9—C8—N1—C11 | 55.7 (3) |
| C3—C4—C5—C6 | 1.2 (4) | C9—C8—N1—S1 | −165.23 (18) |
| S1—C4—C5—C6 | 179.8 (2) | C8—C9—N2—C12 | −175.5 (2) |
| C2—C1—C6—C5 | −1.1 (5) | C8—C9—N2—C10 | 61.1 (3) |
| C7—C1—C6—C5 | 178.4 (3) | C13—C12—N2—C9 | 70.2 (3) |
| C4—C5—C6—C1 | −0.2 (5) | C13—C12—N2—C10 | −166.9 (2) |
| N1—C8—C9—N2 | −57.8 (3) | C11—C10—N2—C9 | −60.8 (3) |
| N2—C10—C11—N1 | 58.1 (3) | C11—C10—N2—C12 | 174.7 (2) |
| N2—C12—C13—C14 | −129.4 (3) | C11—N1—S1—O2 | −177.66 (19) |
| N2—C12—C13—C18 | 53.5 (4) | C8—N1—S1—O2 | 45.1 (2) |
| C18—C13—C14—C15 | 0.0 (4) | C11—N1—S1—O1 | −48.5 (2) |
| C12—C13—C14—C15 | −177.1 (3) | C8—N1—S1—O1 | 174.31 (19) |
| C13—C14—C15—F1 | 179.6 (3) | C11—N1—S1—C4 | 66.8 (2) |
| C13—C14—C15—C16 | −0.3 (4) | C8—N1—S1—C4 | −70.4 (2) |
| F1—C15—C16—C17 | −179.3 (3) | C3—C4—S1—O2 | −28.3 (3) |
| C14—C15—C16—C17 | 0.6 (5) | C5—C4—S1—O2 | 153.0 (2) |
| F1—C15—C16—F2 | 0.2 (4) | C3—C4—S1—O1 | −159.6 (2) |
| C14—C15—C16—F2 | −179.9 (3) | C5—C4—S1—O1 | 21.8 (3) |
| F2—C16—C17—C18 | 179.8 (3) | C3—C4—S1—N1 | 86.1 (2) |
| C15—C16—C17—C18 | −0.7 (5) | C5—C4—S1—N1 | −92.5 (2) |
| C16—C17—C18—C13 | 0.4 (5) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···O1i | 0.93 | 2.67 | 3.380 (4) | 134 |
| C7—H7A···O1ii | 0.96 | 2.66 | 3.400 (4) | 134 |
| C10—H10B···F1iii | 0.97 | 2.66 | 3.585 (3) | 160 |
Symmetry codes: (i) x−1, y, z; (ii) −x, −y+1, −z+2; (iii) x+1, −y+1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ5330).
References
- Bruker (2009). APEX2, SADABS, SAINT-Plus and XPREP Bruker AXS Inc., Madison, Wisconsin, USA.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sreenivasa, S., Anitha, H. C., ManojKumar, K. E., Tonannavar, J., Jayashree, Y., Suchetan, P. A. & Palakshamurthy, B. S. (2013a). Acta Cryst. E69, o239. [DOI] [PMC free article] [PubMed]
- Sreenivasa, S., ManojKumar, K. E., Anitha, H. C., Suchetan, P. A., Palakshamurthy, B. S., Jayashree, Y. & Tonannavar, J. (2013c). Acta Cryst. E69, o782. [DOI] [PMC free article] [PubMed]
- Sreenivasa, S., ManojKumar, K. E., Suchetan, P. A., Tonannavar, J., Chavan, Y. & Palakshamurthy, B. S. (2013b). Acta Cryst. E69, o185. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813016462/sj5330sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813016462/sj5330Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813016462/sj5330Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report